Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste
Abstract
1. Introduction
| Product | Substrate | Inoculum | Reactor | Operation Condition | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Biohydrogen | Synthetic food waste | Clostridium acetobutulicum | Batch fermentation reactor (working volume: 1000 mL) | Temperature: 32 ± 1 °C; pH: 6.85; addition of Fe3O4 (100 mg) and TiO2 (50 mg) | Volume: 3392 mL; yield: 213.66 mL H2/g VS | [35] |
| Biohythane | Synthetic organic wastewater | Anaerobic sludge | The reactor: a feeding tank; two anaerobic reactors (R1 (1.5 L): hydrogen producing reactor, R2 (1.5 L): methane-producing reactor); two gas collectors; a final effluent tank | Sudden shock load phase: OLR: 25.0 g COD/L/d (R1); 15.7 g COD/L/d (R2) | 1.641 mol H2/mol glucose; methane production rate: 1.003 L CH4/L/d | [5] |
| Lactic acid | Coffee waste | Lactiplantibacillus plantarum WiKim0126 | Deman, rogosa and sharpe (MRS) broth | Pretreatment with hydrogen peroxide and acetic acid, along with a combination of Viscozyme L, Celluclast 1.5 L, and Pectinex Ultra SP-L. Lactiplantibacillus plantarum WiKim0126 induced fermentation with a 4% solid loading | Concentration: 22.8 g/L; productivity: 0.95 g/L/h within 24 h | [18] |
| Canteen FW | Enterococcus mundtii CGMCC 22227 | Fermenters with a working volume of 400 mL | Pretreatment of food waste: Glycosylase (2 g/kg FW mixture) was used for saccharification; reactor pH: 6.8 | Concentration: 115 g/L; yield: 0.97 g LA/g total sugar | [19] | |
| Modelled FW | Mixture of commercial yoghurt and solid compost from an industrial platform | Fed-batch fermentation at a pilot scale (12 L) | Temperature: 35 °C; pH: 5; Organic loading rate (OLR): 25 g TS/L/d for the first four days | Concentration: 68 g/L; yield: 0.38 g LA/g TS | [36] | |
| Canteen FW | Indigenous microbiota | Batch fermentation bioreactor (working volume: 5 L) | pH: 6; temperature: 25 °C; mechanically stirred: 120 rpm; TS of FW: 7% | Yield: 0.46 g LA/g TS | [6] | |
| VFAs | Vegetable waste | Anaerobic sludge | Continuous stirred tank reactors (working volume: 1 L) | Hydraulic retention time (HRT): 20 d; OLR: 3 g VS/L/d; a minor pH: 5.8 | VFAs concentration: 29.6 ± 2.1 g/L (47.0 ± 2.1 g COD/L); bioconversion yield: 49.2 ± 2.0% | [37] |
| VFAs and biohythane | Canteen FW | Anaerobic digestion sludge | Microbial electrolysis cell and anaerobic reactor (MEC-AR): working volume: 1 L, the two electrodes: carbon rod (8 × 3 × 50 mm), six square carbon felts (50 × 50 × 5 mm) | Applied voltage: 1.2 V; HRT: 10 d; pH: 5.04 ± 0.10 | Biohythane yield: 2.73 ± 0.05 L/L/d (with 34.80 ± 1.09% CH4, 14.95 ± 0.86% H2); VFAs concentration: 19.39 ± 0.44 g COD/L | [7] |
| MCCAs | Canteen FW | Indigenous microbiota | Batch fermentation (total volume: 500 mL) | VS of reactor FW: 75 g/L; pH: 6 temperature: 37 °C | Caproic acid concentration: 88.24 mM | [38] |
| FW from FW treatment plant | Acclimated pit mud obtained from a CE reactor | Continuous stirred tank reactor (working volume: 1 L) | pH: FW was adjusted to pH 6.0 ± 0.5 before feeding, reactor no control; OLR: 9.24 g COD/L/d; HRT: 20 d | MCCA concentration: 29,886.10 mg COD/L; caproic acid concentration: 28,191.66 mg COD/L | [23] |
2. Materials and Methods
2.1. Materials
2.2. Effects of pH on Food Waste Fermentation
2.3. Effects of Amino Acid Additions on Food Waste Fermentation
2.4. Analytical Methods
2.5. Calculation of the Hydrolysis Rate
2.6. Statistical Analysis of Data
3. Results and Discussion
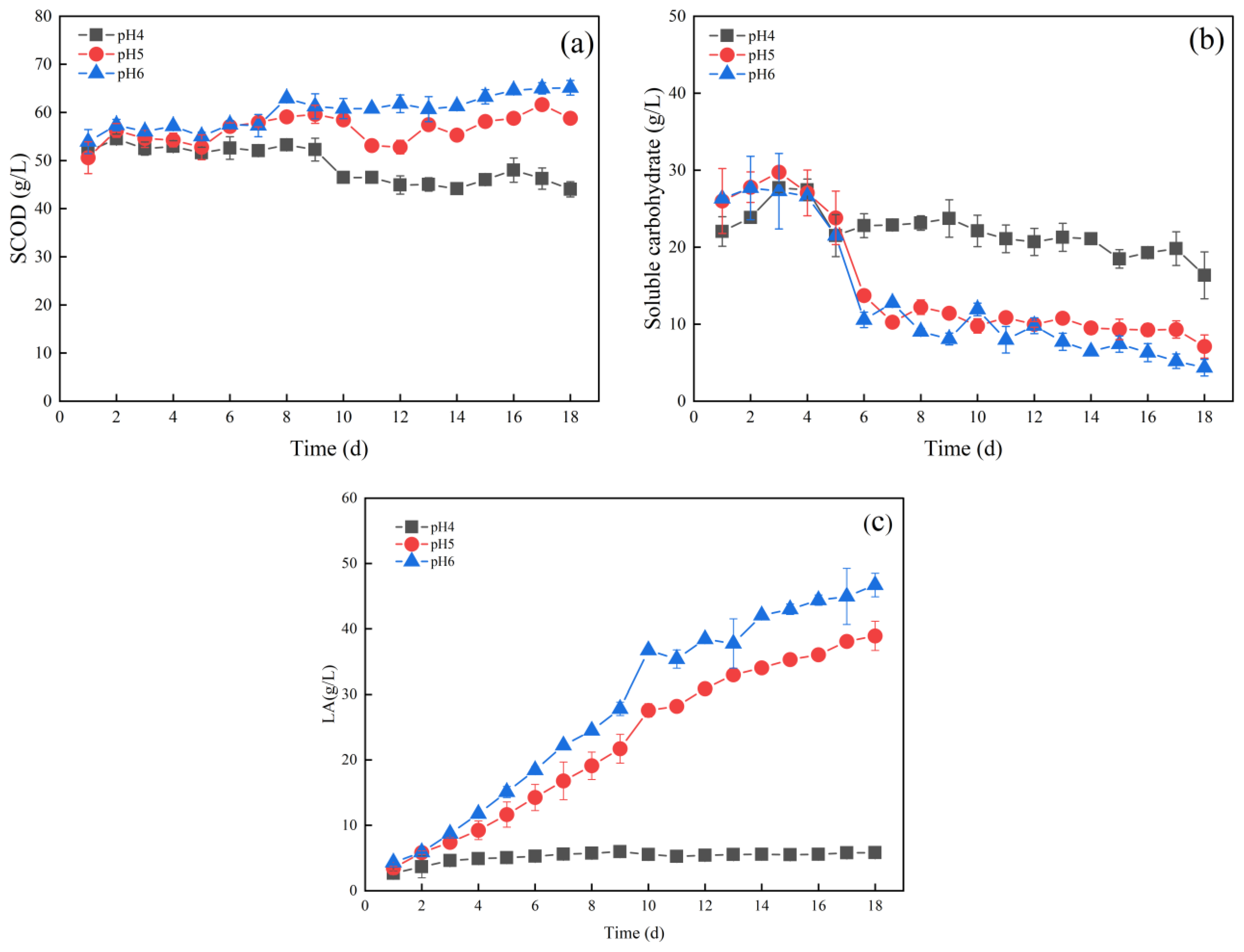
3.1. Effects of pH on Lactic Acid Production from Fermentation of Food Waste
3.1.1. Effect of pH on Hydrolysis and Acid Production
3.1.2. Variations of Amino Acids during Fermentation at pH 5.0
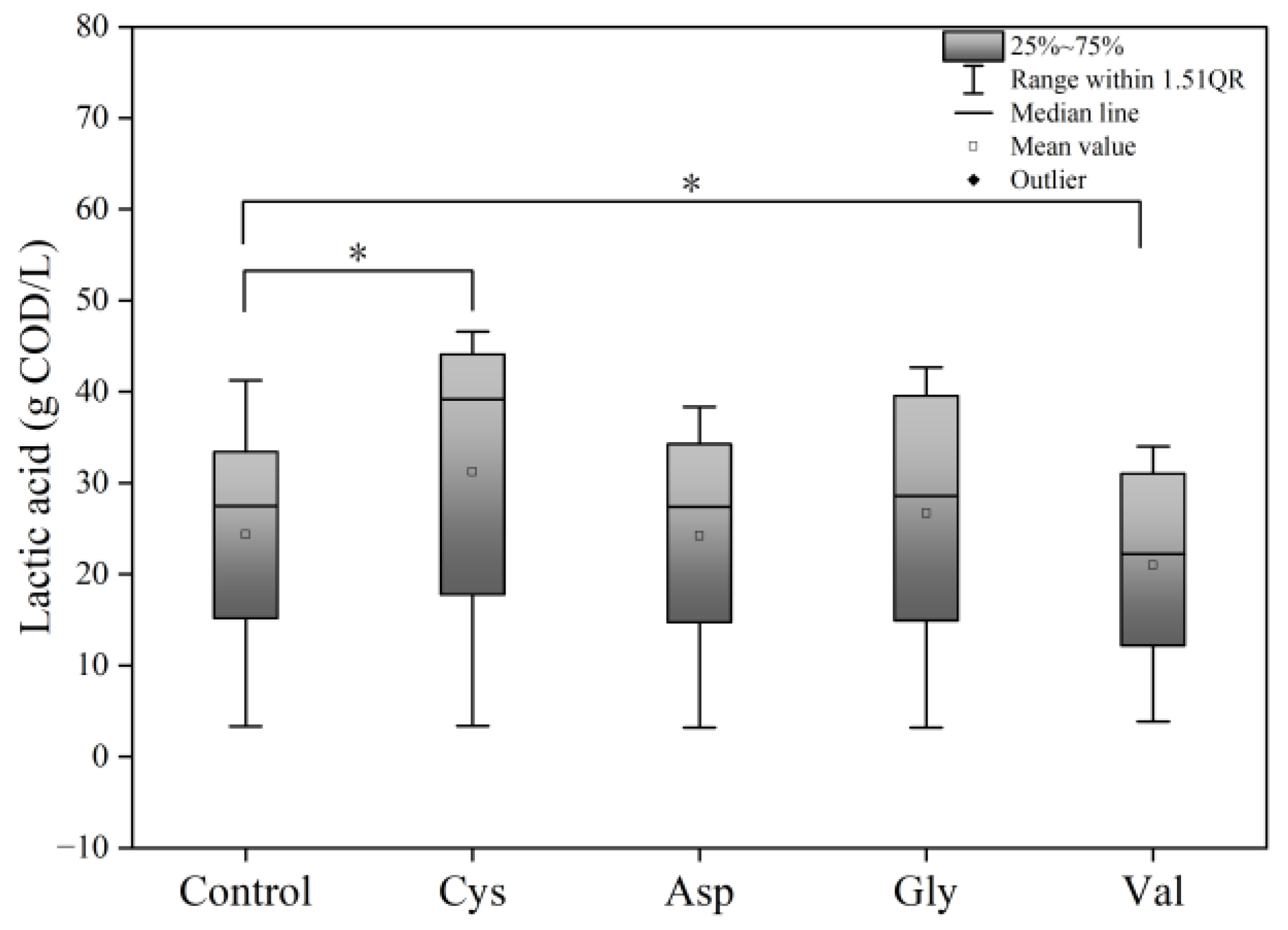
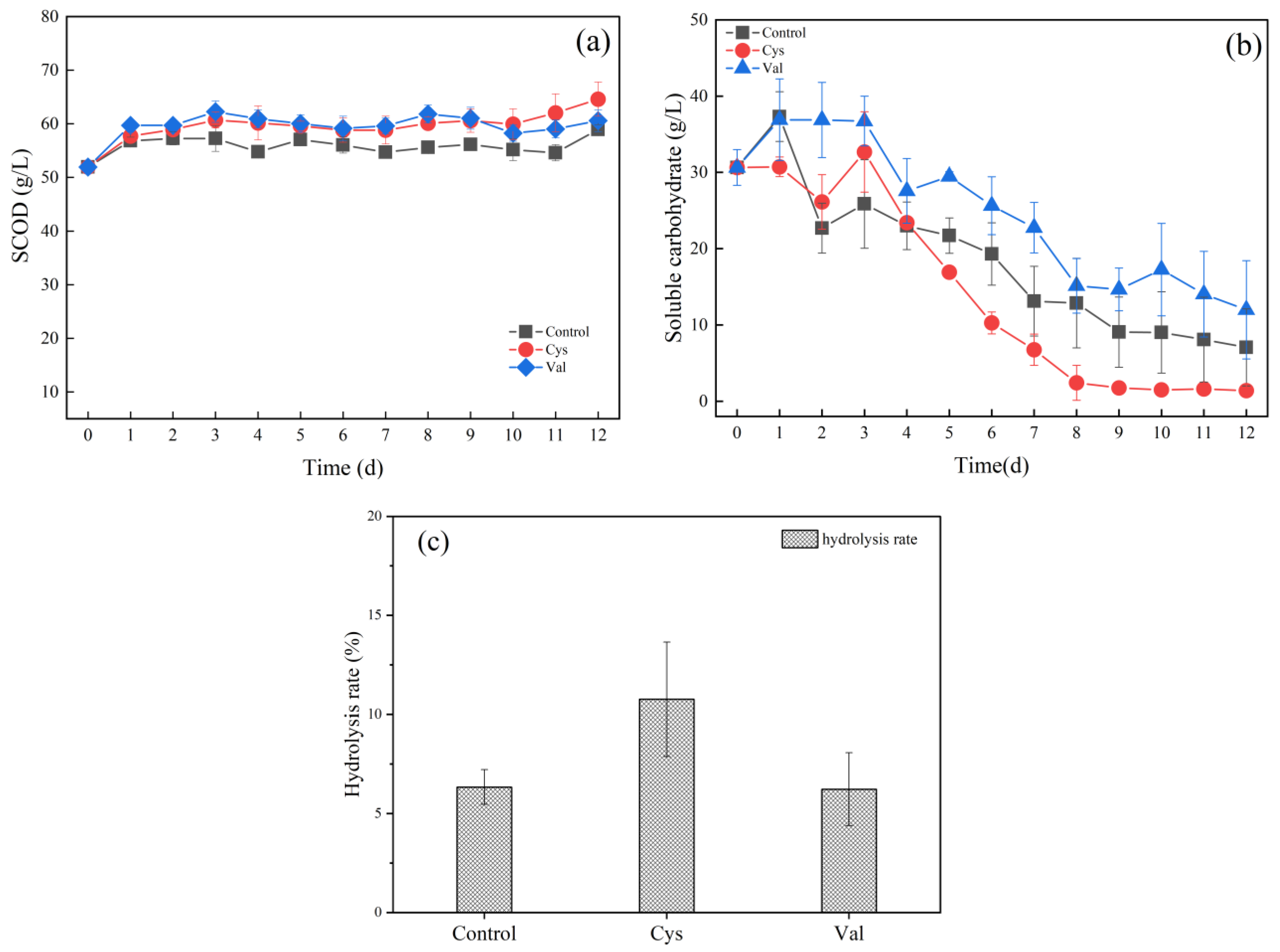
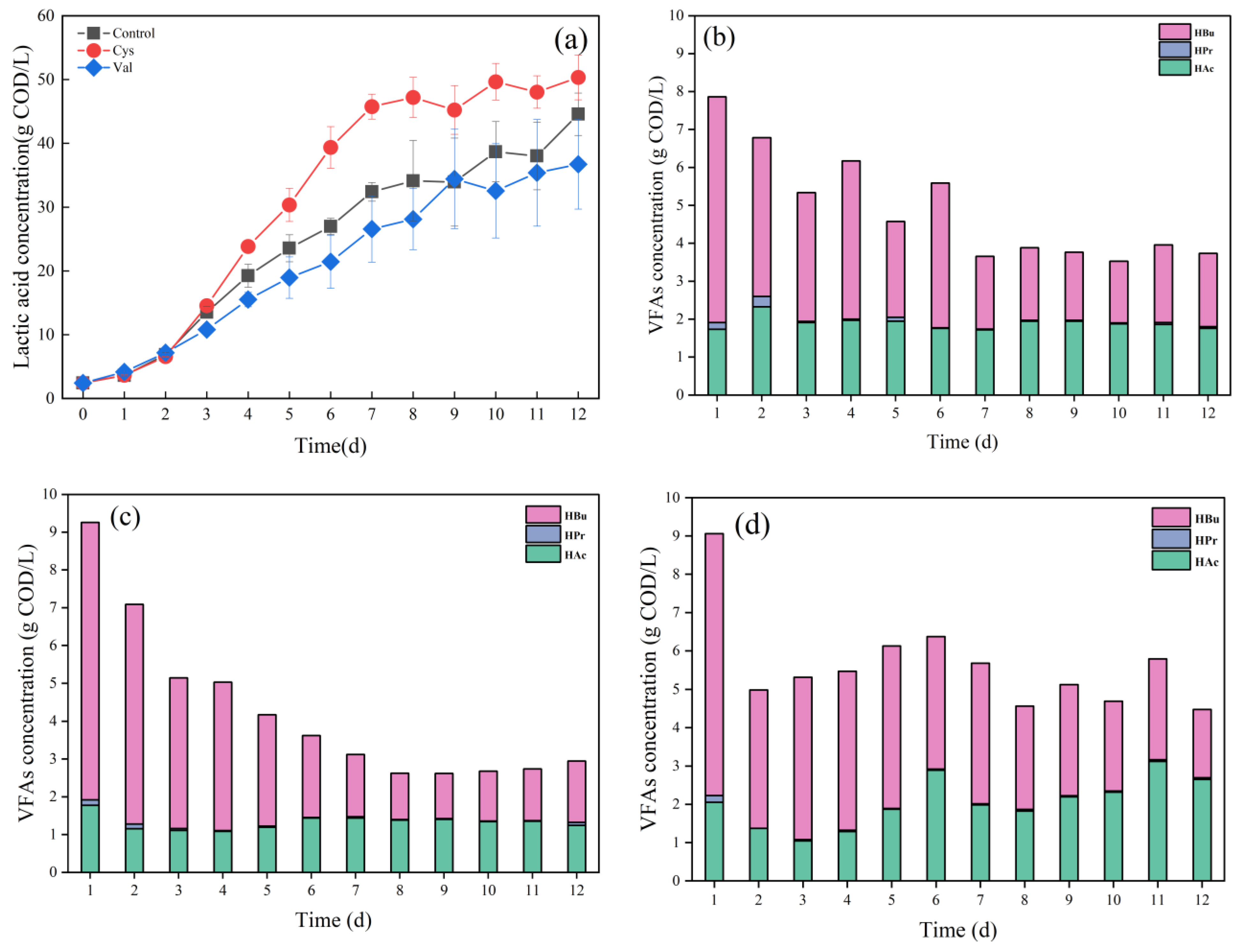
3.2. Effects of Adding Different Amino Acids on Food Waste Fermentation
3.2.1. Effects of Different Amino Acids on Hydrolysis
3.2.2. Effects of Different Amino Acids on Acidification
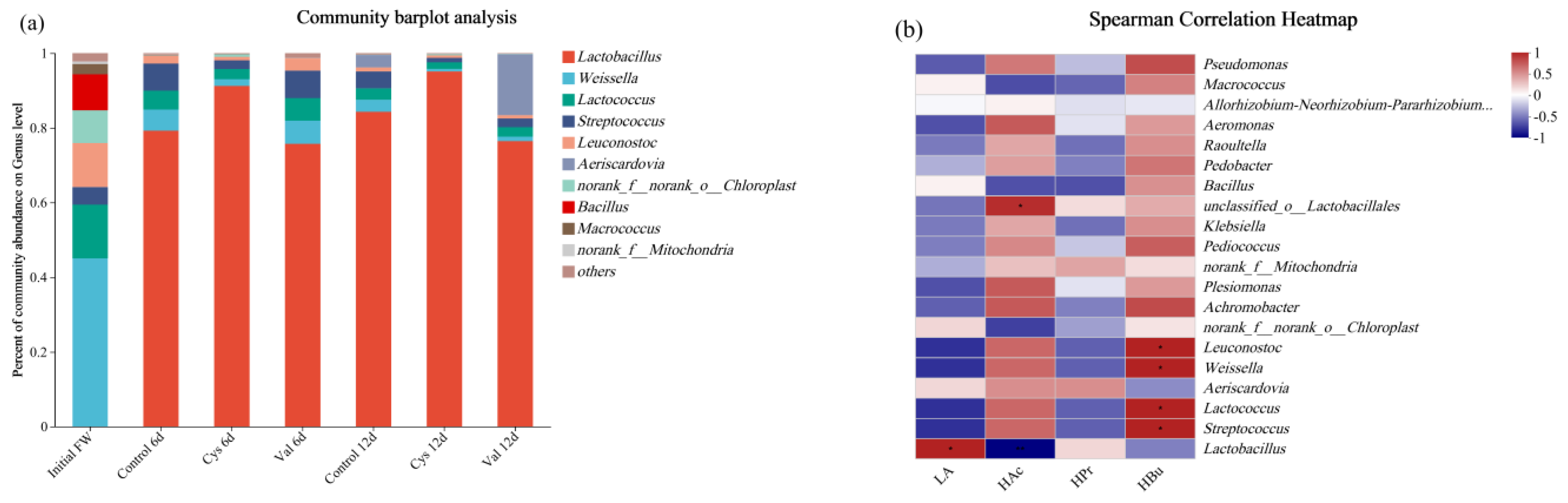
3.2.3. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Li, X.; He, Y.; Xu, X.; Chen, H.; Zhang, A.; Liu, Y.; Xue, G.; Makinia, J. Ammonia amendment promotes high rate lactate production and recovery from semi-continuous food waste fermentation. Bioresour. Technol. 2020, 302, 122881. [Google Scholar] [CrossRef]
- Zhou, M.M.; Yan, B.H.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, K.; Li, H. Nano iron materials enhance food waste fermentation. Bioresour. Technol. 2020, 315, 123804. [Google Scholar] [CrossRef] [PubMed]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Selecting fermentation products for food waste valorisation with HRT and OLR as the key operational parameters. Waste Manag. 2021, 127, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wirasembada, Y.C.; Shin, B.; Shin, J.; Kurniawan, A.; Cho, J. Effects of sudden shock load on simultaneous biohythane production in two-stage anerobic digestion of high-strength organic wastewater. Bioresour. Technol. 2024, 394, 130186. [Google Scholar] [CrossRef]
- Tang, J.L.; Wang, X.C.; Hu, Y.S.; Zhang, Y.M.; Li, Y.Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.L.; Chen, X.Y.; Wang, J.; Xiao, B.Y.; Liu, R.Z.; Li, L.; Liu, J.G. Simultaneous biohythane and volatile fatty acids production from food waste in microbial electrolysis cell-assisted acidogenic reactor. J. Clean. Prod. 2023, 420, 138370. [Google Scholar] [CrossRef]
- Sunhoon, K.; Pyung, C.L.; Eun, G.L.; Yong, K.C.; Nam, C. Production of lactic acid by Lactobacillusrhamnosus with vitamin-supplemented soybean hydrolysate. Enzym. Microb. Technol. 2000, 26, 209–215. [Google Scholar]
- Daly, S.E.; Usack, J.G.; Harroff, L.A.; Booth, J.G.; Keleman, M.P.; Angenent, L.T. Systematic Analysis of Factors That Affect Food-Waste Storage: Toward Maximizing Lactate Accumulation for Resource Recovery. ACS Sustain. Chem. Eng. 2020, 8, 13934–13944. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Li, C.; Wang, N.; Wang, Q.; Sun, X.; Ma, X. Effects of different lignocellulosic wastes on alleviating acidification of L-lactic acid production from food waste fermentation. Bioresour. Technol. 2021, 342, 126043. [Google Scholar] [CrossRef]
- Ye, T.; Li, X.; Zhang, T.; Su, Y.; Zhang, W.; Li, J.; Gan, Y.; Zhang, A.; Liu, Y.; Xue, G. Copper (II) addition to accelerate lactic acid production from co-fermentation of food waste and waste activated sludge: Understanding of the corresponding metabolisms, microbial community and predictive functional profiling. Waste Manag. 2018, 76, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.X.; Cui, M.; Wang, B.; Zhang, C.H.; Zhu, Y.L.; Jin, X.; Wang, R.; Zhang, X.Q. Electrospun Environment-Friendly Poly (L-Lactic Acid)/CO2-Based Polyurea Nanofiber Film for Piezoelectric Sensor. Adv. Sustain. Syst. 2023, 7, 2200441. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Gu, X.; Guo, Z.; Song, J.; Zhu, D.; Liu, Y.; Liu, Y.; Xue, G.; Makinia, J. Stabilizing lactate production through repeated batch fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 300, 122709. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Y.; Feng, H.R.; Guo, G.; Huo, W.J.; Li, Q.H.; Xu, Q.F.; Liu, Q.; Wang, C.; Chen, L. Effects of laccase and lactic acid bacteria on the fermentation quality, nutrient composition, enzymatic hydrolysis, and bacterial community of alfalfa silage. Front. Microbiol. 2022, 13, 1035942. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, H.; Zheng, C.Z. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chuan, S.; Wu, Y.Y.; Han, D.; Li, K.; Ma, J.Y. Effect of pH dynamic control on ethanol-lactic type fermentation (ELTF) performance of glucose. Environ. Technol. 2022, 43, 4102–4114. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Ma, H.L.; Zheng, M.Y.; Wang, K.J. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.C.; Kim, Y.Y.; Yang, J.E.; Lee, H.M.; Hwang, I.M.; Park, H.W.; Kim, H.M. Utilization of coffee waste as a sustainable feedstock for high-yield lactic acid production through microbial fermentation. Sci. Total Environ. 2024, 912, 169521. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.H.; Li, Y.; Zhu, W.B.; Wang, N.H.; Sun, H.S.; Gao, M.; Ma, X.Y. Highly efficient oriented bioconversion of food waste to lactic acid in an open system: Microbial community analysis and biological carbon fixation evaluation. Bioresour. Technol. 2023, 370, 128398. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Li, H.; Deng, Z.; Liu, J. Lactic acid production from mesophilic and thermophilic fermentation of food waste at different pH. J. Environ. Manag. 2022, 304, 114312. [Google Scholar] [CrossRef]
- Bonk, F.; Bastidas, O.J.R.; Yousef, A.F.; Schmidt, J.E.; Bonk, F. Exploring the selective lactic acid production from food waste in uncontrolled pH mixed culture fermentations using different reactor configurations. Bioresour. Technol. 2017, 238, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Montecchio, D.; Gazzola, G.; Gallipoli, A.; Gianico, A.; Braguglia, C.M. Medium chain Fatty acids production from Food Waste via homolactic fermentation and lactate/ethanol elongation: Electron balance and thermodynamic assessment. Waste Manag. 2024, 177, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Bai, J.Z.; Zuo, J.E. Performance and mechanisms of medium-chain fatty acid production by anaerobic fermentation of food waste without external electron donors. Bioresour. Technol. 2023, 374, 128735. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yin, Q.D.; Zhou, X.Z.; Guo, Q.N.; Wu, G.X. Distribution of extracellular amino acids and their potential functions in microbial cross-feeding in anaerobic digestion systems. Bioresour. Technol. 2022, 360, 127535. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.T.; Ma, C.W.; Zhu, Z.H.; Xu, T.C.; Guo, Y.L.; Ye, J.F. Review on the intermediate amino acids and their enantiomers during the anaerobic digestion: The distribution, biofunctions and mechanisms. Rev. Environ. Sci. Bio-Technol. 2022, 21, 469–482. [Google Scholar] [CrossRef]
- Helmstetter, F.; Arnold, P.; Höger, B.; Petersen, L.M.; Beitz, E. Formate-nitrite transporters carrying nonprotonatable amide amino acids instead of a central histidine maintain pH-dependent transport. J. Biol. Chem. 2019, 294, 623–631. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.G. Enhanced Methane Production from Food Waste Using Cysteine To Increase Biotransformation of L-Monosaccharide, Volatile Fatty Acids, and Biohydrogen. Environ. Sci. Technol. 2018, 52, 3777–3785. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Huang, H.; Qin, Z.; Liu, C.; Chen, Y. Amino Acid Configuration Affects Volatile Fatty Acid Production during Proteinaceous Waste Valorization: Chemotaxis, Quorum Sensing, and Metabolism. Environ. Sci. Technol. 2022, 56, 8702–8711. [Google Scholar] [CrossRef]
- Reggiani, R.; Bertani, A. Anaerobic amino acid metabolism. Russ. J. Plant Physiol. 2003, 50, 733–736. [Google Scholar] [CrossRef]
- Ramsay, I.R.; Pullammanappallil, P.C. Protein degradation during anaerobic wastewater treatment: Derivation of stoichiometry. Biodegradation 2001, 12, 247–257. [Google Scholar] [CrossRef]
- Chen, S.S.; Dong, B.; Dai, X.H.; Wang, H.Y.; Li, N.; Yang, D.H. Effects of thermal hydrolysis on the metabolism of amino acids in sewage sludge in anaerobic digestion. Waste Manag. 2019, 88, 309–318. [Google Scholar] [CrossRef]
- Yekta, S.S.; Elreedy, A.; Liu, T.; Hedenström, M.; Isaksson, S.; Fujii, M.; Schnürer, A. Influence of cysteine, serine, sulfate, and sulfide on anaerobic conversion of unsaturated long-chain fatty acid, oleate, to methane. Sci. Total Environ. 2022, 817, 152967. [Google Scholar] [CrossRef]
- Song, L.; Yang, D.; Liu, R.; Liu, S.; Dai, X. The dissolution of polysaccharides and amino acids enhanced lactic acid production from household food waste during pretreatment process. Sci. Total Environ. 2023, 864, 161068. [Google Scholar] [CrossRef]
- Chen, B.Y.; Rupani, P.F.; Azman, S.; Dewil, R.; Appels, L. A redox-based strategy to enhance propionic and butyric acid production during anaerobic fermentation. Bioresour. Technol. 2022, 361, 127672. [Google Scholar] [CrossRef]
- Masihi, F.; Rezaeitavabe, F.; Karimi-Jashni, A.; Riefler, G. Optimization and enhancement of biohydrogen production in a single-stage hybrid (dark/photo) fermentation reactor using Fe3O4 and TiO2 nanoparticles. Int. J. Hydrogen Energy 2024, 52, 295–305. [Google Scholar] [CrossRef]
- Chenebault, C.; Moscoviz, R.; Trably, E.; Escudié, R.; Percheron, B. Lactic acid production from food waste using a microbial consortium: Focus on key parameters for process upscaling and fermentation residues valorization. Bioresour. Technol. 2022, 354, 127230. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; González-Fernández, C.; Greses, S. Long hydraulic retention time mediates stable volatile fatty acids production against slight pH oscillations. Waste Manag. 2024, 176, 140–148. [Google Scholar] [CrossRef]
- Tang, J.L.; Yang, H.; Pu, Y.H.; Hu, Y.S.; Huang, J.; Jin, N.; He, X.R.; Wang, X.C. Caproic acid production from food waste using indigenous microbiota: Performance and mechanisms. Bioresour. Technol. 2023, 387, 129687. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Feng, K.; Li, H.; Deng, Z.; Liu, J. Promote lactic acid production from food waste fermentation using biogas slurry recirculation. Bioresour. Technol. 2021, 337, 125393. [Google Scholar] [CrossRef]
- AHPA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Goel, R.; Mino, T.; Satoh, H.; Matsuo, T. Enzyme activities under anaerobic and aerobic conditions inactivated sludge sequencing batch reactor. Water Res. 1998, 32, 2081–2088. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef]
- Wu, Q.L.; Guo, W.Q.; Zheng, H.S.; Chao, L.H.; Chi, F.X.; Li, Y.R.; Ren, N.Q. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: The mechanism and microbial community analyses. Bioresour. Technol. 2016, 216, 653–660. [Google Scholar] [CrossRef]
- Aljundi, I.H.; Belovich, J.M.; Talu, O. Adsorption of lactic acid from fermentation broth and aqueous solutions on Zeolite molecular sieves. Chem. Eng. Sci. 2005, 60, 5004–5009. [Google Scholar] [CrossRef]
- Dalie, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Ye, J.; Xu, H.; Zhu, Z.; Xu, T. Effects of different amino acids and their configurations on methane yield and biotransformation of intermediate metabolites during anaerobic digestion. J. Environ. Manag. 2021, 296, 113152. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abol-Fotouh, D.; Omer, A.M.; Tamer, T.M.; Abbas, E. Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review. Int. J. Biol. Macromol. 2020, 154, 567–583. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Ding, L.K.; Lin, R.C.; Song, W.L.; Su, H.B.; Zhou, J.H.; Cen, K.F. Substrate consumption and hydrogen production via co-fermentation of monomers derived from carbohydrates and proteins in biomass wastes. Appl. Energy 2015, 139, 9–16. [Google Scholar] [CrossRef]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef]
- Itoh, Y.; Tada, K.; Kanno, T.; Horiuchi, J.I. Selective production of lactic acid in continuous anaerobic acidogenesis by extremely low pH operation. J. Biosci. Bioeng. 2012, 114, 537–539. [Google Scholar] [CrossRef]
- Park, B.H.; Kim, I.S.; Park, J.K.; Zhi, Z.; Lee, H.M.; Kwon, O.W.; Lee, B.C. Probiotic effect of Lactococcus lactis subsp. cremoris RPG-HL-0136 on intestinal mucosal immunity in mice. Appl. Biol. Chem. 2021, 64, 1–9. [Google Scholar]
- Yu, H.S.; Jang, H.J.; Lee, N.K.; Paik, H.D. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT-Food Sci. Technol. 2019, 112, 108229. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | Mass ratio (%) |
|---|---|
| Rice | 21.8 |
| Noodles | 20.0 |
| Cabbage | 12.0 |
| Carrot | 5.6 |
| Onion | 5.0 |
| Potato | 12.0 |
| Egg | 4.6 |
| Tofu | 4.6 |
| Mushroom | 3.0 |
| Pork | 8.2 |
| Spices | 3.2 |
| Index | Food Waste |
|---|---|
| pH | 5.02 ± 0.2 |
| TS (g/L) | 82.92 ± 0.41 |
| VS (g/L) | 81.19 ± 0.35 |
| TCOD (g/L) | 110.40 ± 3.53 |
| SCOD (g/L) | 51.93 ± 0.33 |
| Control | Cys | Val | |
|---|---|---|---|
| α-glucosidase activity (U/g·VS) | 138.56 ± 2.9 | 169.36 ± 2.4 | 134.31 ± 2.4 |
| Lactic acid yield (g/g TS) | 0.50 ± 0.04 | 0.56 ± 0.04 | 0.41 ± 0.08 |
| lactate dehydrogenase activity (μmol/min/104 cell) | 1.76 ± 0.08 | 1.89 ± 0.05 | 1.28 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, X.; Wang, Y.; Liu, H. Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste. Fermentation 2024, 10, 179. https://doi.org/10.3390/fermentation10040179
Zhou Y, Zhang X, Wang Y, Liu H. Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste. Fermentation. 2024; 10(4):179. https://doi.org/10.3390/fermentation10040179
Chicago/Turabian StyleZhou, Yan, Xuedong Zhang, Yue Wang, and Hongbo Liu. 2024. "Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste" Fermentation 10, no. 4: 179. https://doi.org/10.3390/fermentation10040179
APA StyleZhou, Y., Zhang, X., Wang, Y., & Liu, H. (2024). Mechanism and Effect of Amino Acids on Lactic Acid Production in Acidic Fermentation of Food Waste. Fermentation, 10(4), 179. https://doi.org/10.3390/fermentation10040179







