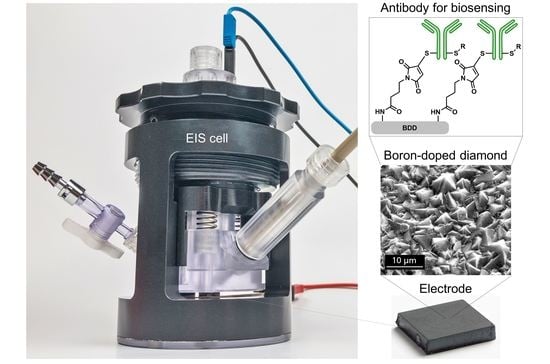
Testing of Diamond Electrodes as Biosensor for Antibody-Based Detection of Immunoglobulin Protein with Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Electrolyte and Protein Analyte
2.3. Electrodes
2.3.1. Electrode Materials and Manufacturing
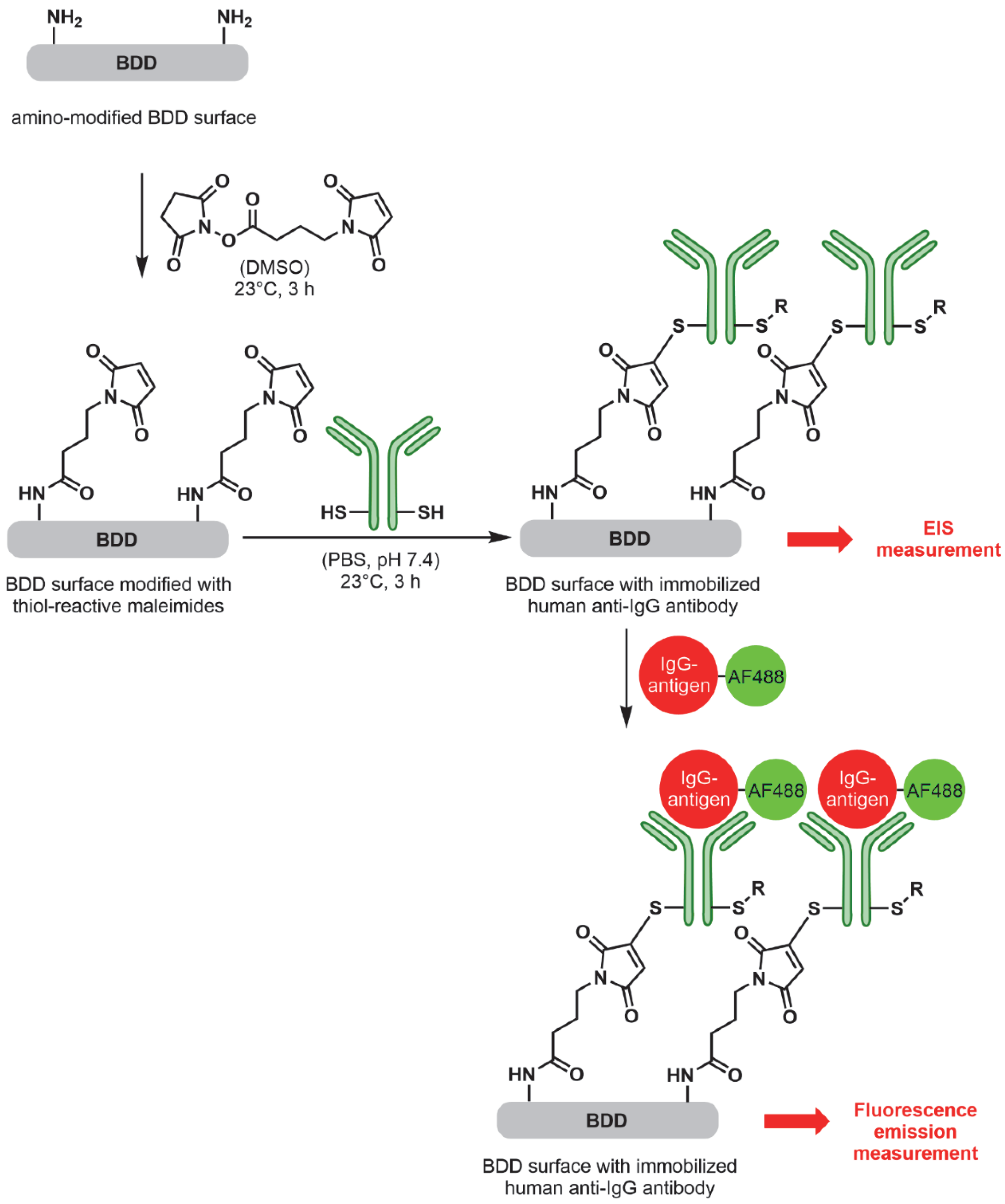
2.3.2. Electrode Functionalisation
2.3.3. Immobilisation of Antibody on Electrodes
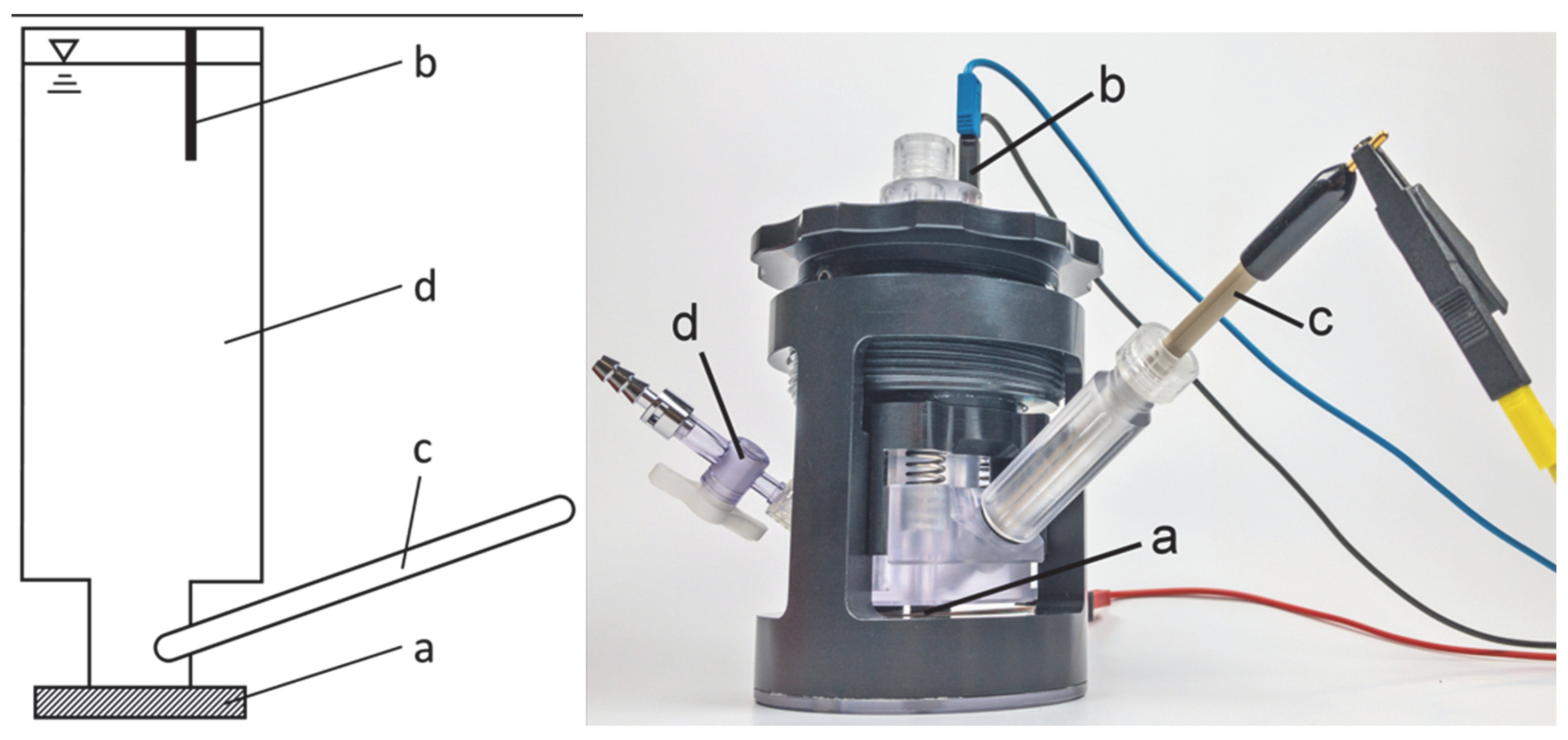
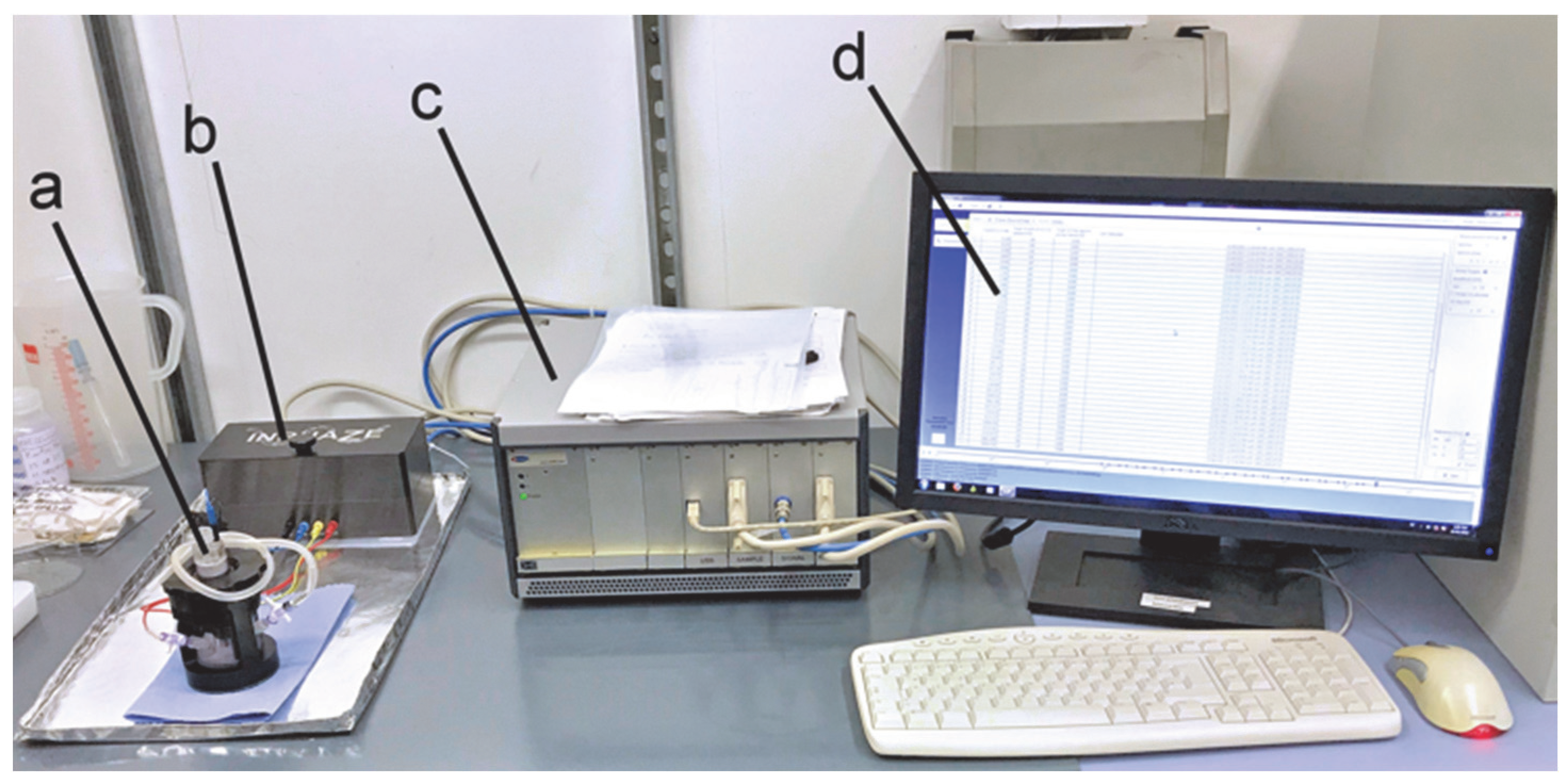
2.4. EIS Cell and Spectrometer
2.5. Spectroscopy Parameters and Procedure
3. Results
3.1. Fluorescence Analysis of Electrode Functionalisation Steps
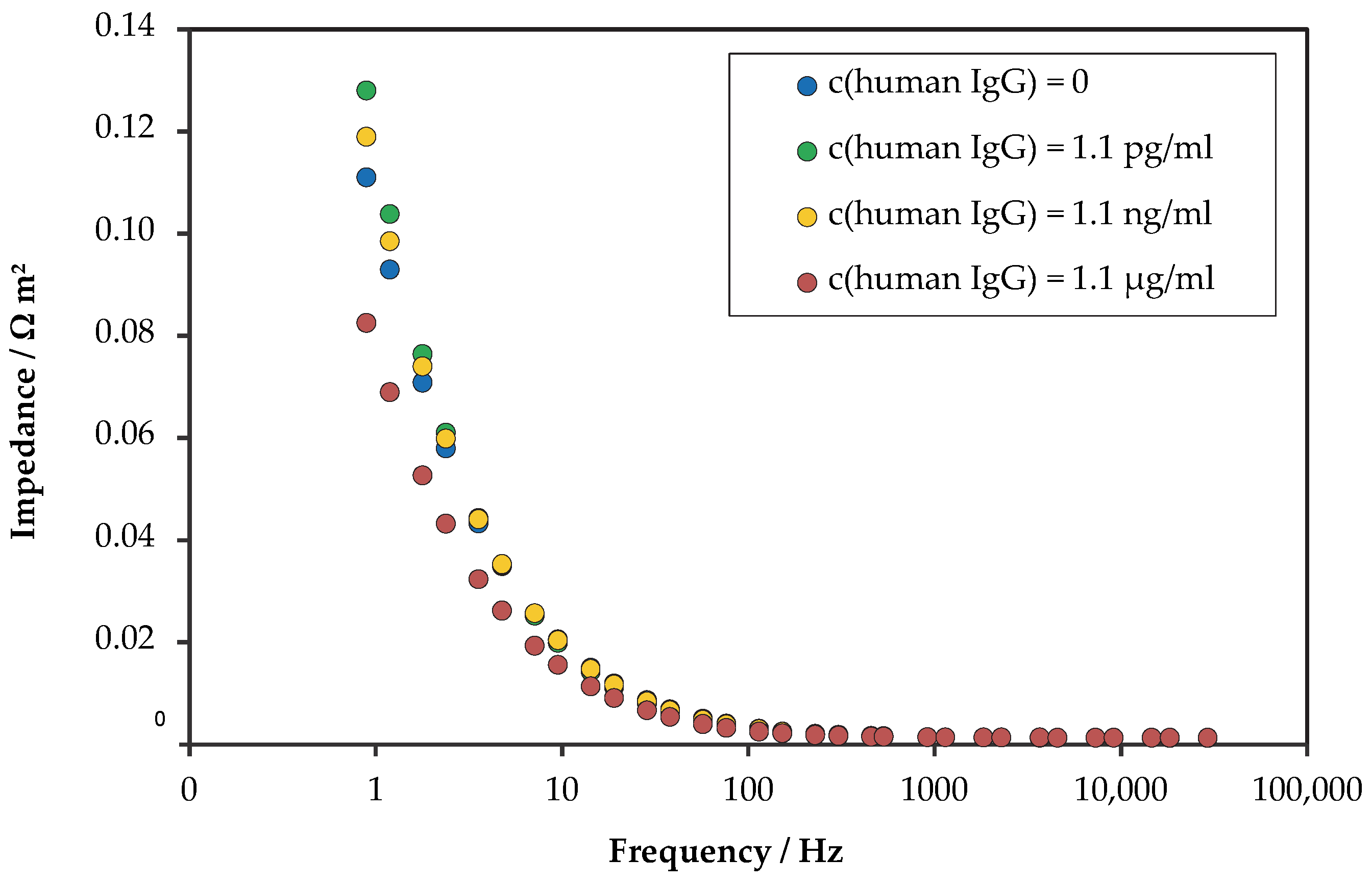
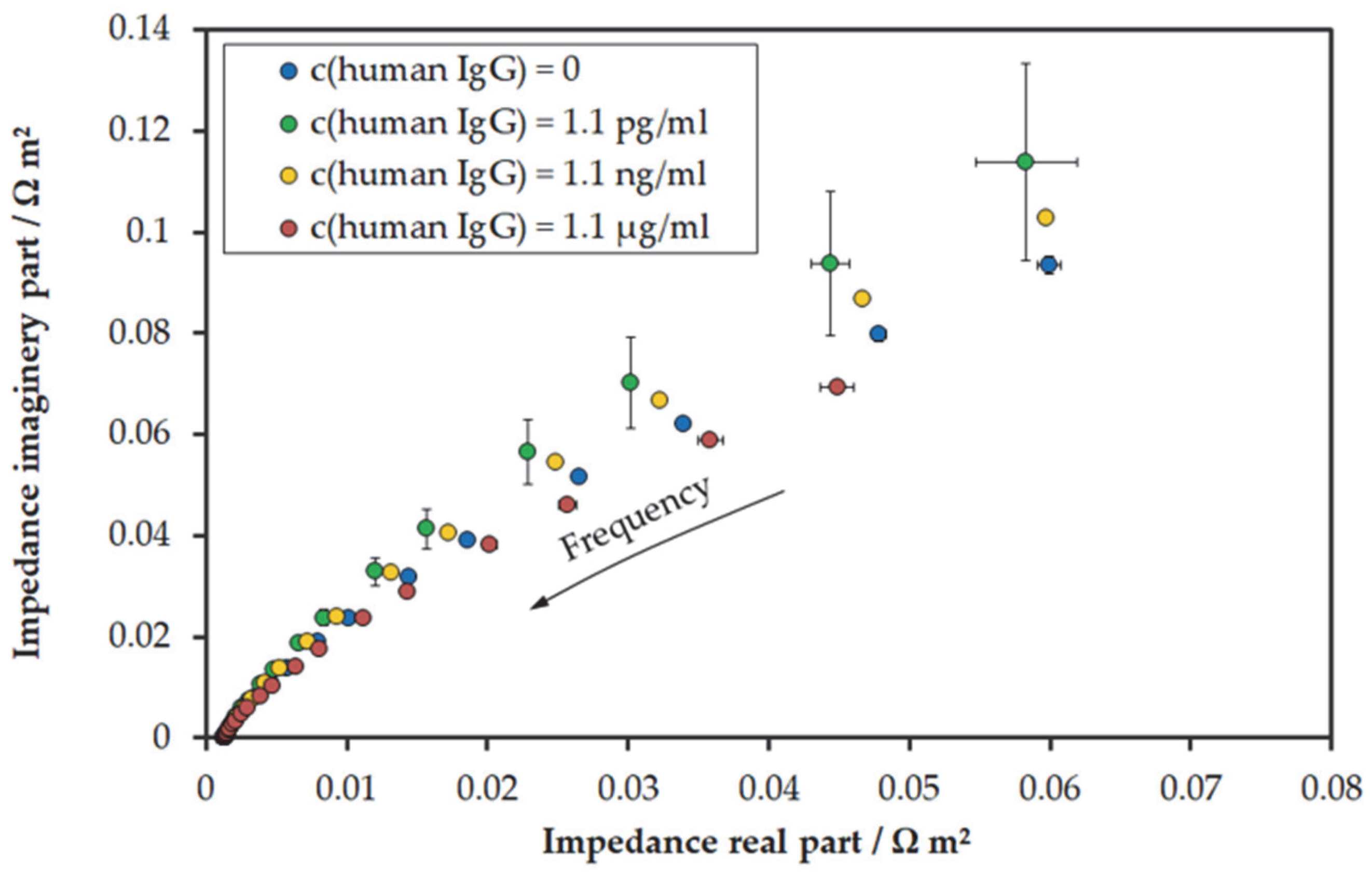
3.2. Electrochemical Impedance Spectroscopy Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Keshky, M.E.S.; Basyouni, S.S.; Al Sabban, A.M. Getting Through COVID-19: The Pandemic’s Impact on the Psychology of Sustainability, Quality of Life, and the Global Economy—A Systematic Review. Front. Psychol. 2020, 11, 585897. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.A.; Wang, J.Y.; Moeser, M.-E.; Starkey, T.; Lee, L.Y.W. A systematic review of the sensitivity and specificity of lateral flow devices in the detection of SARS-CoV-2. BMC Infect. Dis. 2021, 21, 828. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Deng, Z.; Liu, H.; Li, J.; Wang, D.; Yang, Y.; Zhong, S. Current methods and prospects of coronavirus detection. Talanta 2021, 225, 121977. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.H.; Hasan, M.R.; Hossain, S.I.; Ahommed, M.S.; Daizy, M. Ultrasensitive detection of pathogenic viruses with electrochemical biosensor: State of the art. Biosens. Bioelectron. 2020, 166, 112431. [Google Scholar] [CrossRef] [PubMed]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Nidzworski, D.; Siuzdak, K.; Niedziałkowski, P.; Bogdanowicz, R.; Sobaszek, M.; Ryl, J.; Weiher, P.; Sawczak, M.; Wnuk, E.; Goddard, W.A.; et al. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017, 7, 15707. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Ujie, M.; Yamamoto, T.; Akahori, M.; EINAGA, Y.; Sato, T. Highly sensitive detection of influenza virus by boron-doped diamond electrode terminated with sialic acid-mimic peptide. Proc. Natl. Acad. Sci. USA 2016, 113, 8981–8984. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Ujie, M.; Yamamoto, T.; Einaga, Y.; Daidoji, T.; Nakaya, T.; Sato, T. Avian Influenza Virus Detection by Optimized Peptide Termination on a Boron-Doped Diamond Electrode. ACS Sens. 2020, 5, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Siuzdak, K.; Niedziałkowski, P.; Sobaszek, M.; Łęga, T.; Sawczak, M.; Czaczyk, E.; Dziąbowska, K.; Ossowski, T.; Nidzworski, D.; Bogdanowicz, R. Biomolecular influenza virus detection based on the electrochemical impedance spectroscopy using the nanocrystalline boron-doped diamond electrodes with covalently bound antibodies. Sens. Actuators B Chem. 2019, 280, 263–271. [Google Scholar] [CrossRef]
- Witt, S.; Rogien, A.; Werner, D.; Siegenthaler, J.; Lesiyon, R.; Kurien, N.; Rechenberg, R.; Baule, N.; Hardy, A.; Becker, M. Boron doped diamond thin films for the electrochemical detection of SARS-CoV-2 S1 protein. Diam. Relat. Mater. 2021, 118, 108542. [Google Scholar] [CrossRef] [PubMed]
- Białobrzeska, W.; Ficek, M.; Dec, B.; Osella, S.; Trzaskowski, B.; Jaramillo-Botero, A.; Pierpaoli, M.; Rycewicz, M.; Dashkevich, Y.; Łęga, T.; et al. Performance of electrochemical immunoassays for clinical diagnostics of SARS-CoV-2 based on selective nucleocapsid N protein detection: Boron-doped diamond, gold and glassy carbon evaluation. Biosens. Bioelectron. 2022, 209, 114222. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, P.; Balansethupathy, B.; Vezza, V.J.; Butterworth, A.; Macdonald, A.; Blair, E.O.; McAteer, L.; Hannah, S.; Ward, A.C.; Hoskisson, P.A.; et al. SARS-CoV-2 Aptasensors Based on Electrochemical Impedance Spectroscopy and Low-Cost Gold Electrode Substrates. Anal. Chem. 2022, 94, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 37523. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Rucinskiene, A.; Ramanaviciene, A.; Ratautaite, V.; Viter, R.; Chen, C.-F.; Plikusiene, I.; Samukaite-Bubniene, U.; et al. Electrochemical Determination of Interaction between SARS-CoV-2 Spike Protein and Specific Antibodies. Int. J. Mol. Sci. 2022, 23, 6768. [Google Scholar] [CrossRef] [PubMed]
- Lourencao, B.C.; Brocenschi, R.F.; Medeiros, R.A.; Fatibello-Filho, O.; Rocha-Filho, R.C. Analytical Applications of Electrochemically Pretreated Boron-Doped Diamond Electrodes. ChemElectroChem 2020, 7, 1291–1311. [Google Scholar] [CrossRef]
- Fryda, M.; Matthée, T.; Mulcahy, S.; Hampel, A.; Schäfer, L.; Tröster, I. Fabrication and application of Diachem electrodes. Diam. Relat. Mater. 2003, 12, 1950–1956. [Google Scholar] [CrossRef]
- Schäfer, L.; Höfer, M.; Kröger, R. The versatility of hot-filament activated chemical vapor deposition. Thin Solid Film. 2006, 515, 1017–1024. [Google Scholar] [CrossRef]
- Bertoglio, F.; Meier, D.; Langreder, N.; Steinke, S.; Rand, U.; Simonelli, L.; Heine, P.A.; Ballmann, R.; Schneider, K.-T.; Roth, K.D.R.; et al. SARS-CoV-2 neutralizing human recombinant antibodies selected from pre-pandemic healthy donors binding at RBD-ACE2 interface. Nat. Commun. 2021, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.D.R.; Wenzel, E.V.; Ruschig, M.; Steinke, S.; Langreder, N.; Heine, P.A.; Schneider, K.-T.; Ballmann, R.; Fühner, V.; Kuhn, P.; et al. Developing Recombinant Antibodies by Phage Display against Infectious Diseases and Toxins for Diagnostics and Therapy. Front. Cell. Infect. Microbiol. 2021, 11, 697876. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, H.B.; Pedrosa, V.A.; Codognoto, L.; Machado, S.A.; Rocha-Filho, R.C.; Avaca, L.A. Enhanced electrochemical response of boron-doped diamond electrodes brought on by a cathodic surface pre-treatment. Electrochim. Acta 2004, 49, 4021–4026. [Google Scholar] [CrossRef]

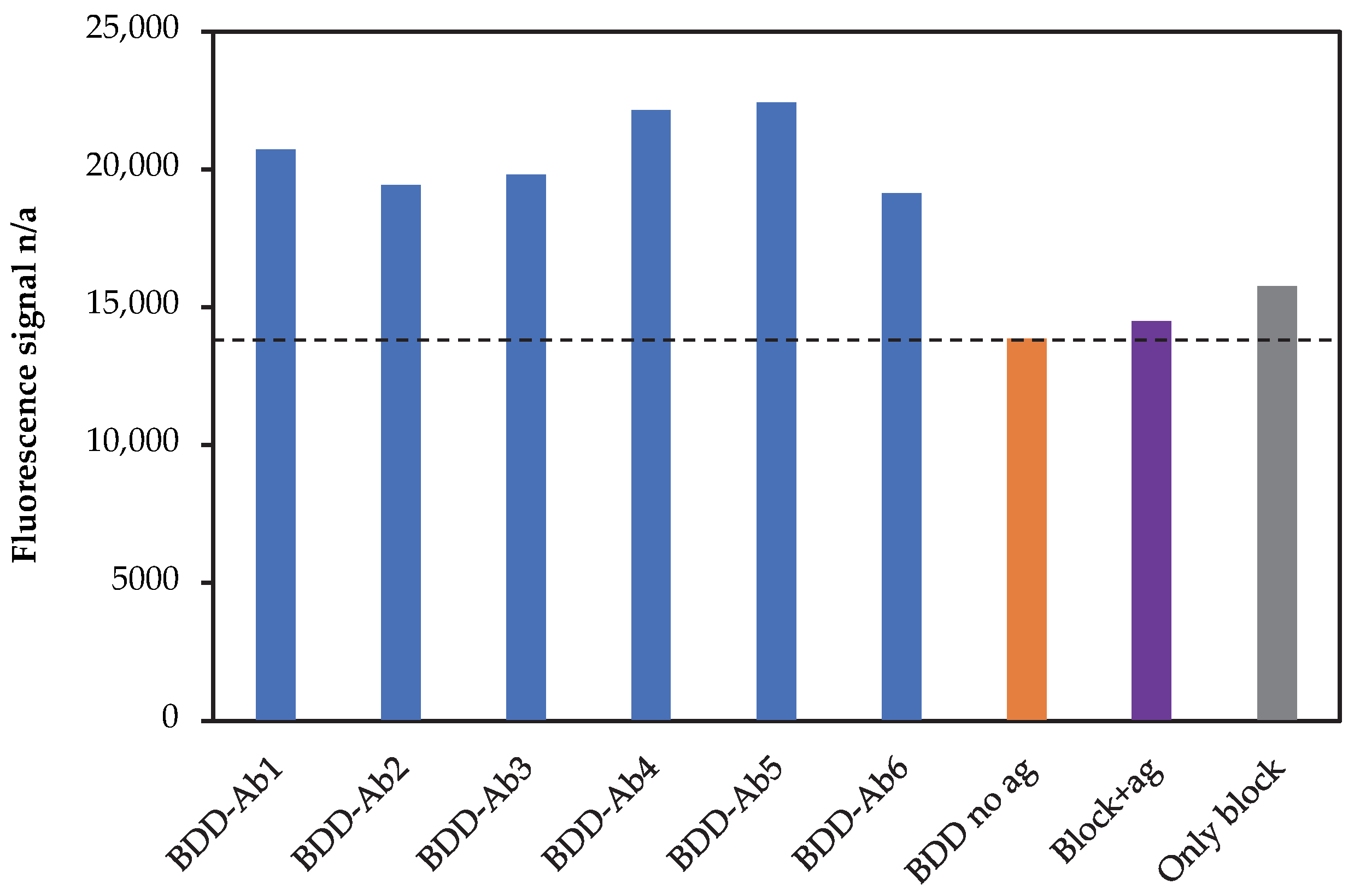
| Parameter | Value |
|---|---|
| Electrolyte volume | 10 mL |
| Frequency range | 0.9 Hz–29 kHz * |
| Number of frequencies within the range | 31 |
| Measurements per frequency | 3 |
| AC amplitude | 10 mV +/− 10% |
| Spectra delay | 1 min |
| DC bias | 0 V |
| Electrolyte temperature | 22 °C (+/−4 °C) |
| Electrolyte volume | 10 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzler, M.; Ganskow, C.S.G.; Ruschig, M.; Moustafa, E.; Sittinger, V.; Lachmann, K.; Wenzel, E.V.; Russo, G.; Klahn, P.; Gäbler, J. Testing of Diamond Electrodes as Biosensor for Antibody-Based Detection of Immunoglobulin Protein with Electrochemical Impedance Spectroscopy. C 2022, 8, 74. https://doi.org/10.3390/c8040074
Menzler M, Ganskow CSG, Ruschig M, Moustafa E, Sittinger V, Lachmann K, Wenzel EV, Russo G, Klahn P, Gäbler J. Testing of Diamond Electrodes as Biosensor for Antibody-Based Detection of Immunoglobulin Protein with Electrochemical Impedance Spectroscopy. C. 2022; 8(4):74. https://doi.org/10.3390/c8040074
Chicago/Turabian StyleMenzler, Martin, Charity S. G. Ganskow, Maximilian Ruschig, Essam Moustafa, Volker Sittinger, Kristina Lachmann, Esther Veronika Wenzel, Giulio Russo, Philipp Klahn, and Jan Gäbler. 2022. "Testing of Diamond Electrodes as Biosensor for Antibody-Based Detection of Immunoglobulin Protein with Electrochemical Impedance Spectroscopy" C 8, no. 4: 74. https://doi.org/10.3390/c8040074
APA StyleMenzler, M., Ganskow, C. S. G., Ruschig, M., Moustafa, E., Sittinger, V., Lachmann, K., Wenzel, E. V., Russo, G., Klahn, P., & Gäbler, J. (2022). Testing of Diamond Electrodes as Biosensor for Antibody-Based Detection of Immunoglobulin Protein with Electrochemical Impedance Spectroscopy. C, 8(4), 74. https://doi.org/10.3390/c8040074