ADM1-Based Modeling of Biohydrogen Production through Anaerobic Co-Digestion of Agro-Industrial Wastes in a Continuous-Flow Stirred-Tank Reactor System
Abstract
1. Introduction
2. Materials and Methods
ADM1 Application for a Hydrogen Production Process
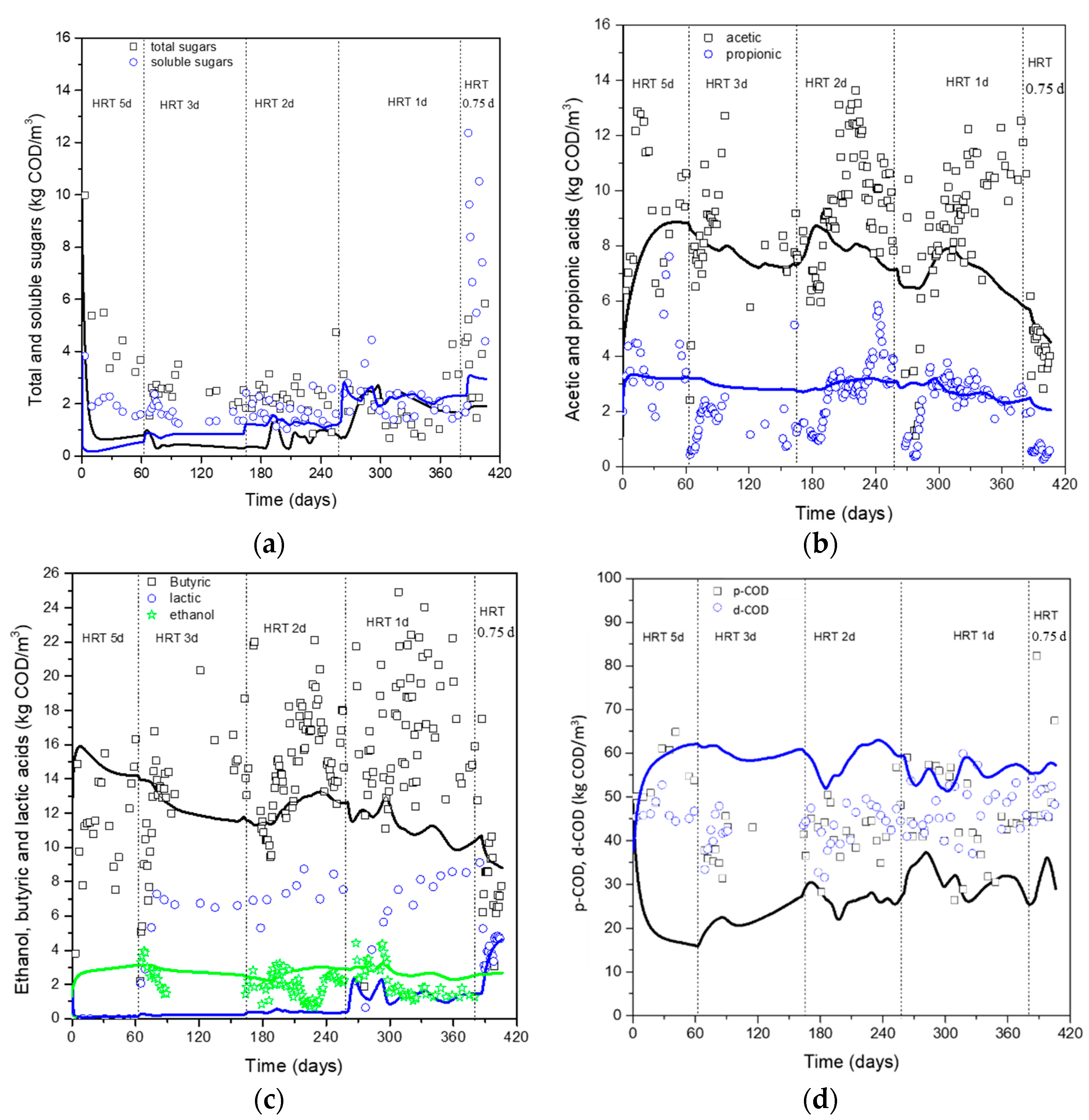
3. Results & Discussion
Estimation of Kinetic and Stoichiometric Parameters–Model Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcoD | Anaerobic Co-Digestion |
| AD | Anaerobic Digestion |
| ADM1 | Anaerobic Digestion Model 1 |
| CM | Cow Manure |
| COD | Chemical Oxygen Demand |
| CSTR | Continuous Stirred Tank Reactor |
| CW | Cheese Whey |
| d-COD | Dissolved Chemical Oxygen Demand |
| HRT | Hydraulic Retention Time |
| OMW | Olive Mill Wastewater |
| p-COD | Particulate Chemical Oxygen Demand |
| TSS | Total Suspended Solids |
| VSS | Volatile Suspended Solids |
Appendix A
| Component → i | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Rate (ρj, kg COD/(m3·d)) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| j | Process ↓ | Ssu | Saa | Sfa | Sva | Sbu | Spro | Sac | Sh2 | Sch4 | SIC | SIN | SI | Seth | Slac | |
| 1 | Disintegration | fSI,XC | ||||||||||||||
| 2 | Hydrolysis of Carbohydrates | 1 | ||||||||||||||
| 3 | Hydrolysis of Proteins | 1 | ||||||||||||||
| 4 | Hydrolysis of Lipids | 1 − ffa,li | ffa,li | |||||||||||||
| 5 | Uptake of Sugars | −1 | (1 − Ysu)·fbu,su | (1 − Ysu)·fpro,su | (1 − Ysu)·fac,su | (1 − Ysu)·fh2,su | (Ysu)·Nbac | (1 − Ysu)·feth,su | ||||||||
| 6 | Uptake of Amino Acids | −1 | (1 − Yaa)·fva,aa | (1 − Yaa)·fbu,aa | (1 − Yaa)·fpro,aa | (1 − Yaa)·fac,aa | (1 − Yaa)·fh2,aa | Naa − (Yaa)·Nbac | ||||||||
| 7 | Uptake of LCFA | −1 | (1 − Yfa)·0.7 | (1 − Yfa)·0.3 | (Yfa)·Nbac | |||||||||||
| 8 | Uptake of Valerate | −1 | (1 − Yc4)·0.54 | (1 − Yc4)·0.31 | (1 − Yc4)·0.15 | (Yc4)·Nbac | ||||||||||
| 9 | Uptake of Butyrate | −1 | (1 − Yc4)·0.8 | (1 − Yc4)·0.2 | (Yc4)·Nbac | |||||||||||
| 10 | Uptake of Propionate | −1 | (1 − Ypro)·0.57 | (1 − Ypro)·0.43 | (Ypro)·Nbac | |||||||||||
| 11 | Uptake of Acetate | −1 | (1 − Yac) | (Yac)·Nbac | ||||||||||||
| 12 | Uptake of Hydrogen | −1 | (1 − Yh2) | (Yh2)·Nbac | ||||||||||||
| 13 | Decay of Xsu | |||||||||||||||
| 14 | Decay of Xaa | |||||||||||||||
| 15 | Decay of Xfa | |||||||||||||||
| 16 | Decay of Xc4 | |||||||||||||||
| 17 | Decay of Xpro | |||||||||||||||
| 18 | Decay of Xac | |||||||||||||||
| 19 | Decay of Xh2 | |||||||||||||||
| 20 | Decay of Xlac | |||||||||||||||
| 21 | Uptake of Lactate | (1 − Ylac)·fbu,lac | (1 − Ylac)·fpro,lac | (1 − Ylac)·fac,lac | (1 − Ylac)·fh2,lac | −1 | ||||||||||
| 22 | Sugars uptake rate by Lactobacillus | (1 − Ysu)·flac,su | ||||||||||||||
| 23 | Decay of Lactobacillus | |||||||||||||||
| Monosaccharides (kg COD m−3) | Amino Acids (kg COD m−3) | Long chain fatty acids (kg COD m−3) | Total valerate (kg COD m−3) | Total butyrate (kg COD m−3) | Total propionate (kg COD m−3) | Total acetate (kg COD m−3) | Hydrogen gas (kg COD m−3) | Methane gas (kg COD m−3) | Inorganic Carbon (kg mole C m−3) | Inorganic Nitrogen (kg mole N m−3) | Soluble Inerts (kg COD m−3) | Total Ethanol (kg COD m−3) | Total Lactate (kg COD m−3) | Inhibition factors |
| Component → i | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | Rate (ρj, kg COD/(m3·d)) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J | Process ↓ | Xc | Xch | Xpr | Xli | Xsu | Xaa | Xfa | Xc4 | Xpro | Xac | Xh2 | XI | Xlac | Xlac,su | |
| 1 | Disintegration | −1 | fch,XC | fpr,XC | fli,XC | fSI,XC | ||||||||||
| 2 | Hydrolysis of Carbohydrates | −1 | ||||||||||||||
| 3 | Hydrolysis of Proteins | −1 | ||||||||||||||
| 4 | Hydrolysis of Lipids | −1 | ||||||||||||||
| 5 | Uptake of Sugars | Ysu | ||||||||||||||
| 6 | Uptake of Amino Acids | Yaa | ||||||||||||||
| 7 | Uptake of LCFA | Yfa | ||||||||||||||
| 8 | Uptake of Valerate | Yc4 | ||||||||||||||
| 9 | Uptake of Butyrate | Yc4 | ||||||||||||||
| 10 | Uptake of Propionate | Ypro | ||||||||||||||
| 11 | Uptake of Acetate | Yac | ||||||||||||||
| 12 | Uptake of Hydrogen | Yh2 | ||||||||||||||
| 13 | Decay of Xsu | 1 | −1 | |||||||||||||
| 14 | Decay of Xaa | 1 | −1 | |||||||||||||
| 15 | Decay of Xfa | 1 | −1 | |||||||||||||
| 16 | Decay of Xc4 | 1 | −1 | |||||||||||||
| 17 | Decay of Xpro | 1 | −1 | |||||||||||||
| 18 | Decay of Xac | 1 | −1 | |||||||||||||
| 19 | Decay of Xh2 | 1 | −1 | |||||||||||||
| 20 | Decay of Xlac | 1 | −1 | |||||||||||||
| 21 | Uptake of Lactate | Ylac | ||||||||||||||
| 22 | Sugars uptake rate by Lactobacillus | Ylac,su | ||||||||||||||
| 23 | Decay of Lactobacillus | 1 | −1 | |||||||||||||
| Composites (kg COD m−3) | Carbohydrates (kg COD m−3) | Proteins (kg COD m−3) | Lipids (kg COD m−3) | Sugar degraders (kg COD m−3) | Amino acid degraders (kg COD m−3) | LCFA degraders (kg COD m−3) | Caproate, valerate and butyrate degraders (kg COD m−3) | Propionate degraders (kg COD m−3) | Acetate degraders (kg COD m−3) | Hydrogen degraders (kg COD m−3) | Particulate inerts (kg mole C m−3) | Lactate Degraders (kg COD m−3) | Lactobacillus (kg COD m−3) | Inhibition factors |
References
- Manthos, G.; Dareioti, M.; Zagklis, D.; Kornaros, M. Using Biochemical Methane Potential Results for the Economic Optimization of Continuous Anaerobic Digestion Systems: The Effect of Substrates’ Synergy. Renew. Energy 2023, 211, 296–306. [Google Scholar] [CrossRef]
- Gomez-Romero, J.; Gonzalez-Garcia, R.A.; Chairez, I.; Torres, L.; García-Peña, E.I. Continuous Two-staged Co-digestion Process for Biohydrogen Production from Agro-industrial Wastes. Int. J. Energy Res. 2016, 40, 257–272. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Trably, E.; Carrere, H.; Lyberatos, G. Continuous Biohydrogen Production from a Food Industry Waste: Influence of Operational Parameters and Microbial Community Analysis. J. Clean. Prod. 2018, 174, 1054–1063. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. A Novel Approach of Modeling Continuous Dark Hydrogen Fermentation. Bioresour. Technol. 2018, 250, 784–792. [Google Scholar] [CrossRef]
- Zappi, A.; Hernandez, R.; Holmes, W.E. A Review of Hydrogen Production from Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2021, 18, 4075–4090. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. Modeling of Fermentative Hydrogen Production from Sweet Sorghum Extract Based on Modified ADM1. Int. J. Hydrogen Energy 2012, 37, 191–208. [Google Scholar] [CrossRef]
- Jurado, E.; Antonopoulou, G.; Lyberatos, G.; Gavala, H.N.; Skiadas, I.V. Continuous Anaerobic Digestion of Swine Manure: ADM1-Based Modelling and Effect of Addition of Swine Manure Fibers Pretreated with Aqueous Ammonia Soaking. Appl. Energy 2016, 172, 190–198. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic Co-Digestion Process for Biogas Production: Progress, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Balaji, R.K.; Rajan, K.P.; Ragula, U.B.R. Modeling & Optimization of Renewable Hydrogen Production from Biomass via Anaerobic Digestion & Dry Reformation. Int. J. Hydrogen Energy 2020, 45, 18226–18240. [Google Scholar]
- Smit, R.; Whitehead, J.; Washington, S. Where Are We Heading with Electric Vehicles? Air Qual. Clim. Chang. 2018, 52, 18–27. [Google Scholar]
- Khadka, A.; Parajuli, A.; Dangol, S.; Thapa, B.; Sapkota, L.; Carmona-Martínez, A.A.; Ghimire, A. Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste. Energies 2022, 15, 834. [Google Scholar] [CrossRef]
- Abudi, Z.N.; Hu, Z.; Sun, N.; Xiao, B.; Rajaa, N.; Liu, C.; Guo, D. Batch Anaerobic Co-Digestion of OFMSW (Organic Fraction of Municipal Solid Waste), TWAS (Thickened Waste Activated Sludge) and RS (Rice Straw): Influence of TWAS and RS Pretreatment and Mixing Ratio. Energy 2016, 107, 131–140. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of Fish Industry Waste for Biogas Production: A Lab Scale Evaluation of Biochemical Methane Potential (BMP) and Kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, P.; Biernacki, P.; Uhlenhut, F.; Cypionka, H.; Steinigeweg, S. Modelling Anaerobic Digestion in a Biogas Reactor: ADM1 Model Development with Lactate as an Intermediate (Part I). J. Environ. Sci. Health Part A 2016, 51, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, P.; Biernacki, P.; Cypionka, H.; Steinigeweg, S. Modelling Anaerobic Digestion in an Industrial Biogas Digester: Application of Lactate-Including ADM1 Model (Part II). J. Environ. Sci. Health Part A 2016, 51, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Guellout, Z.; Clion, V.; Benguerba, Y.; Dumas, C.; Ernst, B. Study of the Dark Fermentative Hydrogen Production Using Modified ADM1 Models. Biochem. Eng. J. 2018, 132, 9–19. [Google Scholar] [CrossRef]
- Couto, P.T.; Eng, F.; Naessens, W.; Nopens, I.; Zaiat, M.; Ribeiro, R. Modelling Sugarcane Vinasse Processing in an Acidogenic Reactor to Produce Hydrogen with an ADM1-Based Model. Int. J. Hydrogen Energy 2020, 45, 6217–6230. [Google Scholar] [CrossRef]
- Shi, E.; Li, J.; Zhang, M. Application of IWA Anaerobic Digestion Model No. 1 to Simulate Butyric Acid, Propionic Acid, Mixed Acid, and Ethanol Type Fermentative Systems Using a Variable Acidogenic Stoichiometric Approach. Water Res. 2019, 161, 242–250. [Google Scholar] [CrossRef]
- Montecchio, D.; Yuan, Y.; Malpei, F. Hydrogen Production Dynamic during Cheese Whey Dark Fermentation: New Insights from Modelization. Int. J. Hydrogen Energy 2018, 43, 17588–17601. [Google Scholar] [CrossRef]
- Ntaikou, I.; Gavala, H.N.; Lyberatos, G. Application of a Modified Anaerobic Digestion Model 1 Version for Fermentative Hydrogen Production from Sweet Sorghum Extract by Ruminococcus Albus. Int. J. Hydrogen Energy 2010, 35, 3423–3432. [Google Scholar] [CrossRef]
- de Araújo Cavalcante, W.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic Fermentation for N-Caproic Acid Production: A Review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of Hydraulic Retention Time (HRT) on the Anaerobic Co-Digestion of Agro-Industrial Wastes in a Two-Stage CSTR System. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of PH on the Anaerobic Acidogenesis of Agroindustrial Wastewaters for Maximization of Bio-Hydrogen Production: A Lab-Scale Evaluation Using Batch Tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Reichert, P. User Manual of AQUASIM 2.0 for the Identification and Simulation of Aquatic Systems; Swiss Federal Institute for Environmental Science and Technology (EAWAG): Dubendorf, Switzerland, 1998. [Google Scholar]
- Mo, R.; Guo, W.; Batstone, D.; Makinia, J.; Li, Y. Modifications to the Anaerobic Digestion Model No. 1 (ADM1) for Enhanced Understanding and Application of the Anaerobic Treatment Processes—A Comprehensive Review. Water Res. 2023, 244, 120504. [Google Scholar] [CrossRef]
- Scoma, A.; Bertin, L.; Fava, F. Effect of Hydraulic Retention Time on Biohydrogen and Volatile Fatty Acids Production during Acidogenic Digestion of Dephenolized Olive Mill Wastewaters. Biomass Bioenergy 2013, 48, 51–58. [Google Scholar] [CrossRef]
- Bułkowska, K.; Białobrzewski, I.; Klimiuk, E.; Pokoj, T. Kinetic Parameters of Volatile Fatty Acids Uptake in the ADM1 as Key Factors for Modeling Co-Digestion of Silages with Pig Manure, Thin Stillage and Glycerine Phase. Renew. Energy 2018, 126, 163–176. [Google Scholar] [CrossRef]
- Kunatsa, T.; Xia, X. A Review on Anaerobic Digestion with Focus on the Role of Biomass Co-Digestion, Modelling and Optimisation on Biogas Production and Enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef]
- Soda, S.; Wada, K.; Okuda, M.; Ike, M. Application of Modified ADM1 to Long-Term Experiments for Methane/Hydrogen Production from Model Organic Waste. Water Pract. Technol. 2011, 6, wpt2011009. [Google Scholar] [CrossRef]
- Feldman, H.; Flores-Alsina, X.; Kjellberg, K.; Jeppsson, U.; Batstone, D.J.; Gernaey, K.V. Model-based Analysis and Optimization of a Full-scale Industrial High-rate Anaerobic Bioreactor. Biotechnol. Bioeng. 2018, 115, 2726–2739. [Google Scholar] [CrossRef]

| Products of Sugars Degradation | Stoichiometric Coefficients, kg COD/kg COD |
|---|---|
| Lactate | flac,su = 0.5 (1 + n1,su) |
| Acetate | fac,su = 0.5 (n3,su) |
| Ethanol | feth,su = 0.5 n2,su |
| Products of Lactate Degradation | Stoichiometric Coefficients, kg COD/kg COD |
|---|---|
| Butyrate | fbu,lac = 0.83 (1 − n1,lac) |
| Acetate | fac,lac = 0.22 n1,lac |
| Propionate | fpro,lac = 0.78 n1,lac |
| Hydrogen | fh2,lac = 0.17 (1 − n1,lac) |
| Coefficient | Estimated Value |
|---|---|
| n1,su | 0.605 |
| n2,su | 0.150 |
| n3,su | 0.245 |
| n1,lac | 0.200 |
| n2,lac | 0.800 |
| Kinetic Parameter | Units | Estimated Value |
|---|---|---|
| km,ac_h2 | kg COD/(kg COD·d) | 2.99 |
| km_lac | kg COD/(kg COD·d) | 2.281 |
| km_lac_su | kg COD/(kg COD·d) | 37.820 |
| ks_ac_h2 | kg COD/m3 | 2.28 × 10−4 |
| ks_lac_su | kg COD/m3 | 13.182 |
| Ylac_su | kg COD/kg COD | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Economou, C.N.; Manthos, G.; Zagklis, D.; Kornaros, M. ADM1-Based Modeling of Biohydrogen Production through Anaerobic Co-Digestion of Agro-Industrial Wastes in a Continuous-Flow Stirred-Tank Reactor System. Fermentation 2024, 10, 138. https://doi.org/10.3390/fermentation10030138
Economou CN, Manthos G, Zagklis D, Kornaros M. ADM1-Based Modeling of Biohydrogen Production through Anaerobic Co-Digestion of Agro-Industrial Wastes in a Continuous-Flow Stirred-Tank Reactor System. Fermentation. 2024; 10(3):138. https://doi.org/10.3390/fermentation10030138
Chicago/Turabian StyleEconomou, Christina N., Georgios Manthos, Dimitris Zagklis, and Michael Kornaros. 2024. "ADM1-Based Modeling of Biohydrogen Production through Anaerobic Co-Digestion of Agro-Industrial Wastes in a Continuous-Flow Stirred-Tank Reactor System" Fermentation 10, no. 3: 138. https://doi.org/10.3390/fermentation10030138
APA StyleEconomou, C. N., Manthos, G., Zagklis, D., & Kornaros, M. (2024). ADM1-Based Modeling of Biohydrogen Production through Anaerobic Co-Digestion of Agro-Industrial Wastes in a Continuous-Flow Stirred-Tank Reactor System. Fermentation, 10(3), 138. https://doi.org/10.3390/fermentation10030138









