Innovative Approaches to Camembert Cheese: Optimizing Prebiotics and Coagulation Conditions for Enhanced Quality and Nutrition
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Biological Material
2.3. Experimental Designs Methodology
2.4. Assessment Methods
2.4.1. Extraction of Okra Fruit Mucilage
2.4.2. Total Flavonoid Content (TFC) Analysis
2.4.3. Method for Determining Clotting Milk Time
2.4.4. Fatty Acid Profile Analysis
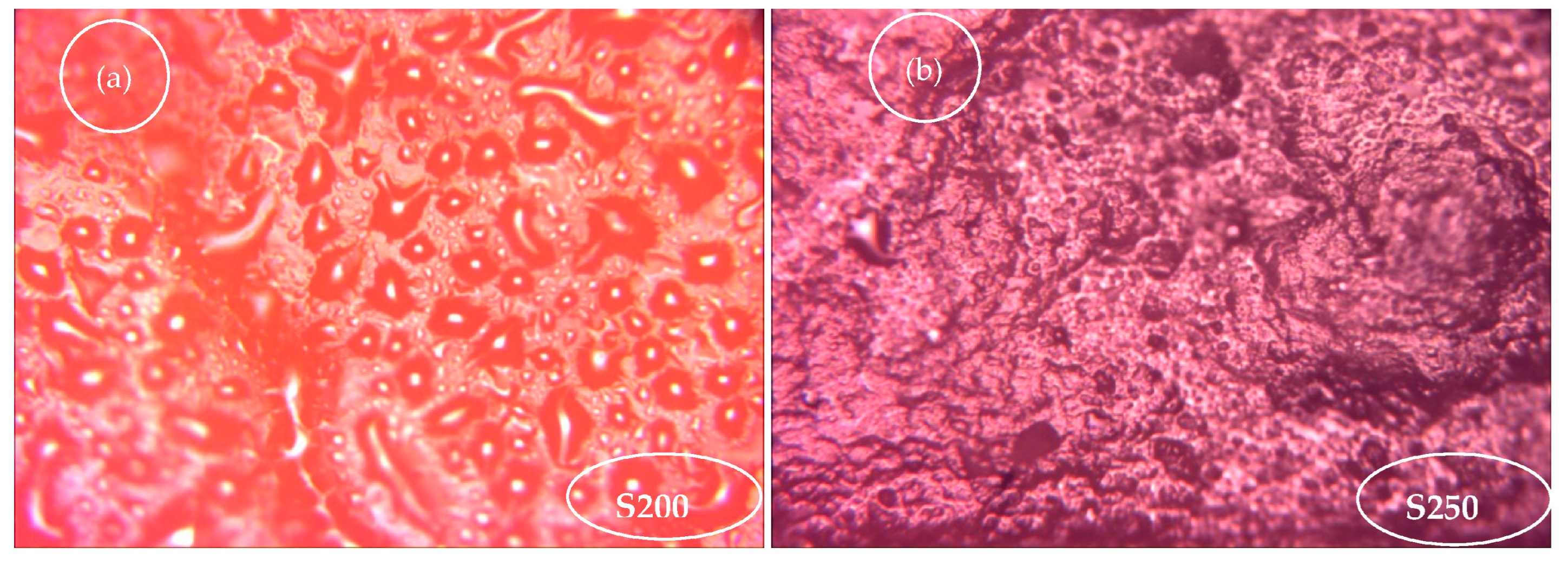
2.4.5. Microscopic Structure
2.4.6. Camembert Cheese Fabrication
2.4.7. Microbiological Analysis
3. Results and Discussion
3.1. First Optimization
3.2. Second Optimization
3.3. Third Optimization
3.4. Optimizations of Milk Coagulation Conditions by RSM
3.5. Stability and Functionality of the Optimized Mix (Prebiotics and Probiotics)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thammarutwasik, P.; Hongpattarakere, T.; Chantachum, S.; Kijroongrojuna, K.; Itharat, A.; Reanmongkol, W.; Tewtrakul, S.; Ooraikul, B. Prebiotics—A Review. J. Sci. Technol. 2009, 31, 401–408. [Google Scholar]
- Bouguerra, A. Evaluation of the Probiotic Potential of Lactic Acid Strains Isolated from Camel Milk. Doctoral Thesis, Ferhat Abbas Setif Universit 1, Setif, Algeria, 2021. [Google Scholar]
- Bruneton, J. Pharmacognosy and Phytochemistry and Phytochemistry, Medicinal Plants, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993; p. 734. [Google Scholar]
- Delzenne, N.; Cherbut, C.; Neyrinck, A. Prebiotics: Actual and potential effects in inflammatory and malignant colonic diseases. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 581–586. [Google Scholar] [CrossRef]
- Rabu, B.A.; Gibson, G.R. Carbohydrates: A limit on bacterial diversity within the colon. Biol. Rev. Camb. Philos. Soc. 2002, 77, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Warrand, J. Structural Study and Properties of Solution of the Polysaccharides Constituting the Mucilage of Flax (Linum usitatissimum L.). Ph.D. Thesis, University of Picardie Jules Verne, Amiens, France, 2004; p. 219. [Google Scholar]
- Martirosyan, D.M.; Leem, C. The bioactive compounds of probiotic foods/supplements and their application in managing mental disorders. Bioact. Compd. Health Dis. 2019, 2, 206–220. [Google Scholar] [CrossRef]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and preventive effects in allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and probiotic Functional Foods to Targett Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Al-Sheraji, H.; Ismail, S.; Manap, A.; Mustafa, M.Y.; Yusof, S.; Hassan, R.M. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Benahmed Djilali, A.; Badrani, F.; Belkhir, F.; Raman, Y. New Way to Develop Mixture of Lactic Leavens and Powder of Cardoon Flowers (Cynara cardunculus) to Produce Yoghurt: Approach to Immobilization. J. Microbiol. Biochem. Technol. 2015, 5, 4. [Google Scholar]
- Djilali, A.B.; Benseddik, A.; Boughellout, H.; Allaf, K.; Nabiev, M. Biological And functional Properties of Vine Leaves. N. Afr. J. Food Nutr. Res. 2021, 5, 43–52. [Google Scholar] [CrossRef]
- Djilali, A.B.; Metahri, M.S.; Benseddik, A.; Arabi, M.; Reniffi, T.; Saada, N.; Tonkin, N.; Allaf, K. Functional Natural Yogurt Tablet Based On Mucilag Okra Fruit And Flexseed Powder As Prebiotic. Carpath. J. Food Sci. Technol. 2023, 15, 159–168. [Google Scholar] [CrossRef]
- Ghedira, K. Flavonoids: Structure, biological properties, prophylactic rol and uses in therapy. Pharmacognosy 2005, 3, 162–169. [Google Scholar]
- Djilali, A.B.; Benseddik, A.; Metahri, M.S.; Lahouazi, D.; Simoud, D.; Nabiev, M.; Allaf, K. Elaboration of New Functional Dairy Dessert Based on Flaxseed Powder. In Proceedings of the 1st International Online Conference on Agriculture—Advances in Agricultural Science and Technology, Online, 10–25 February 2022; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D. Flax mucilage and barley beta-glucan aerogels obtained using supercritical carbon dioxide: Application as flax lignan carriers. Innov. Food Sci. Emer. Technol. 2015, 28, 40–46. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Özkan, T.; Saadat, Y.R. Evidence for health-promoting properties of Lepidium sativum L.: An updated comprehensive review. Turk. J. Pharm. Sci. 2022, 19, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R. Extraction characterization and evaluation of selected mucilage as pharmaceutical excipient. Polim. W Med. 2011, 41, 39–44. [Google Scholar]
- Fabre, J.F.; Lacroux, E.; Valentin, R.; Mouloungui, Z. Ultrasonication as a highly efficient method of flaxseed mucilage extraction. Ind. Crop. Prod. 2015, 65, 354–360. [Google Scholar] [CrossRef]
- Houeijeh, A. Study of the Kinetics of Respiratory Adaptation at Birth Using Magnetic Resonance: The Effcts of Omega Polyunsaturated Fatty Acids on the Perinatal Transition; University of Law and Health II Doctoral School Biology Health: Lille, France, 2018. [Google Scholar]
- Simon, N. Place of Essential Precursor Polyunsaturated Fatty Acids in the Diet of Specific Populations in France. Sci. News Bull. 2019, 1–6. [Google Scholar]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Valorisation of sweet whey by fermentation with mixed yoghurt starter cultures with focus on galactooligosaccharide synthesis. Int. Dairy J. 2021, 119, 105068. [Google Scholar] [CrossRef]
- Dick, M.; Dal Magro, L.; Rodrigues, R.C.; Rios, A.D.O.; Flores, F.H. Valorization of Opuntia monacantha (Willd). Haw. Cladodes to obtain a mucilage with hydrocolloid features physicochemical and functional performance. Int. J. Biol. Macromol. 2019, 130, 198–205. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Vasseur, J.; Gazin, J.C.; Pinkas, M.; Luyckx, M.; Gazin, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneim-Forsch. Drug. Res. 1996, 46, 1086. [Google Scholar]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters. Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- Bourgeois, C.; Malcoste, R. Rapid Methods for Enumerating Microorganisms Analysis and Control Techniques in IAA; Tec & Doc-Lavoisier Publisher: Paris, France, 1991. [Google Scholar]
- Amirdivani, S.; Baba, A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT–Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Walstra, P. On the stability of casein micelles. J. Dairy Sci. 1990, 73, 1965–1979. [Google Scholar] [CrossRef]
- Van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef] [PubMed]
- FAO. Milk and dairy products in human nutrition. In FAO Food and Nutrition Collection; FAO: Rome, Italy, 1995; p. 28. [Google Scholar]
- Chamba, F.J. Application of lactic acid bacteria during cheese production. In Lactic bacteria, from Genetics to Ferments; Corrieu, G., Luquet, F.M., Eds.; Tec & Doc-Lavoisier Publisher: Paris, France, 2008; pp. 787–815. [Google Scholar]
- Schmidt, J.L.; Tourneur, C.; Lenoir, J. Function and Choice of Dairy Lactic Acid Bacteria in Lactic Acid Bacteria; De Roissart, H., Luquet, F.M., Eds.; Lorica: Paris, France, 1994; Volume 2, pp. 37–46. [Google Scholar]
- Guler-Aki, M.B.; Goncu, B.; Akin, M.S. Some properties of probiotic yogurt Ice cream supplemented with carob extract and whey powder. Adv. Microbiol. 2016, 6, 1010–1020. [Google Scholar] [CrossRef]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Otsuka, H.; Yamada, H.; Especik, T. Isolation and partial characertization of biologically active polysaccharides from Plantago major L. Phytother. Res. 1995, 9, 211–218. [Google Scholar] [CrossRef]
- Rubilar, M.; Gutierrez, C.; Verdugo, M.; Shene, C.; Sineiro, J. Flaseed as a source of Functional ingredients. J. Soil Sci. Plant Nutr. 2010, 10, 373–377. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Perego, P.; Oliveira, M.N.; Converti, A. Effect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milk. LWT–Food Sci. Technol. 2011, 44, 520–523. [Google Scholar] [CrossRef]
- Jiang, B.; Yue, H.; Fu, X.; Wang, J.; Feng, Y.; Li, D.; Liu, C.; Feng, Z. One-step high efficiency separation of prolyl endopeptidse from Aspergillus niger and its application. Int. J. Biol. Macromol. 2024, 271, 132582. [Google Scholar]
- Haslam, E.; Lilley, T.H. Natural astringency in foodstuffs—A molecular interpretation. Crit. Rev. Food Sci. Nutr. 1988, 27, 1–40. [Google Scholar] [CrossRef]
- Williamson, M.P.; Trevitt, C.; Noble, J.M. NMR studies of dextran oligomer interactions with model polyphenols. Carbohydr. Res. 1995, 266, 229–235. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; El-Aziz, A.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yogurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosière, M.; Ferlay, A.; Fougère, H.; Graulet, B. Milk fat globule in ruminant: Major and minor compounds, nutritional regulation and differences among species. Eur. J. Lipid Sci. Technol. 2018, 120, 1700039. [Google Scholar] [CrossRef]
- Freitas, A.C.; Malcata, F.X. Lipolysis in Picante cheese: Influence of milk type and ripening time on free fatty acid profile. Milk 1998, 78, 251–258. [Google Scholar] [CrossRef]
- Arman, M.S.I.; Akter, S.; Mahaldar, K.; Tuly, F.A.; Hossain, M.S. Investigation of antiemetic, antimicrobial and anti-radical properties of methanolic extract of Foeniculum vulgare: A medicinal herb. Discov. Phytomed. 2019, 6, 70–76. [Google Scholar]
- Dubrovskii, I.; Arseneva, T.; Evstigneeva, T.; Gorshkova, S.; Bazarnova, Y.; Iakovchenko, N. Development of formulation and technology of yogurt with prolonged shelf life enriched with biologically active substances from fennel seed extract. Agron. Res. 2019, 17, 1313–1323. [Google Scholar]
- El Sayed, H.A.; Magdy, R.S.; El Sayed, A.; Hayfa, H.A.H.; Ashraf, A.; Ehab, K.E. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
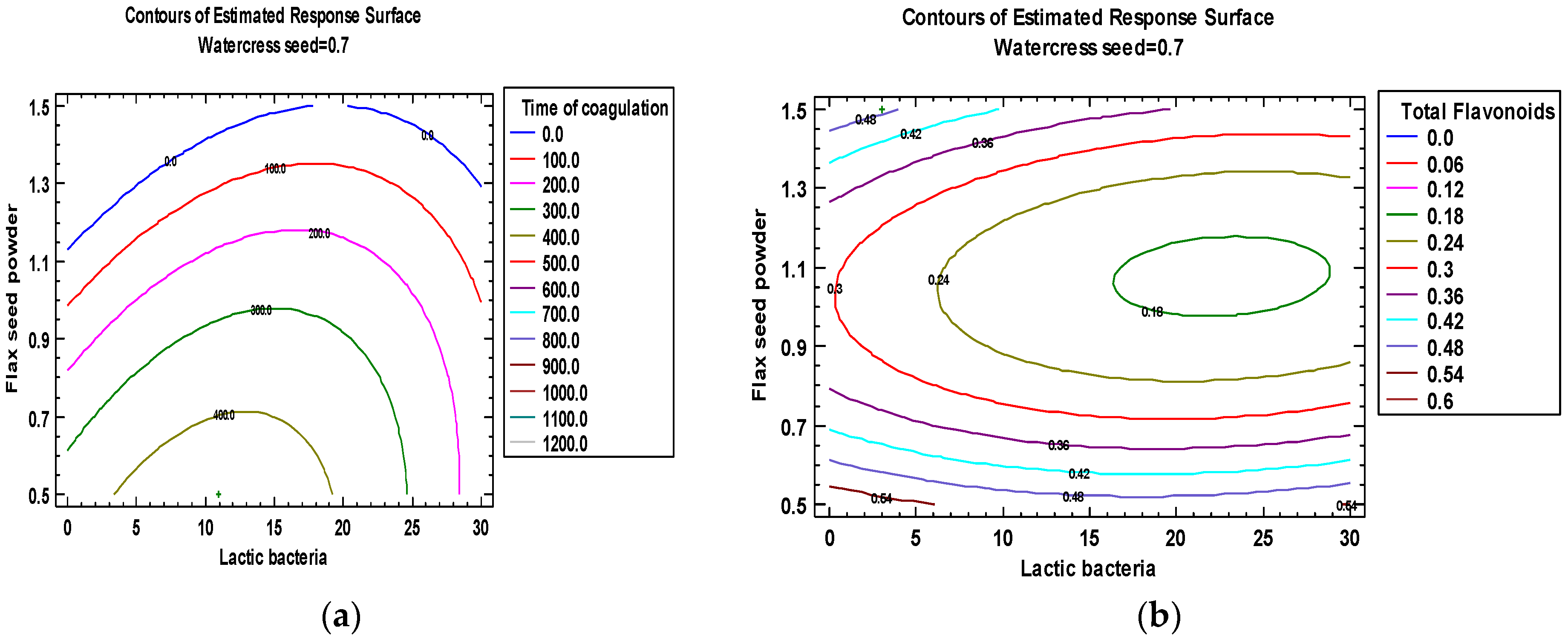
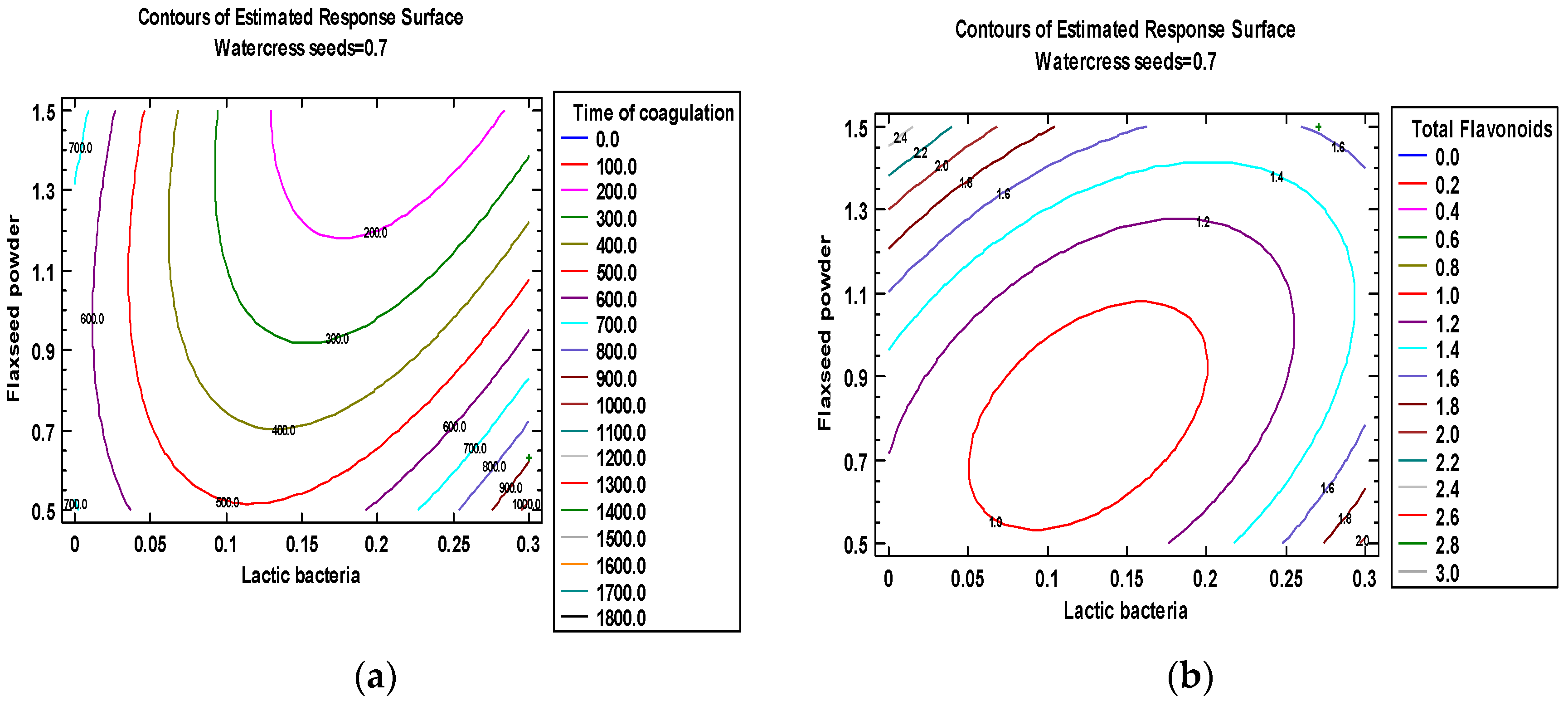
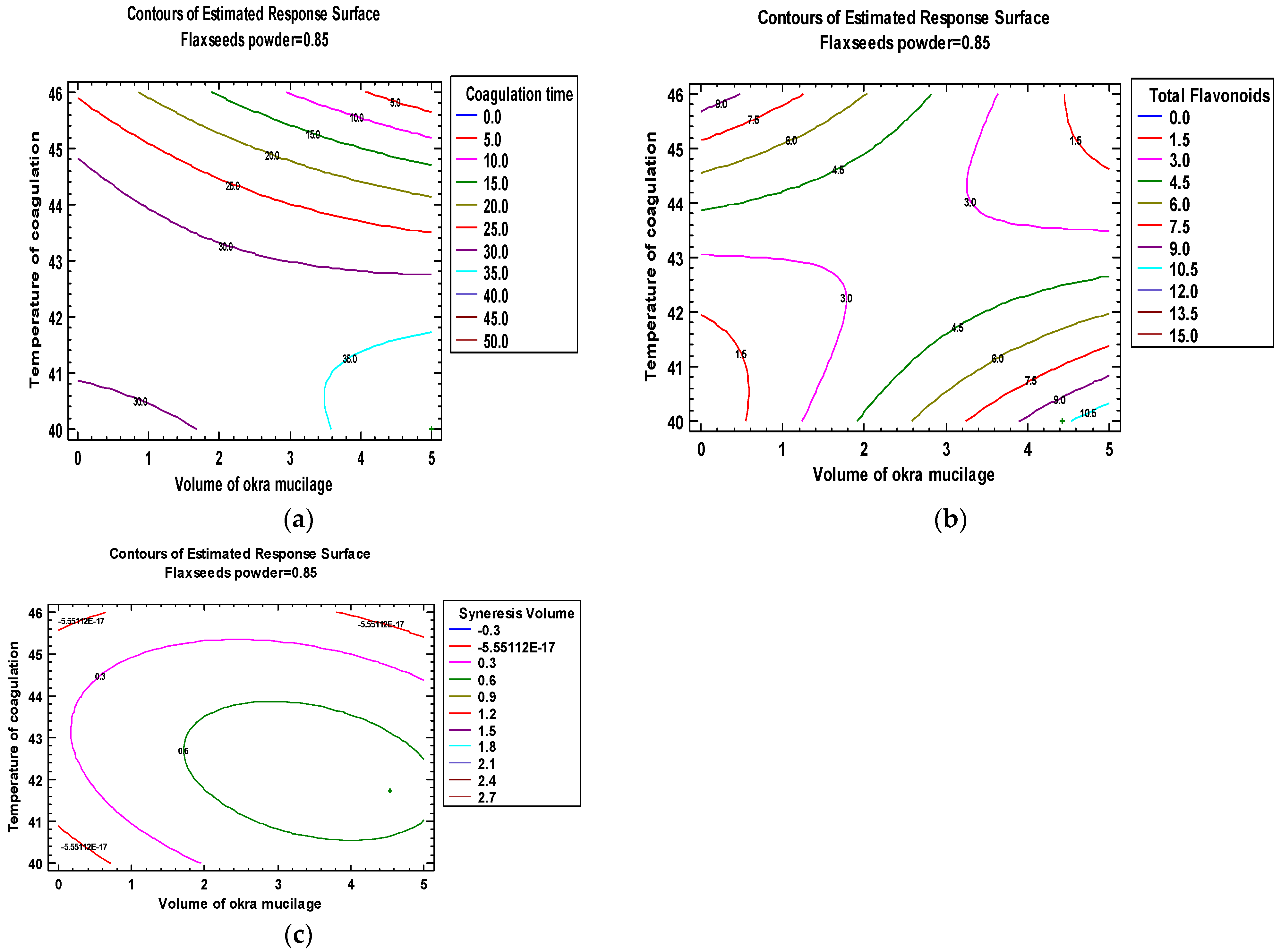

| Run N° | Treatment Parameters (Independent Variables) | Responses (Dependent Variables) | ||||
|---|---|---|---|---|---|---|
| X1 (mg/10 mL of Milk) | X2 (g/10 mL of Milk) | X3 (g/10 mL of Milk) | Y1: tc (S) | Y2: TF (mg Quercetin/g FM) | Y3: SV (mL) | |
| 01 | 24.5 (1) | 0.7 (−1) | 1 (1) | 60 ± 0 | 0.036 ± 0.05 | 0 |
| 02 | 16.5 (0) | 1.5 (1.682) | 0.7 (0) | 30 ± 0 | 0.297 ± 0.02 | 0 |
| 03 | 16.5 (0) | 0.5 (−1.682) | 0.7 (0) | 360 ± 60 | 0.598 ± 0.01 | 0 |
| 04 | 8.47 (−1) | 1.3 (1) | 1 (1) | 60 ± 0 | 0.510 ± 0.05 | 0 |
| 05 | 8.47 (−1) | 0.7 (−1) | 0.4 (−1) | 900 ± 0 | 0.268 ± 0.26 | 0 |
| 06 | 24.5 (1) | 0.7 (−1) | 0.4 (−1) | 690 ± 42.42 | 0.508 ± 0.04 | 0 |
| 07 | 8.47 (−1) | 0.7 (−1) | 1 (1) | 120 ± 0 | 0.428 ± 0.03 | 0 |
| 08 | 8.47 (−1) | 1.3 (1) | 0.4 (−1) | 220 ± 34.64 | 0.147 ± 0.01 | 0 |
| 09 | 30 (1.682) | 1 (0) | 0.7 (0) | 120 ± 0 | 0.226 ± 0.20 | 0 |
| 10 | 16.5 (0) | 1 (0) | 1.2 (1.682) | 28 ± 3.46 | 0.393 ± 0.03 | 0 |
| 11 | 3 (−1.682) | 1 (0) | 0.7 (0) | 105 ± 21.21 | 0.256 ± 0.01 | 0 |
| 12 | 24.5 (1) | 1.3 (1) | 1 (1) | 30 ± 0 | 0.262 ± 0.02 | 0 |
| 13 | 24.5 (1) | 1.3 (1) | 0.4 (−1) | 240 ± 0 | 0.223 ± 0.01 | 0 |
| 14 | 16.5 (0) | 1 (0) | 0.2 (−1.682) | 910 ± 14.14 | 0 | 0 |
| 15 | 16.5 (0) | 1 (0) | 0.7 (0) | 217.5 ± 3.53 | 0.158 ± 0.02 | 0 |
| 16 | 16.5 (0) | 1 (0) | 0.7 (0) | 370 ± 28.284 | 0.199 ± 0.03 | 0 |
| 17 | 16.5 (0) | 1 (0) | 0.7 (0) | 390 ± 42.426 | 0.055 ± 0.01 | 0 |
| 18 | 16.5 (0) | 1 (0) | 0.7 (0) | 185.5 ± 2.121 | 0.319 ± 0.02 | 0 |
| Run N° | Treatment Parameters (Independent Variables) | Responses (Dependent Variables) | ||||
|---|---|---|---|---|---|---|
| X1 (mg/10 mL of Milk) | X2 (g/10 mL of Milk) | X3 (g/10 mL of Milk) | Y1: tc (S) | Y2: TF (mg Quercetin/g FM) | Y3: SV (mL) | |
| 01 | 0.1 (−1) | 1.3 (1) | 0.4 (−1) | 300 ± 0.016 | 1.45 ± 0.028 | 0 |
| 02 | 0.25 (1) | 0.7 (−1) | 1.0 (1) | 60 ± 0 | 1.1 ± 0 | 0 |
| 03 | 0.175 (0) | 1.5 (1.682) | 0.7 (0) | 120 ± 0 | 1.85 ± 0.04 | 0 |
| 04 | 0.1 (−1) | 0.7 (−1) | 0.4 (−1) | 139.8 ± 0.01 | 1.37 ± 0.04 | 0 |
| 05 | 0.05 (−1.682) | 1.0 (0) | 0.7 (0) | 1020 ± 0.03 | 1.12 ± 0.01 | 0 |
| 06 | 0.30 (1.682) | 1.0 (0) | 0.7 (0) | 240 ± 0.016 | 1.22 ± 0.05 | 0 |
| 07 | 0.25 (1) | 1.3 (1) | 1.0 (1) | 120 ± 0 | 1.69 ± 0.01 | 0 |
| 08 | 0.25 (1) | 0.7 (−1) | 0.4 (−1) | 1519.8 ± 0.01 | 2.21 ± 1.05 | 0 |
| 09 | 0.175 (0) | 0.5 (−1.682) | 0.7 (0) | 820.2 ± 0.017 | 0.64 ± 0.05 | 0 |
| 10 | 0.25 (1) | 1.3 (1) | 0.4 (−1) | 900 ± 0.016 | 0.89 ± 0.03 | 0 |
| 11 | 0.1 (−1) | 0.7 (−1) | 1.0 (1) | 319.8 ± 0.007 | 0.95 ± 0.03 | 0 |
| 12 | 0.175 (0) | 1.0 (0) | 1.2 (1.682) | 139.8 ± 0.001 | 1.19 ± 0.02 | 0 |
| 13 | 0.175 (0) | 1.0 (0) | 0.2 (−1.682) | 1419.6 ± 0.02 | 0.51 ± 0.05 | 0 |
| 14 | 0.1 (−1) | 1.3 (1) | 1.0 (1) | 100.2 ± 0.05 | 1.13 ± 0.02 | 0 |
| 15 | 0.175 (0) | 1.0 (0) | 0.7 (0) | 280.2 ± 0.05 | 0.98 ± 0.02 | 0 |
| 16 | 0.175 (0) | 1.0 (0) | 0.7 (0) | 280.2 ± 0.05 | 0.92 ± 0.01 | 0 |
| 17 | 0.175 (0) | 1.0 (0) | 0.7 (0) | 300 ± 0 | 1.04 ± 0.05 | 0 |
| 18 | 0.175 (0) | 1.0 (0) | 0.7 (0) | 160.2 ± 0.05 | 0.98 ± 0.01 | 0 |
| Run N° | Treatment Parameters (Independent Variables) | Responses (Dependent Variables) | ||||
|---|---|---|---|---|---|---|
| X1 (mL/10 mL of Milk) | X2 (°C) | X3 (g/10 mL of Milk) | Y1: tc (S) | Y2: TF (mg Quercetin/g FM) | Y3: SV (mL) | |
| 01 | 2.75 (0) | 42.85 (0) | 0.85 (0) | 31.333 ± 0.026 | 2.77 ± 0.03 | 1.066 ± 0.015 |
| 02 | 1.41 (−1) | 41.16 (−1) | 0.46 (−1) | 26 ± 0.01 | 5.196 ± 0.03 | 1 ± 0.043 |
| 03 | 4.09 (1) | 41.16 (−1) | 0.46 (−1) | 26.666 ± 0.015 | 4.453 ± 0.03 | 1.533 ± 0.025 |
| 04 | 1.41 (−1) | 44.54 (1) | 0.46 (−1) | 20 ± 0.026 | 3.22 ± 0.03 | 1.4 ± 0.05 |
| 05 | 4.09 (1) | 44.54 (1) | 0.46 (−1) | 24.333 ± 0.025 | 6.37 ± 0.036 | 1.066 ± 0.008 |
| 06 | 1.41 (−1) | 41.16 (−1) | 1.24 (1) | 40.666 ± 0.014 | 3.496 ± 0.03 | 0.5 ± 0.02 |
| 07 | 4.09 (1) | 41.16 (−1) | 1.24 (1) | 46.33 ± 0.037 | 12.63 ± 0.01 | 0.133 ± 0.05 |
| 08 | 1.41 (−1) | 44.54 (1) | 1.24 (1) | 22.666 ± 0.041 | 0.3 ± 0.034 | 0.3 ± 0.034 |
| 09 | 4.09 (1) | 44.54 (1) | 1.24 (1) | 17.333 ± 0.028 | 2.013 ± 0.90 | 0.3 ± 0.01 |
| 10 | 2.75 (0) | 42.85 (0) | 0.85 (0) | 13 ± 0.05 | 1.704 ± 0.03 | 0.033 ± 0.057 |
| 11 | 2.75 (0) | 42.85 (0) | 0.85 (0) | 40 ± 0 | 6.326 ± 0.02 | 0.833 ± 0.030 |
| 12 | 0.5 (−1.682) | 42.85 (0) | 0.85 (0) | 30 ± 0 | 1.906 ± 0.05 | 0.133 ± 0.015 |
| 13 | 5 (1.682) | 42.85 (0) | 0.85 (0) | 26.333 ± 0.015 | 2.73 ± 0.016 | 0.7 ± 0.043 |
| 14 | 2.75 (0) | 40 (−1.682) | 0.85 (0) | 27 ± 0.024 | 5.303 ± 0.04 | 0.5 ± 0.036 |
| 15 | 2.75 (0) | 45.7 (1.68) | 0.85 (0) | 13 ± 0.005 | 3.11 ± 0.037 | 0 |
| 16 | 2.75 (0) | 42.85 (0) | 0.2 (−1.682) | 25.333 ± 0.05 | 3.146 ± 0.04 | 2.333 ± 0.007 |
| 17 | 2.75 (0) | 42.85 (0) | 1.5 (1.682) | 33.333 ± 0.028 | 6.376 ± 0.03 | 0.366 ± 0.03 |
| 18 | 2.75 (0) | 42.85 (0) | 0.85 (0) | 40 ± 0 | 3.143 ± 0.02 | 0.866 ± 0.05 |
| Optimization | Optimized Variables Values | Predicted Optimized Response Values | Desirability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic Bacteria (mg/10 mL of Milk) | Flax Seed Powder (g/10 mL of Milk) | Watercress Seed Powder (g/10 mL of Milk) | Volume of Okra Mucilage (mL/10 of Milk) | Temperature of Coagulation (°C) | Tc (s) | TF (mg Quercetin Equivalent/g FM) | SV (mL) | ||
| 1 | 6.53 | 1.05 | 1.196 | - | Fixed at 45 | 5.867 | 0.624 | - | 1 |
| 2 | 0.181 | 1.5 | 1.147 | - | Fixed at 38 | 2 | 2.225 | - | 1 |
| 3 | Fixed at 3 | 1.5 | - | 0.50 | 45.7 | 9.512 | 11.014 | 0.116 | 0.933 |
| Researched Germs | Cheese Made with Optimized Curd | Camembert Cheese from ATTOUCHE MILK Industry | Standards (OJRA, 1998) |
|---|---|---|---|
| Total coliforms at 37 °C | Absent | Absent | 100 |
| Fecal coliforms at 44 °C | Absent | Absent | 10 |
| Mesophilic lactic acid bacteria at 30 °C | 6.3 × 103 | Absent | - |
| Thermophilic lactic acid bacteria at 44 °C | 3.5 × 103 | Non-significant | - |
| Staphylococcus aureus at 37 °C | Absent | Absent | Absent |
| Yeasts and Molds | >300 | Non-significant | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benahmed Djilali, A.; Metahri, M.S.; Lakabi, L.; Tahraoui, H.; Benseddik, A.; Besombes, C.; Allaf, K. Innovative Approaches to Camembert Cheese: Optimizing Prebiotics and Coagulation Conditions for Enhanced Quality and Nutrition. Fermentation 2024, 10, 524. https://doi.org/10.3390/fermentation10100524
Benahmed Djilali A, Metahri MS, Lakabi L, Tahraoui H, Benseddik A, Besombes C, Allaf K. Innovative Approaches to Camembert Cheese: Optimizing Prebiotics and Coagulation Conditions for Enhanced Quality and Nutrition. Fermentation. 2024; 10(10):524. https://doi.org/10.3390/fermentation10100524
Chicago/Turabian StyleBenahmed Djilali, Adiba, Mohammed Said Metahri, Lynda Lakabi, Hichem Tahraoui, Abdelouahab Benseddik, Colette Besombes, and Karim Allaf. 2024. "Innovative Approaches to Camembert Cheese: Optimizing Prebiotics and Coagulation Conditions for Enhanced Quality and Nutrition" Fermentation 10, no. 10: 524. https://doi.org/10.3390/fermentation10100524
APA StyleBenahmed Djilali, A., Metahri, M. S., Lakabi, L., Tahraoui, H., Benseddik, A., Besombes, C., & Allaf, K. (2024). Innovative Approaches to Camembert Cheese: Optimizing Prebiotics and Coagulation Conditions for Enhanced Quality and Nutrition. Fermentation, 10(10), 524. https://doi.org/10.3390/fermentation10100524







