Towards Understanding the Factors behind the Limited Integration of Multispecies Ecotoxicity Assessment in Environmental Risk Characterisation of Graphene-Family Materials—A Bibliometric Review
Abstract
:1. Introduction
2. Bibliometric Analysis
3. Results and Discussion
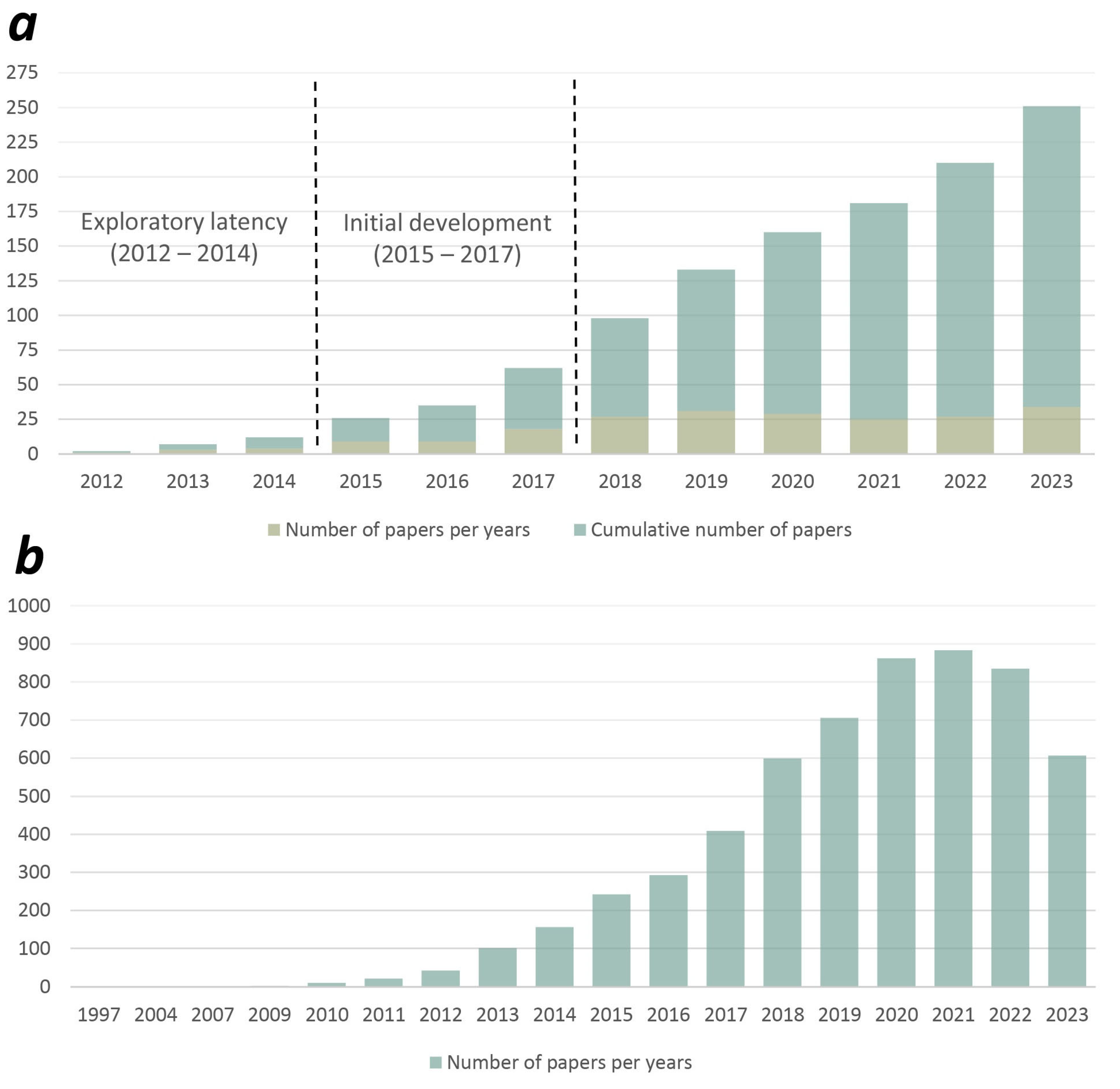
3.1. Temporal Distribution of Studies
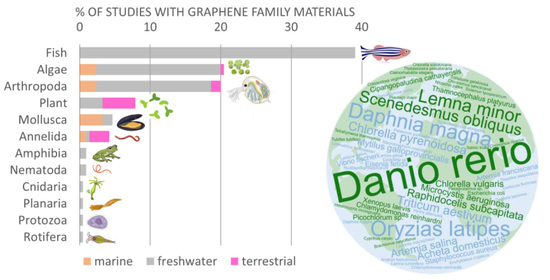
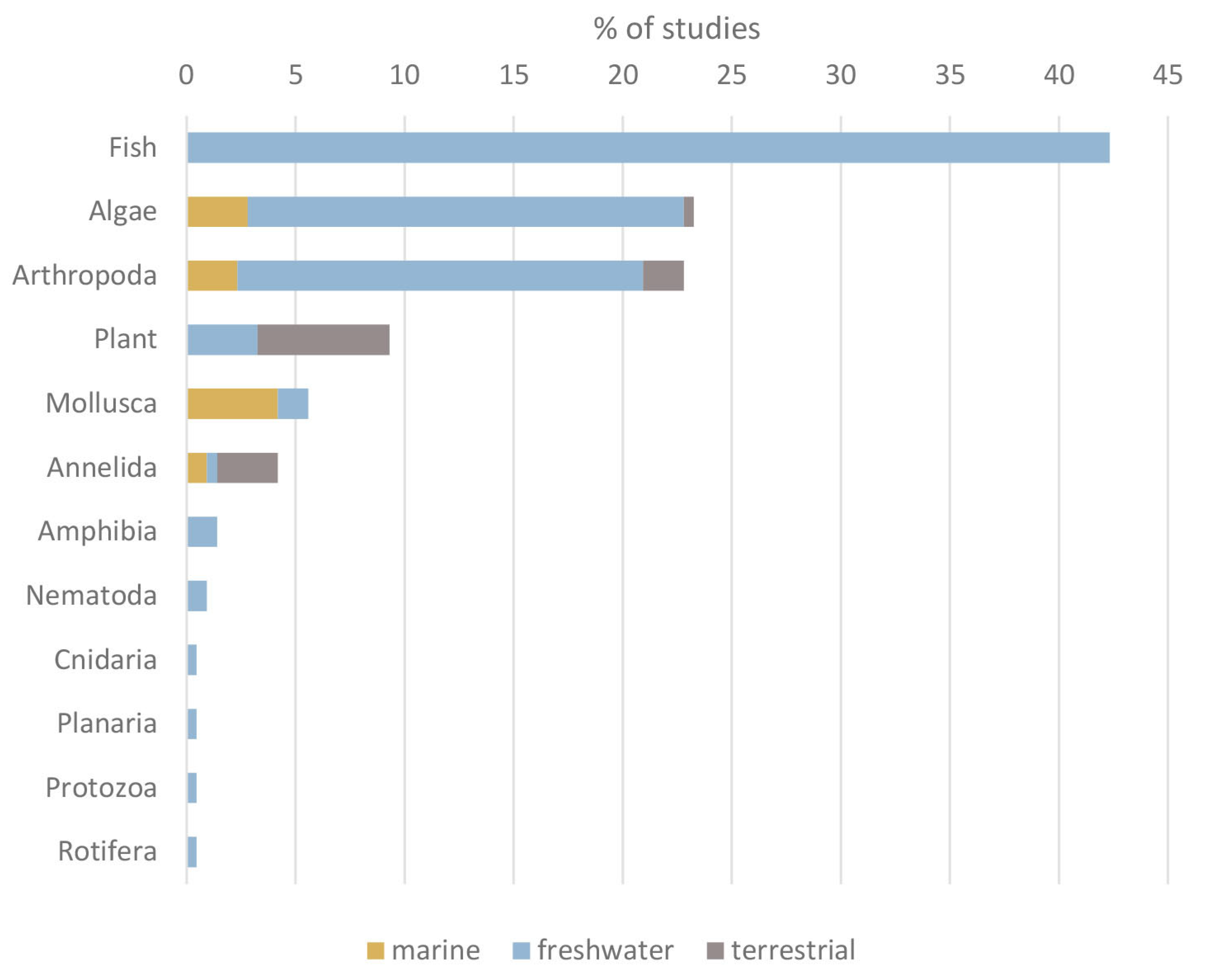
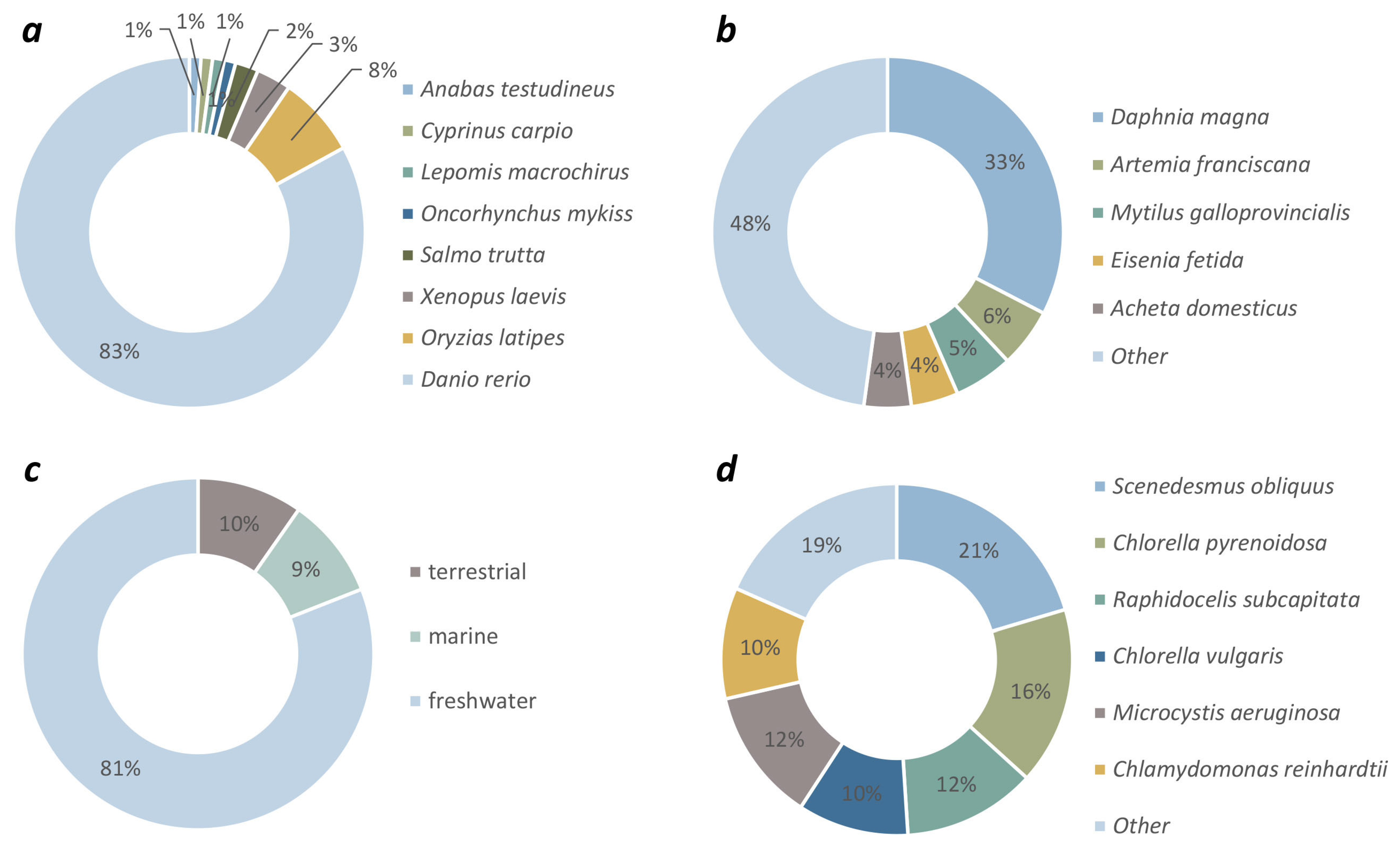
3.2. Target Organism Groups Reported in Ecotoxicity Studies with GFMs
3.3. Ecotoxicological Effects of GFMs in Multispecies Test Systems
3.3.1. Ecotoxicological Effects of GFMs on Bacterial Communities
3.3.2. Ecotoxicological Effects of GFMs in Test Systems with Hierarchical Trophic Organisation
3.3.3. Micro- and Mesocosm Approaches for GFMs Ecotoxicity Characterisation
3.3.4. Trophic Transfer Studies for GFMs Ecotoxicity Characterisation
4. Current Status, Knowledge Gaps and Future Needs
5. Conclusions and Future Perspectives
- Performing more studies on GFMs effects at environmentally relevant concentrations;
- Perform more field and laboratory studies with marine and terrestrial organisms;
- Assess the ecotoxicity of GFMs in more environmentally relevant conditions, such as trophic transfer studies and multispecies exposures in micro- or mesocosms;
- Gaining insights into the interactive effects between GFMs and environmental pollutants;
- Investigate the stability of GFMs in aquatic environments as a function of concentration. Despite the widespread use of GFMs there is limited knowledge about their actual environmental concentrations. Therefore, it is imperative to develop appropriate methods and detection techniques to accurately determine the concentrations of GFMs in the environment;
- The physicochemical characterisation of GFMs should be a critical element of all ecotoxicological investigations throughout the entire test period;
- Encourage the publication of negative results.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvidsson, R.; Boholm, M.; Johansson, M.; De Montoya, M.L. “just Carbon”: Ideas About Graphene Risks by Graphene Researchers and Innovation Advisors. NanoEthics 2018, 12, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Olszyna, A.R. The Ecotoxicity of Graphene Family Materials: Current Status, Knowledge Gaps and Future Needs. J. Nanopart. Res. 2015, 17, 40. [Google Scholar] [CrossRef]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Toxicity Studies on Graphene-based Nanomaterials in Aquatic Organisms: Current Understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef]
- Montagner, A.; Bosi, S.; Tenori, E.; Bidussi, M.; Alshatwi, A.A.; Tretiach, M.; Prato, M.; Syrgiannis, Z. Ecotoxicological Effects of Graphene-Based Materials. 2D Mater. 2016, 4, 12001. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Romero-Toledo, R.; Andrade-Espinoza, B.A. Virginia Viewpoint: How the Graphene Could Help to Decrease Sars-cov-2 Spread? Period. Polytech. Chem. Eng. 2021, 65, 283–291. [Google Scholar] [CrossRef]
- Nassef, B.G.; Nassef, G.A.; Daha, M.A. Graphene and Its Industrial Applications—A Review. Int. J. Mater. Eng. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Berichte Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Z. Anorg. Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of Thermally and Chemically Reduced Graphene Oxides by Thermal Analysis and Raman Spectroscopy. J. Therm. Anal. Calorim. 2020, 142, 331–337. [Google Scholar] [CrossRef]
- Yokwana, K.; Ntsendwana, B.; Nxumalo, E.N.; Mhlanga, S.D. Recent Advances in Nitrogen-doped Graphene Oxide Nanomaterials: Synthesis and Applications in Energy Storage, Sensor Electrochemical Applications and Water Treatment. J. Mater. Res. 2023, 38, 3239–3263. [Google Scholar] [CrossRef]
- Bertóti, I.; Farah, S.; Bulátkó, A.; Farkas, A.; Madarász, J.; Mohai, M.; Sáfrán, G.; László, K. Nitrogen Implantation into Graphene Oxide and Reduced Graphene Oxides Using Radio Frequency Plasma Treatment in Microscale. Carbon 2022, 199, 415–423. [Google Scholar] [CrossRef]
- Feng, L.; Qin, Z.; Huang, Y.; Peng, K.; Wang, F.; Yan, Y.; Chen, Y. Boron-, Sulfur-, and Phosphorus-Doped Graphene for Environmental Applications. Sci. Total Environ. 2020, 698, 134239. [Google Scholar] [CrossRef]
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent Advances in Fluorinated Graphene from Synthesis to Applications: Critical Review on Functional Chemistry and Structure Engineering. Adv. Mater. 2022, 34, 2101665. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Varey, S.A.; Hussain, F.; Haigh, S.J.; Banks, C.E. Electrochemical Properties of CVD Grown Pristine Graphene: Monolayer- vs. Quasi-graphene. Nanoscale 2014, 6, 1607–1621. [Google Scholar] [CrossRef]
- Araújo, M.P.; Soares, O.S.G.P.; Fernandes, A.J.S.; Pereira, M.F.R.; Freire, C. Tuning the Surface Chemistry of Graphene Flakes: New Strategies for Selective Oxidation. RSC Adv. 2017, 7, 14290–14301. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate Chemical Analysis of Oxygenated Graphene-Based Materials Using X-Ray Photoelectron Spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Szabo, T.; Maroni, P.; Szilagyi, I. Size-Dependent Aggregation of Graphene Oxide. Carbon 2020, 160, 145–155. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman Microprobe Studies on Carbon Materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Markets and Markets. Graphene Market Research Report. Report Code: CH 3833. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/graphene-market-83933068.html?gclid=Cj0KCQjwla-hBhD7ARIsAM9tQKus0cHp6Nd4POP33HdI84FZklGGRn7YTcXkSKbtMrBlg6DJPtXdZfIaAj89EALw_wcB (accessed on 4 April 2023).
- United Nations. The 2030 Agenda and the Sustainable Development Goals: An opportunity for Latin America and the Caribbean (LC/G.2681-P/Rev.3); United Nations: Santiago, Chile, 2018. [Google Scholar]
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Pu, Y.; Tang, M.; Zhang, T. Environmental and Health Effects of Graphene-Family Nanomaterials: Potential Release Pathways, Transformation, Environmental Fate and Health Risks. Nano Today 2022, 42, 101379. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Shen, S.J., Jr.; Lyu, Y.; Lankone, R.; Barrios, A.C.; Kabir, S.; Perreault, F.; Wohlleben, W.; Nguyen, T.; Sung, L. Graphene/polymer nanocomposite degradation by ultraviolet light: The Effects of Graphene Nanofillers and Their Potential for Release. Polym. Degrad. Stab. 2020, 182, 109365. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Part, F.; Nowack, B. Prospective Dynamic and Probabilistic Material Flow Analysis of Graphene-based Materials in Europe from 2004 to 2030. Environ. Sci. Technol. 2022, 56, 13798–13809. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review of the Mechanical and Thermal Properties of Graphene and Its Hybrid Polymer Nanocomposites for Structural Applications. J. Mater. Sci. 2019, 54, 5992–6026. [Google Scholar] [CrossRef]
- Saxena, P.; Sangela, V.; Ranjan, S.; Dutta, V.; Dasgupta, N.; Phulwaria, M.; Rathore, D.S. Harish Aquatic Nanotoxicology: Impact of Carbon Nanomaterials on Algal Flora. Energy Ecol. Environ. 2020, 5, 240–252. [Google Scholar] [CrossRef]
- Hjorth, R.; Holden, P.A.; Hansen, S.F.; Colman, B.P.; Grieger, K.; Hendren, C.O. The Role of Alternative Testing Strategies in Environmental Risk Assessment of Engineered Nanomaterials. Environ. Sci. Nano 2017, 4, 292–301. [Google Scholar] [CrossRef]
- Markovic, M.; Andelkovic, I.; Shuster, J.; Janik, L.; Kumar, A.; Losic, D.; McLaughlin, M.J. Addressing Challenges in Providing a Reliable Ecotoxicology Data for Graphene-Oxide (GO) Using an Algae (Raphidocelis subcapitata), and the Trophic Transfer Consequence of GO-Algae Aggregates. Chemosphere 2020, 245, 125640. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Selvakumar, P.; Senthil Kumar, P.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Tamil Elakkiya, V.; Rajeswari, R. Graphene-based Materials for Environmental Applications: A Review. Environ. Chem. Lett. 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Srinivasan, S.; Jeevanantham, S.; Vishnu, M.; Amith, K.V.; Sruthi, R.; Saravanan, R.; Vo, D.-V.N. Insights on Synthesis and Applications of Graphene-Based Materials in Wastewater Treatment: A Review. Chemosphere 2022, 298, 134284. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, X.; Xiong, J.; Zeng, L.; Huang, Y.; Wu, Y.; Cao, G.; Li, W. Nano-Fe3C@PGC as a Novel Low-Cost Anode Electrocatalyst for Superior Performance Microbial Fuel Cells. Biosens. Bioelectron. 2019, 142, 111594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, S.; Zhu, Z.; Wang, S.; Liu, F.; Liu, G. Effects of Oxidation Degree on Photo-Transformation and the Resulting Toxicity of Graphene Oxide in Aqueous Environment. Environ. Pollut. 2019, 249, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Qualhato, G.; Vieira, L.G.; Oliveira, M.; Rocha, T.L. Plastic Microfibers as a Risk Factor for the Health of Aquatic Organisms: A Bibliometric and Systematic Review of Plastic Pandemic. Sci. Total. Environ. 2023, 870, 161949. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.; Slomberg, D.L.; Nassar, M.; Santaella, C.; Masion, A.; Rose, J.; Auffan, M. Aquatic Mesocosm Strategies for the Environmental Fate and Risk Assessment of Engineered Nanomaterials. Environ. Sci. Technol. 2021, 55, 16270–16282. [Google Scholar] [CrossRef] [PubMed]
- Braylé, P.; Pinelli, E.; Gauthier, L.; Mouchet, F.; Barret, M. Graphene-based Nanomaterials and Microbial Communities: A Review of Their Interactions, from Ecotoxicology to Bioprocess Engineering Perspectives. Environ. Sci. Nano 2022, 9, 3725–3741. [Google Scholar] [CrossRef]
- Ahmed, F.; Rodrigues, D.F. Investigation of Acute Effects of Graphene Oxide on Wastewater Microbial Community: A Case Study. J. Hazard. Mater. 2013, 256–257, 33–39. [Google Scholar] [CrossRef]
- Lian, S.; Qu, Y.; Li, S.; Zhang, Z.; Zhang, H.; Dai, C.; Deng, Y. Interaction of Graphene-Family Nanomaterials with Microbial Communities in Sequential Batch Reactors Revealed by High-Throughput Sequencing. Environ. Res. 2020, 184, 109392. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Castro-Wallace, S.L.; Rodrigues, D.F. Acute Toxicity of Graphene Nanoplatelets on Biological Wastewater Treatment Process. Environ. Sci. Nano 2017, 4, 160–169. [Google Scholar] [CrossRef]
- Sha, Y.; Liu, J.; Yu, J.; Xu, S.; Yan, W.; Li, Z.; Shahbaz, M. Effect of Graphene Oxide on the Ammonia Removal and Bacterial Community in a Simulated Wastewater Treatment Process. J. Environ. Eng. 2020, 146, 04020097. [Google Scholar] [CrossRef]
- Dong, S.; Wang, T.; Lu, K.; Zhao, J.; Tong, Y.; Mao, L. Fate of 14c-labeled Few-layer Graphene in Natural Soils: Competitive Roles of Ferric Oxides. Environ. Sci. Nano 2021, 8, 1425–1436. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.-Y.; Wu, S.-H.; Wen, M.-X.; Lu, L.-M.; Ke, F.-Z.; Wu, Q.-S. Effect of Arbuscular Mycorrhizal Fungi on Rhizosphere Organic Acid Content and Microbial Activity of Trifoliate Orange Under Different Low P Conditions. Arch. Agron. Soil Sci. 2019, 65, 2029–2042. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, W.; Liu, S.; Guo, J.; Qi, Z.; Zou, Y.; Wang, L.; Duan, S.-Z.; Zhou, Y.; Lin, C. Impact of Graphene Exposure on Microbial Activity and Community Ecosystem in Saliva. ACS Appl. Bio Mater. 2019, 2, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Duan, C.; Sang, Y.; Wu, S.; Ru, J.; Cui, X. Effects of Graphene on Bacterial Community Diversity and Soil Environments of Haplic Cambisols in Northeast China. Forests 2018, 9, 677. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Teng, Y.; Ren, W.J.; Li, Z.G.; Luo, Y.M. Effects of Graphene on Soil Enzyme Activities and Microbial Communities. Soil 2016, 48, 102–108. [Google Scholar]
- Ru, J.; Chen, G.; Liu, Y.; Sang, Y.; Song, J. Graphene Oxide Influences Bacterial Community and Soil Environments of Cd-polluted Haplic Cambisols in Northeast China. J. For. Res. 2021, 32, 1699–1711. [Google Scholar] [CrossRef]
- Evariste, L.; Mottier, A.; Lagier, L.; Cadarsi, S.; Barret, M.; Sarrieu, C.; Soula, B.; Mouchet, F.; Flahaut, E.; Pinelli, E. Assessment of Graphene Oxide Ecotoxicity at Several Trophic Levels Using Aquatic Microcosms. Carbon 2020, 156, 261–271. [Google Scholar] [CrossRef]
- Evariste, L.; Braylé, P.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Flahaut, E.; Pinelli, E.; Barret, M. Graphene-Based Nanomaterials Modulate Internal Biofilm Interactions and Microbial Diversity. Front. Microbiol. 2021, 12, 623853. [Google Scholar] [CrossRef] [PubMed]
- Urban-Malinga, B.; Jakubowska, M.; Hallmann, A.; Dąbrowska, A. Do the Graphene Nanoflakes Pose a Potential Threat to the Polychaete Hediste diversicolor? Chemosphere 2021, 269, 128685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Bai, X.; Mou, M.; Duo, L. Carbon Nanomaterial Addition Changes Soil Nematode Community in a Tall Fescue Mesocosm. Pedosphere 2022, 32, 777–784. [Google Scholar] [CrossRef]
- Dong, S.; Xia, T.; Yang, Y.; Lin, S.; Mao, L. Bioaccumulation of 14c-labeled Graphene in an Aquatic Food Chain Through Direct Uptake or Trophic Transfer. Environ. Sci. Technol. 2018, 52, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tong, X.; Huang, C.; Chen, J.; Liu, S.; Gao, S.; Mao, L.; Xing, B. Green Algae as Carriers Enhance the Bioavailability of 14c-labeled Few-layer Graphene to Freshwater Snails. Environ. Sci. Technol. 2018, 52, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Malina, T.; Maršálková, E.; Holá, K.; Zbořil, R.; Maršálek, B. The Environmental Fate of Graphene Oxide in Aquatic Environment-Complete itigation of its Acute Toxicity to Planktonic and Benthic Crustaceans by Algae. J. Hazard. Mater. 2020, 399, 123027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Chang, F.; Xie, P.; Liu, Q.; Duan, L.; Wu, H.; Zhang, X.; Peng, W.; Liu, F.; et al. In-situ Responses of Phytoplankton to Graphene Photocatalysis in the Eutrophic Lake Xingyun, Southwestern China. Chemosphere 2021, 278, 130489. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, S.; Gonçalves, S.F.; Gonçalves, G.; Hortiguela, M.J.; Rebelo, S.; Ferro, M.C.; Vila, M. Eco-Friendly Profile of Pegylated Nano-Graphene Oxide at Different Levels of an Aquatic Trophic Chain. Ecotoxicol. Environ. Saf. 2018, 162, 192–200. [Google Scholar] [CrossRef]
- Wahid, M.H.; Eroglu, E.; Chen, X.; Smith, S.M.; Raston, C.L. Entrapment of Chlorella vulgaris Cells Within Graphene Oxide Layers. RSC Adv. 2013, 3, 8180. [Google Scholar] [CrossRef]
- Guo, X.; Dong, S.; Petersen, E.J.; Gao, S.; Huang, Q.; Mao, L. Biological Uptake and Depuration of Radio-Labeled Graphene by Daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Cano, A.M.; Maul, J.D.; Saed, M.; Shah, S.A.; Green, M.J.; Cañas-Carrell, J.E. Bioaccumulation, Stress, and Swimming Impairment in Daphnia magna Exposed to Multiwalled Carbon Nanotubes, Graphene, and Graphene oxide. Environ. Toxicol. Chem. 2017, 36, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, C.; Lu, K.; Su, Y.; Gu, C.; Huang, Q.; Petersen, E.J. Exposure of Few-layer Graphene to Limnodrilus Hoffmeisteri Modifies the Graphene and Changes Its Bioaccumulation by Other Organisms. Carbon 2016, 109, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Petersen, E.J.; Niu, J.; Chang, X.; Wang, P.; Lin, S.; Gao, S.; Mao, L. Biological Uptake, Distribution, and Depuration of Radio-labeled Graphene in Adult Zebrafish: Effects of Graphene Size and Natural Organic Matter. ACS Nano 2017, 11, 2872–2885. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A Mechanism Study on Toxicity of raphene Oxide to Daphnia magna: Direct Link Between Bioaccumulation and Oxidative Stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, I.; Le Menach, K.; Devier, M.H.; Barbarin, I.; Tomovska, R.; Cajaraville, M.P.; Budzinski, H.; Orbea, A. Uptake and Effects of Graphene Oxide Nanomaterials Alone and in Combination with Polycyclic Aromatic Hydrocarbons in Zebrafish. Sci. Total Environ. 2021, 775, 145669. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Liang, D.; Liu, Y.; Zhao, Q.; Huang, P.; Li, X.; Fan, W. Accumulation, Transformation and Subcellular Distribution of Arsenite Associated with Five Carbon Nanomaterials in Freshwater Zebrafish Specific-Tissues. J. Hazard. Mater. 2021, 415, 125579. [Google Scholar] [CrossRef] [PubMed]
- Schwirn, K.; Voelker, D.; Galert, W.; Quik, J.; Tietjen, L. Environmental Risk Assessment of Nanomaterials in the Light of New Obligations Under the REACH Regulation: Which Challenges Remain and How to Approach Them? Integr. Environ. Assess. Manag. 2020, 16, 706–717. [Google Scholar] [CrossRef]
- Auffan, M.; Masion, A.; Mouneyrac, C.; de Garidel-Thoron, C.; Hendren, C.O.; Thiery, A.; Santaella, C.; Giamberini, L.; Bottero, J.-Y.; Wiesner, M.R.; et al. Contribution of Mesocosm Testing to a Single-Step and Exposure-Driven Environmental Risk Assessment of Engineered Nanomaterials. NanoImpact 2019, 13, 66–69. [Google Scholar] [CrossRef]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological Effects of Carbon Based Nanomaterials in Aquatic Organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef]
- Espinasse, B.P.; Geitner, N.K.; Schierz, A.; Therezien, M.; Richardson, C.J.; Lowry, G.V.; Ferguson, L.; Wiesner, M.R. Comparative Persistence of Engineered Nanoparticles in a Complex Aquatic Ecosystem. Environ. Sci. Technol. 2018, 52, 4072–4078. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U. Safety Assessment of Graphene-based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Avant, B.; Bouchard, D.; Chang, X.; Hsieh, H.-S.; Acrey, B.; Han, Y.; Spear, J.; Zepp, R.; Knightes, C.D. Environmental Fate of Multiwalled Carbon Nanotubes and Graphene Oxide Across Different Aquatic Ecosystems. NanoImpact 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Pietro, R.d.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of Pristine Graphene to Marine Organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity Evaluation of Graphene Oxide on Cysts and Three Larval Stages of Artemia salina. Sci. Total Environ. 2017, 595, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Transgenerational Effects of Reduced Graphene Oxide Modified by Au, Ag, Pd, Fe3O4, Co3O4 and SnO2 on Two Generations of Daphnia magna. Carbon 2017, 122, 669–679. [Google Scholar] [CrossRef]
- Dziewięcka, E.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Dziewięcki, M.; Podolec, P.; Rubiś, P. Mortality Risk in Dilated Cardiomyopathy: The Accuracy of Heart Failure Prognostic Models and Dilated Cardiomyopathy-tailored Prognostic Model. ESC Heart Fail. 2020, 7, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Beloin-Saint-Pierre, D.; Hischier, R. Towards a More Environmentally Sustainable Production of Graphene-based Materials. Int. J. Life Cycle Assess. 2021, 26, 327–343. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, C.; Ouyang, S.; Hu, X.; Zhou, Q. Mitigation in Multiple Effects of Graphene Oxide Toxicity in Zebrafish Embryogenesis Driven by Humic Acid. Environ. Sci. Technol. 2015, 49, 10147–10154. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.L.S.S.; Franqui, L.S.; Silva, C.A.; Martinez, D.S.T. Nanotoxicity of Graphene Oxide: Assessing the Influence of Oxidation Debris in the Presence of Humic Acid. Environ. Pollut. 2017, 225, 118–128. [Google Scholar] [CrossRef]
- Castro, V.L.; Clemente, Z.; Jonsson, C.; Silva, M.; Vallim, J.H.; De Medeiros, A.M.Z.; Martinez, D.S.T. Nanoecotoxicity Assessment of Graphene Oxide and Its Relationship with Humic Acid. Environ. Toxicol. Chem. 2018, 37, 1998–2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Guo, X.; Yang, R.; Si, X.; Zhou, J. Humic Acid Alleviates the Ecotoxicity of Graphene-Family Materials on the Freshwater Microalgae Scenedesmus obliquus. Chemosphere 2018, 197, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, T.; Shi, L.; Guo, X.; Si, X.; Yang, R.; Quan, X. The Effects of Humic Acid on the Toxicity of Graphene Oxide to Scenedesmus obliquus and Daphnia magna. Sci. Total Environ. 2019, 649, 163–171. [Google Scholar] [CrossRef]
- Ni, L.; Li, Y. Role of Graphene Oxide in Mitigated Toxicity of Heavy Metal Ions on Daphnia magna. RSC Adv. 2018, 8, 41358–41367. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Shi, L.; Yu, J.; Yao, J.; Sun, J.; Zhao, L.; Sun, J. Enhanced Cd Accumulation by Graphene Oxide (GO) Under Cd Stress in Duckweed. Aquat. Toxicol. 2020, 229, 105579. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhou, Q.; Liu, Z.; Li, Q. Hexavalent Chromium Amplifies the Developmental Toxicity of Graphene Oxide During Zebrafish Embryogenesis. Ecotoxicol. Environ. Saf. 2021, 208, 111487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Yuan, P.; Wu, Z.; Zhaoxin, W.; Wu, W. Graphene Oxide Promoted Chromium Uptake by Zebrafish Embryos with Multiple Effects: Adsorption, Bioenergetic Flux and Metabolism. Sci. Total Environ. 2022, 802, 149914. [Google Scholar] [CrossRef]
- Jurgelene, Z.; Montvydiene, D.; Semcuk, S.; Stankeviciute, M.; Sauliute, G.; Pazusiene, J.; Morkvenas, A.; Butrimiene, R.; Joksas, K.; Pakstas, V.; et al. The Impact of Co-Treatment with Graphene Oxide and Metal Mixture on Salmo trutta at Early Development stages: The Sorption Capacity and Potential Toxicity. Sci. Total Environ. 2022, 838, 156525. [Google Scholar] [CrossRef]
- Almeida, A.R.; Salimian, M.; Ferro, M.; Marques, P.A.A.P.; Goncalves, G.; Titus, E.; Domingues, I. Biochemical and al Responses of Zebrafish Embryos to Magnetic Graphene/Nickel Nanocomposites. Ecotoxicol. Environ. Saf. 2019, 186, 109760. [Google Scholar] [CrossRef]
- Tamanaha-Vegas, C.A.; Zarria-Romero, J.Y.; Greneche, J.M.; Passamani, E.C.; Ramos-Guivar, J.A. Surface Magnetic Properties of a Ternary Nanocomposite and Its Ecotoxicological Properties in Daphnia magna. Adv. Powder Technol. 2022, 33, 103395. [Google Scholar] [CrossRef]
- Zarria-Romero, J.Y.; Ocampo-Anticona, J.A.; Pinotti, C.N.; Passamani, E.C.; Checca-Huaman, N.R.; Castro-Merino, I.L.; Pino, J.; Shiga, B.; Ramos-Guivar, J.A. Ecotoxicological Properties of Functionalized Magnetic Graphene Oxide and Multiwall Carbon Nanotubes in Daphnia magna. Ceram. Int. 2023, 49, 15200–15212. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Zarria-Romero, J.Y.; Canchanya-Huaman, Y.; Guerra, J.A.; Checca-Huaman, N.-R.; Castro-Merino, I.-L.; Passamani, E.C. Raman, TEM, EELS, and Magnetic Studies of a Magnetically Reduced Graphene Oxide Nanohybrid Following Exposure to Daphnia Magna Biomarkers. Nanomaterials 2022, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, É.C.; Da Silva Bruckmann, F.; Schopf, P.F.; Viana, A.R.; Mortari, S.R.; Sagrillo, M.R.; De Vasconcellos, N.J.S.; Da Silva Fernandes, L.; Bohn Rhoden, C.R. In Vitro and in Vivo Safety Profile Assessment of Graphene Oxide Decorated with Different Concentrations of Magnetite. J. Nanopart. Res. 2022, 24, 150. [Google Scholar] [CrossRef]
- Mottier, A.; Mouchet, F.; Pinelli, É.; Gauthier, L.; Flahaut, E. Environmental Impact of Engineered Carbon Nanoparticles: From Releases to Effects on the Aquatic Biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.A.P.; Freitas, R.; Neto, V. An Overview of Graphene Materials: Properties, Applications and Toxicity on Aquatic Environments. Sci. Total Environ. 2018, 631–632, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Ho, S.H. Wastewater treatment nexus: Carbon Nanomaterials Towards Potential Aquatic Ecotoxicity. J. Hazard. Mater. 2021, 417, 125959. [Google Scholar] [CrossRef] [PubMed]
- Munuera, J.; Britnell, L.; Santoro, C.; Cuéllar-Franca, R.; Casiraghi, C. A Review on Sustainable Production of Graphene and Related Life Cycle Assessment. 2D Mater. 2022, 9, 012002. [Google Scholar] [CrossRef]
- Kumar, S.; Himanshi; Prakash, J.; Verma, A.; Suman; Jasrotia, R.; Kandwal, A.; Verma, R.; Kumar Godara, S.; Khan, M.A.M. A Review on Properties and Environmental Applications of Graphene and Its Derivative-based Composites. Catalysts 2023, 13, 111. [Google Scholar] [CrossRef]

| Search Keywords | Number of Results 1 |
|---|---|
| ‘graphene’ AND ‘toxic’ | 5221 |
| ‘graphene’ AND ‘communities’ | 1755 |
| ‘graphene’ AND ‘communities’ AND (‘water’ OR ‘aquatic’) | 453 |
| ‘graphene’ AND ‘communities’ AND ‘wastewater’ | 124 |
| ‘graphene’ AND ‘communities’ AND ‘soil’ | 110 |
| ‘graphene’ AND ‘microcosm’ | 9 |
| ‘graphene’ AND ‘mesocosm’ | 2 |
| Ref. | Test System | Test Organism | Tested Concentration [mg/L or mg/kg] | Exposure Period | Environmental Compartment | Type of GFM | Applied Ecotoxicity Endpoint | |
|---|---|---|---|---|---|---|---|---|
| [56] | trophic transfer study (freshwater) | Escherichia coli | bacterium | 0.05, 0.1, 0.25, 0.5, 1 | 2 h | water | 14C-labeled few-layer graphene | cell density (OD600), cell viability with MTT |
| Tetrahymena thermophila | protozoon | 0.1, 0.25 | 22 h | water | 14C-labeled few-layer graphene | growth | ||
| Daphnia magna | crustacean | 0.005, 0.25 | 20 h | water | 14C-labeled few-layer graphene | graphene uptake | ||
| Danio rerio | fish | 0.001, 0.05 | 4 weeks | water | 14C-labeled few-layer graphene | graphene uptake | ||
| [57] | trophic transfer study (freshwater) | Scenedesmus obliquus | green algae | 0.1, 1 | 24 h | water | 14C-labeled few-layer graphene | FLG bioaccumulation |
| Cipangopaludina cathayensis | mollusca | 48 h | water | 14C-labeled few-layer graphene | FLG uptake | |||
| [52] | microcosm (freshwater) | Nitzschia palea | diatom | 0.05, 0.1 | 6 weeks | water | graphene oxide | growth, abundance |
| Chironomus riparius | insect | 0.05 and 0.1 mg/L | 13 days | water | graphene oxide | mortality, growth and teratogenicity | ||
| Pleurodeles waltii | amphibian | 0.05 and 0.1 mg/L | 10 days | water | graphene oxide | mortality, growth and teratogenicity | ||
| Xenopus laevis | amphibian | - | - | water | graphene oxide | no endpoint (food for newt) | ||
| bacterial consortium | bacterium | 0.05 and 0.1 mg/L | 6 weeks | water, sediment | graphene oxide | species distribution | ||
| [58] | trophic transfer study (freshwater) | Heterocypris incongruens | ostracoda | 0.39, 1.56, 6.25, 25 | 6 days | water | graphene oxide | mortality |
| Thamnocephalus platyurus | crustacean | 0.39, 1.56, 6.25, 25 | 48 h | water | graphene oxide | mortality | ||
| Daphnia magna | crustacean | 0.39, 1.56, 6.25, 25 | 48 h | water | graphene oxide | mortality, oxidative stress | ||
| [33] | trophic transfer study (freshwater) | Raphidocelis subcapitata | green algae | 1, 2, 4, 8, 16, 32 | 96 h | water | graphene oxide | growth |
| Paratya australiensis) | shrimp | 2, 8 | 14 days | water | graphene oxide | survival, molting, food intake | ||
| [53] | microcosm (freshwater) | Nitzschia palea | diatom | 0.1, 1, 10 | 48 h, 144 h | water | graphene oxide and rGO | viability, growth, physiological effects |
| bacterial consortium | bacterium | 0.1, 1, 10 mg/L | 48 h, 144 h | water | graphene oxide and rGO | substrate utilisation pattern, species distribution | ||
| [54] | microcosm (marine) | Hediste diversicolor | annelid worm | 0.4, 4, 40, 400 | 36 h | sediment | graphene multilayer nanoflakes | oxidative stress, behavioural effects, neurotoxicity, cytotoxicity |
| Hediste diversicolor | annelid worm | 4, 40 | 24 days | sediment | graphene multilayer nanoflakes | oxidative stress, behavioural effects, neurotoxicity, cytotoxicity | ||
| phytoplankton community | phytoplankton | 4, 40 | 24 days | water | graphene multilayer nanoflakes | biodiversity indexes, abundance parameters | ||
| [59] | macrocosm (freshwater) | phytoplankton community | phytoplankton | nd | 5 months | water | a graphene photocatalysis nets | abundance, species distribution |
| [55] | mesocosm (terrestrial) | nematodes | nematode | 1 m/m% (10,000 mg/kg) | 130 days | soil | graphene, graphene oxide | biodiversity indexes, abundance parameters |
| Festuca arundinacea | plant | 1 m/m% (10,000 mg/kg) | 130 days | soil | graphene, graphene oxide | dry biomass | ||
| microbial consortium | 1 m/m% (10,000 mg/kg) | 130 days | soil | graphene, graphene oxide | no endpoint | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete-Kertész, I.; László, K.; Molnár, M. Towards Understanding the Factors behind the Limited Integration of Multispecies Ecotoxicity Assessment in Environmental Risk Characterisation of Graphene-Family Materials—A Bibliometric Review. C 2023, 9, 90. https://doi.org/10.3390/c9040090
Fekete-Kertész I, László K, Molnár M. Towards Understanding the Factors behind the Limited Integration of Multispecies Ecotoxicity Assessment in Environmental Risk Characterisation of Graphene-Family Materials—A Bibliometric Review. C. 2023; 9(4):90. https://doi.org/10.3390/c9040090
Chicago/Turabian StyleFekete-Kertész, Ildikó, Krisztina László, and Mónika Molnár. 2023. "Towards Understanding the Factors behind the Limited Integration of Multispecies Ecotoxicity Assessment in Environmental Risk Characterisation of Graphene-Family Materials—A Bibliometric Review" C 9, no. 4: 90. https://doi.org/10.3390/c9040090
APA StyleFekete-Kertész, I., László, K., & Molnár, M. (2023). Towards Understanding the Factors behind the Limited Integration of Multispecies Ecotoxicity Assessment in Environmental Risk Characterisation of Graphene-Family Materials—A Bibliometric Review. C, 9(4), 90. https://doi.org/10.3390/c9040090