Impacts of Harvest Date and Concurrent Alkali Pretreatment and Ensiling on Anaerobic Digestion of Pennycress Biomass
Abstract
1. Introduction
2. Materials and Methods
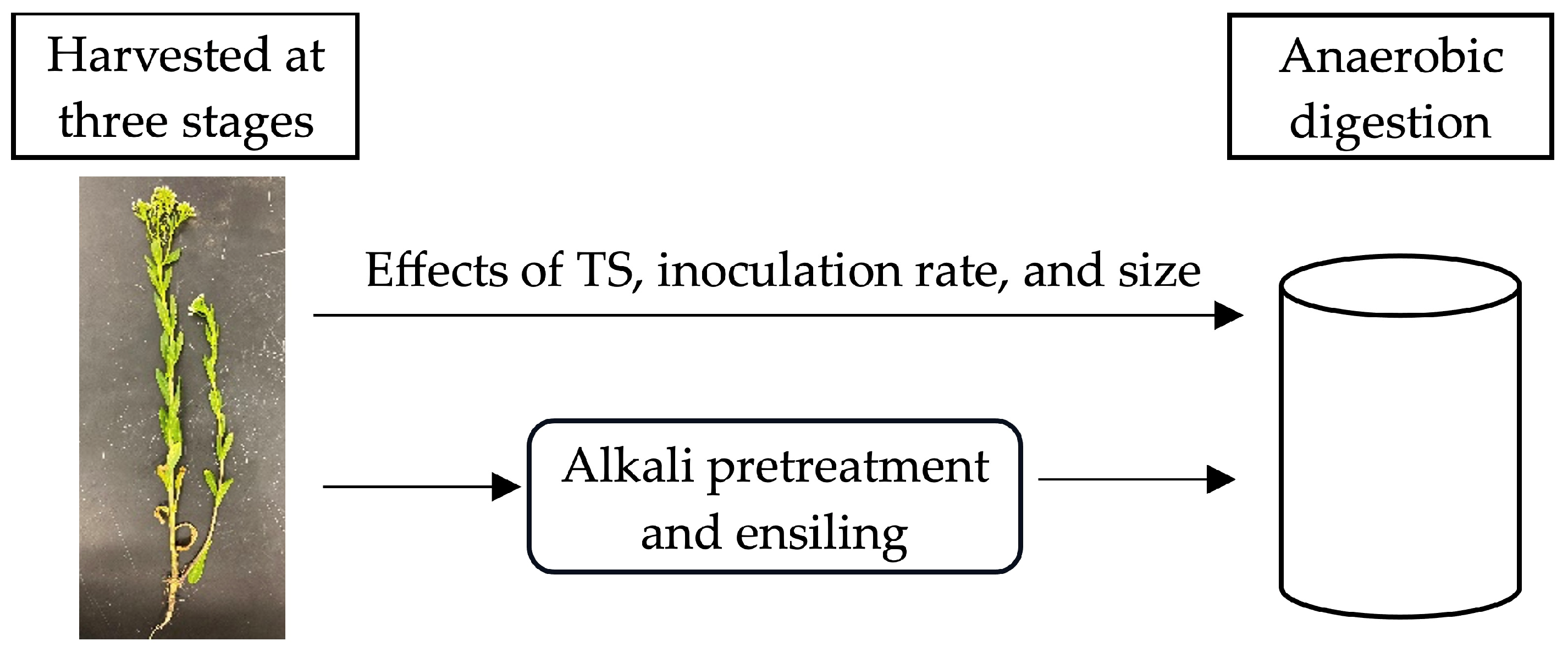
2.1. Overall Experimental Design
2.2. AD Feedstocks, Inoculum, and Micronutrients
2.3. AD Experimental Setup
2.4. Sampling and Analytical Methods
2.5. Data Analysis
3. Results and Discussions
3.1. Effects of Harvest Date on Biomass Characteristics
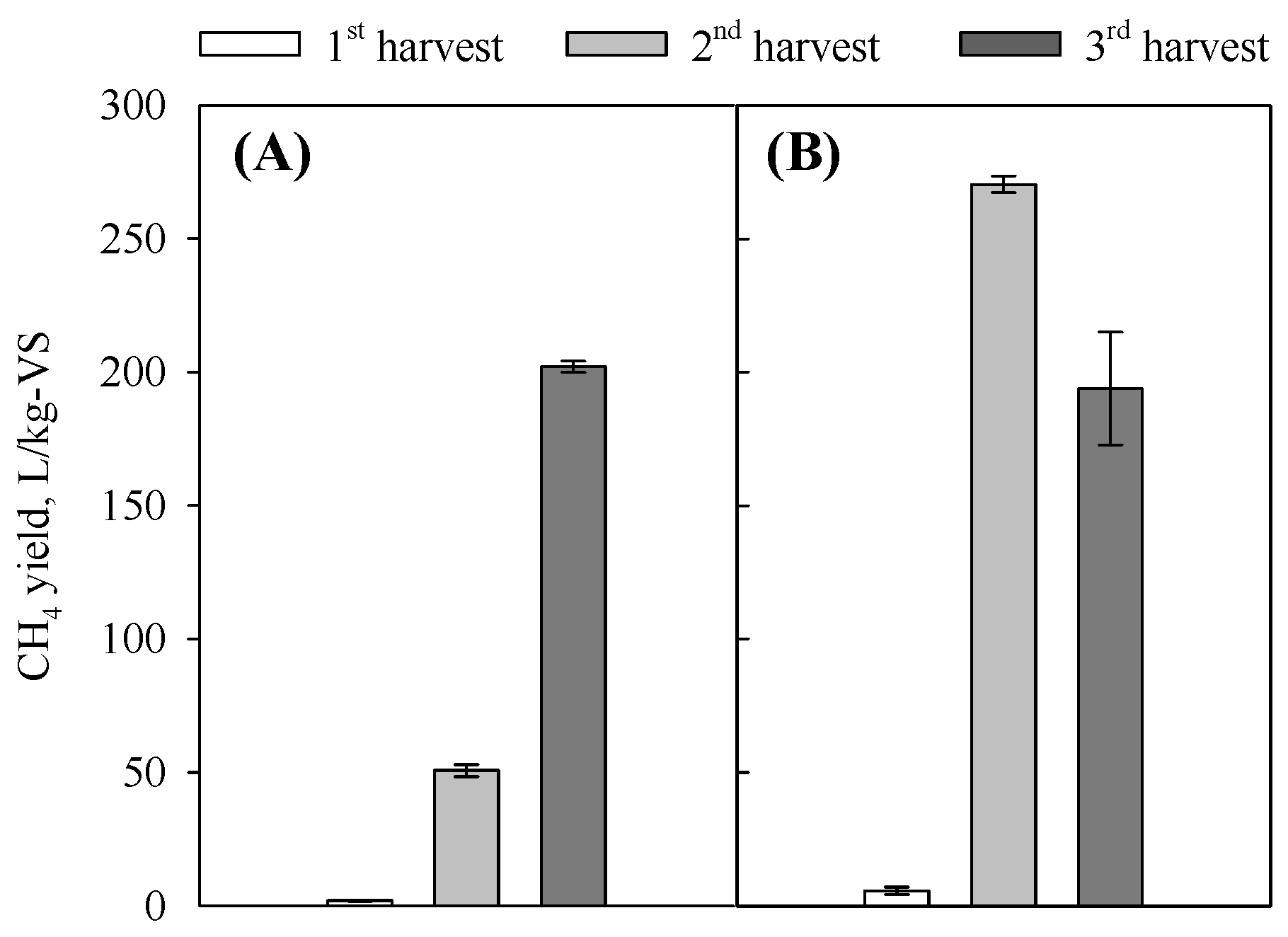
3.2. Effects of Harvest Date, Solids Content, and Particle Size on Methane Yield
3.3. Effects of Inoculation Rate and Concurrent Alkali Pretreatment and Ensiling on Methane Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallander, S.; Smith, D.; Bowman, M.; Classeen, R. Cover Crop Trends, Programs, and Practices in the United States; EIB 222; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2021. [Google Scholar]
- Warwick, S.I.; Francis, A.; Susko, D.J. The Biology of Canadian Weeds. 9. Thlaspi arvense L. (Updated). Can. J. Plant Sci. 2002, 82, 803–823. [Google Scholar] [CrossRef]
- Eerd, L.L.V.; Chahal, I.; Peng, Y.; Awrey, J.C. Influence of Cover Crops at the Four Spheres: A Review of Ecosystem Services, Potential Barriers, and Future Directions for North America. Sci. Total Environ. 2023, 858, 159990. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Rejesus, R.M.; Aglasan, S.; Che, Y.; Hagen, S.C. Payments from Agricultural Conservation Programs and Cover Crop Adoption. Appl. Econ. Perspect. Policy 2023, 45, 984–1007. [Google Scholar] [CrossRef]
- Wittwer, R.; Dorn, B.; Jossi, W.; Heijden, M.V. Cover Crops Support Ecological Intensification of Arable Cropping Systems. Sci. Rep. 2017, 7, 41911. [Google Scholar] [CrossRef]
- Bergtold, J.S.; Duffy, P.A.; Hite, D.; Raper, R.L. Demographic and Management Factors Affecting the Adoption and Perceived Yield Benefit of Winter Cover Crops in the Southeast. J. Agric. Appl. Econ. 2012, 44, 99–116. [Google Scholar] [CrossRef]
- Castellano, M.J.; Helmers, M.J.; Sawyer, J.E.; Barker, D.W.; Christianson, L. Nitrogen, Carbon, and Phosphorus Bal-ances in Iowa Cropping Systems: Sustaining the Soil Resource. In Proceedings of the 2012 Integrated Crop Management Conference, Iowa State University, Ames, IA, USA, 28–29 November 2012; pp. 145–156. [Google Scholar]
- Kladivko, E.J.; Kaspar, T.C.; Jaynes, D.B.; Malone, R.W.; Singer, J.; Morin, X.K.; Searchinger, T. Cover Crops in the Upper Midwestern United States: Potential Adoption and Reduction of Nitrate Leaching in the Mississippi River Basin. J. Soil Water Conserv. 2014, 69, 279–291. [Google Scholar] [CrossRef]
- Snapp, S.; Swinton, S.; Labarta, R.; Mutch, D.; Black, R.; Leep, R.; O’Neil, K. Evaluating Cover Crops for Benefits, Costs and Performance within Cropping System Niches. Agron. J. 2015, 97, 322–332. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon Sequestration in Agricultural Soils via Cultivation of Cover Crops—A Meta-Analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Laloy, E.; Bielders, C. Effect of Intercropping Period Management on Runoff and Erosion in a Maize Cropping System. J. Environ. Qual. 2010, 39, 1001–1008. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using Cover Crops to Mitigate and Adapt to Climate Change. A Review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Myers, R.; Watts, C. Progress and Perspectives with Cover Crops: Interpreting Three Years of Farmer Surveys on Cover Crops. J. Soil Water Conserv. 2015, 70, 125A–129A. [Google Scholar] [CrossRef]
- USEPA. Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 27 November 2023).
- Lemaire, G.; Franzluebbers, A.; Carvalho, P.C.; Dedieu, B. Integrated Crop–Livestock Systems: Strategies to Achieve Synergy between Agricultural Production and Environmental Quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Planisich, A.; Utsumi, S.A.; Larripa, M.; Galli, J.R. Grazing of Cover Crops in Integrated Crop-Livestock Systems. Animal 2021, 15, 100054. [Google Scholar] [CrossRef]
- Finney, D.M.; Creamer, N.G.; Schultheis, J.R.; Wagger, M.G.; Brownie, C. Sorghum Sudangrass as a Summer Cover and Hay Crop for Organic Fall Cabbage Production. Renew. Agric. Food Syst. 2009, 24, 225–233. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Soil Physical Responses to Cattle Grazing Cover Crops under Conventional and No Tillage in the Southern Piedmont USA. Soil Tillage Res. 2008, 100, 141–153. [Google Scholar] [CrossRef]
- Yang, L.; Lamont, L.D.; Liu, S.; Guo, C.; Stoner, S. A Review on Potential Biofuel Yields from Cover Crops. Fermentation 2023, 9, 912. [Google Scholar] [CrossRef]
- Robertson, K.A. Biomass Potential in Sustainable Aviation Fuel Development: Switchgrass Production Optimization and Carinata Oilseed Enterprise Viability Analysis. Master’s Thesis, University of Tennessee, Knoxville, Tennessee, 2020. [Google Scholar]
- Rezki, B.; Essamlali, Y.; Aadil, M.; Semlal, N.; Zahouily, M. Biodiesel production from rapeseed oil and low free fatty acid waste cooking oil using a cesium modified natural phosphate catalyst. RSC Adv. 2020, 10, 41065–41077. [Google Scholar] [CrossRef]
- Fan, J.; Shonnard, D.R.; Kalnes, T.N.; Johnsen, P.B.; Rao, S. A Life Cycle Assessment of Pennycress (Thlaspi arvense L.)—Derived Jet Fuel and Diesel. Biomass Bioenergy 2013, 55, 87–100. [Google Scholar] [CrossRef]
- Trejo-Pech, C.O.; Larson, J.A.; English, B.C.; Yu, T.E. Cost and Profitability Analysis of a Prospective Pennycress to Sustainable Aviation Fuel Supply Chain in Southern USA. Energies 2019, 12, 3055. [Google Scholar] [CrossRef]
- Phippen, W.B.; Phippen, M.E. Soybean seed yield and quality as a response to field pennycress residue. Crop Sci. 2012, 52, 2767. [Google Scholar] [CrossRef]
- Chopra, R.; Johnson, E.B.; Emenecker, R.; Cahoon, E.B.; Lyons, J.; Kliebenstein, D.J. Identification and Stacking of Crucial Traits Required for the Domestication of Pennycress. Nat. Food 2020, 1, 84–91. [Google Scholar] [CrossRef]
- McGinn, M.; Phippen, W.B.; Chopra, R.; Bansal, S.; Jarvis, B.A.; Phippen, M.E. Molecular Tools Enabling Pennycress (Thlaspi arvense) as a Model Plant and Oilseed Cash Cover Crop. Plant Biotechnol. J. 2018, 17, 776–788. [Google Scholar] [CrossRef]
- Marks, M.; Chopra, R.; Sedbrook, J. Technologies enabling rapid crop improvements for sustainable agriculture: Example pennycress (Thlaspi arvense L.). Emerg. Top. Life Sci. 2021, 5, 325–335. [Google Scholar] [CrossRef]
- Jarvis, B.A.; Romsdahl, T.B.; McGinn, M.G.; Nazarenus, T.J.; Cahoon, E.B.; Chapman, K.D.; Sedbrook, J.C. CRISPR/Cas9-Induced Fad2 and Rod1 Mutations Stacked With Fae1 Confer High Oleic Acid Seed Oil in Penny-cress (Thlaspi arvense L.). Front. Plant Sci. 2021, 12, 652319. [Google Scholar] [CrossRef] [PubMed]
- Phippen, W.B.; Rhykerd, R.; Sedbrook, J.C.; Handel, C.; Csonka, S. From Farm to Flight: CoverCress as a Low Carbon Intensity Cash Cover Crop for Sustainable Aviation Fuel Production. A Review of Progress Towards Commercialization. Front. Energy Res. 2022, 10, 793776. [Google Scholar] [CrossRef]
- Moser, B.R.; Knothe, G.; Vaughn, S.F.; Isbell, T.A. Production and Evaluation of Biodiesel from Field Pennycress (Thlaspi arvense L.) Oil. Energy Fuels 2009, 23, 4149–4155. [Google Scholar] [CrossRef]
- Mousavi-Avval, S.H.; Shah, A. Life Cycle Energy and Environmental Impacts of Hydroprocessed Renewable Jet Fuel Production from Pennycress. Appl. Energy 2021, 297, 117098. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Phippen, W.B.; Marks, M.D. New Approaches to Facilitate Rapid Domestication of a Wild Plant to an Oilseed Crop: Example Pennycress (Thlaspi arvense L.). Plant Sci. 2014, 227, 122–132. [Google Scholar] [CrossRef]
- Sun, X.; Boardman, K.; Marks, D.; Wyse, D.L.; Hu, B. Fungal Bioprocessing to Improve Quality of Pennycress Meal as a Potential Feeding Ingredient for Monogastric Animals. Fermentation 2023, 9, 732. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Wang, R.L.; Holser, R.A.; Pesti, G.M. Nutritive Value and the Maximum Inclusion Level of Pennycress Meal for Broiler Chickens. Poult. Sci. 2017, 96, 2281–2293. [Google Scholar] [CrossRef]
- Hartnell, G.F.; Lemke, S.; Moore, D.; Matthews, A.; Nemeth, M.A.; Brister, R.; Liu, S.; Aulbach, C. Performance and health of broiler chickens fed low erucic acid, lower fiber pennycress (CoverCressTM) grain. Poult. Sci. 2023, 102, 102432. [Google Scholar] [CrossRef]
- Mousavi-Avval, S.; Hkanal, S.; Shah, A. Assessment of Potential Pennycress Availability and Suitable Sites for Sustainable Aviation Fuel Refineries in Ohio. Sustainability 2023, 15, 10589. [Google Scholar] [CrossRef]
- Markel, E.; English, B.C.; Hellwinckel, C.; Menard, R.J. Potential for Pennycress to Support a Renewable Jet Fuel Industry. Ecol. Pollut. Environ. Sci. 2018, 4, 95–102. Available online: https://hdl.handle.net/2376/111635 (accessed on 20 October 2023).
- Vaughn, S.F.; Isbell, T.A.; Weisleder, D.; Berhow, M.A. Biofumigant compounds released by field pennycress (Thlaspi arvense) seedmeal. J. Chem. Ecol. 2005, 31, 167–177. [Google Scholar] [CrossRef]
- Moore, S.A.; Wells, M.S.; Gesch, R.W.; Becker, R.L.; Rosen, C.J.; Wilson, M.L. Pennycress as a Cash Cover-Crop: Improving the Sustainability of Sweet Corn Production Systems. Agronomy 2020, 10, 614. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Ampese, L.; Sganzerla, W.G.; Ziero, H.D. Research progress, trends, and updates on anaerobic digestion technology: A bibliometric analysis. J. Clean. Prod. 2022, 331, 130004. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Ervasti, S.; Kostensalo, J.; Tampio, E. Effects of seasonal and local co-feedstocks on the performance of continuous anaerobic digestion of cattle slurry. Bioresour. Technol. Rep. 2022, 19, 101207. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Ge, X.; Yang, L.; Sheet, J.P.; Yu, Z.; Li, Y. Biological conversion of methane to liquid fuels: Status and opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef]
- Verhoff, K.; Phippen, W.; Heller, N.; Lindsey, A. Winter-type oilseed pennycress crop staging guide. Crop Forage Turfgrass Manag. 2022, 8, e20165. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff, S. Methane Yield Potential of Novel Perennial Biogas Crops Influenced by Harvest Date. Ind. Crop. Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Meserszmit, M.; Chrabąszcz, M.; Chylińska, M.; Szymańska-Chargot, M.; Trojanowska-Olichwer, A.; Kącki, Z. The Effect of Harvest Date and the Chemical Characteristics of Biomass from Molinia Meadows on Methane Yield. Biomass Bioenergy 2019, 130, 105391. [Google Scholar] [CrossRef]
- Wendt, L.M.; Zhao, H. Review on Bioenergy Storage Systems for Preserving and Improving Feedstock Value. Front. Bioeng. Biotechnol. 2020, 8, 370. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2015, 199, 42–48. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 43, 273–282. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association: Washington, DC, USA; Water Environment Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef]
- Anderson, G.K.; Yang, G. pH Control in Anaerobic Treatment of Industrial Wastewater. J. Environ. Eng. 1992, 118, 551–567. [Google Scholar] [CrossRef]
- Yang, L.; Lamont, L.; Sedbrook, J.; Heller, N.; Kopsell, D. Anaerobic Digestion of Cereal Rye Cover Crop. Fementation 2022, 8, 617. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Wu, D.; Zhang, P.; Wang, H.; Tao, X.; Ye, J.; Nabi, M. Enzyme pretreatment enhancing biogas yield from corn stover: Feasibility, optimization, and mechanism analysis. J. Agric. Food Chem. 2018, 66, 10026–10032. [Google Scholar] [CrossRef]
- Lizasoain, J.; Trulea, A.; Gittinger, J.; Kral, I. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. [Google Scholar] [CrossRef]
- Singh, R.; Behera, S.; Yadav, Y.K.; Kumar, S. Potential of Wheat Straw for Biogas Production Using Thermophiles; Sardar Swaran Singh National Institute of Renewable Energy: Kapurthala, India, 2014; Volume 3. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, F.; Li, Y. Effects of total ammonia nitrogen concentration on solid-state anaerobic digestion of corn stover. Bioresour. Technol. 2013, 144, 281–287. [Google Scholar] [CrossRef]
- Luo, L.; Qu, Y.; Gong, W.; Qin, L.; Li, W.; Sun, Y. Effect of Particle Size on the Aerobic and Anaerobic Digestion Characteristics of Whole Rice Straw. Energies 2021, 14, 3960. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar]
- Essien, D.; Richard, T.L. Ensiled Wet Storage Accelerates Pretreatment for Bioconversion of Corn Stover. Front. Bioeng. Biotechnol. 2018, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Perschke, Y.; Fontaine, D.; Ward, A.J.; Eriksen, J.; Sorensen, P.; Moller, H.B. Co-ensiling of cover crops and barley straw for biogas production. Renew. Energy 2019, 142, 677–683. [Google Scholar] [CrossRef]
- Vlierberghe, C.V.; Escudie, R.; Bernet, N.; Santa-Catalina, G.; Frederic, S.; Carrere, H. Conditions for efficient alkaline storage of cover crops for biomethane production. Bioresour. Technol. 2022, 348, 126722. [Google Scholar] [CrossRef] [PubMed]
- Vlierberghe, C.V.; Escudie, R.; Bernet, N.; Frederic, S.; Carrere, H. Long term alkaline storage and pretreatment process of cover crops for anaerobic digestion. Bioresour. Technol. 2021, 330, 124986. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of kinetic parameters with process parameters and operational conditions during anaerobic digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Franco, R.T.; Buffiere, P.; Bayard, R. Ensiling for biogas production: Critical parameters. A review. Biomass Bioenergy 2016, 94, 94–104. [Google Scholar] [CrossRef]
- Phippen, W.B.; Gallant, J.V.; Phippen, M.E. Evaluation of planting method and seeding rates with field pennycress (Thlaspi arvense L.). In Proceedings of the 22nd AAIC Annual Meeting, Fort Collins, CO, USA, 18–22 September 2010. [Google Scholar]
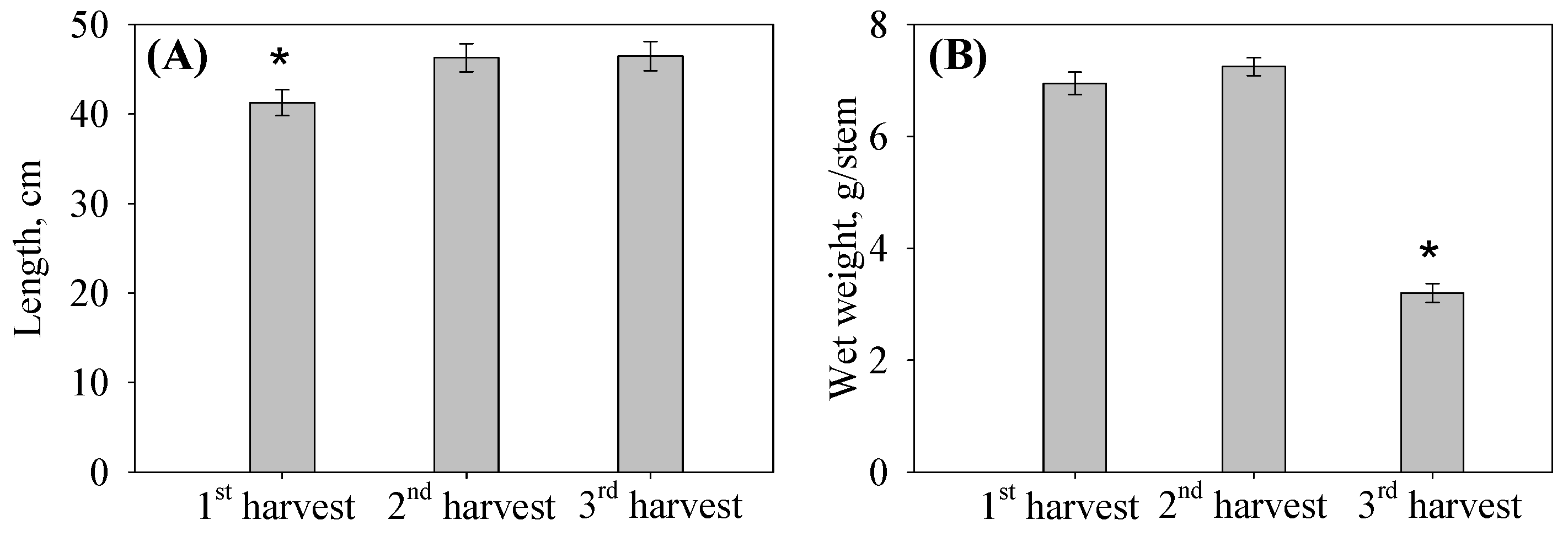


| Composition | 1st Harvest | 2nd Harvest | 3rd Harvest |
|---|---|---|---|
| Length, cm | 41.28 ± 1.46 | 46.29 ± 1.59 | 46.48 ± 1.64 |
| Wet wt, g/stem | 6.95 ± 0.20 | 7.25 ± 0.16 | 3.20 ± 0.17 |
| Dry matter, g/stem | 1.65 ± 0.04 | 1.67 ± 0.04 | 1.62 ± 0.09 |
| Total solids, % | 23.81 ± 0.35 | 23.03 ± 0.13 | 50.55 ±1.13 |
| Volatile solids, % | 21.62 ± 0.31 | 21.34 ± 0.11 | 45.97 ± 1.02 |
| Protein, % | 13.56 ± 0.91 | 14.44 ± 0.01 | 13.19 ± 0.28 |
| ADF, % | 32.69 ± 1.40 | 37.84 ± 0.58 | 41.47 ± 1.50 |
| NDF, % | 36.59 ± 0.01 | 43.85 ± 1.29 | 44.95 ± 0.01 |
| Lignin, % | 6.81 ± 0.17 | 8.71 ± 0.01 | 8.95 ± 0.02 |
| C, % | 40.70 ± 1.10 | 43.65 ± 0.25 | 49.00 ± 0.10 |
| N, % | 2.09 ± 0.07 | 2.31 ± 0.01 | 2.12 ± 0.05 |
| 2.54 cm Biomass | 1.27 cm Biomass | |||||
|---|---|---|---|---|---|---|
| TS | 1st Harvest | 2nd Harvest | 3rd Harvest | 1st Harvest | 2nd Harvest | 3rd Harvest |
| pH | pH | |||||
| 4% | 5.94 ± 0.07 | 7.24 ± 0.61 | 8.13 ± 0.14 | 5.02 ± 0.04 | 7.27 ± 0.90 | 7.91 ± 0.20 |
| 6% | 6.22 ± 0.02 | 6.47 ± 0.30 | 8.12 ± 0.06 | 5.41 ± 0.02 | 6.46 ± 1.17 | 8.31 ± 0.13 |
| 8% | 6.48 ± 0.14 | 7.35 ± 0.59 | 8.27 ± 0.27 | 5.49 ± 0.02 | 6.39 ± 0.98 | 7.95 ± 0.10 |
| Alkalinity, mg/L CaCO3 | Alkalinity, mg/L CaCO3 | |||||
| 4% | 1854 ± 0 | 2414 ± 177 | 3058 ± 243 | 1052 ± 219 | 2679 ± 612 | 2710 ± 254 |
| 6% | 3507 ± 157 | 3240 ± 197 | 3616 ± 143 | 3244 ± 79 | 2995 ± 658 | 3461 ± 56 |
| 8% | 4665 ± 91 | 4457 ± 379 | 4331 ± 34 | 3469 ± 5 | 3713 ± 425 | 4293 ± 76 |
| NH4-N, mg/L | NH4-N, mg/L | |||||
| 4% | 787 ± 34 | 692 ± 34 | 617 ± 29 | 663 ± 3 | 675 ± 25 | 608 ± 18 |
| 6% | 1067 ± 44 | 1035 ± 51 | 842 ± 25 | 1220 ± 0 | 953 ± 28 | 903 ± 43 |
| 8% | 1475 ± 13 | 1353 ± 34 | 1190 ± 30 | 1220 ± 35 | 1228 ± 18 | 1163 ± 23 |
| Biomass, AD Conditions | P (L/kg-VS) | Rm (L/kg-VS.d) | λ (d) |
|---|---|---|---|
| 1.27 cm, 3rd harvest, F/I = 2, TS = 4% | 130 ± 6.09 | 6.24 ± 0.49 | 12.8 ± 0.73 |
| 2.54 cm 3rd-harvest biomass, F/I = 2, TS = 4% | 181 ± 4.5 | 9.15 ± 0.50 | 20.6 ± 0.51 |
| 2.54 cm 3rd-harvest biomass, F/I = 1, TS = 4% | 200 ± 8.2 | 8.22 ± 0.78 | 5.92 ± 1.13 |
| 2.54 cm, 3rd harvest, F/I = 2, TS = 4%, pretreated | 191 ± 3.31 | 11.7 ± 0.81 | 0.28 ± 0.63 |
| 2.54 cm, 2nd harvest, F/I = 2, TS = 4%, pretreated | 266 ± 3.01 | 21.2 ± 1.08 | 1.35 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Lubna, T.Y.; Moklak, M.A.; Gautam, B.; Heller, N.J.; Rhykerd, R.L.; Kopsell, D.E.; Sedbrook, J.C. Impacts of Harvest Date and Concurrent Alkali Pretreatment and Ensiling on Anaerobic Digestion of Pennycress Biomass. Fermentation 2024, 10, 96. https://doi.org/10.3390/fermentation10020096
Yang L, Lubna TY, Moklak MA, Gautam B, Heller NJ, Rhykerd RL, Kopsell DE, Sedbrook JC. Impacts of Harvest Date and Concurrent Alkali Pretreatment and Ensiling on Anaerobic Digestion of Pennycress Biomass. Fermentation. 2024; 10(2):96. https://doi.org/10.3390/fermentation10020096
Chicago/Turabian StyleYang, Liangcheng, Tuba Yasmin Lubna, Michael A. Moklak, Barsanti Gautam, Nicholas J. Heller, Robert L. Rhykerd, David E. Kopsell, and John C. Sedbrook. 2024. "Impacts of Harvest Date and Concurrent Alkali Pretreatment and Ensiling on Anaerobic Digestion of Pennycress Biomass" Fermentation 10, no. 2: 96. https://doi.org/10.3390/fermentation10020096
APA StyleYang, L., Lubna, T. Y., Moklak, M. A., Gautam, B., Heller, N. J., Rhykerd, R. L., Kopsell, D. E., & Sedbrook, J. C. (2024). Impacts of Harvest Date and Concurrent Alkali Pretreatment and Ensiling on Anaerobic Digestion of Pennycress Biomass. Fermentation, 10(2), 96. https://doi.org/10.3390/fermentation10020096







