Activated Carbon for Sepsis Prevention and Intervention: A Modern Way of Utilizing Old Therapies
Abstract
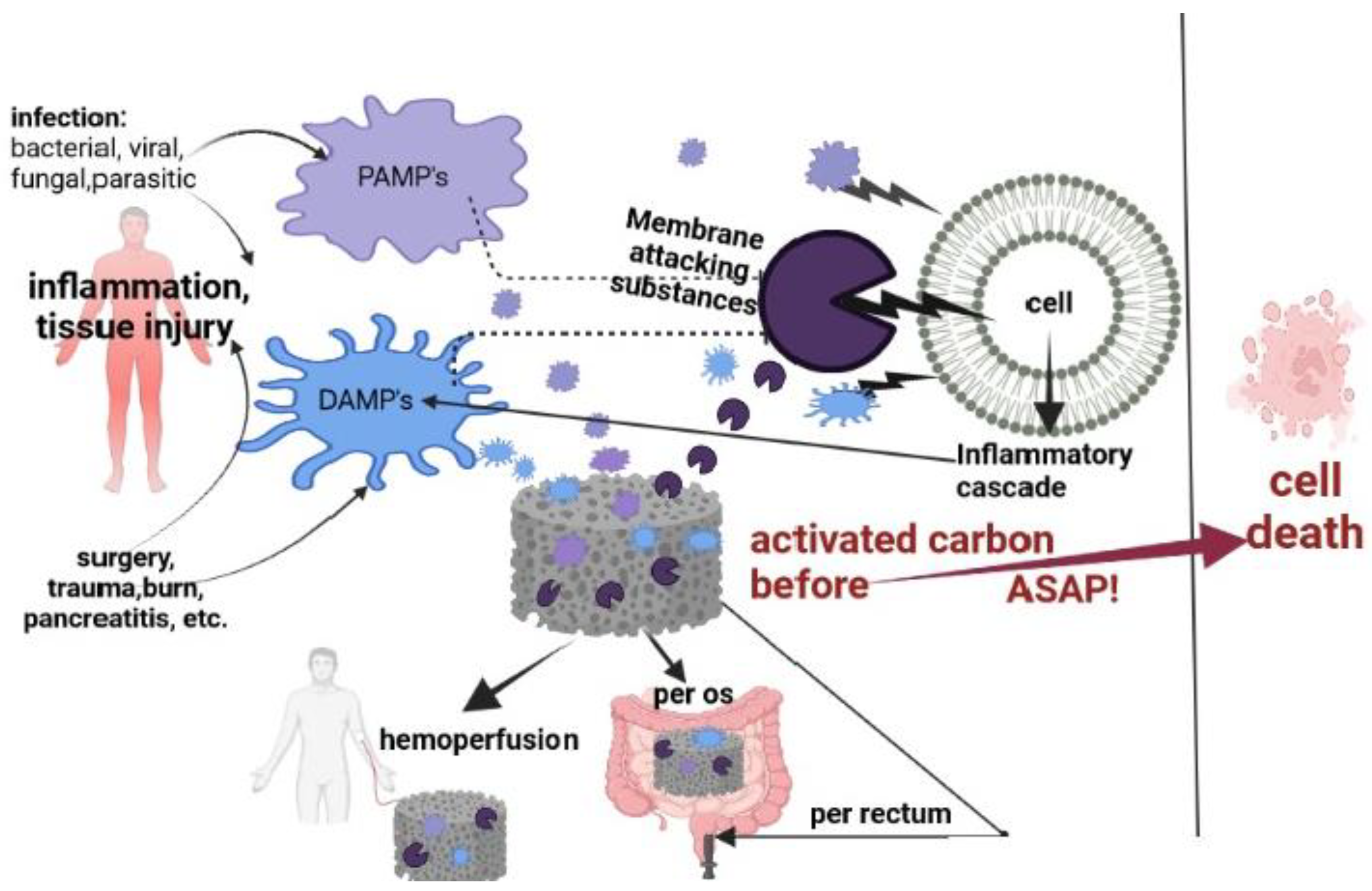
1. Introduction
- -
- identification of the pathological states with an abnormal level of inflammation markers—PAMPs and DAMPs, in which AC administration is beneficial;
- -
- identification of homeostasis parameters, compromised by inflammation and/or sepsis, which can be ameliorated by AC therapy;
- -
- delineation of the known and potential mechanisms of action of AC therapy;
- -
- identification of the challenges, and future areas/options of AC applications to maximize its efficiency.
2. First Application of AC for Infection Prevention and Treatment
3. AC for Uncontrolled Inflammation Treatment
3.1. AC Application for a Decrease PAMPs and DAMPs Elevated Levels
3.2. Adsorption and AC Modification
3.3. Per Oral Administration of Activated Charcoal (Enterosorption)
3.3.1. AC in Anti-Diarrhea Treatment
3.3.2. AC Enterosorption in Renal Dysfunction
Uremic Toxins
Mechanistic Aspects of Enterosorption Action in Renal Dysfunction
Clinical Interventions
3.3.3. AC Enterosorption in Liver Failure
Clinical Study
3.4. AC Application per Rectum
3.5. Hemoperfusion (HP)
3.5.1. The Strategy of Membrane-Damage Substance Removal in Sepsis
3.5.2. Combination of Selective and Non-Selective Adsorption for HP
4. Positive Effect of AC on Some Parameters of Homeostasis Compromised by Sepsis
4.1. Bone Marrow Index and Oxidative Stress
4.2. Serum Albumin
4.3. Microbiome
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Ayala, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.-M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.S.; Drechsler, S.; et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An International Expert Consensus Initiative for Improvement of Animal Modeling in Sepsis. Shock 2018, 50, 377–380. [Google Scholar] [CrossRef]
- Iskander, K.N.; Osuchowski, M.F.; Stearns-Kurosawa, D.J.; Kurosawa, S.; Stepien, D.; Valentine, C.; Remick, D.G. Sepsis: Multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 2013, 93, 1247–1288. [Google Scholar] [CrossRef]
- Global Sepsis Alliance. WHA Adopts Resolution on Sepsis. Available online: https://www.global-sepsis-alliance.org/news/2017/5/26/wha-adopts-resolution-on-sepsis (accessed on 15 February 2018).
- Angelo, M.; Jaewoo, L. Scavenging Damage and Pathogen Associated Molecules. Curr. Trends Biomed. Eng. Biosci. 2017, 2, 555–578. [Google Scholar] [CrossRef]
- Rai, V.; Mathews, G.; Agrawal, D.K. Translational and Clinical Significance of DAMPs, PAMPs, and PRRs in Trauma-induced Inflammation. Arch. Clin. Biomed. Res. 2022, 6, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-X.; Zhang, W.-W.; Wu, C.-H.; Wang, S.-S.; Smith, F.G.; Jin, S.-W.; Zhang, P.-H. The Novel Role of Metabolism-Associated Molecular Patterns in Sepsis. Front. Cell Infect. Microbiol. 2022, 12, 915099. [Google Scholar] [CrossRef]
- Moriyama, K.; Nishida, O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar] [CrossRef] [PubMed]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Drüner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar] [CrossRef]
- Gruda, M.C.; Ruggeberg, K.-G.; O’sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; De Souza, H.S.P. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 2018, 24, 4622–4634. [Google Scholar] [CrossRef]
- Bianco, A.; Chen, Y.; Frackowiak, E.; Holzinger, M.; Koratkar, N.; Meunier, V.; Mikhailovsky, S.; Strano, M.; Tascon, J.M.D.; Terrones, M. Carbon science perspective in 2020: Current research and future challenges. Carbon 2020, 161, 373–391. [Google Scholar] [CrossRef]
- Tetta, C.; Cavaillon, J.M.; Schulze, M.; Ronco, C.; Ghezzi, P.M.; Camussi, G.; Serra, A.M.; Curti, F.; Lonnemann, G. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol. Dial. Transplant. 1998, 13, 1458–1464. [Google Scholar] [CrossRef][Green Version]
- Shoji, H.; Ferrer, R. Potential survival benefit and early recovery from organ dysfunction with polymyxin B hemoperfusion: Perspectives from a real-world big data analysis and the supporting mechanisms of action. J. Anesth. Analg. Crit. Care 2022, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F. The Molecular Adsorbent Recirculating System (MARS) in the intensive care unit: A rescue therapy for patients with hepatic failure. Crit. Care 2006, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Ebeyer-Masotta, M.; Eichhorn, T.; Weiss, R.; Semak, V.; Lauková, L.; Fischer, M.B.; Weber, V. Heparin-Functionalized Adsorbents Eliminate Central Effectors of Immunothrombosis, including Platelet Factor 4, High-Mobility Group Box 1 Protein and Histones. Int. J. Mol. Sci. 2022, 23, 1823. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.G.; Sarnatskaya, S.V.; Mikhalovsky, A.S.; Sidorenko, K.I.; Bardakhivskaya, E.A.; Snezhkova, L.A.; Yushko, A.; Kozinchenko, L.A.; Sakhno, V.N.; Maslenny, A.V.; et al. Deliganding Carbonic Adsorbents for Simultaneous Removal of Protein-Bound Toxins, Bacterial Toxins and Inflammatory Cytokines. In Biodefence; NATO Science for Peace and Security Series A: Chemistry and Biology, 2011; Mikhalovsky, S., Khajibaev, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 289–305. [Google Scholar] [CrossRef]
- Snezkova, E.A.; Muller, D.; Bardakhivskaya, K.I.; Mikhalovsky, S.V.; Nikolaev, V.G. High-porosity activated carbon as a possible matrix for native DNA and dextran-sulfate immobilization. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 529–537. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Matsui, A.; Hasegawa, K.; Tokuyama, H.; Hayashi, K.; Itoh, H. Oral adsorbent AST-120 ameliorates gut environment and protects against the progression of renal impairment in CKD rats. Clin. Exp. Nephrol. 2018, 22, 1069–1078. [Google Scholar] [CrossRef]
- Zawadzki, A.; Johnson, L.B.; Bohe, M.; Johansson, C.; Ekelund, M.; Nielsen, O.H. An open prospective study evaluating efficacy and safety of a new medical device for rectal application of activated carbon in the treatment of chronic, uncomplicated perianal fistulas. Int. J. Color. Dis. 2017, 32, 509–512. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, J.C.; Plata-Menchaca, E.P.; Chiscano-Camón, L.; Ruiz-Sanmartin, A.; Ferrer, R. Blood purification in sepsis and COVID-19: What´s new in cytokine and endotoxin hemoadsorption. J. Anesth. Analg. Crit. Care 2022, 2, 15. [Google Scholar] [CrossRef]
- Lake, C.D. Egyptian Medical papyrus, in Hieratic language, called Ebers Medical papyrus, 1550 BCE. In The Old Egyptian Medical Papyri; University of Kansas Press: Laurence, KS, USA, 1952; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjx64PIx5CAAxV4g_0HHfgnCPwQFnoECDkQAQ&url=https%3A%2F%2Fkuscholarworks.ku.edu%2Fbitstream%2Fhandle%2F1808%2F6339%2Fupk.old_egyptian_medical_papyri.pdf%3Fsequence%3D1&usg=AOvVaw2oHMtB-nvFRdCXYwy8rrNT&opi=89978449 (accessed on 16 June 2023).
- Hippocrates. 450-370 BC. The medical Works of Hippocrates: A New Translation from the Original Greek Made Especially for English Readers by the Collaboration of John Chadwick and W. N. Mann; Blackwell: Oxford, UK, 1950. (In Greek) [Google Scholar]
- Kühn, K.G. (Ed.) Claudii Galeni Opera Omnia; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Senderovich, H.; Vierhout, M.J. Is there a role for charcoal in palliative diarrhea management? Curr. Med. Res. Opin. 2018, 34, 1253–1259. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated Carbon Surface Modifications by Adsorption of Bacteria and Their Effect on Aqueous Lead Adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Poli, E.C.; Rimmelé, T.; Schneider, A.G. Hemoadsorption with CytoSorb®. Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Keizo, K.; Shozo, K.; Yoshiji, Y.; Saburo, H.; Hiroshi, S.; Joji, O.; Hyoe, I.; Gengo, O.; Satoru, F. Clinical evaluation of AST-120 on suppression of progression of chronic renal failure. Multi-center, double-blind study in comparison with placebo. Clin. Eval. 1987, 15, 527–564. [Google Scholar]
- de Gunzburg, J.; Ghozlane, A.; Ducher, A.; Le Chatelier, E.; Duval, X.; Ruppé, E.; Armand-Lefevre, L.; Sablier-Gallis, F.; Burdet, C.; Alavoine, L.; et al. Protection of the Human Gut Microbiome from Antibiotics. J. Infect. Dis. 2018, 217, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Macnaughtan, J.; Albillos, A.; Kerbert, A.; Vargas, V.; Durand, F.; Gines, P.; Sola, E.; Edwards, L.; Eaton, S.; Cox, J.; et al. O09 A double blind, randomised, placebo-controlled study to assess safety and tolerability of oral enterosorbent Carbalive (Yaq-001) in cirrhotic patients. Gut 2021, 70, A5–A6. [Google Scholar] [CrossRef]
- Su, P.Y.; Lee, Y.H.; Kuo, L.N.; Chen, Y.C.; Chen, C.; Kang, Y.N.; Chang, E.H. Efficacy of AST-120 for Patients with Chronic Kidney Disease: A Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 676345. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Khazaeli, M.; Masuda, Y.; Ichii, H.; Liu, S. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am. J. Nephrol. 2013, 37, 518–525. [Google Scholar] [CrossRef]
- Howell, C.A.; Mikhalovsky, S.V.; Markaryan, E.N.; Khovanov, A.V. Investigation of the adsorption capacity of the enterosorbent Enterosgel for a range of bacterial toxins, bile acids and pharmaceutical drugs. Sci. Rep. 2019, 9, 5629. [Google Scholar] [CrossRef]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Jaishankar, D.; Hopkins, J.; Thakkar, N.; Koujah, L.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef]
- Natori, Y.; Kinase, Y.; Ikemoto, N.; Spaziani, F.; Kojima, T.; Kakuta, H.; Fujita, J.; Someya, K.; Tatenuma, K.; Yabuta, T.; et al. Activated Carbon Impregnated with Elementary Iodine: Applications against Virus- and Bacteria-Related Issues. C 2021, 7, 86. [Google Scholar] [CrossRef]
- Antonieti, C.C.; Ginoris, Y.P. Removal of Cylindrospermopsin by Adsorption on Granular Activated Carbon, Selection of Carbons and Estimated Fixed-Bed Breakthrough. Water 2022, 14, 1630. [Google Scholar] [CrossRef]
- Nagaki, M.; Hughes, R.D.; Lau, J.Y.N.; Williams, R. Removal of Endotoxin and Cytokines by Adsorbents and the Effect of Plasma Protein Binding. Int. J. Artif. Organs 1991, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ruggeberg, K.G.; O’Sullivan, P.; Kovacs, T.J.; Dawson, K.; Capponi, V.J.; Chan, P.P.; Golobish, T.D.; Gruda, M.C. Hemoadsorption Improves Survival of Rats Exposed to an Acutely Lethal Dose of Aflatoxin B1. Sci. Rep. 2020, 10, 799. [Google Scholar] [CrossRef]
- Webster, J.M.; Darling, A.L.; Uversky, V.N.; Blair, L.J. Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease. Front. Pharmacol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Ivanov, A.E.; Kozynchenko, O.P.; Mikhalovska, L.I.; Tennison, S.R.; Jungvid, H.; Gun’ko, V.M.; Mikhalovsky, S.V. Activated carbons and carbon-containing poly(vinyl alcohol) cryogels: Characterization, protein adsorption and possibility of myoglobin clearance. Phys. Chem. Chem. Phys. 2012, 14, 16267–16278. [Google Scholar] [CrossRef]
- Inoue, S.; Kiriyama, K.; Hatanaka, Y.; Kanoh, H. Adsorption properties of an activated carbon for 18 cytokines and HMGB1 from inflammatory model plasma. Colloids Surf. B Biointerfaces 2015, 126, 58–62. [Google Scholar] [CrossRef]
- Macnaughtan, J.; Soeda, J.; Mouralidarane, A.; Sandeman, S.; Howell, C.; Mikhalovsky, S.; Kozynchenko, S.; Tennison, S.; Davies, N.; Oben, J.; et al. Gut decontamination using nanoporous carbons reduces portal pressure and prevents liver failure in bile-duct ligated cirrhotic animals by reducing kupffer cell activation. J. Hepatol. 2012, 56, S230–S231. [Google Scholar] [CrossRef]
- Kazner, C.; Lehnberg, K.; Kovalova, L.; Wintgens, T.; Melin, T.; Hollender, J.; Dott, W. Removal of endocrine disruptors and cytostatics from effluent by nanofiltration in combination with adsorption on powdered activated carbon. Water Sci. Technol. 2008, 58, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Snezhkova, E.A.; Tridon, A.; Evrard, B.; Nikolaev, V.G.; Uvarov, V.Y.; Tsimbalyuk, R.S.; Ivanuk, A.A.; Komov, V.V.; Sakhno, L.A. Binding Potency of Heparin Immobilized on Activated Charcoal for DNA Antibodies. Bull. Exp. Biol. Med. 2016, 160, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Parent-Robitaille, C.; Anderson, K.; Tremblay, M.; Rose, C.F. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology 2011, 53, 1995–2002. [Google Scholar] [CrossRef]
- Seankham, S.; Novalin, S.; Pruksasri, S. Kinetics and adsorption isotherm of lactic acid from fermentation broth onto activated charcoal. Chem. Ind. Chem. Eng. Q. 2017, 23, 515–521. [Google Scholar] [CrossRef]
- Cupisti, A.; Piccoli, G.B.; Gallieni, M. Charcoal for the management of pruritus and uremic toxins in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 71–79. [Google Scholar] [CrossRef]
- Sandeman, S.R.; Howell, C.A.; Phillips, G.J.; Zheng, Y.; Standen, G.; Pletzenauer, R.; Davenport, A.; Basnayake, K.; Boyd, O.; Holt, S.; et al. An adsorbent monolith device to augment the removal of uraemic toxins during haemodialysis. J. Mater. Sci. Mater. Med. 2014, 25, 1589–1597. [Google Scholar] [CrossRef]
- Park, S.; Islam, M.I.; Jeong, J.H.; Cho, N.J.; Song, H.Y.; Lee, E.Y.; Gil, H.W. Hemoperfusion leads to impairment in hemostasis and coagulation process in patients with acute pesticide intoxication. Sci. Rep. 2019, 9, 13325. [Google Scholar] [CrossRef] [PubMed]
- Burgelman, M.; Vandendriessche, C.; Vandenbroucke, R.E. Extracellular Vesicles: A Double-Edged Sword in Sepsis. Pharmaceuticals 2021, 14, 829. [Google Scholar] [CrossRef]
- Wisgrill, L.; Lamm, C.; Hell, L.; Thaler, J.; Berger, A.; Weiss, R.; Weber, V.; Rinoesl, H.; Hiesmayr, M.J.; Spittler, A.; et al. Influence of hemoadsorption during cardiopulmonary bypass on blood vesicle count and function. J. Transl. Med. 2020, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Fendl, B.; Weiss, R.; Eichhorn, T.; Linsberger, I.; Afonyushkin, T.; Puhm, F.; Binder, C.J.; Fischer, M.B.; Weber, V. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci. Rep. 2021, 11, 6996. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry. Special Issue: Hierarchically-Ordered Materials. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Gauden, P.A.; Kowalczyk, P. Fractal geometry concept in physical adsorption on solids. Arab. J. Sci. Eng. 2003, 28, 133–167. [Google Scholar]
- Veldeman, L.; Vanmassenhove, J.; Van Biesen, W.; Massy, Z.A.; Liabeuf, S.; Glorieux, G.; Vanholder, R. Evolution of protein-bound uremic toxins indoxyl sulphate and p-cresyl sulphate in acute kidney injury. Int. Urol. Nephrol. 2019, 51, 293–302. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Wang, X.; Ji, F.; Ronco, C.; Tian, J.; Yin, Y. Gut-liver crosstalk in sepsis-induced liver injury. Crit. Care 2020, 24, 614. [Google Scholar] [CrossRef]
- Mitzner, S.R.; Stange, J.; Klammt, S.; Peszynski, P.; Schmidt, R.; Nöldge-Schomburg, G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J. Am. Soc. Nephrol. 2001, 28 (Suppl. S17), S75–S82. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ito, T.; Sato, M.; Goto, S.; Kazama, J.J.; Gejyo, F.; Narita, I. Adsorption of Protein-Bound Uremic Toxins Using Activated Carbon through Direct Hemoperfusion in vitro. Blood Purif. 2019, 48, 215–222. [Google Scholar] [CrossRef]
- Howell, C.A.; Sandeman, S.R.; Zheng, Y.; Mikhalovsky, S.V.; Nikolaev, V.G.; Sakhno, L.A.; Snezhkova, E.A. New dextran coated activated carbons for medical use. Carbon 2016, 97, 134–146. [Google Scholar] [CrossRef]
- Beloglasov, V.A.; Snezhkova, E.A.; Nikolaev, V.G. Hemoperfusion through DNA-coated and uncoated synthetic activated charcoals as an additive to the bronchial asthma traditional treatment. Artif. Cells Blood Substit. Immobil. Biotechnol. 1998, 2, 191–197. [Google Scholar] [CrossRef]
- Burchacka, E.; Pstrowska, K.; Beran, E.; Fałtynowicz, H.; Chojnacka, K.; Kułażyński, M. Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens 2021, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.U.; Lian, Q.; Zappi, M.E.; Buchireddy, P.R.; Gang, D.D. Adsorptive removal of resorcinol on a novel ordered mesoporous carbon (OMC) employing COK-19 silica scaffold: Kinetics and equilibrium study. J. Environ. Sci. 2019, 75, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef]
- Zhang, J.; Ankawi, G.; Sun, J.; Digvijay, K.; Yin, Y.; Rosner, M.H.; Ronco, C. Gut-kidney crosstalk in septic acute kidney injury. Crit. Care 2018, 22, 117. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Generoso, J.S.; Lence, L.; Candiotto, G.; Streck, E.; Petronilho, F.; Pillai, A.; Sharshar, T.; Dal-Pizzol, F.; Barichello, T. A crosstalk between gut and brain in sepsis-induced cognitive decline. J. Neuroinflammation 2022, 19, 114. [Google Scholar] [CrossRef]
- Sarmin, M.; Begum, M.; Islam, F.; Afroze, F.; Shahrin, L.; Sharifuzzaman; Alam, T.; Bin Shahid, A.S.M.S.; Ahmed, T.; Chisti, M.J. Factors associated with severe sepsis in diarrheal adults and their outcome at an urban hospital, Bangladesh: A retrospective analysis. PLoS ONE 2021, 16, e0257596. [Google Scholar] [CrossRef]
- Kocsár, L.T.; Bertók, L.; Várterész, V. Effect of bile acids on the intestinal absorption of endotoxin in rats. J. Bacteriol. 1969, 100, 220–223. [Google Scholar] [CrossRef]
- Wedlake, L.; A’hern, R.; Russell, D.; Thomas, K.; Walters, J.R.F.; Andreyev, H.J.N. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 30, 707–717. [Google Scholar] [CrossRef]
- Bereswill, S.; Mousavi, S.; Weschka, D.; Heimesaat, M.M. Disease-Alleviating Effects of Peroral Activated Charcoal Treatment in Acute Murine Campylobacteriosis. Microorganisms 2021, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Zemskov, A.M.; Zemskov, V.M.; Zoloedov, V.I. Non-pharmacological immunocorrection. J. Immunopathol. Allergol. Infectology 2003, 4, 12–16. [Google Scholar] [CrossRef][Green Version]
- Konorev, M.R. Clinical pharmacology of enterosorbents of new generation. Vestn. Pharm. 2013, 4, 79–85. [Google Scholar]
- Alobaidi, R.; Basu, R.K.; Goldstein, S.L.; Bagshaw, S.M. Sepsis-associated acute kidney injury. Semin. Nephrol. 2015, 35, 2–11. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Tonelli, M.; Unsworth, L.D. Adsorption-Based Strategies for Removing Uremic Toxins from Blood. Microporous Mesoporous Mater. 2021, 319, 111035. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, D.; Giasafaki, D.; Charalambopoulou, G.; Van Geffen, E.; Gerritsen, K.G.F.; Steriotis, T.; Stamatialis, D. Carbon Adsorbents with Dual Porosity for Efficient Removal of Uremic Toxins and Cytokines from Human Plasma. Sci. Rep. 2017, 7, 14914. [Google Scholar] [CrossRef] [PubMed]
- Mitome, T.; Uchida, Y.; Egashira, Y.; Hayashi, K.; Nishiura, A.; Nishiyama, N. Adsorption of indole on KOH-activated mesoporous carbon. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 89–95. [Google Scholar] [CrossRef]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Toyoda, S.; Hashimoto, R.; Tezuka, T.; Sakuma, M.; Abe, S.; Ishikawa, T.; Taguchi, I.; Inoue, T. Antioxidative effect of an oral adsorbent, AST-120, and long-term outcomes in chronic kidney disease patients with cardiovascular disease. Hypertens. Res. 2020, 43, 1128–1131. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H.; Maruyama, T.; Narita, I. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.-H.; Ko, Y.-F.; Hwang, T.-L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Schulman, G.; Vanholder, R.; Niwa, T. AST-120 for the management of progression of chronic kidney disease. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 49–56. [Google Scholar] [CrossRef]
- Sato, E.; Hosomi, K.; Sekimoto, A.; Mishima, E.; Oe, Y.; Saigusa, D.; Ito, S.; Abe, T.; Sato, H.; Kunisawa, J.; et al. Effects of the oral adsorbent AST-120 on fecal p-cresol and indole levels and on the gut microbiota composition. Biochem. Biophys Res. Commun. 2020, 525, 773–779. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kubota, T.; Katoh, M.; Horai, Y.; Suzuki, H.; Yamashita, Y.; Hirata, R.; Moroi, M. AST-120 Treatment Alters the Gut Microbiota Composition and Suppresses Hepatic Triglyceride Levels in Obese Mice. Endocr. Res. 2021, 46, 178–185. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Su, S.-C.; Chang, L.-C.; Yang, K.-J.; Lee, C.-C.; Hsu, H.-J.; Chen, Y.-T.; Sun, C.-Y.; Wu, I.-W. Oral Absorbent AST-120 Is Associated with Compositional and Functional Adaptations of Gut Microbiota and Modification of Serum Short and Medium-Chain Fatty Acids in Advanced CKD Patients. Biomedicines 2022, 10, 2234. [Google Scholar] [CrossRef]
- Yu, M.; Kim, Y.J.; Kang, D.-H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef]
- Sarnatskaya, V.V.; Lindup, W.E.; Niwa, T.; Ivanov, A.I.; Yushko, L.A.; Tjia, J.; Maslenny, V.N.; Korneeva, L.N.; Nikolaev, V.G. Effect of protein-bound uraemic toxins on the thermodynamic characteristics of human albumin. Biochem. Pharmacol. 2002, 63, 1287–1296. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Li, Y.; Lv, C.; Wang, Z. Effects of oral activated charcoal on hyperphosphatemia and vascular calcification in Chinese patients with stage 3–4 chronic kidney disease. J. Nephrol. 2019, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Majid, H.; Abid, S. Update on clinical and research application of fecal biomarkers for gastrointestinal diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 39–46. [Google Scholar] [CrossRef]
- Langhorst, J.; Boone, J.; Lauche, R.; Rueffer, A.; Dobos, G. Faecal Lactoferrin, Calprotectin, PMN-elastase, CRP, and White Blood Cell Count as Indicators for Mucosal Healing and Clinical Course of Disease in Patients with Mild to Moderate Ulcerative Colitis: Post Hoc Analysis of a Prospective Clinical Trial. J. Crohn’s Colitis 2016, 10, 786–794. [Google Scholar] [CrossRef]
- Zerbato, V.; Di Bella, S.; Giuffrè, M.; Jaracz, A.W.; Gobbo, Y.; Luppino, D.; Macor, P.; Segat, L.; Koncan, R.; D’Agaro, P.; et al. High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study. World J. Gastroenterol. 2021, 27, 3130–3137. [Google Scholar] [CrossRef]
- Kanki, T.; Kuwabara, T.; Morinaga, J.; Fukami, H.; Umemoto, S.; Fujimoto, D.; Mizumoto, T.; Hayata, M.; Kakizoe, Y.; Izumi, Y.; et al. The predictive role of serum calprotectin on mortality in hemodialysis patients with high phosphoremia. BMC Nephrol. 2020, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; van den Berg, E.H.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; van Goor, H.; Abdulle, A.E. Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. Int. J. Mol. Sci. 2022, 23, 15708. [Google Scholar] [CrossRef] [PubMed]
- Khatib-Massalha, E.; Michelis, R.; Trabelcy, B.; Gerchman, Y.; Kristal, B.; Ariel, A.; Sela, S. Free circulating active elastase contributes to chronic inflammation in patients on hemodialysis. Am. J. Physiol. Ren. Physiol. 2018, 314, F203–F209. [Google Scholar] [CrossRef]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Shimizu, M.; Kikuchi, M.; Shobu, Y. Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin. Exp. Nephrol. 2018, 22, 299–308. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Kim, S.W.; Hur, K.Y.; Cha, B.-S.; Kim, I.J.; Park, T.S.; Baik, S.H.; Yoon, K.H.; Lee, K.W.; Lee, I.K.; et al. Predictive Factors for Efficacy of AST-120 Treatment in Diabetic Nephropathy: A Prospective Single-Arm, Open-Label, Multi-Center Study. J. Korean Med. Sci. 2019, 34, e117. [Google Scholar] [CrossRef]
- Tomin, T.; Schittmayer, M.; Birner-Gruenberger, R. Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues. Metabolites 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Hundertmark, J.; Guillot, A.; Tacke, F. Molecular and Cellular Mediators of the Gut-Liver Axis in the Progression of Liver Diseases. Front. Med. 2021, 8, 725390. [Google Scholar] [CrossRef] [PubMed]
- Macnaughtan, J.; Ranchal, I.; Soeda, J.; Sawhney, R.; Oben, J.; Davies, N.; Mookerjee, R.; Marchesi, J.; Cox, J.; Jalan, R. PTH-095. Oral carbon therapy is associated with a selective modulation of the microbiome in cirrhotic rats which is associated with a significant reduction in inflammatory activation. Gut 2015, 64 (Suppl. S1), A449–A450. [Google Scholar] [CrossRef]
- Chiara, F.D.; Li, J.; Lu, H.; Davies, N.; Mookerjee, R.; Jalan, R.; Macnaughtan, J. FRI-231. Characterization of the protective effects of yaq-001on organ injury in cirrhosis. J. Hepatol. 2018, 68, S463–S465. [Google Scholar] [CrossRef]
- Macnaughtan, J.; Soeda, J.; Mouralidarane, A.; Sandeman, S.; Howell, C.; Milkhalovsky, S.; Kozynchenko, O.; Tennison, S.; Davies, N.; Mookerjee, R.; et al. PMO-128 effects of oral nanoporous carbon therapy in leptin null mice as a model of non-alcoholic steatohepatitis. Gut 2012, 61 (Suppl. S2). [Google Scholar] [CrossRef]
- Okamoto, N.; Ohama, H.; Matsui, M.; Fukunishi, S.; Higuchi, K.; Asai, A. Hepatic F4/80+CD11b+CD68– cells influence the antibacterial response in irradiated mice with sepsis by Enterococcus faecalis. J. Leukoc. Biol. 2021, 109, 943–952. [Google Scholar] [CrossRef]
- Contijoch, E.J.; Britton, G.J.; Yang, C.; Mogno, I.; Li, Z.; Ng, R.; Llewellyn, S.R.; Hira, S.; Johnson, C.; Rabinowitz, K.M.; et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife 2019, 8, 1–26. [Google Scholar]
- Malyarchuk , A.R.; Koval, D.B.; Koshovska, D.O. Effect of Enterosgel and Carboline (Activated Carbon) Food Additive on Intestinal Microflora. Achiev. Clin. Exp. Med. 2021, 4, 122–126. [Google Scholar] [CrossRef]
- Stass, H.; Kubitza, D.; Moller, J.-G.; Delesen, H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br. J. Clin. Pharmacol. 2005, 59, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Travis, S.; Hanauer, S.; Wang, H.; Shara, N.; Harris, M.S. AST-120 (spherical carbon adsorbent) in the treatment of perianal fistulae in mild-to-moderate Crohn’s disease: FHAST-1, a phase 3, multicenter, placebo-controlled study. Inflamm. Bowel Dis. 2014, 20, 872–881. [Google Scholar] [CrossRef][Green Version]
- Fukuda, Y.; Takazoe, M.; Sugita, A.; Kosaka, T.; Kinjo, F.; Otani, Y.; Fujii, H.; Koganei, K.; Makiyama, K.; Nakamura, T.; et al. Oral spherical adsorptive carbon for the treatment of intractable anal fistulas in Crohn’s disease: A multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 2008, 103, 1721–1729. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef]
- Supady, A.; Brodie, D.; Wengenmayer, T. Extracorporeal haemoadsorption: Does the evidence support its routine use in critical care? Lancet Respir. Med. 2022, 10, 307–312. [Google Scholar] [CrossRef]
- Feng, Y.; Peng, J.-Y.; Peng, Z. Blood purification in sepsis and systemic inflammation. Curr. Opin. Crit. Care 2021, 27, 582–586. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Grillari, J. Pathogenesis of Multiple Organ Failure: The Impact of Systemic Damage to Plasma Membranes. Front. Med. 2022, 9, 806462. [Google Scholar] [CrossRef]
- Dias, C.; Nylandsted, J. Plasma membrane integrity in health and disease: Significance and therapeutic potential. Cell Discov. 2021, 7, 4. [Google Scholar] [CrossRef]
- Boye, T.L.; Nylandsted, J. Annexins in plasma membrane repair. Biol. Chem. 2016, 397, 961–969. [Google Scholar] [CrossRef]
- Erkurt, M.A.; Sarici, A.; Özer, A.B.; Kuku, I.; Biçim, S.; Aydogan, M.S.; Kose, A.; Memisoglu, F.; Ince, V.; Otan, E.; et al. The effect of HA330 hemoperfusion adsorbent method on inflammatory markers and end-organ damage levels in sepsis: A retrospective single center study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8112–8117. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dash, P.K. Molecular details of secretory phospholipase A2 from flax (Linum usitatissimum L.) provide insight into its structure and function. Sci. Rep. 2017, 7, 11080. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.M.; Roberts, E.F.; Manetta, J.; Putnam, J.E. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J. Biol. Chem. 1991, 266, 5268–5272. [Google Scholar] [CrossRef] [PubMed]
- DiScipio, R.G.; Smith, C.A.; Muller-Eberhard, H.J.; Hugli, T.E. The activation of human complement component C5 by a fluid phase C5 convertase. J. Biol. Chem. 1983, 258, 10629–10636. [Google Scholar] [CrossRef]
- Podack, E.R.; Kolb, W.P.; Esser, A.F.; Müller-Eberhard, H.J. Structural similarities between C6 and C7 of human complement. J. Immunol. 1979, 123, 1071–1077. [Google Scholar] [CrossRef]
- Jones, J.; Laffafian, I.; Morgan, B.P. Purification of C8 and C9 from rat serum. Complement Inflamm. 1990, 7, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, A.; Sekizawa, Y.; Emoto, K.; Sakuraba, H.; Inoue, K.; Kobayashi, H.; Umeda, M. Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 1998, 273, 5300–5306. [Google Scholar] [CrossRef]
- Gouaux, E.; Hobaugh, M.; Song, L. Alpha-Hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: Distant in sequence but similar in structure. Protein Sci. 1997, 6, 2631–2635. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef]
- Divyakolu, S.; Chikkala, R.; Ratnakar, K.S.; Sritharan, V. Hemolysins of Staphylococcus aureus—An Update on Their Biology, Role in Pathogenesis and as Targets for Anti-Virulence Therapy. Adv. Infect. Dis. 2019, 9, 80–104. [Google Scholar] [CrossRef]
- Prévost, G.; Cribier, B.; Couppié, P.; Petiau, P.; Supersac, G.; Finck-Barbançon, V.; Monteil, H.; Piemont, Y. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 1995, 63, 4121–4129. [Google Scholar] [CrossRef]
- Tarek, B.; Bruggisser, J.; Cattalani, F.; Posthaus, H. Platelet Endothelial Cell Adhesion Molecule 1 (CD31) Is Essential for Clostridium perfringens Beta-Toxin Mediated Cytotoxicity in Human Endothelial and Monocytic Cells. Toxins 2021, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, M.; Sun, S. Pore-Forming Toxins During Bacterial Infection: Molecular Mechanisms and Potential Therapeutic Targets. Drug Des. Devel. Ther. 2021, 15, 3773–3781. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Avigad, L.S. Partial characterization of aerolysin, a lytic exotoxin from Aeromonas hydrophila. Infect. Immun. 1974, 9, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Kisovec, M.; Anderluh, G. Molecular mechanism of pore formation by aerolysin-like proteins. Philos. Trans. R Soc. Lond B Biol. Sci. 2017, 372, 20160209. [Google Scholar] [CrossRef]
- Lu, N.-F.; Jiang, L.; Zhu, B.; Yang, D.-G.; Zheng, R.-Q.; Shao, J.; Xi, X.-M. Elevated plasma histone H4 level predicts increased risk of mortality in patients with sepsis. Ann. Palliat. Med. 2020, 9, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.-N.; Deluna, X.; White, M.R.; Hartshorn, K.L. Histone H4 directly stimulates neutrophil activation through membrane permeabilization. J. Leukoc. Biol. 2021, 109, 763–775. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tawaramoto-Sasanuma, M.; Kawaguchi, S.; Ohta, T.; Yoda, K.; Kurumizaka, H.; Yokoyama, S. Expression and purification of recombinant human histones. Methods 2004, 33, 3–11. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ito, T.; Yasuda, T.; Furubeppu, H.; Kamikokuryo, C.; Yamada, S.; Maruyama, I.; Kakihana, Y. Circulating histone H3 levels in septic patients are associated with coagulopathy, multiple organ failure, and death: A single-center observational study. Thromb. J. 2019, 17, 1. [Google Scholar] [CrossRef]
- Li, Y.; Wan, D.; Luo, X.; Song, T.; Wang, Y.; Yu, Q.; Jiang, L.; Liao, R.; Zhao, W.; Su, B. Circulating Histones in Sepsis: Potential Outcome Predictors and Therapeutic Targets. Front. Immunol. 2021, 12, 650184. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.; Demonchy, J.; Arrii, E.; Luperto, M.; Lion, J.; Fodil, S.; Pons, S.; Mooney, N.; Zafrani, L. Endothelial Cells Activated by Extracellular Histones Promote Foxp3+ Suppressive Treg Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 4527. [Google Scholar] [CrossRef]
- Zlatina, K.; Galuska, S.P. Polysialic Acid Modulates Only the Antimicrobial Properties of Distinct Histones. ACS Omega 2019, 4, 1601–1610. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef]
- Shafer, W.M.; Martin, L.E.; Spitznagel, J.K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. 1984, 45, 29–35. [Google Scholar] [CrossRef]
- Fisher, J.; Linder, A. Heparin-binding protein: A key player in the pathophysiology of organ dysfunction in sepsis. J. Intern. Med. 2017, 281, 562–574. [Google Scholar] [CrossRef]
- Zhang, H.; Rodriguez, S.; Wang, L.; Wang, S.; Serezani, C.H.; Kapur, R.; Cardoso, A.A.; Carlesso, N. Sepsis Induces Hematopoietic Stem Cell Exhaustion and Myelosuppression through Distinct Contributions of TRIF and MYD88. Stem Cell Rep. 2016, 6, 940–956. [Google Scholar] [CrossRef]
- Belok, S.H.; Bosch, N.A.; Klings, E.S.; Walkey, A.J. Evaluation of leukopenia during sepsis as a marker of sepsis-defining organ dysfunction. PLoS ONE 2021, 16, e0252206. [Google Scholar] [CrossRef]
- Barthlen, W.; Zantl, N.; Pfeffer, K.; Heidecke, C.-D.; Holzmann, B.; Stadler, J. Impact of experimental peritonitis on bone marrow cell function. Surgery 1999, 126, 41–47. [Google Scholar] [CrossRef]
- Naismith, E.; Pangrazzi, L. The impact of oxidative stress, inflammation, and senescence on the maintenance of immunological memory in the bone marrow in old age. Biosci. Rep. 2019, 39, BSR20190371. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.Y.; Kung, C.T.; Su, C.M.; Lai, Y.R.; Huang, C.C.; Tsai, N.W.; Wang, H.C.; Cheng, B.C.; Su, Y.J.; Lin, W.C.; et al. Impact of oxidative stress on treatment outcomes in adult patients with sepsis: A prospective study. Medicine 2020, 99, e20872. [Google Scholar] [CrossRef] [PubMed]
- Karapetsa, M.; Pitsika, M.; Goutzourelas, N.; Stagos, D.; Becker, A.T.; Zakynthinos, E. Oxidative status in ICU patients with septic shock. Food Chem. Toxicol. 2013, 61, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Snezhkova, E.; Rodionova, N.; Bilko, D.; Silvestre-Albero, J.; Sydorenko, A.; Yurchenko, O.; Pakharenko, M.; Alavijeh, M.; Bardakhivska, K.; Riabchenko, N.; et al. Orally Administered Activated Charcoal as a Medical Countermeasure for Acute Radiation Syndrome in Rats. Appl. Sci. 2021, 11, 3174. [Google Scholar] [CrossRef]
- Shevchuk, O.; Snezhkova, E.; Sarnatskaya, V.; Mikhailenko, V.; Glavin, A.; Makovetska, L.; Bardakhivska, K.; Birchenko, I.; Kozynchenko, O.; Nikolaev, V. Effect of Primary and Secondary Beads of Carbon Enterosorbent on Haematological Parameters and Oxidative Stress Development Caused by Melphalan in Rats. Medicina 2019, 55, 557. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, O.; Posokhova, K.A.; Sidorenko, A.S.; Bardakhivskaya, K.I.; Maslenny, V.M.; Yushko, L.A.; Chekhun, V.F.; Nikolaev, V. The influence of enterosorption on some haematological and biochemical indices of the normal rats after single injection of melphalan. Exp. Oncol. 2014, 36, 94–100. [Google Scholar]
- Sarnatskaya, V.; Mikhailenko, V.; Prokopenko, I.; Gerashchenko, B.I.; Shevchuk, O.; Yushko, L.; Glavin, A.; Makovetska, L.; Sakhno, L.; Sydorenko, O.; et al. The effect of two formulations of carbon enterosorbents on oxidative stress indexes and molecular conformation of serum albumin in experimental animals exposed to CCl4. Heliyon 2020, 6, e03126. [Google Scholar] [CrossRef]
- Badawy, M.A.; Yasseen, B.A.; El-Messiery, R.M.; Abdel-Rahman, E.A.; Elkhodiry, A.A.; Kamel, A.G.; El-Sayed, H.; Shedra, A.M.; Hamdy, R.; Zidan, M.; et al. Neutrophil-mediated oxidative stress and albumin structural damage predict COVID-19-associated mortality. eLife 2021, 10, e6941. [Google Scholar] [CrossRef]
- Sarnatskaya, V.; Shlapa, Y.; Lykhova, A.; Brieieva, O.; Prokopenko, I.; Sidorenko, A.; Solopan, S.; Kolesnik, D.; Belous, A.; Nikolaev, V. Structure and biological activity of particles produced from highly activated carbon adsorbent. Heliyon 2022, 8, e09163. [Google Scholar] [CrossRef]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 2020, 24, 278. [Google Scholar] [CrossRef]
- Agudelo-Ochoa, G.M.; Valdés-Duque, B.E.; Giraldo-Giraldo, N.A.; Jaillier-Ramírez, A.M.; Giraldo-Villa, A.; Acevedo-Castaño, I.; Yepes-Molina, M.A.; Barbosa-Barbosa, J.; Benítez-Paéz, A. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes 2020, 12, 1707610. [Google Scholar] [CrossRef] [PubMed]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, R.F.; Wiersinga, W.J.; Haak, B.W. Gut microbiota and sepsis: From pathogenesis to novel treatments. Curr. Opin. Gastroenterol. 2021, 37, 578–585. [Google Scholar] [CrossRef]
- Watarai, S.; Tana. Eliminating the carriage of Salmonella enterica serovar Enteritidis in domestic fowls by feeding activated charcoal from bark containing wood vinegar liquid (Nekka-Rich). Poult. Sci. 2005, 84, 515–521. [Google Scholar] [CrossRef]
- Ryu, D.-Y.; Nakabayashi, K.; Shimohara, T.; Morio, U.; Mochida, I.; Miyawaki, J.; Jeon, Y.; Park, J.-I.; Yoon, S.-H. Behaviors of Cellulose-Based Activated Carbon Fiber for Acetaldehyde Adsorption at Low Concentration. Appl. Sci. 2020, 10, 25. [Google Scholar] [CrossRef]
- Chen, D.; Wan, P.; Cai, B.; Ye, Z.; Chen, H.; Chen, X.; Sun, H.; Pan, J. Trimethylamine Adsorption Mechanism on Activated Carbon and Removal in Water and Oyster Proteolytic Solution. J. Ocean. Univ. China 2021, 20, 1578–1586. [Google Scholar] [CrossRef]
- Silvestre-Albero, A.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; Rodriguez-Reinoso, F. Ethanol Removal Using Activated Carbon: Effect of Porous Structure and Surface Chemistry. Microporous Mesoporous Mater. 2009, 120, 62–68. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Seubsai, A.; Roddecha, S.; Yudistira, A.; Wiharto, A.D. Isotherm adsorption of free fatty acid in waste cooking oil used activated carbon of banana peel as bio-adsorbent. J. Phys. Conf. Ser. 2021, 1918, 032008. [Google Scholar] [CrossRef]
- Pédron, T.; Mulet, C.; Dauga, C.; Frangeul, L.; Chervaux, C.; Grompone, G.; Sansonetti, P.J. A crypt-specific core microbiota resides in the mouse colon. Mbio 2012, 3, e00116-12. [Google Scholar] [CrossRef]
- Zaborin, A.; Krezalek, M.; Hyoju, S.; Defazio, J.R.; Setia, N.; Belogortseva, N.; Bindokas, V.P.; Guo, Q.; Zaborina, O.; Alverdy, J.C. Critical role of microbiota within cecal crypts on the regenerative capacity of the intestinal epithelium following surgical stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G112–G122. [Google Scholar] [CrossRef]
- Krajina, B.A.; Tropini, C.; Zhu, A.; DiGiacomo, P.; Sonnenburg, J.L.; Heilshorn, S.C.; Spakowitz, A.J. Dynamic Light Scattering Microrheology Reveals Multiscale Viscoelasticity of Polymer Gels and Precious Biological Materials. ACS Cent. Sci. 2017, 3, 1294–1303. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Rashidi, A.; Karuppiah, S.; Ebadi, M.; Shanley, R.; Khoruts, A.; Weisdorf, D.J.; Staley, C. A dose-finding safety and feasibility study of oral activated charcoal and its effects on the gut microbiota in healthy volunteers not receiving antibiotics. PLoS ONE 2022, 17, e0269986. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiol. Open 2022, 11, e1260. [Google Scholar] [CrossRef]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory from Infection to Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar]
- Chen, J.; Han, W.; Chen, J.; Zong, W.; Wang, W.; Wang, Y.; Cheng, G.; Li, C.; Ou, L.; Yu, Y. High performance of a unique mesoporous polystyrene-based adsorbent for blood purification. Regen. Biomater. 2017, 4, 31–37. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Q.; Zhao, T.; Sui, L.; Wang, S.; Xiao, Z.; Nan, Y.; Ai, K. Nanotherapies for sepsis by regulating inflammatory signals and reactive oxygen and nitrogen species: New insight for treating COVID-19. Redox Biol. 2021, 45, 102046. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R. Sepsis Management, Controversies, and Advancement in Nanotechnology: A Systematic Review. Cureus 2022, 14, e22112. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Guo, S.; Bianco, A.; Ménard-Moyon, C. Carbon nanomaterials applied for the treatment of inflammatory diseases: Preclinical evidence. Adv. Ther. 2020, 3, 2000051. [Google Scholar] [CrossRef]
- Kiss, A. Inflammation in Focus: The Beginning and the End. Pathol. Oncol. Res. 2022, 27, 1610136. [Google Scholar] [CrossRef]
| Substances | Adsorbed, [Reference Number] | MW, kDa | Adsorbed Potentially * | |
|---|---|---|---|---|
| PAMPs: | Toxic Shock Syndrome Toxin-1 (TSST-1) | - | 24 | * |
| Streptococcal cysteine protease (SpeB) | - | 40 | * | |
| Bacteria and viruses | [33,34,35] | |||
| Shiga toxin (Stx-2B) | [33] | |||
| Clostridium difficile toxins: TcDA, and TcDB | [33] | |||
| Cyanotoxins | [36] | |||
| Endotoxin | [33,37] | |||
| Mycotoxins: aflatoxin B1-312 Da and T-2 toxin | [38] | |||
| Calprotectin | - | 36.5 | * | |
| DAMPs: | PCT (procalcitonin) | - | 14.5 | * |
| high-mobility group protein 1 (HMGB-1) | - | 25 | * | |
| S100-family protein | - | 9–12 | * | |
| Small heat shock proteins | [39] | |||
| Histones | - | 11–22 | ||
| Myoglobin | [40] | |||
| Azurocidin | - | 37 | * | |
| Plasminogen activator inhibitor1 (PAI-1) | - | 43 | * | |
| Cytokines pro-inflammatory & anti-inflammatory | C3a | [13] | ||
| C5a | [13,27] | |||
| IFN- α, IFN-ꝩ, TNF-α, IL-1β, IL-6 | [37] | |||
| G-CSF | - | 18.8 | * | |
| GM-CSF | - | 14–35 | ||
| IL-9 | - | 14 | * | |
| IL-1 | [37] | |||
| IL-2, IL-3, IL-8 | [13,41] | |||
| IL-18 | - | 18.2 | * | |
| IL-4, IL-10 | [42] | |||
| IL-1ra | - | 22–25 | * | |
| IL-13 | - | 12.5 | * | |
| Drugs | Cytostatic | [43] | ||
| Antibiotics | [29] | |||
| Heparin | [44] | |||
| Chemokines | Eotaxin | - | 8.4 | * |
| CXCL-1 | - | 11 | * | |
| MCP-1 | - | 11–13 | * | |
| MIP-1alpha | - | 7.8 | * | |
| Hormone | T3 Cortisol | - | 0.362 | * |
| Metabolization product | Ammonia | [30,45] | ||
| Creatinine | [17] | |||
| Lactic acid | [46] | |||
| Bile acids | [42] | |||
| Protein-bound substances | Unconjugated bilirubin, uremic toxin (CMPF), indoxyl sulfate, hippuric acid | [17] | ||
| Indoles, scatoles, phenols, polyamines, advanced glycation end’s product (AGES) | [47] | |||
| Cells/cellular component | Blood cells: platelets, neutrophils, monocytes | [48,49] | ||
| Extracellular vesicles (EVs) | ## | |||
| Substances Upregulated in Serum or Plasma | MW (kDa) | pI | Reference |
|---|---|---|---|
| Secretory phospholipase A2 (sPLA 2) group IIa | 13–17 | 6.68 | [119] |
| cytosolic PLA 2 (cPLA2) | 80–85 group IV | 5.0 | [120] |
| C5 (MAC) | 120(ɑ) + 175(β) = 195 | 4.7–5.5 | [121] |
| C5a (MAC) | 11 | 8.6 | [121] |
| C 5b (MAC) | 171 | 4.5–5.3 | [121] |
| C6 (MAC) | 90–100 | 5.6–6.1 | [122] |
| C7(MAC) | 110 | 5.6–6.1 | [122] |
| C8 (MAC) | 64 (ɑ) + 64(β)+ 22(γ) = 150 | 7.4–7.9 | [123] |
| Lysenin PFTs | 41 | 6.5 | [124] |
| Alpha-hemolysin (αHL) secreted from Staphylococcus aureus PFTs | 33–37 | 7.94 | [125,126,127] |
| γ-Hemolysin, from Staphylococcus aureus PFTs | 31.81 | 9.52 | [125,128] |
| Delta-hemolysin from Staphylococcus aureus | 3 | - | [127] |
| Beta toxin from Clostridium perfringens (CPB) β-PFTs | 27.6 | 5.6–6 | [129] |
| Leucocidin A/B (LukAB) from Staphylococcus aureus PFTs | 32.39 | 8.77 | [125,127,130] |
| Aerolysin PFTs | 50–53 | 5–7 | [131,132] |
| Histone H4 | 11 | 8–9 | [133,134,135] |
| Positive Effects of Activated Charcoal (AC) Administration on: | Proven/Hypothesized Mechanism of Action(s) | Gaps |
|---|---|---|
| PAMPs & DAMPs in biological fluids | Increasing AC surface & porosity and its selectivity towards PAMPs & DAMPs; by a combination of selective and broad-range AC adsorption; by modifying biological fluids e.g.,by transfusion, and special drug use to enhance PAMPs & DAMPs adsorption by AC. | a; adsorption of benefic substances |
| Bone marrow and oxidative stress | Early initiation of AC administration; study of the mechanisms of AC action. | b |
| Serum albumin | Increasing AC surface & porosity and selectivity towards protein-bound toxins. | a |
| Microbiome | Combination of different routes of AC administration; AC modification; study of mechanisms of AC action. | b; e |
| Diarrhea | Enhance AC biocompatibility and adsorptive capacity towards e.g., bacteria, viruses, and their toxins; study of the mechanisms of AC action. | a–d |
| Renal dysfunction | Early initiation of AC administration; combination of different routes of AC administration; amelioration of AC biocompatibility and adsorptive capacity towards uremic especially protein-bound toxins; study of the mechanisms of AC action. | b; d; e |
| Liver failure | Early initiation of AC administration; combination of different ways of AC administration; amelioration of AC biocompatibility and adsorptive capacity towards hepatic toxins; study of the mechanisms of AC action. | b; d; e |
| Perianal fistula | Combination of AC per oral administration with those per rectum; study of the mechanisms of AC action. | Begin of clinical application; b |
| Sepsis | Early initiation of AC administration before an irreparable membrane; a combination of different routes of AC administration; development of biocompatible AC combining selective and broad-range adsorption; study of the mechanisms of AC action. | a–d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snezhkova, E.; Redl, H.; Grillari, J.; Osuchowski, M. Activated Carbon for Sepsis Prevention and Intervention: A Modern Way of Utilizing Old Therapies. C 2023, 9, 72. https://doi.org/10.3390/c9030072
Snezhkova E, Redl H, Grillari J, Osuchowski M. Activated Carbon for Sepsis Prevention and Intervention: A Modern Way of Utilizing Old Therapies. C. 2023; 9(3):72. https://doi.org/10.3390/c9030072
Chicago/Turabian StyleSnezhkova, Elisaveta, Heinz Redl, Johannes Grillari, and Marcin Osuchowski. 2023. "Activated Carbon for Sepsis Prevention and Intervention: A Modern Way of Utilizing Old Therapies" C 9, no. 3: 72. https://doi.org/10.3390/c9030072
APA StyleSnezhkova, E., Redl, H., Grillari, J., & Osuchowski, M. (2023). Activated Carbon for Sepsis Prevention and Intervention: A Modern Way of Utilizing Old Therapies. C, 9(3), 72. https://doi.org/10.3390/c9030072







