Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges
Abstract
1. Introduction
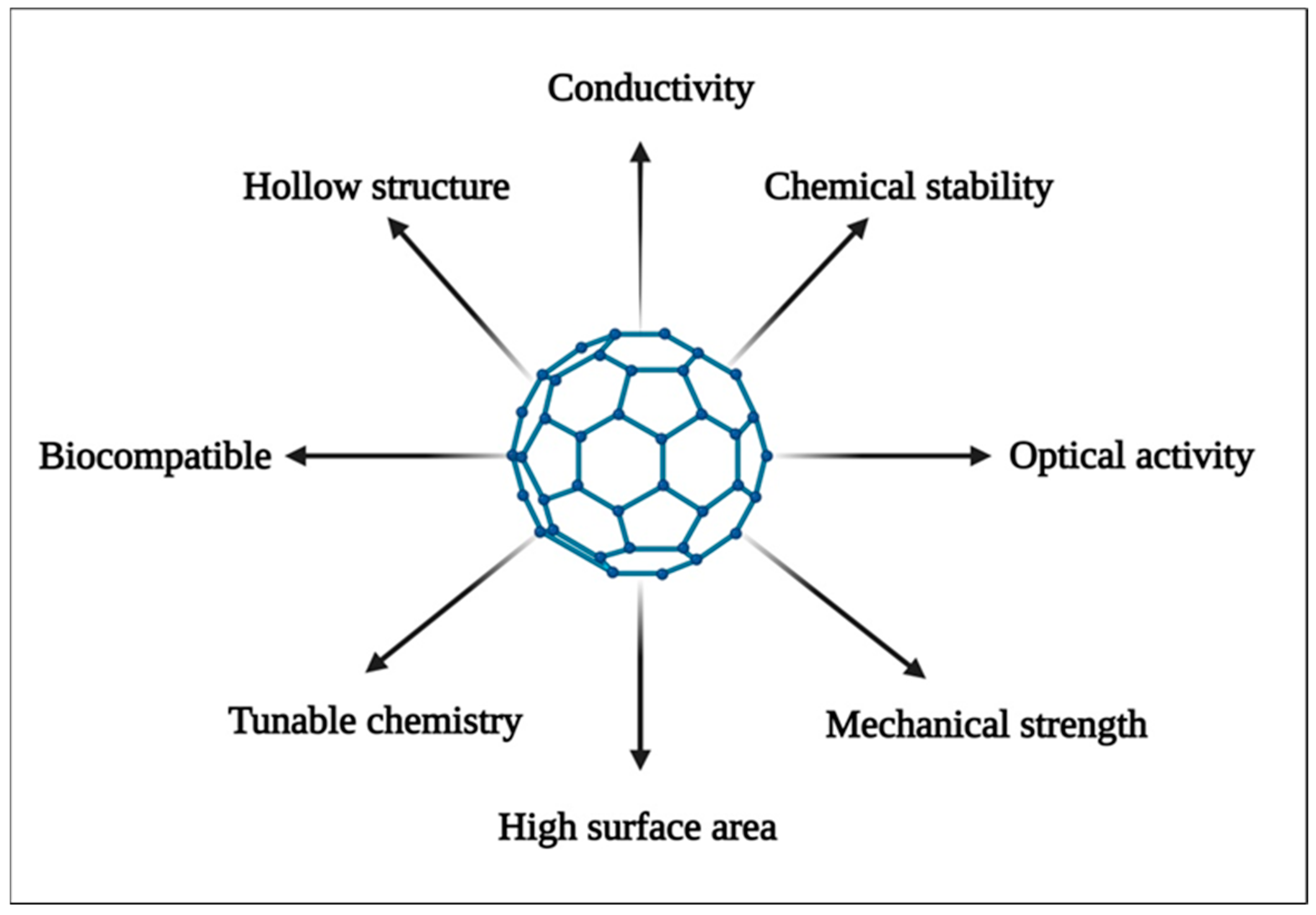
2. Properties of Carbon Nanostructures
3. Green Approaches for Synthesis of Carbon Nanostructures
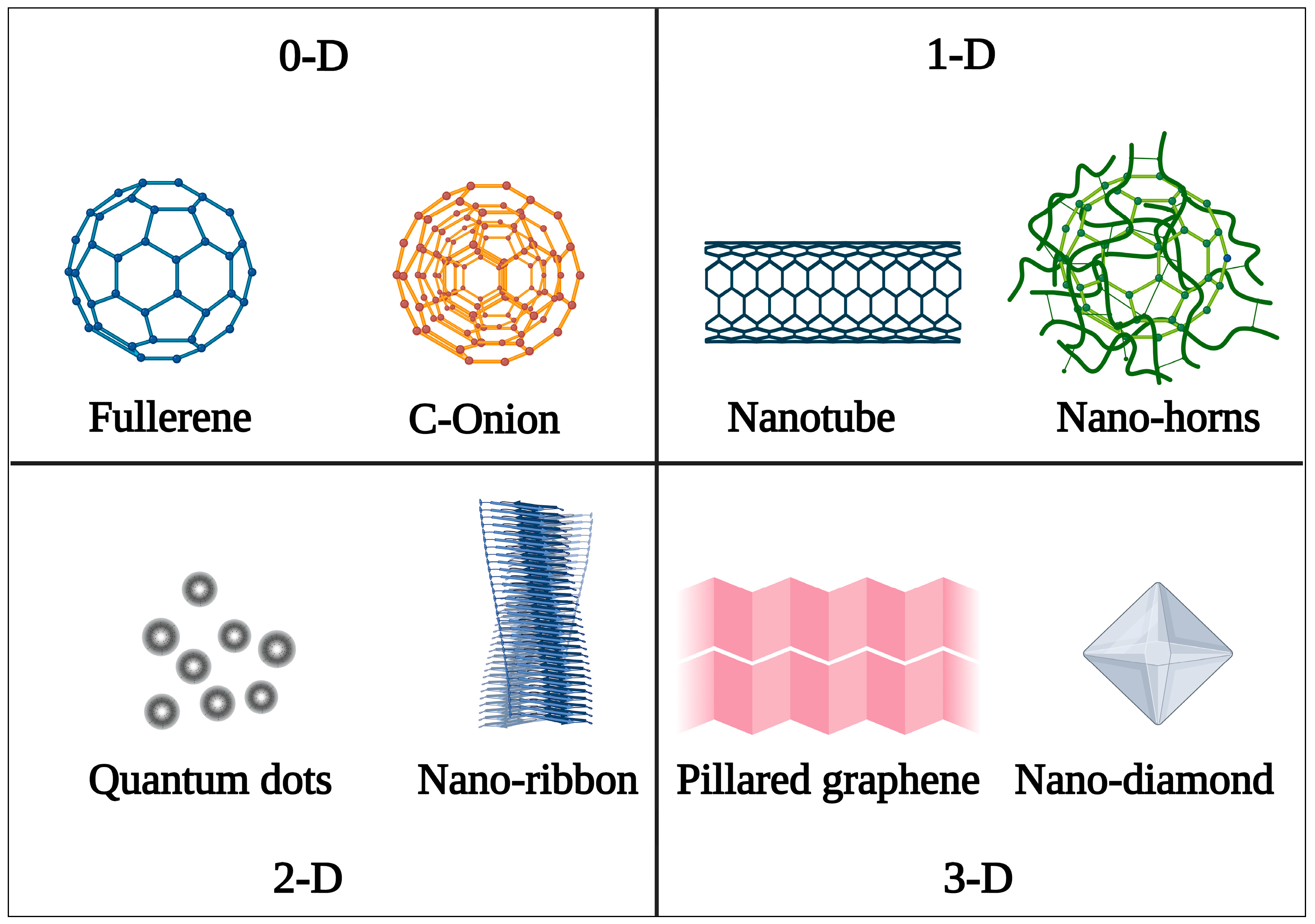
4. Classification of Carbon Nanostructures
4.1. Fullerene
4.1.1. Synthesis
4.1.2. Functionalization
4.1.3. Applications
Biosensing
Drug Delivery
4.2. Carbon Nano-Onions
4.2.1. Synthesis
4.2.2. Functionalization
4.2.3. Applications
Biosensing
Drug Delivery
4.3. Carbon Quantum Dots
4.3.1. Synthesis
4.3.2. Functionalization
4.3.3. Applications
Bioimaging
Drug Delivery
4.4. Carbon Nanotubes
4.5. A. Single-Walled Carbon Nanotubes
4.6. B. Multiple-Walled Carbon Nanotubes
4.6.1. Synthesis
4.6.2. Functionalization
4.6.3. Applications
Theranostics
Biosensing
Drug Delivery
4.7. Nanodiamonds
4.7.1. Synthesis
4.7.2. Functionalization
4.7.3. Applications
Bioimaging
Gene Therapy
Drug Delivery
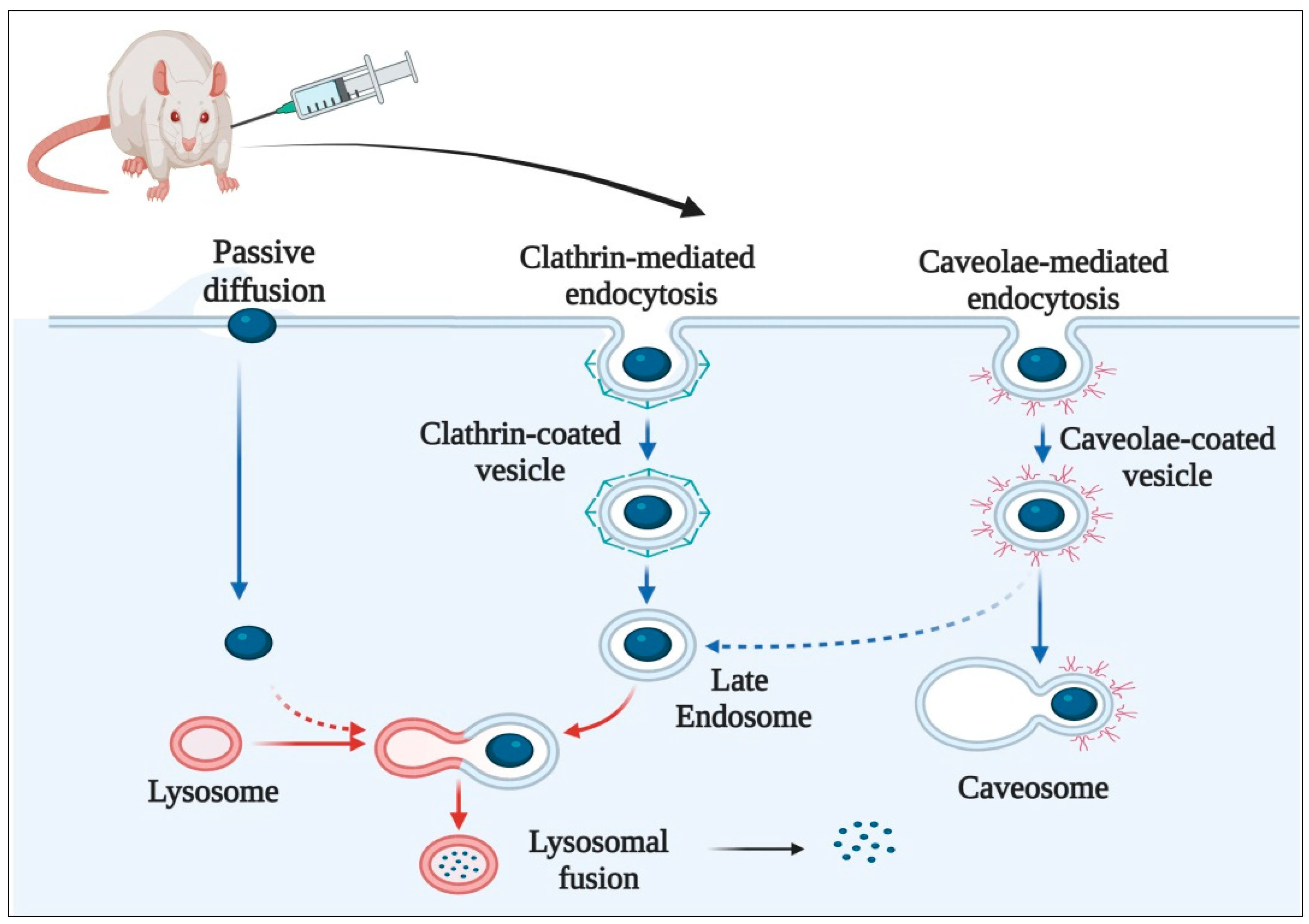
5. Mechanism of Cellular Uptake of Carbon Nanostructures
6. Pharmacokinetic Considerations
7. Toxicity of Carbon Nanostructures
8. Recent Patents
9. Challenges and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhiman, S.; Kaur, A.; Sharma, M. Fullerenes For Anticancer Drug Targeting: Teaching An Old Dog A New Trick. Mini-Rev. Med. Chem. 2022, 22, 2872–2888. [Google Scholar] [CrossRef]
- Saliev, T. The Advances in Biomedical Applications of Carbon Nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef]
- Ahmed, W.; Elhissi, A.; Dhanak, V.; Subramani, K. Carbon Nanotubes: Applications in Cancer Therapy and Drug Delivery Research. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 371–389. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart Carbon Nanotubes for Drug Delivery System: A Comprehensive Study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon Nanotubes in Biomedical Applications: Current Status, Promises, and Challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical Nanotechnology: From the Bench to the Market. Future J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Suttee, A.; Singh, G.; Yadav, N.; Pratap Barnwal, R.; Singla, N.; Prabhu, K.S.; Mishra, V. A Review on Status of Nanotechnology in Pharmaceutical Sciences. Int. J. Drug Deliv. Technol. 2019, 9, 98–103. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Mishra, V.; Sriram, P.; Suttee, A. Potential Approaches of Nanotechnology for Cancer Therapy: An Insight. Int. J. Drug Deliv. Technol. 2021, 11, 24. [Google Scholar] [CrossRef]
- Kaushik, N.; Borkar, S.B.; Nandanwar, S.K.; Panda, P.K.; Choi, E.H.; Kaushik, N.K. Nanocarrier Cancer Therapeutics with Functional Stimuli-Responsive Mechanisms. J. Nanobiotechnol. 2022, 20, 152. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, G.; Yadav, N.; Pratap Barnwal, R.; Singla, N.; Prabhu, K.S.; Suttee, A. Biomedical Potential of Graphene Oxide Based Nanoformulations: An Overview. Int. J. Drug Deliv. Technol. 2019, 9, 109–113. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Piperno, A. Carbon Nanomaterials for Therapy, Diagnosis and Biosensing. Nanomaterials 2022, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, J.; Qiao, S.; Zhang, W. Carbon-Based Nanomaterials for Bone and Cartilage Regeneration: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4718–4735. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and Behavior of Carbon Nanomaterials When Interfacing Neuronal Cells: How Far Have We Come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Geckeler, K.E.; Premkumar, T. Carbon Nanotubes: Are They Dispersed or Dissolved in Liquids? Nanoscale Res. Lett. 2011, 6, 136. [Google Scholar] [CrossRef]
- Samadishadlou, M.; Farshbaf, M.; Annabi, N.; Kavetskyy, T.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A.; Mousavi, S. Magnetic Carbon Nanotubes: Preparation, Physical Properties, and Applications in Biomedicine. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1314–1330. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Green Synthesis of Nanomaterials from Sustainable Materials for Biosensors and Drug Delivery. Sens. Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green Approaches to Carbon Nanostructure-Based Biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Ankamwar, B.; Kirtiwar, S.; Shukla, A.C. Plant-Mediated Green Synthesis of Nanoparticles. In Advance in Pharmaceutical Biotechnology; Springer: Singapore, 2020; pp. 221–234. [Google Scholar] [CrossRef]
- Tripathi, N.; Pavelyev, V.; Islam, S.S. Synthesis of Carbon Nanotubes Using Green Plant Extract as Catalyst: Unconventional Concept and Its Realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef]
- Damera, D.P.; Manimaran, R.; Krishna Venuganti, V.V.; Nag, A. Green Synthesis of Full-Color Fluorescent Carbon Nanoparticles from Eucalyptus Twigs for Sensing the Synthetic Food Colorant and Bioimaging. ACS Omega 2020, 5, 19905–19918. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yao, H.F.; Chai, M.H.; He, W.; Huang, Y.P.; Liu, Z.S. Green Synthesis of Carbon Nanotubes-Reinforced Molecularly Imprinted Polymer Composites for Drug Delivery of Fenbufen. AAPS PharmSciTech 2018, 19, 3895–3906. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, S.; Mayyas, M.; Sahajwalla, V. Nano-Carbons from Waste Tyre Rubber: An Insight into Structure and Morphology. Waste Manag. 2017, 69, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Joseph Berkmans, A.; Jagannatham, M.; Priyanka, S.; Haridoss, P. Synthesis of Branched, Nano Channeled, Ultrafine and Nano Carbon Tubes from PET Wastes Using the Arc Discharge Method. Waste Manag. 2014, 34, 2139–2145. [Google Scholar] [CrossRef]
- Irmania, N.; Dehvari, K.; Gedda, G.; Tseng, P.J.; Chang, J.Y. Manganese-Doped Green Tea-Derived Carbon Quantum Dots as a Targeted Dual Imaging and Photodynamic Therapy Platform. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1616–1625. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Zhang, Y.; Kong, H.; Wang, S.; Luo, J.; Qu, H.; Zhao, Y. Green Synthesis of Zingiberis Rhizoma-Based Carbon Dots Attenuates Chemical and Thermal Stimulus Pain in Mice. Nanomedicine 2020, 15, 851–869. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Laíño, R.B.; Calzón, J.A.G.; García, M.E.D. Facile Synthesis of Water-Soluble Carbon Nano-Onions under Alkaline Conditions. Beilstein J. Nanotechnol. 2016, 7, 758–766. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Yang, G.W. Nanodiamonds from Coal under Ambient Conditions. Nanoscale 2015, 7, 6114–6125. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Z.; Li, S.; Zhang, Y.; He, B.; Peng, D.; Tian, J.; Zhao, M.; Wang, X.; Zhang, Q. The Interactions of Single-Wall Carbon Nanohorns with Polar Epithelium. Int. J. Nanomed. 2017, 12, 4177–4194. [Google Scholar] [CrossRef][Green Version]
- Mashino, T. Development of Bio-Active Fullerene Derivatives Suitable for Drug. Yakugaku Zasshi 2022, 142, 165–179. [Google Scholar] [CrossRef]
- Khamatgalimov, A.R.; Kovalenko, V.I. Substructural Approach for Assessing the Stability of Higher Fullerenes. Int. J. Mol. Sci. 2021, 22, 3760. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Kuznetsov, V. Stereochemistry of Simple Molecules inside Nanotubes and Fullerenes: Unusual Behavior of Usual Systems. Molecules 2020, 25, 2437. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, H.; Mozafari, M. Fullerene-Based Delivery Systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Korzuch, J.; Rak, M.; Balin, K.; Zubko, M.; Głowacka, O.; Dulski, M.; Musioł, R.; Madeja, Z.; Serda, M. Towards Water-Soluble [60]Fullerenes for the Delivery of SiRNA in a Prostate Cancer Model. Sci. Rep. 2021, 11, 10565. [Google Scholar] [CrossRef] [PubMed]
- Moussa, F. [60]Fullerene and Derivatives for Biomedical Applications. In Nanobiomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 113–136. [Google Scholar] [CrossRef]
- Nimibofa, A.; Newton, E.A.; Cyprain, A.Y.; Donbebe, W. Fullerenes: Synthesis and Applications. J. Mater. Sci. Res. 2018, 7, 22. [Google Scholar] [CrossRef]
- Omri, N.; Moussa, F.; Bu, Y. Functionalization of [60]Fullerene through Photochemical Reaction for Fulleropyrrolidine Nanovectors Synthesis: Experimental and Theoretical Approaches. Colloids Surf. B Biointerfaces 2021, 198, 111457. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Liu, M.T.H.; Nagase, S.; Akasaka, T. New Horizons in Chemical Functionalization of Endohedral Metallofullerenes. Molecules 2020, 25, 3626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Patil, A.; Mishra, V.; Thakur, S.; Riyaz, B.; Kaur, A.; Khursheed, R.; Patil, K.; Sathe, B. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Nanosci. 2018, 15, 137–146. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Cevher, S.C.; Toppare, L.; Cirpan, A.; Soylemez, S. Electrochemical Biosensor Based on Three Components Random Conjugated Polymer with Fullerene (C60). Bioelectrochemistry 2022, 147, 108219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Raza, K. C60-Fullerenes as Drug Delivery Carriers for Anticancer Agents: Promises and Hurdles. Pharm. Nanotechnol. 2017, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.I. Fullerene Derivatives for Drug Delivery against COVID-19: A Molecular Dynamics Investigation of Dendro [60]Fullerene as Nanocarrier of Molnupiravir. Nanomaterials 2022, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Thotakura, N.; Kumar, P.; Joshi, M.; Bhushan, S.; Bhatia, A.; Kumar, V.; Malik, R.; Sharma, G.; Guru, S.K.; et al. C60-Fullerenes for Delivery of Docetaxel to Breast Cancer Cells: A Promising Approach for Enhanced Efficacy and Better Pharmacokinetic Profile. Int. J. Pharm. 2015, 495, 551–559. [Google Scholar] [CrossRef]
- Engelberth, S.A.; Hempel, N.; Bergkvist, M. Development of Nanoscale Approaches for Ovarian Cancer Therapeutics and Diagnostics. Crit. Rev. Oncog. 2014, 19, 281. [Google Scholar] [CrossRef]
- Bilyalov, A.I.; Shanazarov, N.A.; Zinchenko, S.V. Photodynamic Therapy as Alternative Method of Treatment of Metastatic Ovarian Cancer with Many Recurrence: Case Report. BioNanoScience 2020, 10, 807–810. [Google Scholar] [CrossRef]
- Guo, L.; He, N.; Zhao, Y.; Liu, T.; Deng, Y. Autophagy Modulated by Inorganic Nanomaterials. Theranostics 2020, 10, 3206–3222. [Google Scholar] [CrossRef]
- Soldà, A.; Cantelli, A.; Di Giosia, M.; Montalti, M.; Zerbetto, F.; Rapino, S.; Calvaresi, M. C60@lysozyme: A New Photosensitizing Agent for Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 6608–6615. [Google Scholar] [CrossRef]
- Wang, P.; Yan, G.; Zhu, X.; Du, Y.; Chen, D.; Zhang, J. Heterofullerene Mc59 (M = b, Si, Al) as Potential Carriers for Hydroxyurea Drug Delivery. Nanomaterials 2021, 11, 115. [Google Scholar] [CrossRef]
- Bagheri Novir, S.; Aram, M.R. Quantum Mechanical Simulation of Chloroquine Drug Interaction with C60 Fullerene for Treatment of COVID-19. Chem. Phys. Lett. 2020, 757, 137869. [Google Scholar] [CrossRef]
- Illescas, B.M.; Pérez-Sánchez, A.; Mallo, A.; Martín-Domenech, Á.; Rodríguez-Crespo, I.; Martín, N. Multivalent Cationic Dendrofullerenes for Gene Transfer: Synthesis and DNA Complexation. J. Mater. Chem. B 2020, 8, 4505–4515. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Politenkova, S.; Afanasieva, K.; Korolovych, V.; Bogutska, K.; Sivolob, A.; Skivka, L.; Evstigneev, M.; Kostjukov, V.; Prylutskyy, Y.; et al. A Nanocomplex of C60 Fullerene with Cisplatin: Design, Characterization and Toxicity. Beilstein J. Nanotechnol. 2017, 8, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro. Nanoscale Res. Lett. 2019, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Bartkowski, M.; Giordani, S. Supramolecular Chemistry of Carbon Nano-Onions. Nanoscale 2020, 12, 9352–9358. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Delgadillo, R.M.V.; Ortiz, A.G.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Giordani, S.; Camisasca, A.; Maffeis, V. Carbon Nano-Onions: A Valuable Class of Carbon Nanomaterials in Biomedicine. Curr. Med. Chem. 2019, 26, 6915–6929. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon Nanomaterials for Targeted Cancer Therapy Drugs: A Critical Review. Chem. Rec. 2019, 19, 502–522. [Google Scholar] [CrossRef]
- Guo, A.; Bao, K.; Sang, S.; Zhang, X.; Shao, B.; Zhang, C.; Wang, Y.; Cui, F.; Yang, X. Soft-Chemistry Synthesis, Solubility and Interlayer Spacing of Carbon Nano-Onions. RSC Adv. 2021, 11, 6850–6858. [Google Scholar] [CrossRef]
- Orozco-Ic, M.; Sundholm, D. Magnetic Response Properties of Carbon Nano-Onions. Phys. Chem. Chem. Phys. 2022, 24, 22487–22496. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E. Carbon Nano-Onions: A Review of Recent Progress in Synthesis and Applications. ChemNanoMat 2019, 5, 568–580. [Google Scholar] [CrossRef]
- D’amora, M.; Maffeis, V.; Brescia, R.; Barnes, D.; Scanlan, E.; Giordani, S. Carbon Nano-Onions as Non-Cytotoxic Carriers for Cellular Uptake of Glycopeptides and Proteins. Nanomaterials 2019, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Das, A. The n → Π* Interaction: A Rapidly Emerging Non-Covalent Interaction. Phys. Chem. Chem. Phys. 2015, 17, 9596–9612. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Agrawal, A.; Pandey, G.; Kumar, S.; Awasthi, K.; Awasthi, A. Carbon Nano-Onion-Decorated ZnO Composite-Based Enzyme-Less Electrochemical Biosensing Approach for Glucose. ACS Omega 2022, 7, 37748–37756. [Google Scholar] [CrossRef] [PubMed]
- Sok, V.; Fragoso, A. Preparation and Characterization of Alkaline Phosphatase, Horseradish Peroxidase, and Glucose Oxidase Conjugates with Carboxylated Carbon Nano-Onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Masoudi Asil, S.; Guillama Barroso, G.; Nurunnabi, M.; Narayan, M. Application of Carbon Nano Onions in the Biomedical Field: Recent Advances and Challenges. Biomater. Sci. 2021, 9, 626–644. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-Onions Reinforced Poly (ε-Caprolactone) Composite Nanofibers for PH-Responsive Drug Release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef]
- Mamidi, N.; Villela Castrejón, J.; González-Ortiz, A. Rational Design and Engineering of Carbon Nano-Onions Reinforced Natural Protein Nanocomposite Hydrogels for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103696. [Google Scholar] [CrossRef]
- d’Amora, M.; Camisasca, A.; Boarino, A.; Giordani, S.; Arpicco, S. Supramolecular Functionalization of Carbon Nano-Onions with Hyaluronic Acid-Phospholipid Conjugates for Selective Targeting of Cancer Cells. Colloids Surf. B Biointerfaces 2020, 188, 110779. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Czyrko, J.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon Nano-Onion Composites: Physicochemical Characteristics and Biological Activity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 185–192. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of Carbon Nano-Onion Bioconjugates for Biomedical Applications. Mater. Sci. Eng. C 2021, 120, 111698. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.H. The Advanced Role of Carbon Quantum Dots in Nanomedical Applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. A Review on Nanostructured Carbon Quantum Dots and Their Applications in Biotechnology, Sensors, and Chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.H.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon Dot Therapeutic Platforms: Administration, Distribution, Metabolism, Excretion, Toxicity, and Therapeutic Potential. Small 2022, 18, 2106342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Yin, Z.; Sun, L. A Review on Carbon Quantum Dots: Synthesis, Photoluminescence Mechanisms and Applications. Luminescence 2022, 37, 1612–1638. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.T. Carbon Dots: Advances in Nanocarbon Applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon Dots: Synthesis, Properties and Biomedical Applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Sharma, S.K.; Micic, M.; Li, S.; Hoar, B.; Paudyal, S.; Zahran, E.M.; Leblanc, R.M. Conjugation of Carbon Dots with β-Galactosidase Enzyme: Surface Chemistry and Use in Biosensing. Molecules 2019, 24, 3275. [Google Scholar] [CrossRef]
- Yoosefian, M.; Fouladi, M.; Atanase, L.I. Molecular Dynamics Simulations of Docetaxel Adsorption on Graphene Quantum Dots Surface Modified by PEG-b-PLA Copolymers. Nanomaterials 2022, 12, 926. [Google Scholar] [CrossRef]
- Arsalani, N.; Nezhad-Mokhtari, P.; Jabbari, E. Microwave-Assisted and One-Step Synthesis of PEG Passivated Fluorescent Carbon Dots from Gelatin as an Efficient Nanocarrier for Methotrexate Delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 540–547. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Nayak, A.; Gadnayak, A.; Sahoo, M.; Dave, S.; Mohanty, P.; Mohanty, J.N.; Das, J. Quantum Dots Enabled Point-of-Care Diagnostics: A New Dimension to the Nanodiagnosis. In Advance Nanomaterials for Point of Care Diagnosis and Therapy; Elesevier: Amsterdam, The Netherlands, 2022; pp. 43–52. [Google Scholar] [CrossRef]
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon Dots: Emerging Theranostic Nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Gao, N.; Yang, W.; Nie, H.; Gong, Y.; Jing, J.; Gao, L.; Zhang, X. Turn-on Theranostic Fluorescent Nanoprobe by Electrostatic Self-Assembly of Carbon Dots with Doxorubicin for Targeted Cancer Cell Imaging, in Vivo Hyaluronidase Analysis, and Targeted Drug Delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly Fluorescent Nitrogen-Doped Carbon Dots Derived from Phyllanthus Acidus Utilized as a Fluorescent Probe for Label-Free Selective Detection of Fe3+ Ions, Live Cell Imaging and Fluorescent Ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Zhang, Z.; Lei, J.H.; Liu, T.M.; Xing, G.; Deng, C.X.; Tang, Z.; Qu, S. One Step Synthesis of Efficient Red Emissive Carbon Dots and Their Bovine Serum Albumin Composites with Enhanced Multi-Photon Fluorescence for in Vivo Bioimaging. Light Sci. Appl. 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Anpalagan, K.K.; Karakkat, J.V.; Lai, D.T.H.; Apostolopoulos, V.; Nurgali, K.; Truskewycz, A.; Cole, I. A Pilot Study on Carbon Quantum Dots for Bioimaging of Muscle Myoblasts. In Proceedings of the IEEE 20th International Conference on Bioinformatics and Bioengineering BIBE 2020, Cincinnati, OH, USA, 26–28 October 2020; pp. 630–636. [Google Scholar] [CrossRef]
- Ou, S.F.; Zheng, Y.Y.; Lee, S.J.; Chen, S.T.; Wu, C.H.; Hsieh, C.T.; Juang, R.S.; Peng, P.Z.; Hsueh, Y.H. N-Doped Carbon Quantum Dots as Fluorescent Bioimaging Agents. Crystals 2021, 11, 789. [Google Scholar] [CrossRef]
- Karthik, S.; Saha, B.; Ghosh, S.K.; Pradeep Singh, N.D. Photoresponsive Quinoline Tethered Fluorescent Carbon Dots for Regulated Anticancer Drug Delivery. Chem. Commun. 2013, 49, 10471–10473. [Google Scholar] [CrossRef]
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic Therapy of Melanoma Skin Cancer Using Carbon Dot—Chlorin E6—Hyaluronate Conjugate. Acta Biomater. 2015, 26, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Ma, Y.H.; Gao, G.; Chen, X.; Jia, H.R.; Li, Y.H.; Chen, Z.; Wu, F.G. Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of Carbon Quantum Dots- Quinic Acid for Drug Delivery of Gemcitabine to Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Guo, L.; Duan, Q.; Wu, G.; Zhang, B.; Huang, L.; Xue, J.; Li, P.; Sang, S.; Wei, X. Novel Multifunctional Delivery System for Chondrocytes and Articular Cartilage Based on Carbon Quantum Dots. Sens. Actuators B Chem. 2022, 356, 131348. [Google Scholar] [CrossRef]
- Camlik, G.; Ozakca, I.; Bilakaya, B.; Ozcelikay, A.T.; Velaro, A.J.; Wasnik, S.; Degim, I.T. Development of Composite Carbon Quantum Dots-Insulin Formulation for Oral Administration. J. Drug Deliv. Sci. Technol. 2022, 76, 103833. [Google Scholar] [CrossRef]
- Ardestani, M.S.; Zaheri, Z.; Mohammadzadeh, P.; Bitarafan-Rajabi, A.; Ghoreishi, S.M. Novel Manganese Carbon Quantum Dots as a Nano-Probe: Facile Synthesis, Characterization and Their Application in Naproxen Delivery (Mn/CQD/SiO2@naproxen). Bioorg. Chem. 2021, 115, 105211. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, M.; Nayak, P.; Sriram, P.; Suttee, A. Carbon Nanotubes as Emerging Nanocarriers in Drug Delivery: An Overview. Int. J. Pharm. Qual. Assur. 2020, 11, 373–378. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 15. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon Nanotubes: A Review on Properties, Synthesis Methods and Applications in Micro and Nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon Nanotubes: Current Perspectives on Diverse Applications in Targeted Drug Delivery and Therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Pennetta, C.; Floresta, G.; Graziano, A.C.E.; Cardile, V.; Rubino, L.; Galimberti, M.; Rescifina, A.; Barbera, V. Functionalization of Single and Multi-Walled Carbon Nanotubes with Polypropylene Glycol Decorated Pyrrole for the Development of Doxorubicin Nano-Conveyors for Cancer Drug Delivery. Nanomaterials 2020, 10, 1073. [Google Scholar] [CrossRef]
- Singh, M.; Nayak, P.; Mishra, V. Carbon Nanotubes In Treatment Of Arthritis: An Overview. Eur. J. Mol. Clin. Med. 2020, 7, 4366–4372. [Google Scholar]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-Based Drug Delivery Carriers for Cancer Therapy. Arch. Pharm. Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Ali Shah, A.u.H.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C 2019, 5, 3. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22, 7726. [Google Scholar] [CrossRef] [PubMed]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danleé, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of Carbon Nanotubes by Arc-Discharge and Chemical Vapor Deposition Method with Analysis of Its Morphology, Dispersion and Functionalization Characteristics. Cogent Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Mehra, N.K.; Mishra, V.; Jain, N.K. A Review of Ligand Tethered Surface Engineered Carbon Nanotubes. Biomaterials 2014, 35, 1267–1283. [Google Scholar] [CrossRef]
- Hou, P.X.; Zhang, F.; Zhang, L.; Liu, C.; Cheng, H.M. Synthesis of Carbon Nanotubes by Floating Catalyst Chemical Vapor Deposition and Their Applications. Adv. Funct. Mater. 2022, 32, 2108541. [Google Scholar] [CrossRef]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef]
- Golubewa, L.; Timoshchenko, I.; Romanov, O.; Karpicz, R.; Kulahava, T.; Rutkauskas, D.; Shuba, M.; Dementjev, A.; Svirko, Y.; Kuzhir, P. Single-Walled Carbon Nanotubes as a Photo-Thermo-Acoustic Cancer Theranostic Agent: Theory and Proof of the Concept Experiment. Sci. Rep. 2020, 10, 22174. [Google Scholar] [CrossRef]
- Song, J.; Wang, F.; Yang, X.; Ning, B.; Harp, M.G.; Culp, S.H.; Hu, S.; Huang, P.; Nie, L.; Chen, J.; et al. Gold Nanoparticle Coated Carbon Nanotube Ring with Enhanced Raman Scattering and Photothermal Conversion Property for Theranostic Applications. J. Am. Chem. Soc. 2016, 138, 7005–7015. [Google Scholar] [CrossRef] [PubMed]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-Carbons in Biosensor Applications: An Overview of Carbon Nanotubes (CNTs) and Fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
- Christwardana, M.; Ji, J.; Chung, Y.; Kwon, Y. Highly Sensitive Glucose Biosensor Using New Glucose Oxidase Based Biocatalyst. Korean J. Chem. Eng. 2017, 34, 2916–2921. [Google Scholar] [CrossRef]
- Singh, A.; Choudhary, M.; Kaur, S.; Singh, S.P.; Arora, K. Molecular Functionalization of Carbon Nanomaterials for Immuno-Diagnosis of Cancer. Mater. Today Proc. 2016, 2, 157–161. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, H.; Dong, Y.; Han, C.; Sui, X.; Jian, B. Multi-walled Carbon Nanotube-based Systems for Improving the Controlled Release of Insoluble Drug Dipyridamole. Exp. Ther. Med. 2019, 17, 4610–4616. [Google Scholar] [CrossRef]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef]
- You, Y.; Wang, N.; He, L.; Shi, C.; Zhang, D.; Liu, Y.; Luo, L.; Chen, T. Designing Dual-Functionalized Carbon Nanotubes with High Blood–Brain-Barrier Permeability for Precise Orthotopic Glioma Therapy. Dalton Trans. 2019, 48, 1569–1573. [Google Scholar] [CrossRef]
- Khazaee, M.; Ye, D.; Majumder, A.; Baraban, L.; Opitz, J.; Cuniberti, G. Non-Covalent Modified Multi-Walled Carbon Nanotubes: Dispersion Capabilities and Interactions with Bacteria. Biomed. Phys. Eng. Express 2016, 2, 055008. [Google Scholar] [CrossRef]
- Zhang, P.; Yi, W.; Hou, J.; Yoo, S.; Jin, W.; Yang, Q. A Carbon Nanotube-Gemcitabine-Lentinan Three-Component Composite for Chemo-Photothermal Synergistic Therapy of Cancer. Int. J. Nanomed. 2018, 13, 3069–3080. [Google Scholar] [CrossRef]
- Komane, P.P.; Kumar, P.; Marimuthu, T.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Dexamethasone-Loaded, PEGylated, Vertically Aligned, Multiwalled Carbon Nanotubes for Potential Ischemic Stroke Intervention. Molecules 2018, 23, 1406. [Google Scholar] [CrossRef]
- Suo, N.; Wang, M.; Jin, Y.; Ding, J.; Gao, X.; Sun, X.; Zhang, H.; Cui, M.; Zheng, J.; Li, N.; et al. Magnetic Multiwalled Carbon Nanotubes with Controlled Release of Epirubicin: An Intravesical Instillation System for Bladder Cancer. Int. J. Nanomed. 2019, 14, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Chegeni, M.; Rozbahani, Z.S.; Ghasemian, M.; Mehri, M. Synthesis and Application of the Calcium Alginate/SWCNT-Gl as a Bio-Nanocomposite for the Curcumin Delivery. Int. J. Biol. Macromol. 2020, 156, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Foo, J.B.; Fakurazi, S.; Hussein, M.Z. Release Behaviour and Toxicity Evaluation of Levodopa from Carboxylated Single-Walled Carbon Nanotubes. Beilstein J. Nanotechnol. 2015, 6, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Yang, Y.; Sun, L.; Han, D.; Li, H.; Wang, C. Pharmacological and Toxicological Target Organelles and Safe Use of Single-Walled Carbon Nanotubes as Drug Carriers in Treating Alzheimer Disease. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as Novel Nanomaterials for Biomedical Applications: Drug Delivery and Imaging Systems. Int. J. Nanomed. 2013, 8, 203. [Google Scholar] [CrossRef]
- Boruah, A.; Saikia, B.K. Synthesis, Characterization, Properties, and Novel Applications of Fluorescent Nanodiamonds. J. Fluoresc. 2022, 32, 863–885. [Google Scholar] [CrossRef]
- Terada, D.; Sotoma, S.; Harada, Y.; Igarashi, R.; Shirakawa, M. One-Pot Synthesis of Highly Dispersible Fluorescent Nanodiamonds for Bioconjugation. Bioconjug. Chem. 2018, 29, 2786–2792. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with Powerful Ability for Drug Delivery and Biomedical Applications: Recent Updates on in Vivo Study and Patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Chang, S.L.Y.; Reineck, P.; Krueger, A.; Mochalin, V.N. Ultrasmall Nanodiamonds: Perspectives and Questions. ACS Nano 2022, 16, 8513–8524. [Google Scholar] [CrossRef]
- Reina, G.; Zhao, L.; Bianco, A.; Komatsu, N. Chemical Functionalization of Nanodiamonds: Opportunities and Challenges Ahead. Angew. Chem. Int. Ed. Engl. 2019, 58, 17918–17929. [Google Scholar] [CrossRef]
- Jariwala, D.H.; Patel, D.; Wairkar, S. Surface Functionalization of Nanodiamonds for Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110996. [Google Scholar] [CrossRef] [PubMed]
- Perevedentseva, E.; Lin, Y.C.; Cheng, C.L. A Review of Recent Advances in Nanodiamond-Mediated Drug Delivery in Cancer. Expert Opin. Drug Deliv. 2021, 18, 369–382. [Google Scholar] [CrossRef]
- Jung, H.S.; Neuman, K.C. Surface Modification of Fluorescent Nanodiamonds for Biological Applications. Nanomaterials 2021, 11, 153. [Google Scholar] [CrossRef]
- Bondon, N.; Raehm, L.; Charnay, C.; Boukherroub, R.; Durand, J.O. Nanodiamonds for Bioapplications, Recent Developments. J. Mater. Chem. B 2020, 8, 10878–10896. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, K.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds for In Vivo Applications. Small 2018, 14, e1703838. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.A.; Włodarczyk, E. Fluorescent Nanodiamonds in Biomedical Applications. J. AOAC Int. 2018, 101, 1297–1307. [Google Scholar] [CrossRef]
- Mohan, N.; Chen, C.S.; Hsieh, H.H.; Wu, Y.C.; Chang, H.C. In Vivo Imaging and Toxicity Assessments of Fluorescent Nanodiamonds in Caenorhabditis Elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef]
- Barone, F.C.; Marcinkiewicz, C.; Li, J.; Sternberg, M.; Lelkes, P.I.; Dikin, D.A.; Bergold, P.J.; Gerstenhaber, J.A.; Feuerstein, G. Pilot Study on Biocompatibility of Fluorescent Nanodiamond-(NV)-Z~800 Particles in Rats: Safety, Pharmacokinetics, and Bio-Distribution (Part III). Int. J. Nanomed. 2018, 13, 5449. [Google Scholar] [CrossRef]
- Merson, T.D.; Turnley, A.M.; Turbic, A.; Aharonovich, I.; Castelletto, S.; Kilpatrick, T.J. Nanodiamonds with Silicon Vacancy Defects for Nontoxic Photostable Fluorescent Labeling of Neural Precursor Cells. Opt. Lett. 2013, 38, 4170–4173. [Google Scholar] [CrossRef]
- Zurbuchen, M.A.; Lake, M.P.; Kohan, S.A.; Leung, B.; Bouchard, L.S. Nanodiamond Landmarks for Subcellular Multimodal Optical and Electron Imaging. Sci. Rep. 2013, 3, 2668. [Google Scholar] [CrossRef]
- Alhaddad, A.; Durieu, C.; Dantelle, G.; Le Cam, E.; Malvy, C.; Treussart, F.; Bertrand, J.R. Influence of the Internalization Pathway on the Efficacy of SiRNA Delivery by Cationic Fluorescent Nanodiamonds in the Ewing Sarcoma Cell Model. PLoS ONE 2012, 7, e52207. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, M.; Hooi, L.; Toh, T.B.; Thng, D.K.H.; Lim, J.J.; Chow, E.K.H. Enhanced Penetrative SiRNA Delivery by a Nanodiamond Drug Delivery Platform against Hepatocellular Carcinoma 3D Models. Nanoscale 2021, 13, 16131–16145. [Google Scholar] [CrossRef] [PubMed]
- Al Qtaish, N.; Gallego, I.; Paredes, A.J.; Villate-Beitia, I.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Sainz-Ramos, M.; Lopez-Mendez, T.B.; Fernández, E.; Puras, G.; et al. Nanodiamond Integration into Niosomes as an Emerging and Efficient Gene Therapy Nanoplatform for Central Nervous System Diseases. ACS Appl. Mater. Interfaces 2022, 14, 13665–13677. [Google Scholar] [CrossRef] [PubMed]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Rehman, A.; Houshyar, S.; Wang, X. Nanodiamond in Composite: Biomedical Application. J. Biomed. Mater. Res. A 2020, 108, 906–922. [Google Scholar] [CrossRef]
- Tian, Y.; Nusantara, A.C.; Hamoh, T.; Mzyk, A.; Tian, X.; Perona Martinez, F.; Li, R.; Permentier, H.P.; Schirhagl, R. Functionalized Fluorescent Nanodiamonds for Simultaneous Drug Delivery and Quantum Sensing in HeLa Cells. ACS Appl. Mater. Interfaces 2022, 14, 39265–39273. [Google Scholar] [CrossRef]
- Ali, M.S.; Metwally, A.A.; Fahmy, R.H.; Osman, R. Chitosan-Coated Nanodiamonds: Mucoadhesive Platform for Intravesical Delivery of Doxorubicin. Carbohydr. Polym. 2020, 245, 116528. [Google Scholar] [CrossRef]
- Zhong, B.; Mateu-Roldán, A.; Fanarraga, M.L.; Han, W.; Muñoz-Guerra, D.; González, J.; Tao Weng, L.; Ricardo Ibarra, M.; Marquina, C.; Lun Yeung, K. Graphene-Encapsulated Magnetic Nanoparticles for Safe and Steady Delivery of Ferulic Acid in Diabetic Mice. Chem. Eng. J. 2022, 435, 134466. [Google Scholar] [CrossRef]
- Ryu, T.K.; Kang, R.H.; Jeong, K.Y.; Jun, D.R.; Koh, J.M.; Kim, D.; Bae, S.K.; Choi, S.W. Bone-Targeted Delivery of Nanodiamond-Based Drug Carriers Conjugated with Alendronate for Potential Osteoporosis Treatment. J. Control. Release 2016, 232, 152–160. [Google Scholar] [CrossRef]
- Volnova, A.B.; Gordeev, S.K.; Lenkov, D.N. Targeted Delivery of 4-Aminopyridine Into the Rat Brain by Minicontainers from Carbon-Nanodiamonds Composite. J. Neurosci. Neuroeng. 2014, 2, 569–573. [Google Scholar] [CrossRef]
- Alawdi, S.H.; Eidi, H.; Safar, M.M.; Abdel-Wahhab, M.A. Loading Amlodipine on Diamond Nanoparticles: A Novel Drug Delivery System. Nanotechnol. Sci. Appl. 2019, 12, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Singh, R.N. Polyethyleneimine-Functionalized Magnetic Fe₃O₄ and Nanodiamond Particles as a Platform for Amoxicillin Delivery. J. Nanosci. Nanotechnol. 2020, 20, 3957–3970. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; de Barros, A.L.B.; Soares, D.C.F.; Moss, S.N.; Alisaraie, L. Functionalized Single-Walled Carbon Nanotubes: Cellular Uptake, Biodistribution and Applications in Drug Delivery. Int. J. Pharm. 2017, 524, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, M.; Morimoto, T.; Tajima, N.; Ichiraku, K.; Fujita, K.; Iijima, S.; Yudasaka, M.; Okazaki, T. Size-Dependent Cell Uptake of Carbon Nanotubes by Macrophages: A Comparative and Quantitative Study. Carbon N. Y. 2018, 127, 93–101. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z. PEGylated Carbon Nanotubes Decorated with Silver Nanoparticles: Fabrication, Cell Cytotoxicity and Application in Photo Thermal Therapy. Iran. J. Pharm. Res. IJPR 2021, 20, 91. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Strano, M.S. Single-Particle Tracking of Endocytosis and Exocytosis of Single-Walled Carbon Nanotubes in NIH-3T3 Cells. Nano Lett. 2008, 8, 1577–1585. [Google Scholar] [CrossRef]
- Wang, M.; Yu, S.; Wang, C.; Kong, J. Tracking the Endocytic Pathway of Recombinant Protein Toxin Delivered by Multiwalled Carbon Nanotubes. ACS Nano 2010, 4, 6483–6490. [Google Scholar] [CrossRef]
- Wang, Z.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells. ACS Nano 2009, 3, 4110. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Li, X.; Nie, C.; Liang, T.; Xie, F.; Liu, K.; Peng, X.; Xie, J. Highly Hydrophilic Carbon Nanoparticles: Uptake Mechanism by Mammalian and Plant Cells. RSC Adv. 2018, 8, 35246–35256. [Google Scholar] [CrossRef]
- Raffa, V.; Ciofani, G.; Vittorio, O.; Riggio, C.; Cuschieri, A. Physicochemical Properties Affecting Cellular Uptake of Carbon Nanotubes. Nanomedicine 2009, 5, 89–97. [Google Scholar] [CrossRef]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.W.; Al-Jamal, K.T. Functionalised Carbon Nanotubes: From Intracellular Uptake and Cell-Related Toxicity to Systemic Brain Delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Luo, J.; Zhou, Q.; Wang, H. Pharmacokinetics, Metabolism and Toxicity of Carbon Nanotubes for Biomedical Purposes. Theranostics 2012, 2, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon Nanotubes Degraded by Neutrophil Myeloperoxidase Induce Less Pulmonary Inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, I.; Sar, D.; Mukherjee, P.; Schwartz-Duval, A.S.; Huang, Z.; Jaramillo, C.; Civantos, A.; Tripathi, I.; Allain, J.P.; Bhargava, R.; et al. Enzyme-Catalysed Biodegradation of Carbon Dots Follows Sequential Oxidation in a Time Dependent Manner. Nanoscale 2019, 11, 8226–8236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, Y. The Application of Carbon Nanotubes in Target Drug Delivery Systems for Cancer Therapies. Nanoscale Res. Lett. 2011, 6, 1–22. [Google Scholar] [CrossRef]
- Riviere, J.E. Pharmacokinetics of Nanomaterials: An Overview of Carbon Nanotubes, Fullerenes and Quantum Dots. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 26–34. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, S.T.; Wang, H.; Liu, Y. Advances in Biodistribution Study and Tracing Methodology of Carbon Nanotubes. J. Nanosci. Nanotechnol. 2010, 10, 8469–8481. [Google Scholar] [CrossRef]
- Sandova, J.; Ventura-Sobrevilla, J.; Boone-Villa, D.; Ramos-González, R.; Velázquez, M.; Silva-Belmares, Y.; Cobos-Puc, L.; Aguilar, C. Carbon Nanomaterials as Pharmaceutic Forms for Sustained and Controlled Delivery Systems. In Nanomaterials for Drug Delivery Therepy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–434. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-Based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Koiranen, T.; Nevalainen, T.; Virkki-Hatakka, T.; Aalto, H.; Murashko, K.; Backfolk, K.; Kraslawski, A.; Pyrhönen, J. The Risk Assessment of Potentially Hazardous Carbon Nanomaterials for Small Scale Operations. Appl. Mater. Today 2017, 7, 104–111. [Google Scholar] [CrossRef]
- Parwez, K.; Budihal, S.V.; Parwez, K.; Budihal, S.V. Quality Control and Risk Management of Carbon Nanomaterials. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato, M.; Bianco, A.; Kostarelos, K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotube Radiotracers. Proc. Natl. Acad. Sci. USA 2006, 103, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Deng, X.; Sun, H.; Shi, Z.; Gu, Z.; Liu, Y.; Zhao, Y. Biodistribution of Carbon Single-Wall Carbon Nanotubes in Mice. J. Nanosci. Nanotechnol. 2004, 4, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Malina, T.; Poláková, K.; Hirsch, C.; Svoboda, L.; Zbořil, R. Toxicity of Carbon Nanomaterials—Towards Reliable Viability Assessment via New Approach in Flow Cytometry. Int. J. Mol. Sci. 2021, 22, 7750. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaraprasad, K.; Kumara, B.N. Ocular Drug Delivery and Tracking Comprising Photoluminescent Carbon Nanodots. IN202041056414, 1 July 2022. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN367958415&_cid=P12-LAK2D2-88868-1 (accessed on 17 November 2022).
- Hazis, N.U.A.; Aneja, N.; Rajabalaya, R.; David, S.R. Systematic Patent Review of Nanoparticles in Drug Delivery and Cancer Therapy in the Last Decade. Recent Adv. Drug Deliv. Formul. 2021, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Casteignau, F.; Beré, K.E.; Martel, R.; Allard, C.; Aissou, T.E.; Veilleux, J. Method of Manufacturing Carbon Nanohorns and the Carbon Nanohorns Thus Produced. Wo/2022/160055 2022. Available online: https://patentscope.wipo.int/search/ja/detail.jsf;jsessionid=138B47F362C76798BF3DA90BEB78CA8A.wapp1nA?docId=WO2022160055&_gid=202231 (accessed on 20 November 2022).
- Cross, B.; Kirby, L.H.; Bailey, T.F. Surface Area and Porosity for Catenated Carbon Nano-Onions (CNOS). U.S. Patent 20,200,385,272, 10 December 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US312970919&_cid=P12-LAK3P4-01353-1 (accessed on 17 November 2022).
- Bhowmick, M.; Bhowmick, P.; Dama, G.Y.; Joshi, S.A.; Mantry, S.; Pethakar, S.R. Targeted Release of Camptothecin for Cancer Chemotherapy Using Carbon Nanotubes. AU2021104363, 19 August 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=AU333857057&_cid=P11-LAP07A-39198-6 (accessed on 20 November 2022).
- Lung, H.; Neumann, K.C. Polydopamine-Encapsulated Nanodiamonds and Methods. U.S. Patent 20,200,276,126, 3 September 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US305251502&_cid=P11-LAP0NU-41271-1 (accessed on 20 November 2022).
| Nanostructure | Source of Carbon | Green Pathway | Application | Reference |
|---|---|---|---|---|
| Quantum dots | Green tea | Hydrothermal | Photodynamic therapy | [27] |
| Quantum dots | Zingiberis rhizoma | Pyrolysis | Analgesic | [28] |
| Nano onions | Carbonization | Tomatoes | Theranostics | [29] |
| Nanodiamonds | Coal | Laser ablation | Bioimaging | [30] |
| Nano horns | UV/H2O2 oxidation | Cellulose | Drug delivery | [31] |
| Nano System | Functionalized With | Technique Used | Drug Used | Application | Reference |
|---|---|---|---|---|---|
| Heterofullerene BC59, SiC59, and AlC59 | Pristine C60 and heterofullerene | Conjugation | Hydroxyurea | Treatment of myeloid leukemia | [52] |
| Al and Si doped fullerene | Pristine C60 and chloroquine | Chemical interaction | Chloroquine | Enhanced efficacy against COVID-19 | [53] |
| C60-Dex-NH2 | TPFE | Conjugation | siRNA | Treatment of prostate cancer | [31,54] |
| C60-nano complex | Cisplatin | Conjugation | Cisplatin | Enhanced penetration in tumor cells | [55] |
| C60-DOX | DOX-complex | Covalent interaction | Doxorubicin | Enhanced in vitro cytotoxicity in cancer cells | [56] |
| Nanosystem | Drug Used | Application | Reference |
|---|---|---|---|
| Polycaprolactone/fts i nanocomposite fibers | Doxorubicin | pH dependant release of drugs at the tumor site | [69] |
| f8DIN CSL_CIT composite hydrogels | 5-FU | Sustained and site-specific release of 5-FU | [70] |
| HADIN CSL_CI | HA-Phospholipid | Targeting of overexpressed CD44 cancer cells | [71] |
| CTAB-CNOs | CTAB | Antimicrobial action against Escherichia coli) | [72] |
| PHPMA-CNOs = f-CNOs | Doxorubicin | Thermosensitive, prolonged, and site-specific release of drug | [73] |
| Nanosystem | Method | Application | Reference |
|---|---|---|---|
| CQD-RhB-Si Nps | Surface functionalization | In vivo imaging of Cu2+ in body tissues | [34] |
| N-P-CQDs | In situ hydrothermal synthesis | Imaging of Fe3+ ions and cell imaging | [90] |
| FA-CQDs | Solvothermal process | Fluorescent imaging of blood vessels | [91] |
| B1,B2-CQDs | Thermal carbonization | Imaging of myoblasts (mouse satellite) | [92] |
| N-CQDs | Hydrothermal process | Fluorescent staining of E. coli and S. aureus | [93] |
| Nanosystem | Drug | Application | Reference |
|---|---|---|---|
| FA-Gd-CQD | Doxorubicin | Anti-cancer effect against Hela cell lines | [96] |
| QA-N-CQDs | Gemcitabine | Theranostic system for breast cancer cell lines | [97] |
| m-CQD-HA | Hyaluronic acid | Site-specific delivery of HA to chondrocytes | [98] |
| N-CQD-Insulin | Insulin | Oral delivery of insulin | [99] |
| Mn/CQD/SiO2- naproxen | Naproxen | Drug delivery and tracer system | [100] |
| Nanosystem | Drug | Application | Reference |
|---|---|---|---|
| MWCNTs/Gemcitabine (Ge)/Lentinan-Le | Gemcitabine-Lentinan | Chemophotothermal therapy for cancer | [124] |
| PEGylated-MWCNTs | Dexamethasone | CNS delivery of dexamethasone for ischemic stroke | [125] |
| Magnetic MWCNTs-Epirubicin | Epirubicin | Site-specific and prolonged release of epirubicin for bladder cancer | [126] |
| CA/SWCNT-Gl | Curcumin | Antibacterial activity against B. cereus and E. coli | [127] |
| SWCNT-COOH- Levodopa | Levodopa | pH-dependent CNS release of levodopa | [128] |
| SWCNT-Acetylcholine | Acetylcholine | CNS delivery of Ach, restoring memory in mice | [129] |
| Nanosystem | Drug | Application | Reference |
|---|---|---|---|
| ND-DOX | Doxorubicin | Inducing cytotoxicity in colorectal carcinoma cells | [130] |
| ND-Alen | Alendronate | Enhancing osteogenic effect in bones | [154] |
| ND-4-AP | 4-Aminopyridine | CNS delivery of epileptogenic drugs | [155] |
| ND-Aml | Amlodipine | CNS delivery of amlodipine for neurological disorders | [156] |
| ND-PEI-Fe3O4Amox | Amoxycillin | Magnet-responsive delivery of antibiotics at the target site | [157] |
| Patent No. | Publication Date | Classification | Title | Inventors | Organization | Reference |
|---|---|---|---|---|---|---|
| EP1558524B1 | 16 January 2019 | Carbon nanotubes | CNTs synthesis by CVD | Milo Sebastian Peter Shaffer, Alan H. Windle, et al. | Zeon corp. | [104] |
| IN202041056414 | 1 July 2022 | Carbon nanodots | Ocular drug delivery and tracking comprising photoluminescent CNTs | K. Sudhakaraprasad, Kumara. B. N., et al. | Yenepoya (Deemed To Be University) | [181] |
| WO 2017/029501 Al | 23 February 2017 | Carbon nanodots | Conjugated quantum dots | Naasani, Imad | NANOCO TECHNOLOGIES LTD | [182] |
| WO/2022/160055 | 4 August 2022 | Carbon nanohorns | Method of manufacturing carbon nanohorns and the carbon nanohorns thus produced | Braidy, Nadi Casteignau, Fanny, et al. | UNIVERSITÉ DE MONTRÉAL | [183] |
| US20200385272 | 6 July 2020 | Carbon nano-onion | Porosity for catenated carbon nano-onions and surface area | Danny Cross, Larry Herbert Kirby, Thomas Frank Bailey, et al. | Structured Nano Carbon LLC | [184] |
| AU2021104363 | 19 August 2021 | Carbon nanotubes | CNTs for camptothecin targeted release in cancer chemotherapy | Bhowmick, Mithun, Bhowmick, Pratibha, et al. | Individual | [185] |
| US20200276126 | 3 August 2020 | Carbon nanodiamonds | Polydopamine-encapsulated nanodiamonds and methods | Haksung Jung and Keir Cajal Neuman | NIH, USA | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Nayak, P.; Singh, G.; Khandai, M.; Sarangi, R.R.; Kar, M.K. Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges. C 2023, 9, 3. https://doi.org/10.3390/c9010003
Singh J, Nayak P, Singh G, Khandai M, Sarangi RR, Kar MK. Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges. C. 2023; 9(1):3. https://doi.org/10.3390/c9010003
Chicago/Turabian StyleSingh, Jagtar, Pallavi Nayak, Gurdeep Singh, Madhusmruti Khandai, Rashmi Ranjan Sarangi, and Mihir Kumar Kar. 2023. "Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges" C 9, no. 1: 3. https://doi.org/10.3390/c9010003
APA StyleSingh, J., Nayak, P., Singh, G., Khandai, M., Sarangi, R. R., & Kar, M. K. (2023). Carbon Nanostructures as Therapeutic Cargoes: Recent Developments and Challenges. C, 9(1), 3. https://doi.org/10.3390/c9010003






