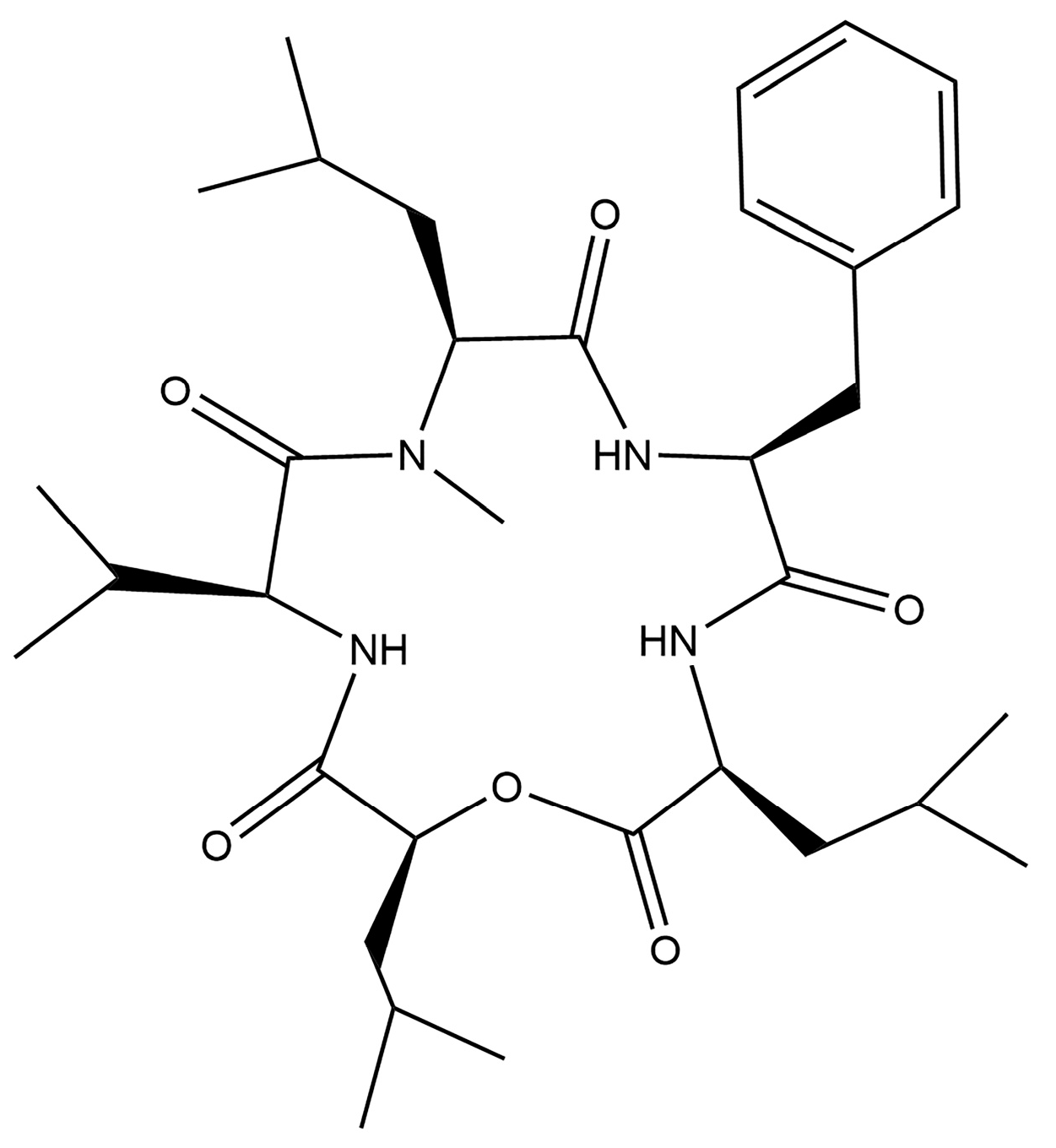
Optimization of Stationary Liquid Fermentation Conditions for N-Methylsansalvamide Production by the Endophytic Strain Fusarium sp. R1
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Cultivation
2.2. Fermentation Process
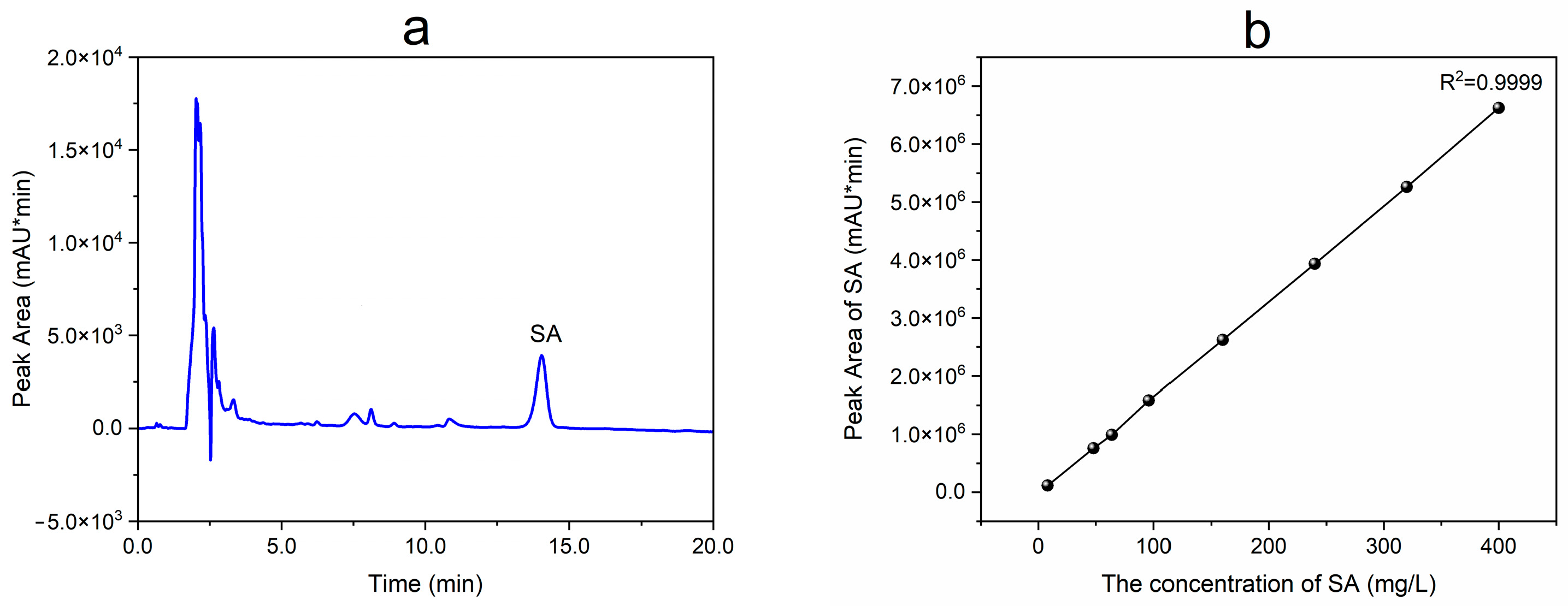
2.3. Analytical Method for SA
2.4. Effects of Fermentation Conditions on SA Yield
2.5. Effects of Carbon and Nitrogen Sources on SA Yield
2.6. Determination of Central Values for Response Surface Design
2.7. Response Surface Methodology for Optimization of Medium Compositions
2.8. Experimental Data Processing
2.9. Verification Experiments
3. Results
3.1. Effects of Fermentation Conditions on SA Yield
3.1.1. Effect of Fermentation Time on SA Production
3.1.2. Effect of Temperature on SA Production
3.1.3. Effect of Initial pH on SA Production
3.1.4. Effect of Seed Age on SA Production
3.1.5. Effect of Inoculum Size on SA Production
3.1.6. Effect of Loading Volume on SA Production
3.1.7. Effect of Sea Salt Concentration on SA Production
3.2. Effect of Carbon and Nitrogen Sources on SA Production
3.3. Central Values Analysis
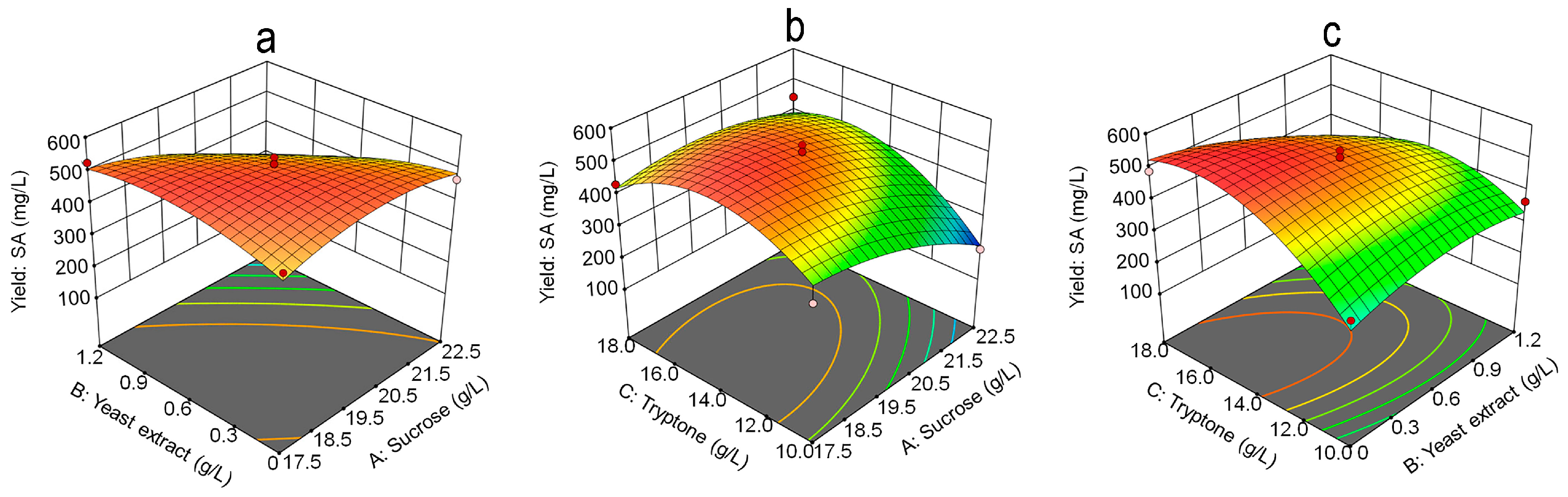
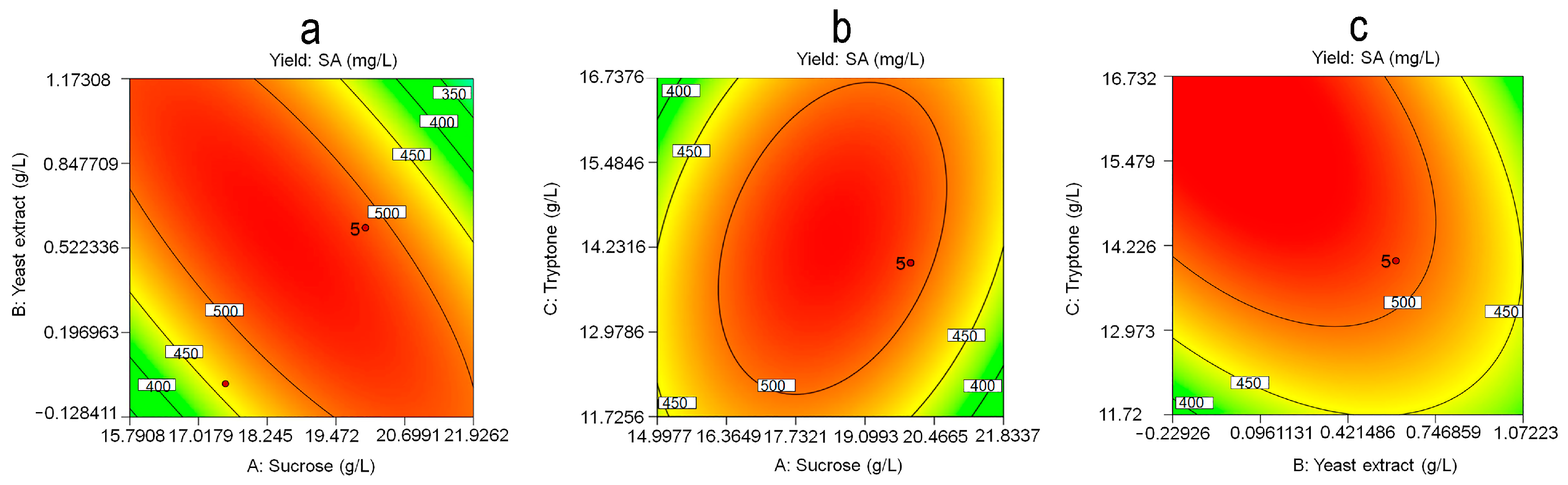
3.4. Response Surface Methodology Analysis
3.5. Verification Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gangadevi, V.; Muthumary, J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J. Microbiol. Biotechnol. 2007, 24, 717–724. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. A novel endophytic Taxol-producing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl. Biochem. Biotechnol. 2009, 158, 675–684. [Google Scholar] [CrossRef]
- Toghueo, R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Li, M.; Yu, R.; Bai, X.; Wang, H.; Zhang, H. Fusarium: A treasure trove of bioactive secondary metabolites. Nat. Prod. Rep. 2020, 37, 1568–1588. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gu, W.; Lo, D.; Ding, X.Z.; Ujiki, M.; Adrian, T.E.; Soff, G.A.; Silverman, R.B. N-methylsansalvamide a peptide analogues. Potent new antitumor agents. J. Med. Chem. 2005, 48, 3630–3638. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Jensen, P.R.; Fenical, W. Sansalvamide: A new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999, 40, 2913–2916. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Fenical, W. N-methylsansalvamide, a cytotoxic cyclic depsipeptide from a marine fungus of the genus Fusarium. Phytochemistry 2000, 55, 223–226. [Google Scholar] [CrossRef]
- Park, J.; Moon, S.K.; Lee, C. N-methylsansalvamide elicits antitumor effects in colon cancer cells in vitro and in vivo by regulating proliferation, apoptosis, and metastatic capacity. Front. Pharmacol. 2023, 14, 1146966. [Google Scholar] [CrossRef] [PubMed]
- Kheiralla, Z.H.; El-Gendy, N.S.; Ahmed, H.A.; Shaltout, T.H.; Hussein, M.M.D. One-factor-at-a-time (OFAT) optimization of hemicellulases production from Fusarium moniliformein submerged fermentation. Energ. Source Part A 2018, 40, 1877–1885. [Google Scholar] [CrossRef]
- Guo, F.Z.; Zheng, H.R.; Cheng, Y.W.; Song, S.; Zheng, Z.X.; Jia, S. Medium optimization for epsilon-poly-L-lysine production by Streptomyces diastatochromogenes using response surface methodology. Lett. Appl. Microbiol. 2018, 66, 124–131. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xu, M.J.; Tang, Y.Q.; Shao, Y.L.; Wang, H.; Zhang, H.W. Genome features and antiSMASH analysis of an endophytic strain Fusarium sp. R1. Metabolites 2022, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, J.; Chen, X.; Tao, X.; Xie, J.; Shi, X.; Huang, Z. Comparative transcriptome analysis of Monascus purpureus at different fermentation times revealed candidate genes involved in exopolysaccharide biosynthesis. Food Res. Int. 2022, 160, 111700. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J. Temperature and the catalytic activity of enzymes: A fresh understanding. FEBS Lett. 2013, 587, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chu, X.; Du, B.; Huang, C.; Xie, C.; Zhang, Z.; Jiang, L. Functional characterization of a novel violacein biosynthesis operon from Janthinobacterium sp. B9-8. Appl. Microbiol. Biotechnol. 2022, 106, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhao, X.; Li, Y. Factors affecting the efficiency of a bioelectrochemical system: A review. RSC Adv. 2019, 9, 19748–19761. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Yang, W.; Huang, G. Optimization of fermentation conditions for the production of epothilone B. Chem. Biol. Drug Des. 2020, 96, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.U.; Flashman, E.; Mohlin, S.; Licausi, F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 2020, 370, eaba3512. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K. Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol. 2016, 7, 2087. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Understanding and application of Bacillus nitrogen regulation: A synthetic biology perspective. J. Adv. Res. 2023, 49, 1–14. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, E.S. Recent advances in heterologous expression of natural product biosynthetic gene clusters in Streptomyces hosts. Curr. Opin. Biotechnol. 2021, 69, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Phat, C.; Nam, W.S.; Lee, C. Optimization of culture conditions of Fusarium solani for the production of neoN-methylsansalvamide. Biosci. Biotechnol. Biochem. 2014, 78, 1421–1427. [Google Scholar] [CrossRef] [PubMed]


| Factor | Code | Levels (g/L) | |
|---|---|---|---|
| −1 | 1 | ||
| Sucrose | A | 10.0 | 20.0 |
| Yeast Extract | B | 1.0 | 3.0 |
| Tryptone | C | 2.0 | 10.0 |
| Run | Sucrose (g/L) | Yeast Extract (g/L) | Tryptone (g/L) |
|---|---|---|---|
| 1 | 15.0 | 2.0 | 6.0 |
| 2 | 17.5 | 1.3 | 10.0 |
| 3 | 20.0 | 0.6 | 14.0 |
| 4 | 22.5 | 0.0 | 18.0 |
| 5 | 25.0 | 0.0 | 22.0 |
| Factor | Code | Levels (g/L) | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Sucrose | A | 17.5 | 20.0 | 22.5 |
| Yeast Extract | B | 0.0 | 0.6 | 1.2 |
| Tryptone | C | 10.0 | 14.0 | 18.0 |
| Run | Sucrose (g/L) | Yeast Extract (g/L) | Tryptone (g/L) | SA Yield (mg/L) |
|---|---|---|---|---|
| 1 | 1 | −1 | 1 | 252.16 ± 47.92 |
| 2 | −1 | 1 | 1 | 166.16 ± 36.95 |
| 3 | −1 | 1 | −1 | 117.98 ± 7.26 |
| 4 | 1 | 1 | −1 | 78.42 ± 3.37 |
| 5 | 1 | −1 | −1 | 51.87 ± 15.95 |
| 6 | −1 | −1 | 1 | 193.20 ± 40.28 |
| 7 | −1 | 1 | 1 | 166.16 ± 36.95 |
| 8 | −1 | −1 | −1 | 62.70 ± 4.22 |
| 9 | −1 | −1 | −1 | 62.72 ± 4.30 |
| 10 | 1 | 1 | −1 | 78.46 ± 2.78 |
| 11 | 1 | 1 | 1 | 127.88 ± 12.66 |
| 12 | 1 | −1 | 1 | 252.09 ± 20.33 |
| Code | t Test | p | Significance |
|---|---|---|---|
| Sucrose | 0.51 | 0.625 | 3 |
| Yeast Extract | −0.99 | 0.352 | 2 |
| Tryptone | 4.99 | 0.001 | 1 |
| Run | Sucrose (g/L) | Yeast Extract (g/L) | Tryptone (g/L) | SA Yield (mg/L) |
|---|---|---|---|---|
| 1 | 15.0 | 2.0 | 6.0 | 124.46 ± 29.43 |
| 2 | 17.5 | 1.3 | 10.0 | 235.21 ± 10.30 |
| 3 | 20.0 | 0.6 | 14.0 | 385.33 ± 14.72 |
| 4 | 22.5 | 0.0 | 18.0 | 380.16 ± 10.02 |
| 5 | 25.0 | 0.0 | 22.0 | 174.23 ± 36.71 |
| Run | Sucrose (g/L) | Yeast Extract (g/L) | Tryptone (g/L) | SA Yield (mg/L) |
|---|---|---|---|---|
| 1 | 1 | −1 | 0 | 466.08 ± 40.67 |
| 2 | 1 | 0 | 1 | 445.36 ± 0.75 |
| 3 | −1 | 0 | 1 | 432.88 ± 48.14 |
| 4 | 0 | 0 | 0 | 515.68 ± 21.21 |
| 5 | 0 | 0 | 0 | 471.36 ± 14.85 |
| 6 | 0 | −1 | −1 | 328.08 ± 21.44 |
| 7 | 0 | 0 | 0 | 517.52 ± 18.83 |
| 8 | −1 | −1 | 0 | 484.64 ± 11.47 |
| 9 | 0 | 1 | −1 | 367.68 ± 27.35 |
| 10 | −1 | 0 | −1 | 364.72 ± 24.64 |
| 11 | 0 | −1 | 1 | 488.40 ± 5.72 |
| 12 | −1 | 1 | 0 | 524.72 ± 7.55 |
| 13 | 0 | 0 | 0 | 537.36 ± 18.32 |
| 14 | 0 | 1 | 1 | 268.00 ± 19.87 |
| 15 | 1 | 0 | −1 | 198.88 ± 2.78 |
| 16 | 1 | 1 | 0 | 230.96 ± 21.64 |
| 17 | 0 | 0 | 0 | 517.52 ± 28.04 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.757 × 105 | 9 | 19,519.75 | 10.03 | 0.0030 | significant |
| A | 27,107.23 | 1 | 27,107.23 | 13.93 | 0.0073 | |
| B | 17,656.96 | 1 | 17,656.96 | 9.08 | 0.0196 | |
| C | 17,604.38 | 1 | 17,604.38 | 9.05 | 0.0197 | |
| AB | 18,933.76 | 1 | 18,933.76 | 9.73 | 0.0168 | |
| AC | 7949.51 | 1 | 7949.51 | 4.09 | 0.0829 | |
| BC | 16,900.00 | 1 | 16,900.00 | 8.69 | 0.0215 | |
| A2 | 8127.14 | 1 | 8127.14 | 4.18 | 0.0802 | |
| B2 | 7200.65 | 1 | 7200.65 | 3.70 | 0.0958 | |
| C2 | 48,652.46 | 1 | 48,652.46 | 25.01 | 0.0016 | |
| Residual | 13,617.54 | 7 | 1945.36 | |||
| Lack of fit | 11,248.38 | 3 | 3749.46 | 6.33 | 0.0533 | not significant |
| Pure Error | 2369.16 | 4 | 592.29 | |||
| Cor Total | 1.893 × 108 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Bai, Y.; Cai, Z.; Pu, N.; Zhang, H. Optimization of Stationary Liquid Fermentation Conditions for N-Methylsansalvamide Production by the Endophytic Strain Fusarium sp. R1. Fermentation 2024, 10, 140. https://doi.org/10.3390/fermentation10030140
Shao Y, Bai Y, Cai Z, Pu N, Zhang H. Optimization of Stationary Liquid Fermentation Conditions for N-Methylsansalvamide Production by the Endophytic Strain Fusarium sp. R1. Fermentation. 2024; 10(3):140. https://doi.org/10.3390/fermentation10030140
Chicago/Turabian StyleShao, Yilan, Yifan Bai, Zhehui Cai, Nan Pu, and Huawei Zhang. 2024. "Optimization of Stationary Liquid Fermentation Conditions for N-Methylsansalvamide Production by the Endophytic Strain Fusarium sp. R1" Fermentation 10, no. 3: 140. https://doi.org/10.3390/fermentation10030140
APA StyleShao, Y., Bai, Y., Cai, Z., Pu, N., & Zhang, H. (2024). Optimization of Stationary Liquid Fermentation Conditions for N-Methylsansalvamide Production by the Endophytic Strain Fusarium sp. R1. Fermentation, 10(3), 140. https://doi.org/10.3390/fermentation10030140






