Electronic and Magnetic Properties of FeCl3 Intercalated Bilayer Graphene
Abstract
:1. Introduction
2. Computational Detail
3. Results
3.1. Structural Properties
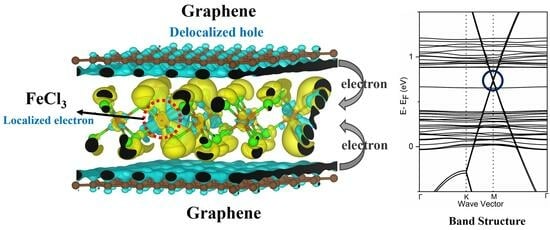
3.2. Magnetic and Electronic Properties
3.3. Pressure Dependence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| BLG | Bilayer graphene |
| SLG | Single-layer graphene |
| FLG | Few-layer graphene |
| GICs | Graphite intercalation compounds |
| DOS | Density of state |
Appendix A

References
- Rüdorff, W. Graphite intercalation compounds. In Advances in Inorganic Chemistry and Radiochemistry; Elsevier: Amsterdam, The Netherlands, 1959; Volume 1, pp. 223–266. [Google Scholar]
- Selig, H.; Ebert, L.B. Graphite intercalation compounds. In Advances in Inorganic Chemistry and Radiochemistry; Elsevier: Amsterdam, The Netherlands, 1980; Volume 23, pp. 281–327. [Google Scholar]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-principles study of alkali metal-graphite intercalation compounds. J. Power Sources 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Dresselhaus, M.S. Intercalation in layered materials. MRS Bull. 1987, 12, 24–28. [Google Scholar] [CrossRef]
- Besenhard, J. The electrochemical preparation and properties of ionic alkali metal-and NR4-graphite intercalation compounds in organic electrolytes. Carbon 1976, 14, 111–115. [Google Scholar] [CrossRef]
- Pruvost, S.; Hérold, C.; Hérold, A.; Lagrange, P. Co-intercalation into graphite of lithium and sodium with an alkaline earth metal. Carbon 2004, 42, 1825–1831. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G. Intercalation compounds of graphite. Adv. Phys. 2002, 51, 1–186. [Google Scholar] [CrossRef]
- Noel, M.; Santhanam, R. Electrochemistry of graphite intercalation compounds. J. Power Sources 1998, 72, 53–65. [Google Scholar] [CrossRef]
- Jiang, H. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Moon, S.Y.; Bang, S.Y.; Choi, B.G.; Ham, H.; Sekino, T.; Shim, K.B. Fabrication of graphene layers from multiwalled carbon nanotubes using high dc pulse. Appl. Phys. Lett. 2009, 95, 083103. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Wang, H.; Strait, J.H.; George, P.A.; Shivaraman, S.; Shields, V.B.; Chandrashekhar, M.; Hwang, J.; Rana, F.; Spencer, M.G.; Ruiz-Vargas, C.S.; et al. Ultrafast relaxation dynamics of hot optical phonons in graphene. Appl. Phys. Lett. 2010, 96, 081917. [Google Scholar] [CrossRef]
- Kampfrath, T.; Perfetti, L.; Schapper, F.; Frischkorn, C.; Wolf, M. Strongly coupled optical phonons in the ultrafast dynamics of the electronic energy and current relaxation in graphite. Phys. Rev. Lett. 2005, 95, 187403. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single-and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Lui, C.H.; Li, Z.; Chen, Z.; Klimov, P.V.; Brus, L.E.; Heinz, T.F. Imaging stacking order in few-layer graphene. Nano Lett. 2011, 11, 164–169. [Google Scholar] [CrossRef]
- Wei, D.; Wu, B.; Guo, Y.; Yu, G.; Liu, Y. Controllable chemical vapor deposition growth of few layer graphene for electronic devices. Accounts Chem. Res. 2013, 46, 106–115. [Google Scholar] [CrossRef]
- Orsu, P.; Koyyada, A. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: A comprehensive review. Mater. Today Commun. 2020, 22, 100823. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, P.X. Fabrication and characterization of few-layer graphene. Carbon 2010, 48, 359–364. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; He, C.; Dai, L.; Liu, J.; Wang, L. Rationally designed surfactants for few-layered graphene exfoliation: Ionic groups attached to electron-deficient π-conjugated unit through alkyl spacers. ACS Nano 2014, 8, 6663–6670. [Google Scholar] [CrossRef] [PubMed]
- Nijamudheen, A.; Sarbapalli, D.; Hui, J.; Rodríguez-López, J.; Mendoza-Cortes, J.L. Impact of surface modification on the lithium, sodium, and potassium intercalation efficiency and capacity of few-layer graphene electrodes. ACS Appl. Mater. Interfaces 2020, 12, 19393–19401. [Google Scholar] [CrossRef]
- McCann, E.; Koshino, M. The electronic properties of bilayer graphene. Rep. Prog. Phys. 2013, 76, 056503. [Google Scholar] [CrossRef]
- Ohta, T.; Bostwick, A.; Seyller, T.; Horn, K.; Rotenberg, E. Controlling the electronic structure of bilayer graphene. Science 2006, 313, 951–954. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of few-layer graphite flakes with FeCl3: Raman determination of Fermi level, layer by layer decoupling, and stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef]
- Sun, Y.; Han, F.; Zhang, C.; Zhang, F.; Zhou, D.; Liu, H.; Fan, C.; Li, X.; Liu, J. FeCl3 Intercalated microcrystalline graphite enables high volumetric capacity and good cycle stability for lithium-ion batteries. Energy Technol. 2019, 7, 1801091. [Google Scholar] [CrossRef]
- Millman, S.; Holmes, B.; Zimmerman, G. Magnetic susceptibility study of magnetic properties in low stage FeCl3 intercalated graphite. Solid State Commun. 1982, 43, 903–906. [Google Scholar] [CrossRef]
- Kim, N.; Kim, K.S.; Jung, N.; Brus, L.; Kim, P. Synthesis and electrical characterization of magnetic bilayer graphene intercalate. Nano Lett. 2011, 11, 860–865. [Google Scholar] [CrossRef]
- Nathaniel, J.; Wang, X.Q. Tunable electron and hole doping in FeCl3 intercalated graphene. Appl. Phys. Lett. 2012, 100, 213112. [Google Scholar] [CrossRef]
- Bonacum, J.P.; O’Hara, A.; Bao, D.L.; Ovchinnikov, O.S.; Zhang, Y.F.; Gordeev, G.; Arora, S.; Reich, S.; Idrobo, J.C.; Haglund, R.F.; et al. Atomic-resolution visualization and doping effects of complex structures in intercalated bilayer graphene. Phys. Rev. Mater. 2019, 3, 064004. [Google Scholar] [CrossRef]
- Zhan, D.; Sun, L.; Ni, Z.H.; Liu, L.; Fan, X.F.; Wang, Y.; Yu, T.; Lam, Y.M.; Huang, W.; Shen, Z.X. FeCl3-based few-layer graphene intercalation compounds: Single linear dispersion electronic band structure and strong charge transfer doping. Adv. Funct. Mater. 2010, 20, 3504–3509. [Google Scholar] [CrossRef]
- Zou, X.; Zhan, D.; Fan, X.; Lee, D.; Nair, S.K.; Sun, L.; Ni, Z.; Luo, Z.; Liu, L.; Yu, T.; et al. Ultrafast carrier dynamics in pristine and FeCl3-intercalated bilayer graphene. Appl. Phys. Lett. 2010, 97, 141910. [Google Scholar] [CrossRef]
- Li, Y.; Yue, Q. First-principles study of electronic and magnetic properties of FeCl3-based graphite intercalation compounds. Phys. B Condens. Matter 2013, 425, 72–77. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Aryasetiawan, F.; Lichtenstein, A. First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA+ U method. J. Phys. Condens. Matter 1997, 9, 767. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+ U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [PubMed]



| Material | Stacking Pattern | Magnetic Configuration | Lattice Constant a (Å) | Energy (eV) | (eV) | Magnetic Momentum () | (Å) | (Å) | Charge Transfer (e) |
|---|---|---|---|---|---|---|---|---|---|
| Graphene | - | - | 12.339 | −462.874 | - | 0.000 | - | 0 | - |
| BLG | AA | - | 12.337 | −927.804 | −2.056 | 0.000 | 3.656 | 0 | - |
| AB | - | 12.336 | −928.065 | −2.308 | 0.000 | 3.486 | 0 | - | |
| Graphite | AA | - | 12.337 | −928.351 | −2.604 | 0.000 | 3.621 | 0 | - |
| AB | - | 12.336 | −928.939 | −3.120 | 0.000 | 3.485 | 0 | - | |
| FeCl | - | FM | 12.316 | −122.800 | - | 40.000 | - | - | - |
| AFM | 12.316 | −122.817 | - | 0.000 | - | - | - | ||
| FeCl-BLG | AA | FM | 12.331 | −1053.586 | −2.982 | 39.006 | 9.550 | 0.007/0.011 | 0.982 |
| AFM | 12.332 | −1053.571 | −2.950 | 1.001 | 9.549 | 0.007/0.010 | 0.975 | ||
| AB | FM | 12.332 | −1053.579 | −2.714 | 39.005 | 9.567 | 0.010/0.026 | 0.984 | |
| AFM | 12.332 | −1053.572 | −2.690 | 1.001 | 9.563 | 0.006/0.025 | 0.975 | ||
| FeCl-GIC | AB | FM | 12.333 | −2112.323 | −4.422 | 78.001 | 9.510 | 0.005/0.035 | 2.003 |
| AFM | 12.333 | −2112.323 | −4.405 | 0.000 | 9.510 | 0.005/0.035 | 1.985 | ||
| AFM2 | 12.333 | −2112.357 | −4.439 | 2.000 | 9.510 | 0.005/0.034 | 1.968 |
| (Å) | (eV) | Shift of Dirac Point/ Charge Transfer Value | (eV) | Shift of Dirac Point/ Charge Transfer Value |
|---|---|---|---|---|
| 9.5 | −1053.572 | 0.7579/0.9907 | −1053.549 | 0.6919/0.9799 |
| 9.4 | −1053.521 | 0.7648/0.9988 | −1053.498 | 0.7021/0.9865 |
| 9.3 | −1053.448 | 0.7666/1.0144 | −1053.429 | 0.7065/0.9993 |
| 9.2 | −1053.330 | 0.7734/1.0247 | −1053.306 | 0.7180/1.0033 |
| 9.1 | −1053.151 | 0.7740/1.0391 | −1053.124 | 0.7249/1.0134 |
| 9.0 | −1052.915 | 0.7753/1.0579 | −1052.886 | 0.7259/1.0307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Yadav, S.; Paulus, B. Electronic and Magnetic Properties of FeCl3 Intercalated Bilayer Graphene. C 2023, 9, 95. https://doi.org/10.3390/c9040095
Dai J, Yadav S, Paulus B. Electronic and Magnetic Properties of FeCl3 Intercalated Bilayer Graphene. C. 2023; 9(4):95. https://doi.org/10.3390/c9040095
Chicago/Turabian StyleDai, Jiajun, Shilpa Yadav, and Beate Paulus. 2023. "Electronic and Magnetic Properties of FeCl3 Intercalated Bilayer Graphene" C 9, no. 4: 95. https://doi.org/10.3390/c9040095
APA StyleDai, J., Yadav, S., & Paulus, B. (2023). Electronic and Magnetic Properties of FeCl3 Intercalated Bilayer Graphene. C, 9(4), 95. https://doi.org/10.3390/c9040095