RF Sensor with Graphene Film for HRP Concentration Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene and Film Characterization
2.2. Sensor Fabrication and Measurement Setup
3. Results
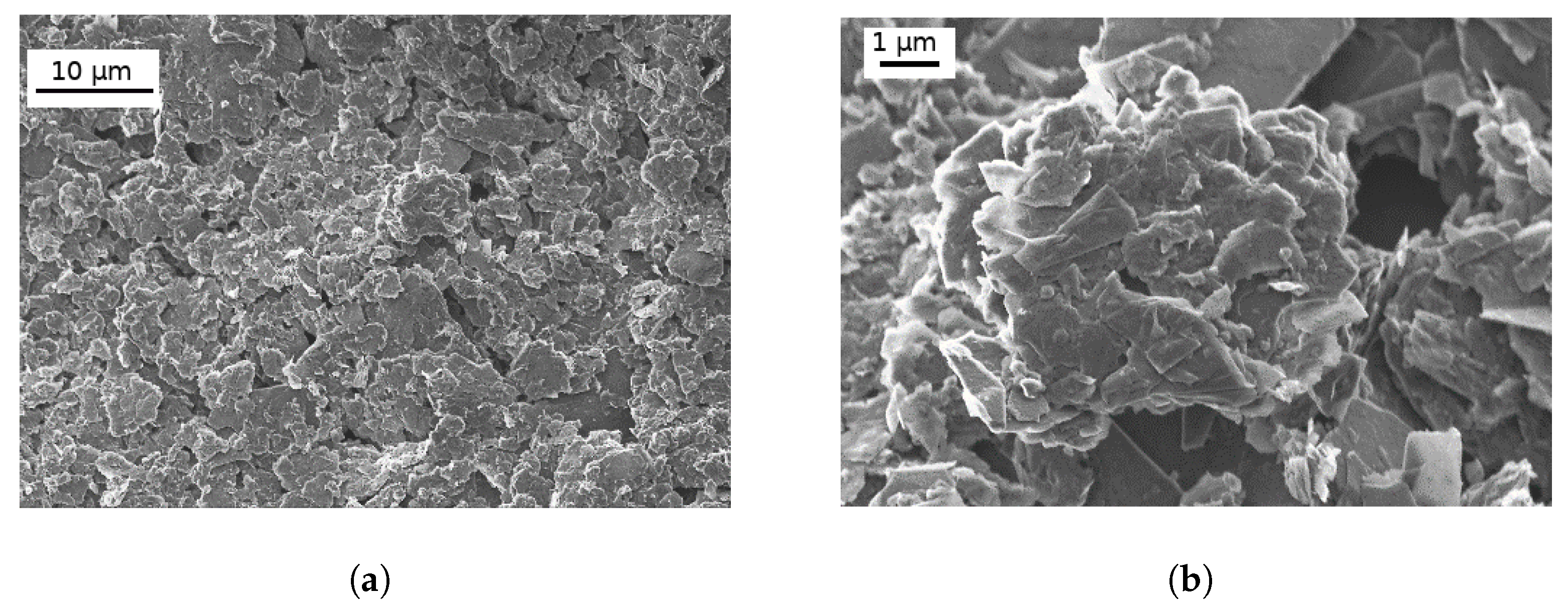
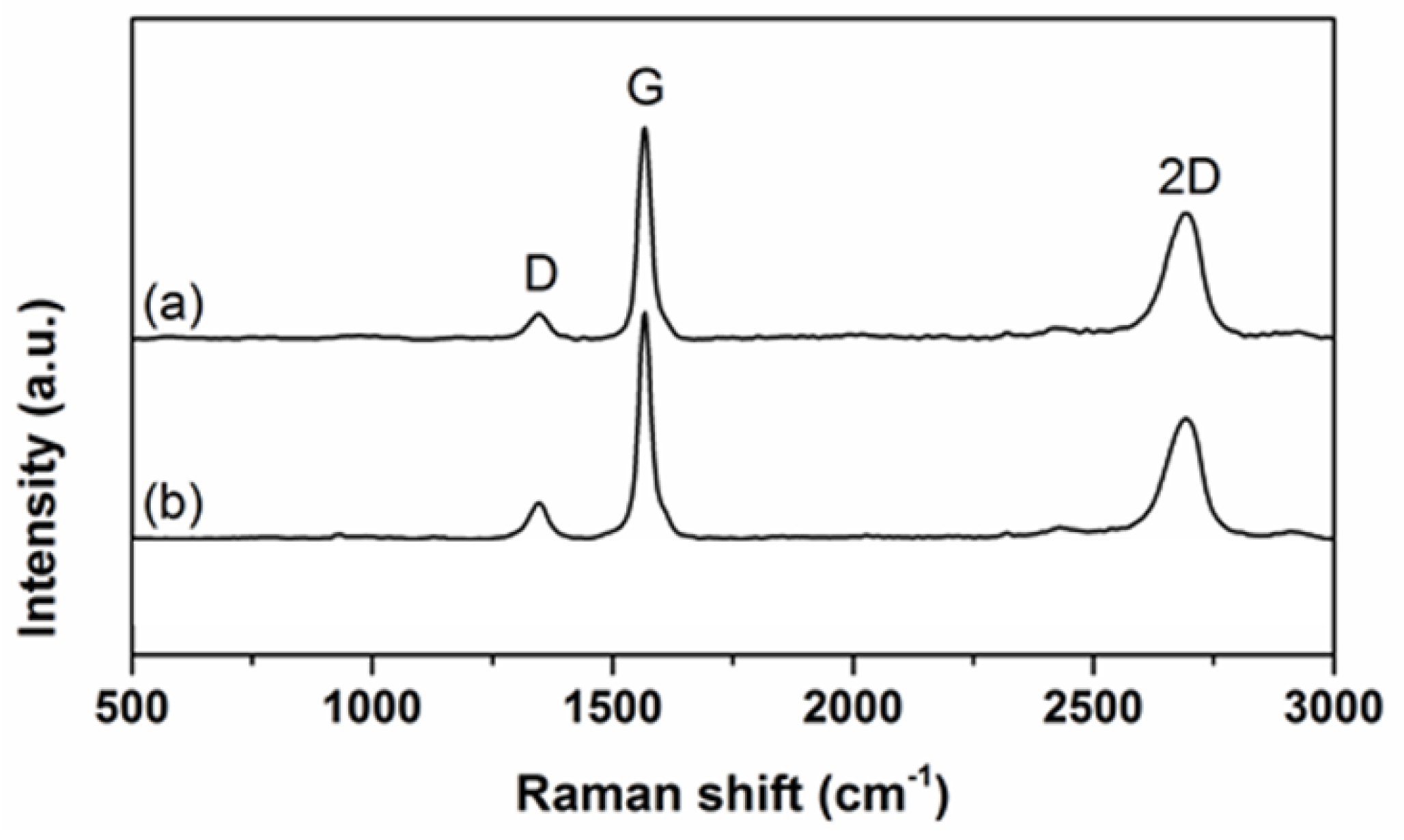
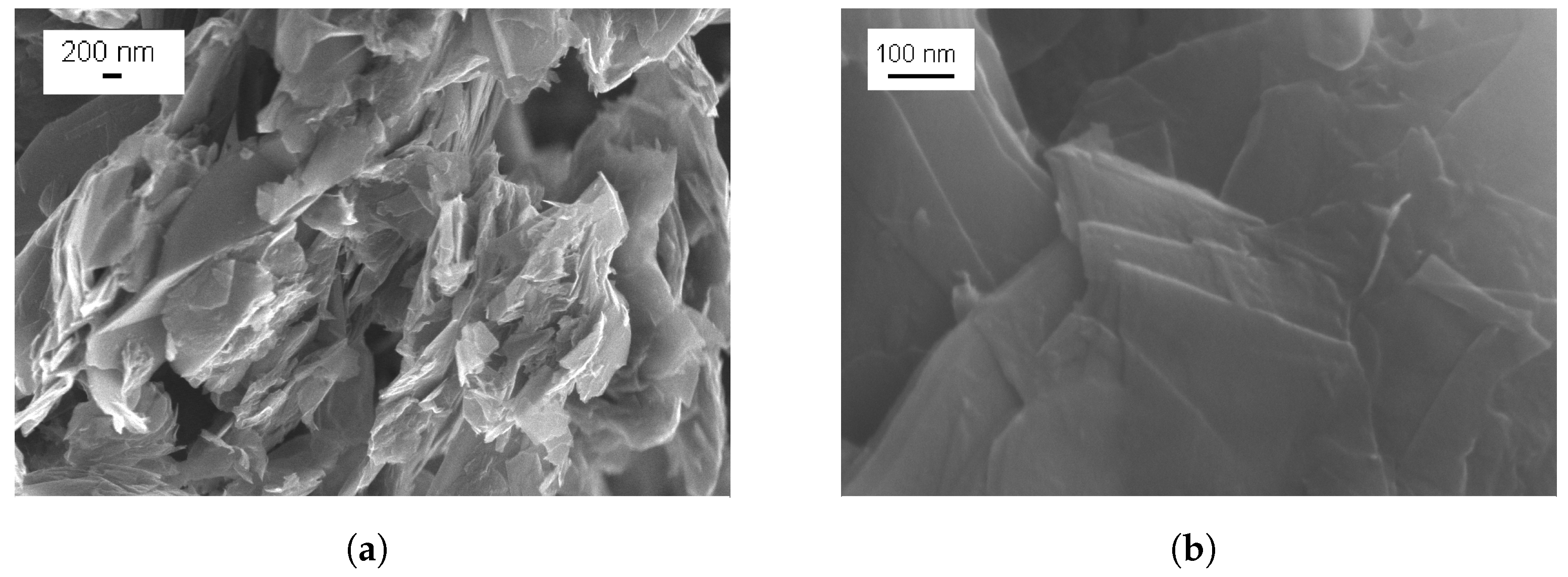
3.1. Characterization of Graphene Nanoplatelets and Its Composite Films
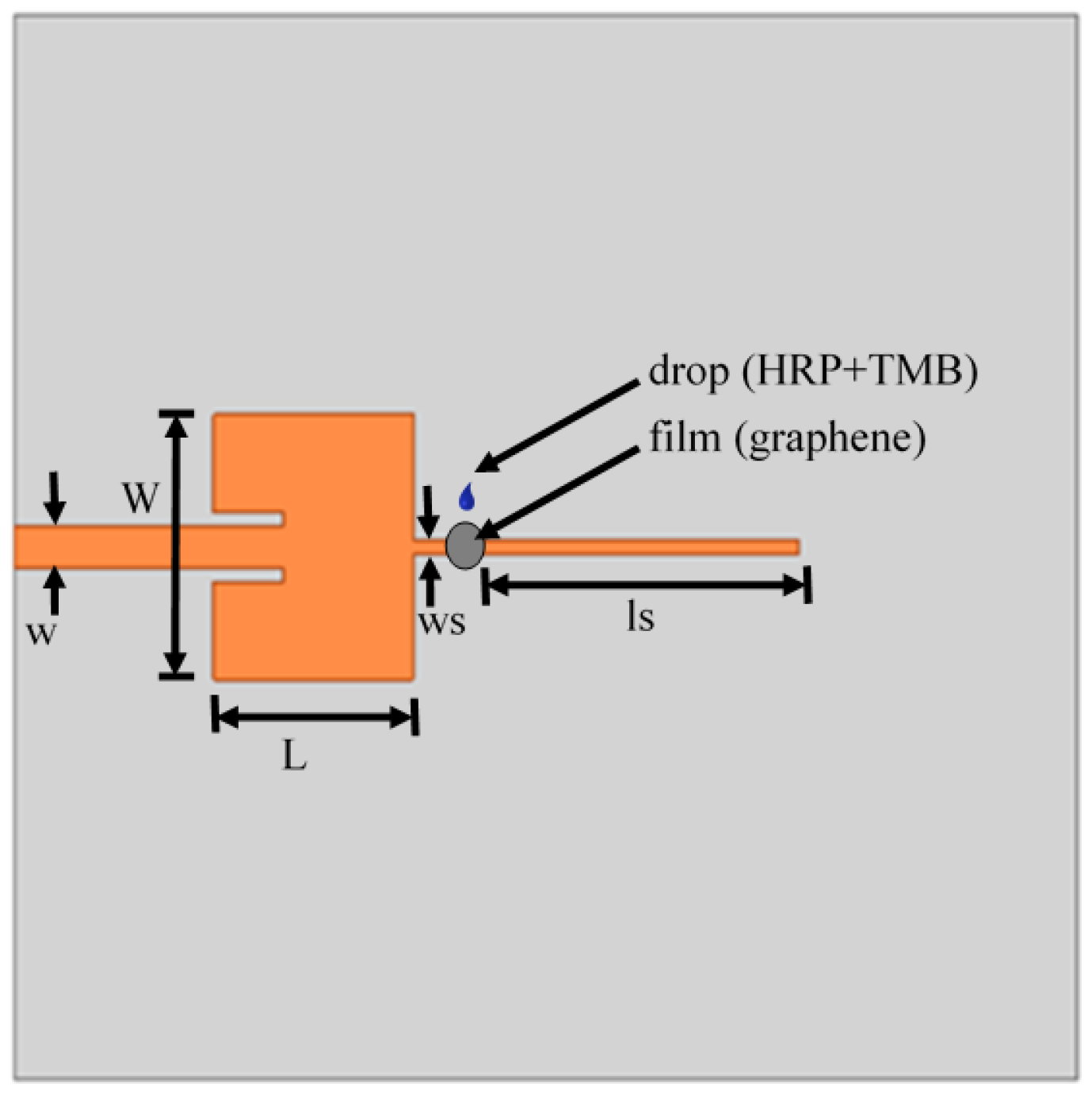
3.2. Sensor Design and Realization
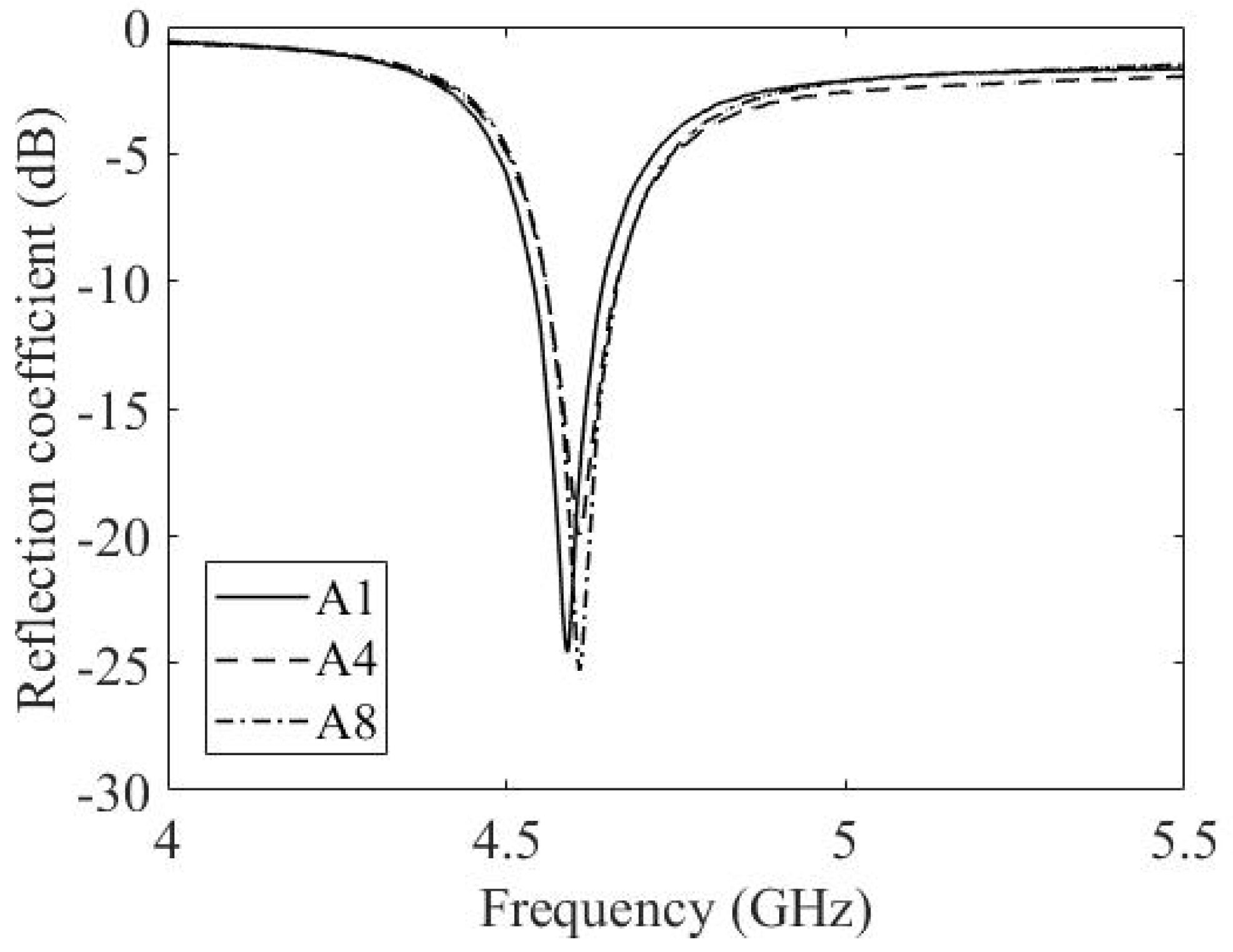
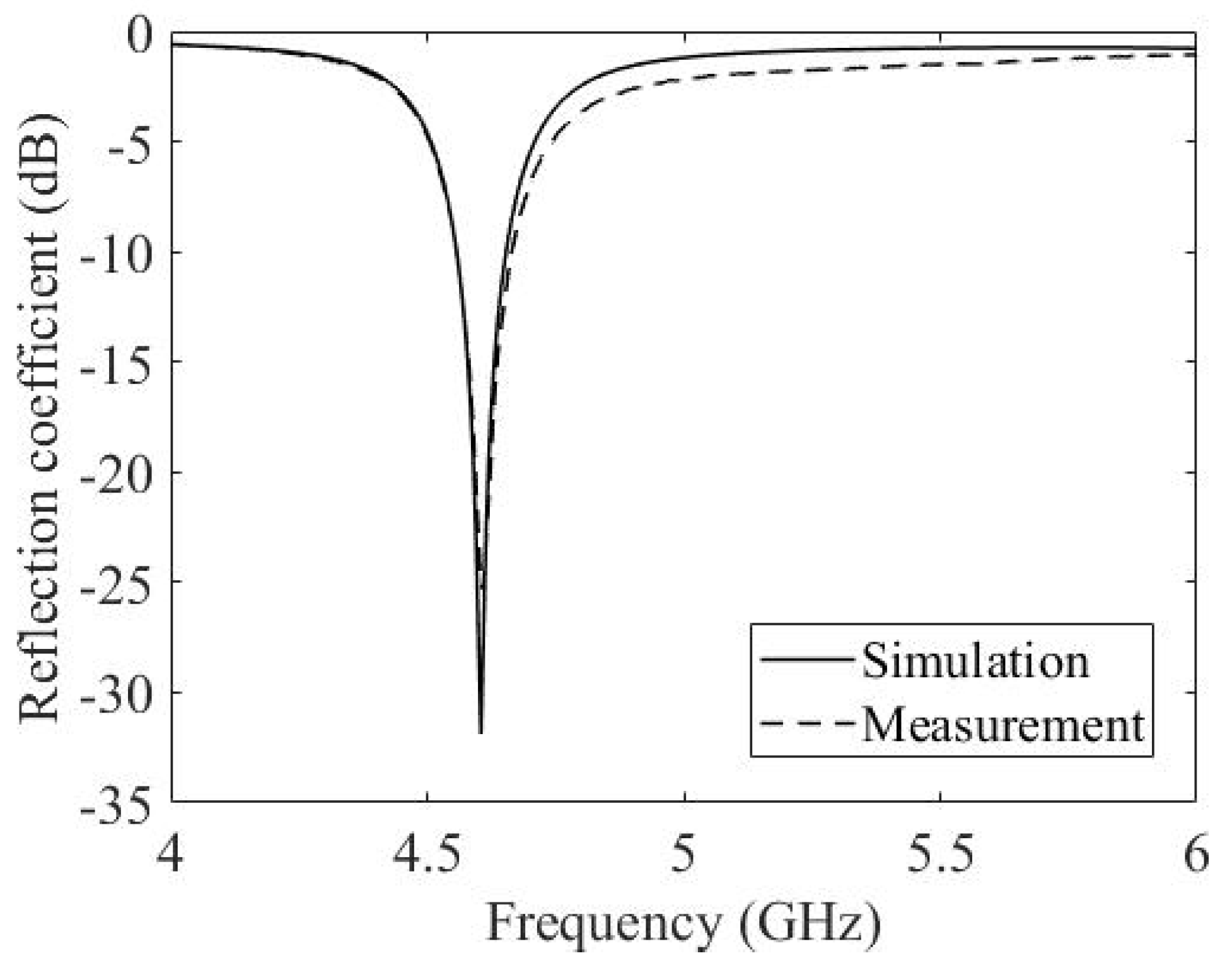
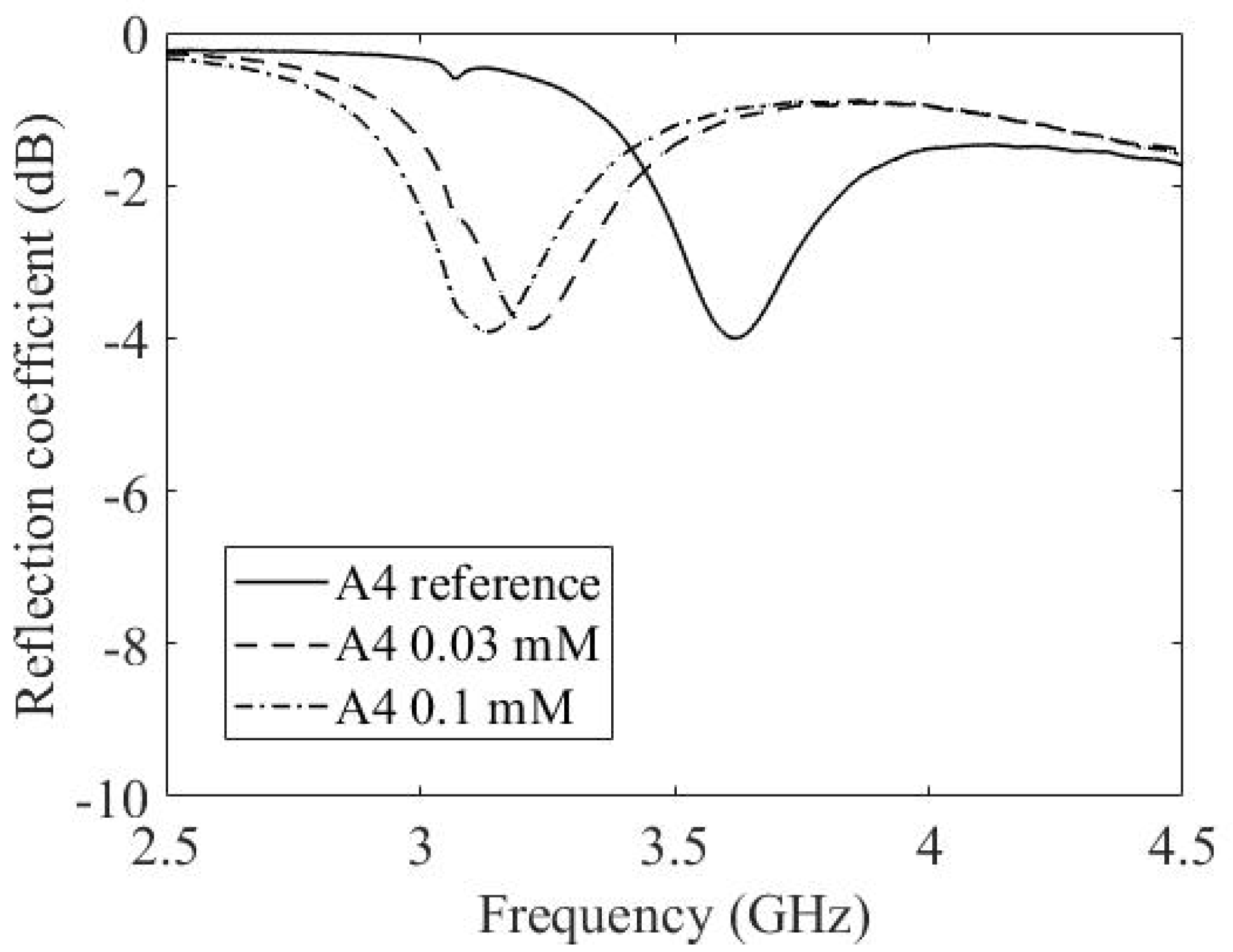
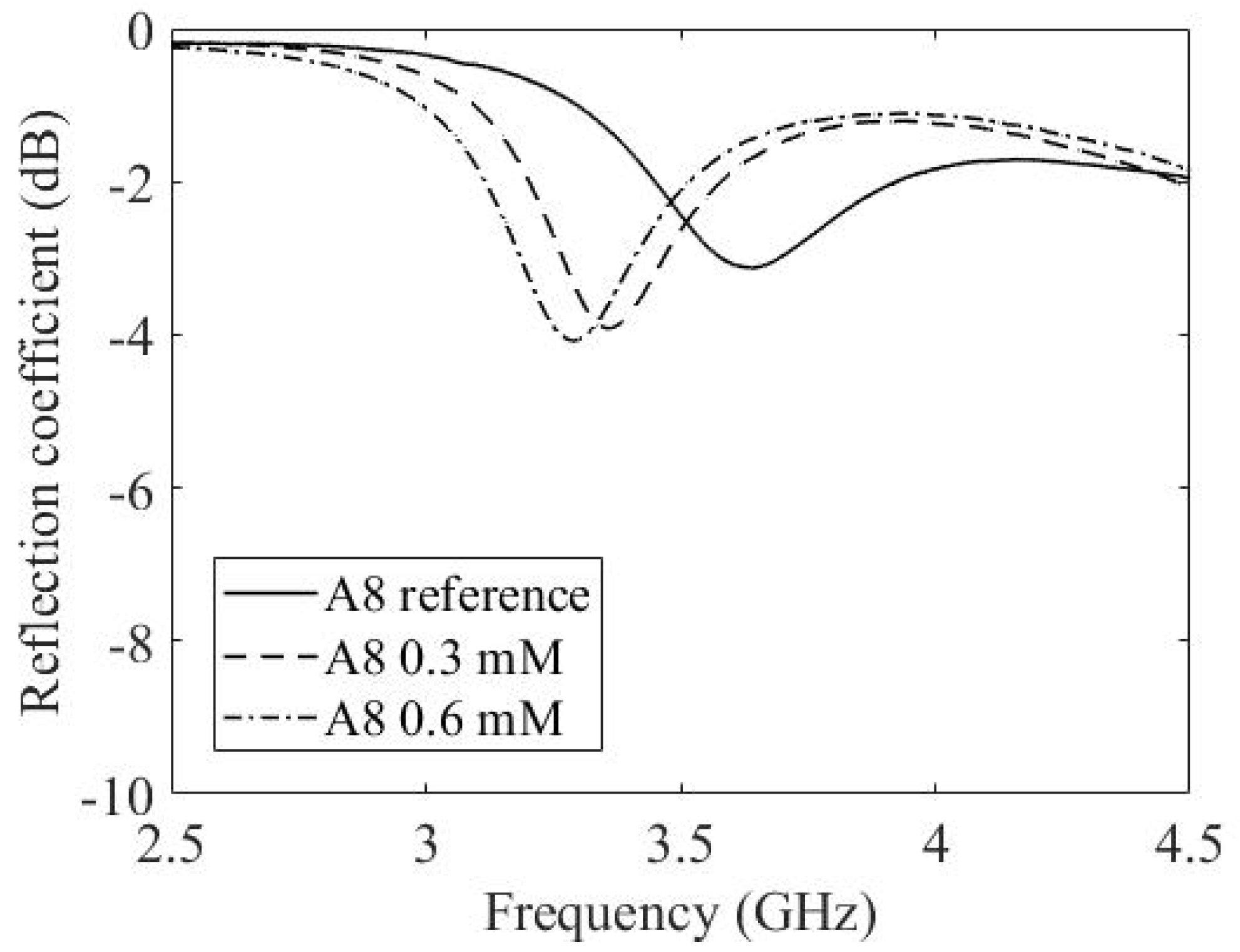
3.3. Experimental Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Zhong, W.H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Hill, E.W.; Vijayaragahvan, A.; Novoselov, K. Graphene sensors. IEEE Sens. J. 2011, 11, 3161–3170. [Google Scholar] [CrossRef]
- Kymakis, E.; Stratakis, E.; Stylianakis, M.; Koudoumas, E.; Fotakis, C. Spin coated graphene films as the transparent electrode in organic photovoltaic devices. Thin Solid Film. 2011, 520, 1238–1241. [Google Scholar] [CrossRef]
- Novikov, S.; Lebedeva, N.; Pierz, K.; Satrapinski, A. Fabrication and study of large-area QHE devices based on epitaxial graphene. IEEE Trans. Instrum. Meas. 2015, 64, 1533–1538. [Google Scholar] [CrossRef]
- Kiraly, B.; Jacobberger, R.M.; Mannix, A.J.; Campbell, G.P.; Bedzyk, M.J.; Arnold, M.S.; Hersam, M.C.; Guisinger, N.P. Electronic and mechanical properties of graphene–germanium interfaces grown by chemical vapor deposition. Nano Lett. 2015, 15, 7414–7420. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-resolution patterning of graphene by screen printing with a silicon stencil for highly flexible printed electronics. Adv. Mater. 2015, 27, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Novikov, S.; Satrapinski, A.; Lebedeva, N.; Iisakka, I. Sensitivity optimization of epitaxial graphene-based gas sensors. IEEE Trans. Instrum. Meas. 2013, 62, 1859–1864. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Elmquist, R.E.; Huang, L.I.; Yang, Y.; Liu, F.H.; Kaneko, N.h. Controlling the fermi level in a single-layer graphene QHE device for resistance standard. IEEE Trans. Instrum. Meas. 2015, 64, 1451–1454. [Google Scholar] [CrossRef]
- Leng, X.; Li, W.; Luo, D.; Wang, F. Differential structure with graphene oxide for both humidity and temperature sensing. IEEE Sens. J. 2017, 17, 4357–4364. [Google Scholar] [CrossRef]
- Sanaeepour, M.; Abedi, A.; Sharifi, M.J. Performance analysis of nanoscale single layer graphene pressure sensors. IEEE Trans. Electron Devices 2017, 64, 1300–1304. [Google Scholar] [CrossRef]
- Shi, G.; Liu, T.; Kopecki, Z.; Cowin, A.; Lee, I.; Pai, J.H.; Lowe, S.E.; Zhong, Y.L. A multifunctional wearable device with a graphene/silver nanowire nanocomposite for highly sensitive strain sensing and drug delivery. C 2019, 5, 17. [Google Scholar] [CrossRef]
- Dragoman, M.; Neculoiu, D.; Dragoman, D.; Deligeorgis, G.; Konstantinidis, G.; Cismaru, A.; Coccetti, F.; Plana, R. Graphene for microwaves. IEEE Microw. Mag. 2010, 11, 81–86. [Google Scholar] [CrossRef]
- Bozzi, M.; Pierantoni, L.; Bellucci, S. Applications of graphene at microwave frequencies. Radioengineering 2015, 24, 661–669. [Google Scholar] [CrossRef]
- Pierantoni, L.; Mencarelli, D.; Bozzi, M.; Moro, R.; Moscato, S.; Perregrini, L.; Micciulla, F.; Cataldo, A.; Bellucci, S. Broadband microwave attenuator based on few layer graphene flakes. IEEE Trans. Microw. Theory Tech. 2015, 63, 2491–2497. [Google Scholar] [CrossRef]
- Savi, P.; Naishadam, K.; Bayat, A.; Giorcelli, M.; Quaranta, S. Multi-walled carbon nanotube thin film loading for tuning microstrip patch antennas. In Proceedings of the 2016 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–3. [Google Scholar]
- Yasir, M.; Savi, P.; Bistarelli, S.; Cataldo, A.; Bozzi, M.; Perregrini, L.; Bellucci, S. A planar antenna with voltage-controlled frequency tuning based on few-layer graphene. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 2380–2383. [Google Scholar] [CrossRef]
- Yuan, M.; Alocilja, E.C.; Chakrabartty, S. A novel biosensor based on silver-enhanced self-assembled radio-frequency antennas. IEEE Sens. J. 2013, 14, 941–942. [Google Scholar] [CrossRef]
- Yuan, M.; Alocilja, E.C.; Chakrabartty, S. Self-powered wireless affinity-based biosensor based on integration of paper-based microfluidics and self-assembled RFID antennas. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 799–806. [Google Scholar] [CrossRef]
- Su, W.; Xu, J.; Ding, X. An electrochemical pH sensor based on the amino-functionalized graphene and polyaniline composite film. IEEE Trans. Nanobiosci. 2016, 15, 812–819. [Google Scholar] [CrossRef]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Wu, H.W. Label-free and antibody-free wideband microwave biosensor for identifying the cancer cells. IEEE Trans. Microw. Theory Tech. 2016, 64, 982–990. [Google Scholar] [CrossRef]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef]
- Purr, F.; Lowe, R.D.; Stehr, M.; Singh, M.; Burg, T.P.; Dietzel, A. Biosensing based on optimized asymmetric optofluidic nanochannel gratings. Micro Nano Eng. 2020, 8, 100056. [Google Scholar] [CrossRef]
- Nikoleli, G.P.; Siontorou, C.G.; Nikolelis, D.P.; Bratakou, S.; Karapetis, S.; Tzamtzis, N. Biosensors based on lipid modified graphene microelectrodes. C 2017, 3, 9. [Google Scholar] [CrossRef]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Aguas, H.; Fortunato, E.; Martins, R. Inkjet printed and “doctor blade” TiO2 photodetectors for DNA biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef]
- Marzo, A.M.L.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Pogăcean, F.; Varodi, C.; Măgeruşan, L.; Pruneanu, S. Highly Sensitive Graphene-Based Electrochemical Sensor for Nitrite Assay in Waters. Nanomaterials 2023, 13, 1468. [Google Scholar] [CrossRef]
- Jang, C.; Lee, H.J.; Yook, J.G. Radio-frequency biosensors for real-time and continuous glucose detection. Sensors 2021, 21, 1843. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yook, J.G. Recent research trends of radio-frequency biosensors for biomolecular detection. Biosens. Bioelectron. 2014, 61, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Park, H.G.; Ji, J.h.; Cho, J.; Jun, S.C. A reduced graphene oxide based radio frequency glucose sensing device using multi-dimensional parameters. Micromachines 2016, 7, 136. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Dao, J.; Guo, J.; Gao, Y. A colorimetric sensing platform for the determination of H2O2 using 2D–1D MoS2-CNT nanozymes. RSC Adv. 2022, 12, 28349–28358. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef] [PubMed]
- Umasankar, Y.; Unnikrishnan, B.; Chen, S.M.; Ting, T.W. Graphene impregnated with horseradish peroxidase multimer for the determination of hydrogen peroxide. Anal. Methods 2012, 4, 3653–3660. [Google Scholar] [CrossRef]
- Bocanegra-Rodríguez, S.; Jornet-Martínez, N.; Molins-Legua, C.; Campíns-Falcó, P. New reusable solid biosensor with covalent immobilization of the horseradish peroxidase enzyme: In situ liberation studies of hydrogen peroxide by portable chemiluminescent determination. ACS Omega 2020, 5, 2419–2427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, B.; Zhang, S.; Jiang, M.; Fu, W.; Sun, W. Preparation and application of electrochemical horseradish peroxidase sensor based on a black phosphorene and single-walled carbon nanotubes nanocomposite. Molecules 2022, 27, 8064. [Google Scholar] [CrossRef]
- Natsuki, T.; Yiwada, A.; Natsuki, J. Influence of temperature on vibrational frequency of graphene sheet used as nano-scale sensing. C 2017, 3, 4. [Google Scholar] [CrossRef]
- Chrouda, A.; Ayed, D.; Elamin, M.B.; Ali, S.M.A.; Alhaidari, L.M.; Bessueille, F.; Jaffrezic-Renault, N. Design of a Boron-Doped Diamond Microcell Grafted with HRP for the Sensitive and Selective Detection of Ochratoxin A. Chemosensors 2023, 11, 176. [Google Scholar] [CrossRef]
- Chaudhary, K.; Kumar, K.; Venkatesu, P.; Masram, D.T. Protein immobilization on graphene oxide or reduced graphene oxide surface and their applications: Influence over activity, structural and thermal stability of protein. Adv. Colloid Interface Sci. 2021, 289, 102367. [Google Scholar] [CrossRef]
- Evtugyn, G.A.; Goldfarb, O.; Budnikov, H.; Ivanov, A.; Vinter, V. Amperometric DNA-peroxidase sensor for the detection of pharmaceutical preparations. Sensors 2005, 5, 364–376. [Google Scholar] [CrossRef]
- Sharafeldin, M.; McCaffrey, K.; Rusling, J.F. Influence of antibody immobilization strategy on carbon electrode immunoarrays. Analyst 2019, 144, 5108–5116. [Google Scholar] [CrossRef]
- Koposova, E.; Liu, X.; Kisner, A.; Ermolenko, Y.; Shumilova, G.; Offenhäusser, A.; Mourzina, Y. Bioelectrochemical systems with oleylamine-stabilized gold nanostructures and horseradish peroxidase for hydrogen peroxide sensor. Biosens. Bioelectron. 2014, 57, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Chiadò, A.; Palmara, G.; Ricciardi, S.; Frascella, F.; Castellino, M.; Tortello, M.; Ricciardi, C.; Rivolo, P. Optimization and characterization of a homogeneous carboxylic surface functionalization for silicon-based biosensing. Colloids Surf. B Biointerfaces 2016, 143, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Palmara, G. Microcantilever-Based Sensing Arrays for Evaluation of Biomolecular Interactions. Ph.D. Thesis, Politecnico di Torino, Piedmont, Italy, 2016. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Frascella, F.; Ricciardi, C. Functionalization protocols of silicon micro/nano-mechanical biosensors. In Nanomaterial Interfaces in Biology: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; pp. 109–115. [Google Scholar]
- Roppolo, I.; Chiappone, A.; Bejtka, K.; Celasco, E.; Chiodoni, A.; Giorgis, F.; Sangermano, M.; Porro, S. A powerful tool for graphene functionalization: Benzophenone mediated UV-grafting. Carbon 2014, 77, 226–235. [Google Scholar] [CrossRef]
- Yasir, M.; Savi, P. Dynamically Tunable Phase Shifter with Commercial Graphene Nanoplatelets. Micromachines 2020, 11, 600. [Google Scholar] [CrossRef]
- Yasir, M.; Savi, P. Commercial graphene nanoplatelets-based tunable attenuator. Electron. Lett. 2020, 56, 184–187. [Google Scholar] [CrossRef]
- Yasir, M.; Fatikow, S.; Haenssler, O.C. Amplitude-Phase Variation in a Graphene-Based Microstrip Line. Micromachines 2022, 13, 1039. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.; Park, S. Estimation of number of graphene layers using different methods: A focused review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Yasir, M.; Savi, P.; Palmara, G.; Frascella, F.; Chiado, A.; Zaccagnini, P. Detection of HRP at microwave frequency with functionalized graphene film. In Proceedings of the 2022 International Conference on Electromagnetics in Advanced Applications (ICEAA), Cape Town, South Africa, 5–9 September 2022; pp. 323–325. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peinetti, F.; Yasir, M.; Savi, P. RF Sensor with Graphene Film for HRP Concentration Detection. C 2023, 9, 63. https://doi.org/10.3390/c9030063
Peinetti F, Yasir M, Savi P. RF Sensor with Graphene Film for HRP Concentration Detection. C. 2023; 9(3):63. https://doi.org/10.3390/c9030063
Chicago/Turabian StylePeinetti, Fabio, Muhammad Yasir, and Patrizia Savi. 2023. "RF Sensor with Graphene Film for HRP Concentration Detection" C 9, no. 3: 63. https://doi.org/10.3390/c9030063
APA StylePeinetti, F., Yasir, M., & Savi, P. (2023). RF Sensor with Graphene Film for HRP Concentration Detection. C, 9(3), 63. https://doi.org/10.3390/c9030063







