Fabrication of Metal/Graphene Composites via Cold Spray Process: State-of-the-Art and the Way Forward
Abstract
1. Introduction
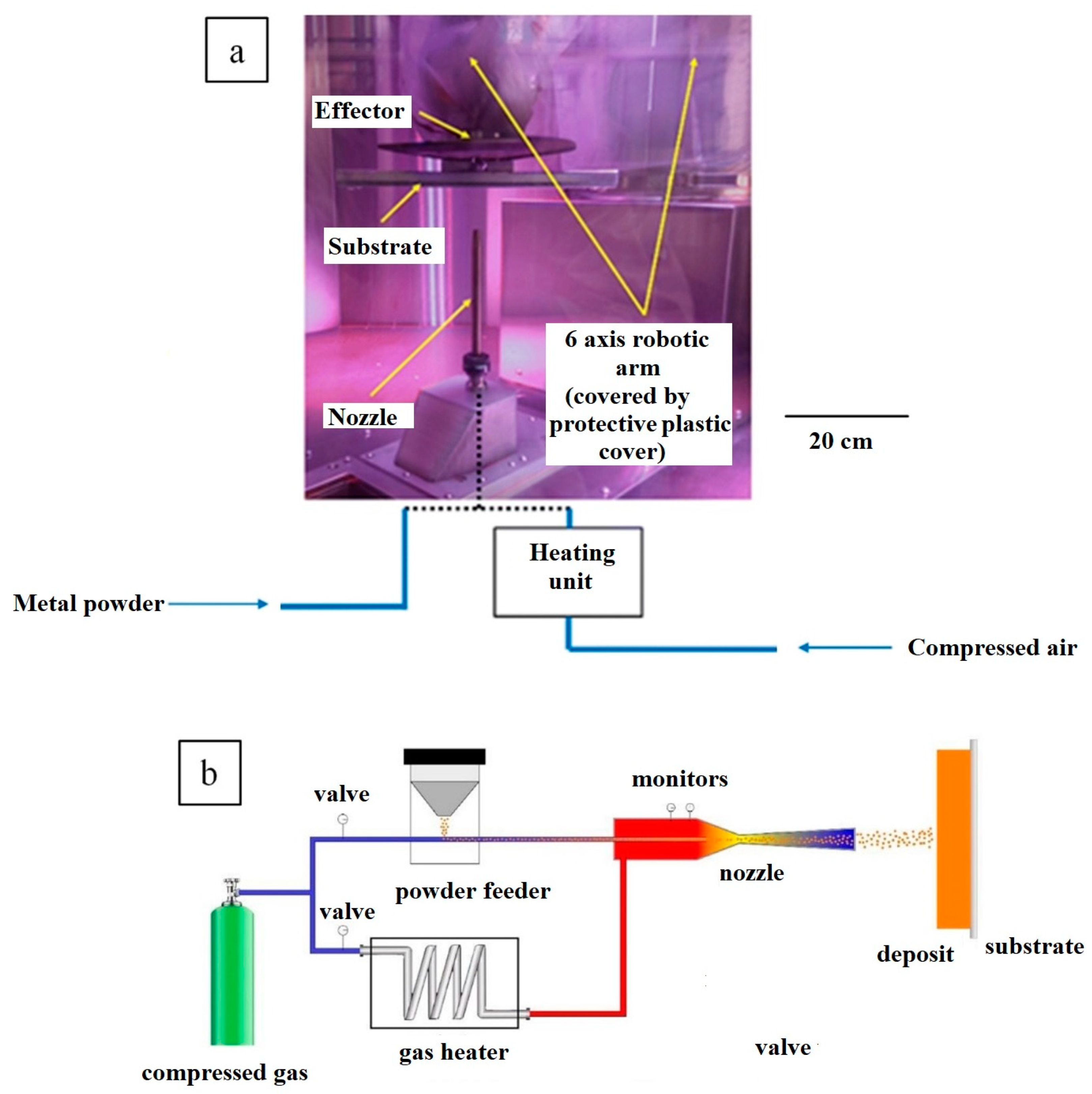
2. CS Fabrication of Metal–Graphene Composites
2.1. Ball Milling
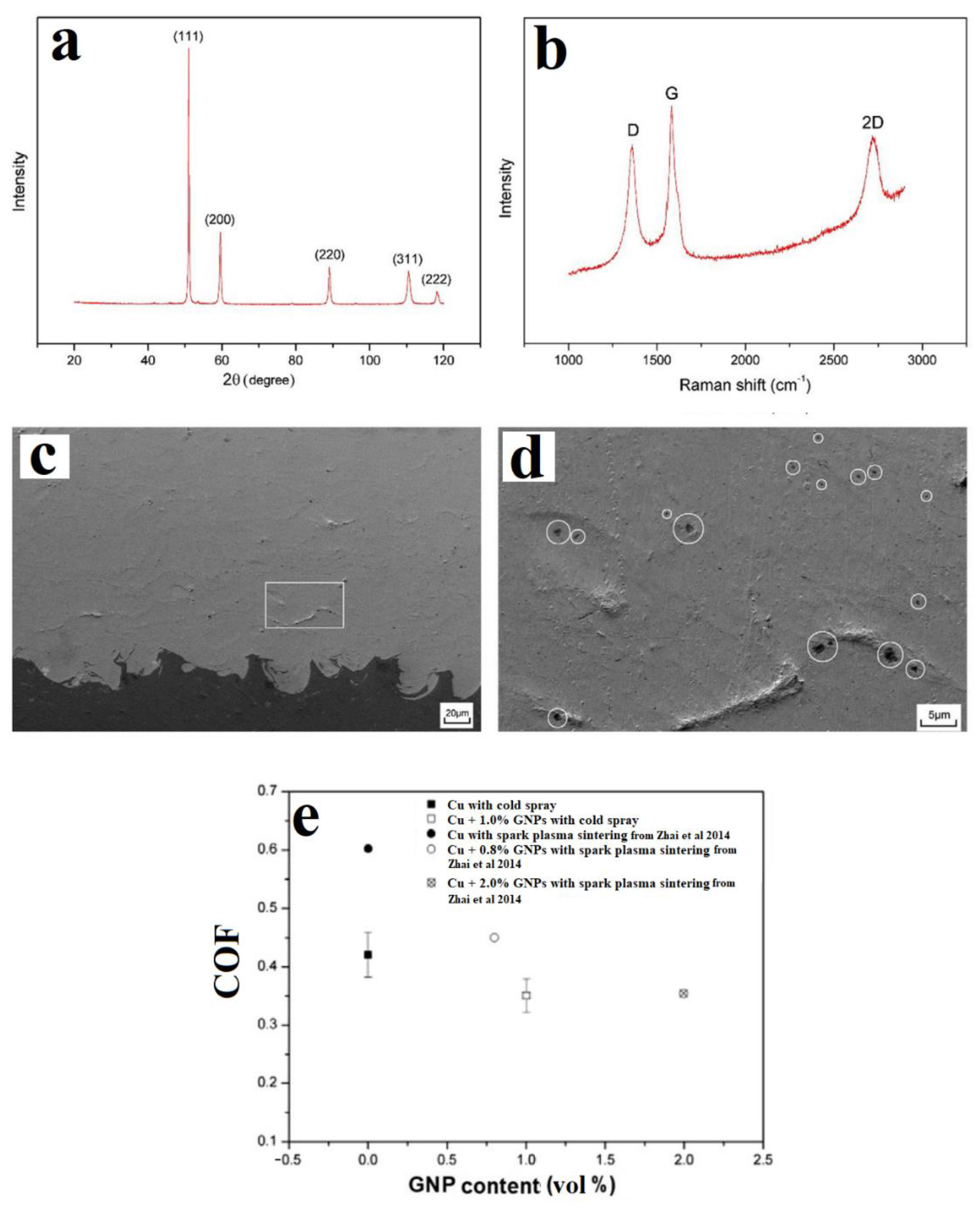
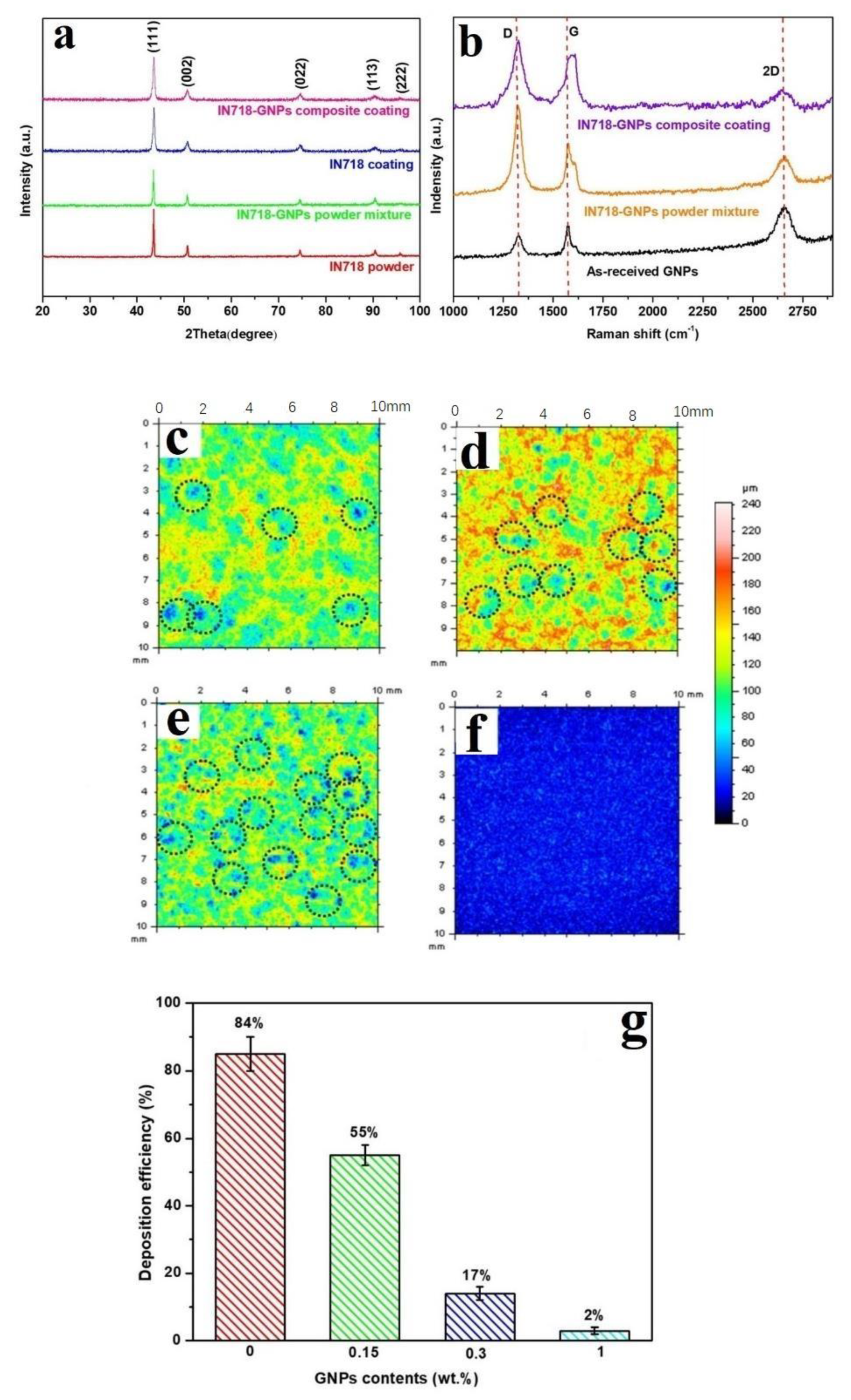
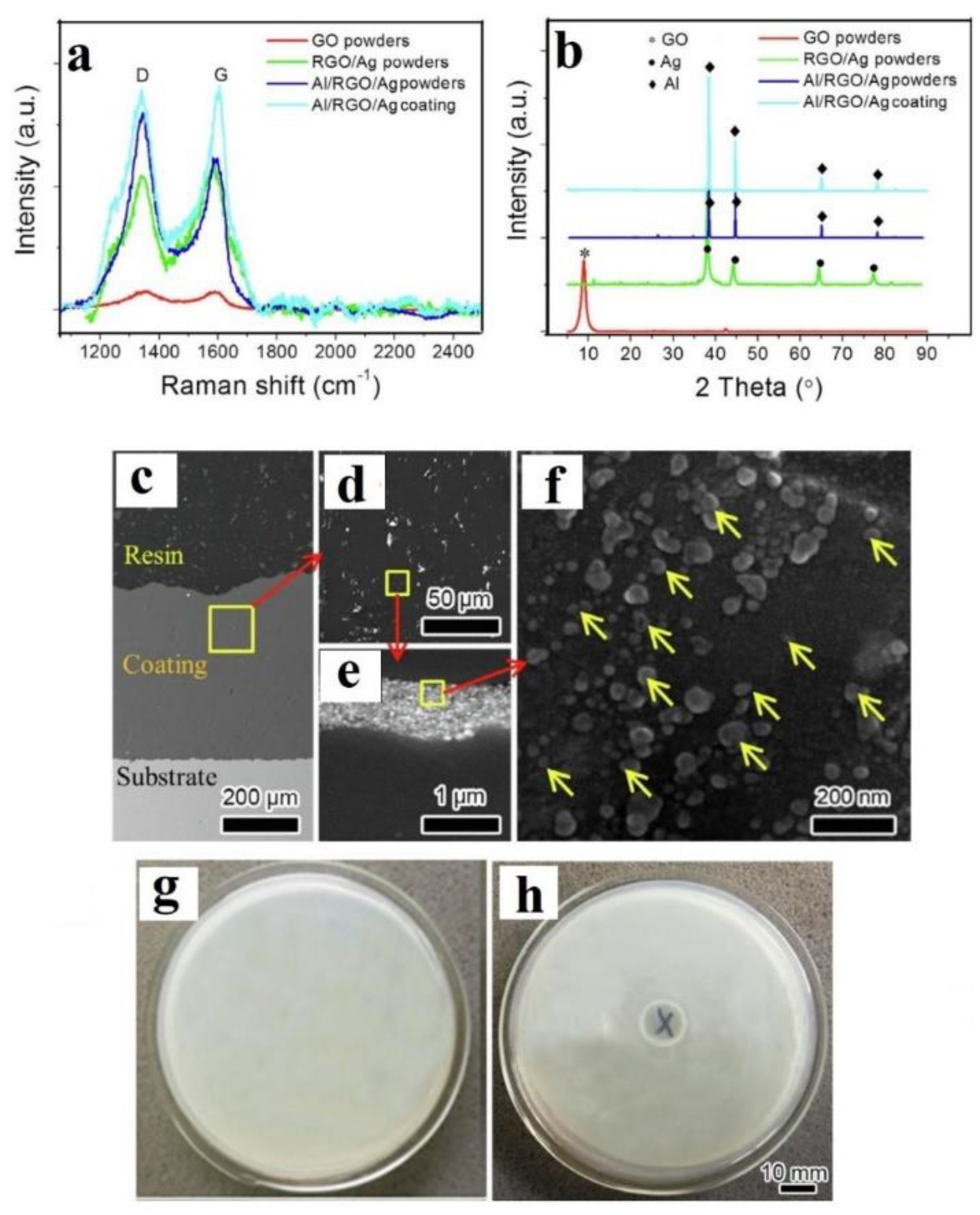
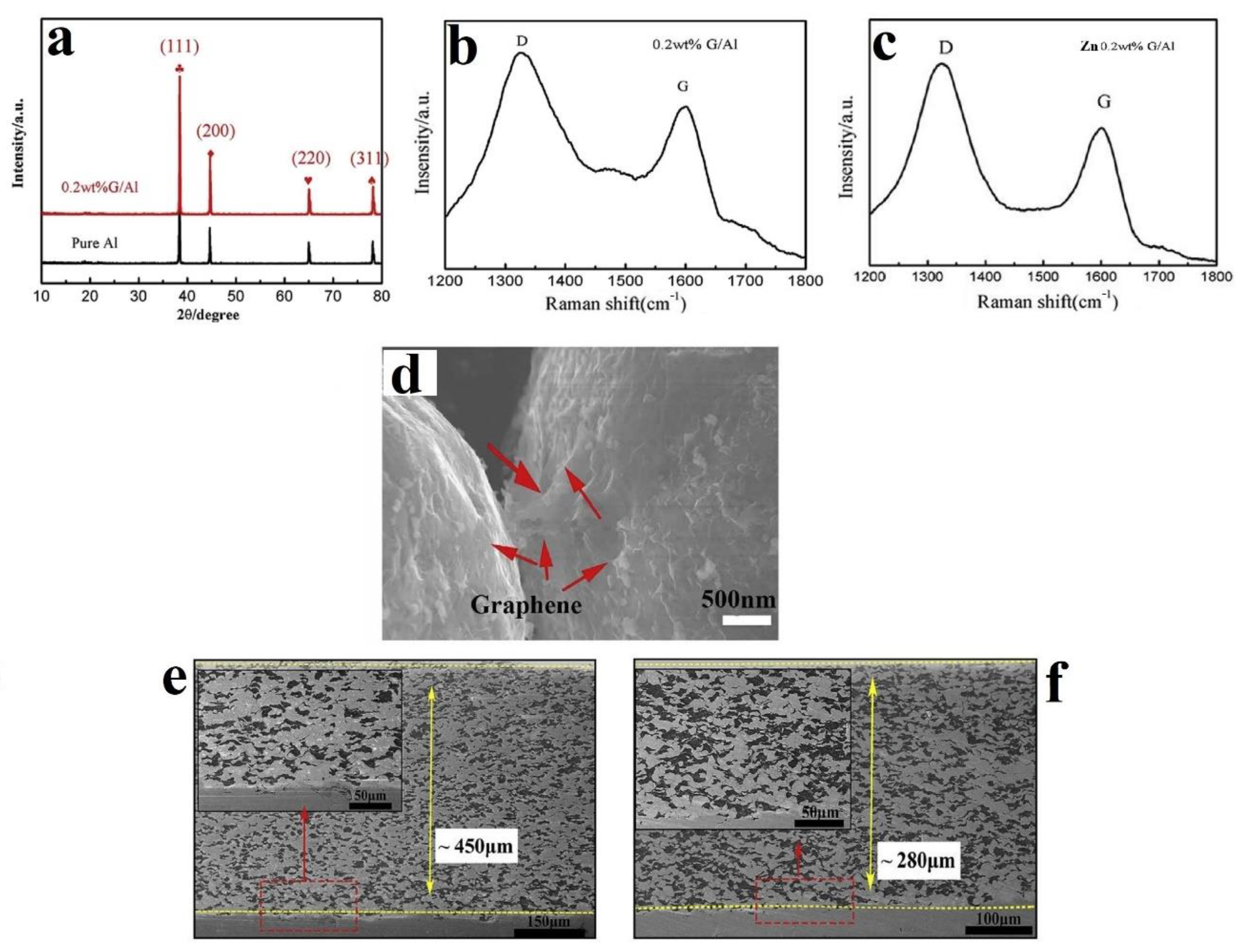
2.2. In-Situ Deposition/Reduction
2.3. Other Methods
3. Discussion and Summary
4. Research Gaps and Future Works
- (i)
- Obtaining a uniform dispersion of metal and GNP powders. There are significant technical issues to achieving a homogeneous/uniform dispersion of graphene nano-particulates with metal powders via either ball milling or in situ reduction composite-powder fabrication techniques.
- (ii)
- Establishing optimised metal/GNP composite powders wherein the GNP particulates retain their original structure as much as possible. Often, the high-energy ball milling process causes damage to the morphology of the graphene powders, which have high aspect ratios, and the in situ reduction process does not yield sufficient graphene nano-layers from metal powders.
- (iii)
- Achieving sufficient interfacial bonding between graphene nano-particulates and the metal powders. This involves understanding interfacial bonding in metal/GNP composites wherein the results from thermal, microscopic, and crystallographic analyses can be combined to achieve a holistic view of the metal/GNP interface. This can then provide information on how to fabricate the most effective feedstock by understanding the critical interaction between these two-phased particle mixtures and their subsequent effects on the cold spray printing process.
- (i)
- Establishing optimised cold spray process parameters to deposit/fabricate metal/GNP coatings/parts. The CS process could yield highly dense composite parts with the fewest defects. The effects of process parameters such as spray pressure, nozzle temperature, powder flow rate, standoff distance between the spray nozzle and the substrate plate, carrier gas, etc. on the print-part quality and properties are not well understood.
- (ii)
- Evaluating the effect of the volume fraction of graphene nano-particulate powders within the feedstock on the print-part properties. It is possible that there is a limit to the volume of GNPs that can provide observable differences in the part properties.
- (iii)
- Identifying and applying suitable post-heat treatment to bulk components to further improve functionality and performance without affecting the GNPs embedded in the printed structures. CS fabrication often requires the use of post-manufacture annealing procedures to improve the mechanical performance to the level achieved using conventional manufacturing techniques.
- (iv)
- Printing and validating a fully functional composite component for a real-world application to demonstrate that the CS process is an economically viable method to fabricate bulk metal/GNP composite components with a tailored suite of properties for different applications. This will involve characterisation of printed coupons for various properties including, but not limited to, electrical and thermal conductivity, mechanical properties in terms of tensile and compression performance, tribological properties in terms of friction and wear performance, and corrosion resistance. Moreover, an effective comparison of the properties achieved from CS coatings with self-standing CS parts needs to be analysed.
- (v)
- Developing novel techniques to spray metal/GNP composite coatings with self-healing behaviour. This behaviour can be leveraged into printing high-mechanical-performance self-standing structures and, with effective CS printing strategies, can eliminate the need for annealing and other post-processing steps, thus streamlining the CS process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranjan, R.; Bajpai, V. Graphene-based metal matrix nanocomposites: Recent development and challenges. J. Compos. Mater. 2021, 55, 2369–2413. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Itapu, B.; Jayatissa, A. A Review in Graphene/Polymer Composites. Chem. Sci. Int. J. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef]
- Gao, Q.; Qin, J.; Guo, B.; Fan, X.; Wang, F.; Zhang, Y.; Xiao, R.; Huang, F.; Shi, X.; Zhang, G. High-performance electromagnetic interference shielding epoxy/Ag nanowire/thermal annealed graphene aerogel composite with bicontinuous three-dimensional conductive skeleton. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106648. [Google Scholar] [CrossRef]
- Hameed, N.; Dumée, L.F.; Allioux, F.-M.; Reghat, M.; Church, J.S.; Naebe, M.; Magniez, K.; Parameswaranpillai, J.; Fox, B.L. Graphene based room temperature flexible nanocomposites from permanently cross-linked networks. Sci. Rep. 2018, 8, 2803. [Google Scholar] [CrossRef]
- Mirabedini, A.; Anderson, L.; Antiohos, D.; Ang, A.; Nikzad, M.; Fuss, F.K.; Hameed, N. Scalable Production and Thermoelectrical Modeling of Infusible Functional Graphene/Epoxy Nanomaterials for Engineering Applications. Ind. Eng. Chem. Res. 2022, 61, 5141–5157. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Yan, X.; Gao, W.; Chen, H.; Chen, Q.; Bai, Y. Functional & enhanced graphene/polyamide 6 composite fiber constructed by a facile and universal method. Compos. Part A Appl. Sci. Manuf. 2019, 123, 149–157. [Google Scholar]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y. Vertically aligned silicon carbide nanowires/reduced graphene oxide networks for enhancing the thermal conductivity of silicone rubber composites. Compos. Part A Appl. Sci. Manuf. 2020, 133, 105873. [Google Scholar] [CrossRef]
- Markandan, K.; Chin, J.K.; Tan, M.T.T. Recent progress in graphene based ceramic composites: A review. J. Mater. Res. 2017, 32, 84–106. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, F.; Yusoff, P.S.M.M.; Muhamad, N.; Oñate, E.; Raza, M.R.; Malik, K. A review of graphene reinforced Cu matrix composites for thermal management of smart electronics. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106357. [Google Scholar] [CrossRef]
- Amini, A.; Cheng, C.; Naebe, M.; Church, J.S.; Hameed, N.; Asgari, A.; Will, F. Temperature variations at nano-scale level in phase transformed nanocrystalline NiTi shape memory alloys adjacent to graphene layers. Nanoscale 2013, 5, 6479–6484. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Hameed, N.; Church, J.S.; Cheng, C.; Asgari, A.; Will, F. Effect of graphene layers on the thermomechanical behaviour of a NiTi shape memory alloy during the nanoscale phase transition. Scr. Mater. 2013, 68, 420–423. [Google Scholar] [CrossRef]
- Navasingh, R.J.H.; Kumar, R.; Marimuthu, K.; Planichamy, S.; Khan, A.; Asiri, A.M.; Asad, M. 6–Graphene-based nano metal matrix composites: A review. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin, Asiri, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 153–170. [Google Scholar]
- Shinde, S.K.; Kim, D.Y.; Kumar, M.; Murugadoss, G.; Ramesh, S.; Tamboli, A.M.; Yadav, H.M. MOFs-Graphene Composites Synthesis and Application for Electrochemical Supercapacitor: A Review. Polymers 2022, 14, 511. [Google Scholar] [CrossRef]
- Yang, G.; Han, Y.; Lu, A.; Guo, Q. Enhanced damping capacity of nanolaminated graphene (reduced graphene oxide)/Al-Mg-Si composite. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106887. [Google Scholar] [CrossRef]
- Yu, M.; Shao, D.; Lu, F.; Sun, X.; Sun, H.; Hu, T.; Wang, G.; Sawyer, S.; Qiu, H.; Lian, J. ZnO/graphene nanocomposite fabricated by high energy ball milling with greatly enhanced lithium storage capability. Electrochem. Commun. 2013, 34, 312–315. [Google Scholar] [CrossRef]
- Li, X.; Zheng, X.; Shao, J.; Gao, T.; Shi, Q.; Qu, Q. Synergistic Ternary Composite (Carbon/Fe3O4 @Graphene) with Hollow Microspherical and Robust Structure for Li-Ion Storage. Chemistry 2016, 22, 376–381. [Google Scholar] [CrossRef]
- Kim, D.Y.; Joshi, B.N.; Park, J.J.; Lee, J.G.; Cha, Y.H.; Seong, T.Y.; In Noh, S.; Ahn, H.J.; Al-Deyabe, S.S.; Yoon, S.S. Graphene–titania films by supersonic kinetic spraying for enhanced performance of dye-sensitized solar cells. Ceram. Int. 2014, 40 Pt B, 11089–11097. [Google Scholar] [CrossRef]
- Anderson, L.; Govindaraj, P.; Ang, A.; Mirabedini, A.; Hameed, N. Modelling, fabrication and characterization of graphene/polymer nanocomposites for electromagnetic interference shielding applications. Carbon Trends 2021, 4, 100047. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Kumar, H.G.P.; Xavior, M.A. Graphene Reinforced Metal Matrix Composite (GRMMC): A Review. Procedia Eng. 2014, 97, 1033–1040. [Google Scholar] [CrossRef]
- Mirabedini, A.; Ang, A.; Nikzad, M.; Fox, B.; Lau, K.-T.; Hameed, N. Evolving Strategies for Producing Multiscale Graphene-Enhanced Fiber-Reinforced Polymer Composites for Smart Structural Applications. Adv. Sci. 2020, 7, 1903501. [Google Scholar] [CrossRef] [PubMed]
- Poyato, R.; Vasiliev, A.L.; Padture, N.P.; Tanaka, H.; Nishimura, T. Aqueous colloidal processing of single-wall carbon nanotubes and their composites with ceramics. Nanotechnology 2006, 17, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Hutasoit, N.; Rahman Rashid, R.A.; Palasinamy, S.; Duguid, A. Effect of build orientation and post-build heat treatment on the mechanical properties of cold spray additively manufactured copper parts. Int. J. Adv. Manuf. Technol. 2020, 110, 1–17. [Google Scholar]
- Jiang, X.; Overman, N.; Smith, C.; Ross, K. Microstructure, hardness and cavitation erosion resistance of different cold spray coatings on stainless steel 316 for hydropower applications. Mater. Today Commun. 2020, 25, 101305. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H. A comprehensive review on sustainable cold spray additive manufacturing: State of the art, challenges and future challenges. J. Clean. Prod. 2021, 310, 127606. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, Q.; Chen, J.; Ramachandran, C.S. Abrasion, sliding wear, corrosion, and cavitation erosion characteristics of a duplex coating formed on AZ31 Mg alloy by sequential application of cold spray and plasma electrolytic oxidation techniques. Mater. Today Commun. 2021, 26, 101978. [Google Scholar] [CrossRef]
- Wang, X.; Feng, F.; Klecka, M.A.; Mordasky, M.D.; Garofano, J.K.; El-Wardany, T.; Nardi, A.; Champagne, V.K. Characterization and modeling of the bonding process in cold spray additive manufacturing. Addit. Manuf. 2015, 8, 149–162. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, N.; Zhou, Z.; Tong, Z. Influence of heating temperature and holding time on the formation sequence of iron aluminides at the interface of Fe/Al coatings. Mater. Today Commun. 2021, 28, 102516. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Rahman Rashid, R.A.; Barr, C.J.; Palanisamy, S.; Nazari, K.A.; Orchowski, N.; Matthews, N.; Dargusch, M.S. Effect of clad orientation on the mechanical properties of laser-clad repaired ultra-high strength 300M steel. Surf. Coat. Technol. 2019, 380, 125090. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Masood, S.H.; Ruan, D.; Palanisamy, S.; Rashid, R. High strain rate dynamic behaviour of AlSi12 alloy processed by selective laser melting. Int. J. Adv. Manuf. Technol. 2018, 97, 1023–1035. [Google Scholar] [CrossRef]
- Nazari, K.A.; Rahman Rashid, R.A.; Palanisamy, S.; Xia, K.; Dargusch, M.S. A novel Ti-Fe composite coating deposited using laser cladding of low cost recycle d nano-crystalline titanium powder. Mater. Lett. 2018, 229, 301–304. [Google Scholar] [CrossRef]
- Prasad, K.; Khalik, M.A.; Hutasoit, N.; Rashid, R.A.R.; Rashid, R.; Duguid, A.; Palanisamy, S. Printability of low-cost pre-heat-treated ball milled Al7075 powders using compressed air assisted cold spray additive manufacturing. Addit. Manuf. Lett. 2022, 3, 100046. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Sinha-Ray, S.; Park, J.; Lee, J.-G.; Cha, Y.-H.; Bae, S.-H.; Ahn, J.-H.; Jung, Y.C.; Kim, S.M.; Yarin, A.L. Supersonic spray creates high-quality graphene layer. Adv. Funct. Mater. 2014, 24, 4986–4995. [Google Scholar] [CrossRef]
- Kim, S.D.; Lee, J.-G.; Kim, T.-G.; Rana, K.; Jeong, J.Y.; Park, J.H.; Yoon, S.S.; Ahn, J.-H. Additive-free electrode fabrication with reduced graphene oxide using supersonic kinetic spray for flexible lithium-ion batteries. Carbon 2018, 139, 195–204. [Google Scholar] [CrossRef]
- Lee, K.D.; Park, M.J.; Kim, D.-Y.; Kim, S.M.; Kang, B.; Kim, S.; Kim, H.; Lee, H.-S.; Kang, Y.; Yoon, S.S. Graphene quantum dot layers with energy-down-shift effect on crystalline-silicon solar cells. ACS Appl. Mater. Interfaces 2015, 7, 19043–19049. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, D.-Y.; Mali, M.G.; Al-Deyab, S.S.; Swihart, M.T.; Yoon, S.S. Supersonically blown nylon-6 nanofibers entangled with graphene flakes for water purification. Nanoscale 2015, 7, 19027–19035. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. SARS-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hutasoit, N.; Topa, S.H.; Javed, M.A.; Rahman Rashid, R.A.; Palombo, E.; Palanisamy, S. Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli. Materials 2021, 14, 6744. [Google Scholar] [CrossRef] [PubMed]
- Hutasoit, N.; Javed, M.A.; Rashid, R.A.R.; Wade, S.; Palanisamy, S. Effects of build orientation and heat treatment on microstructure, mechanical and corrosion properties of Al6061 aluminium parts built by cold spray additive manufacturing process. Int. J. Mech. Sci. 2021, 204, 106526. [Google Scholar] [CrossRef]
- Zahiri, S.H.; Antonio, C.I.; Jahedi, M. Elimination of porosity in directly fabricated titanium via cold gas dynamic spraying. J. Mater. Process. Technol. 2009, 209, 922–929. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yu, Y.H.; Lee, Y.C.; Hong, Y.P.; Ko, K.H. Cold spray of SiC and Al2O3 with soft metal incorporation: A technical contribution. J. Therm. Spray Technol. 2004, 13, 184–189. [Google Scholar] [CrossRef]
- Irissou, E.; Legoux, J.-G.; Arsenault, B.; Moreau, C. Investigation of Al-Al2O3 Cold Spray Coating Formation and Properties. J. Therm. Spray Technol. 2007, 16, 661–668. [Google Scholar] [CrossRef]
- Feng, C.; Guipont, V.; Jeandin, M.; Amsellem, O.; Pauchet, F.; Saenger, R.; Bucher, S.; Iacob, C. B4C/Ni Composite Coatings Prepared by Cold Spray of Blended or CVD-Coated Powders. J. Therm. Spray Technol. 2012, 21, 561–570. [Google Scholar] [CrossRef]
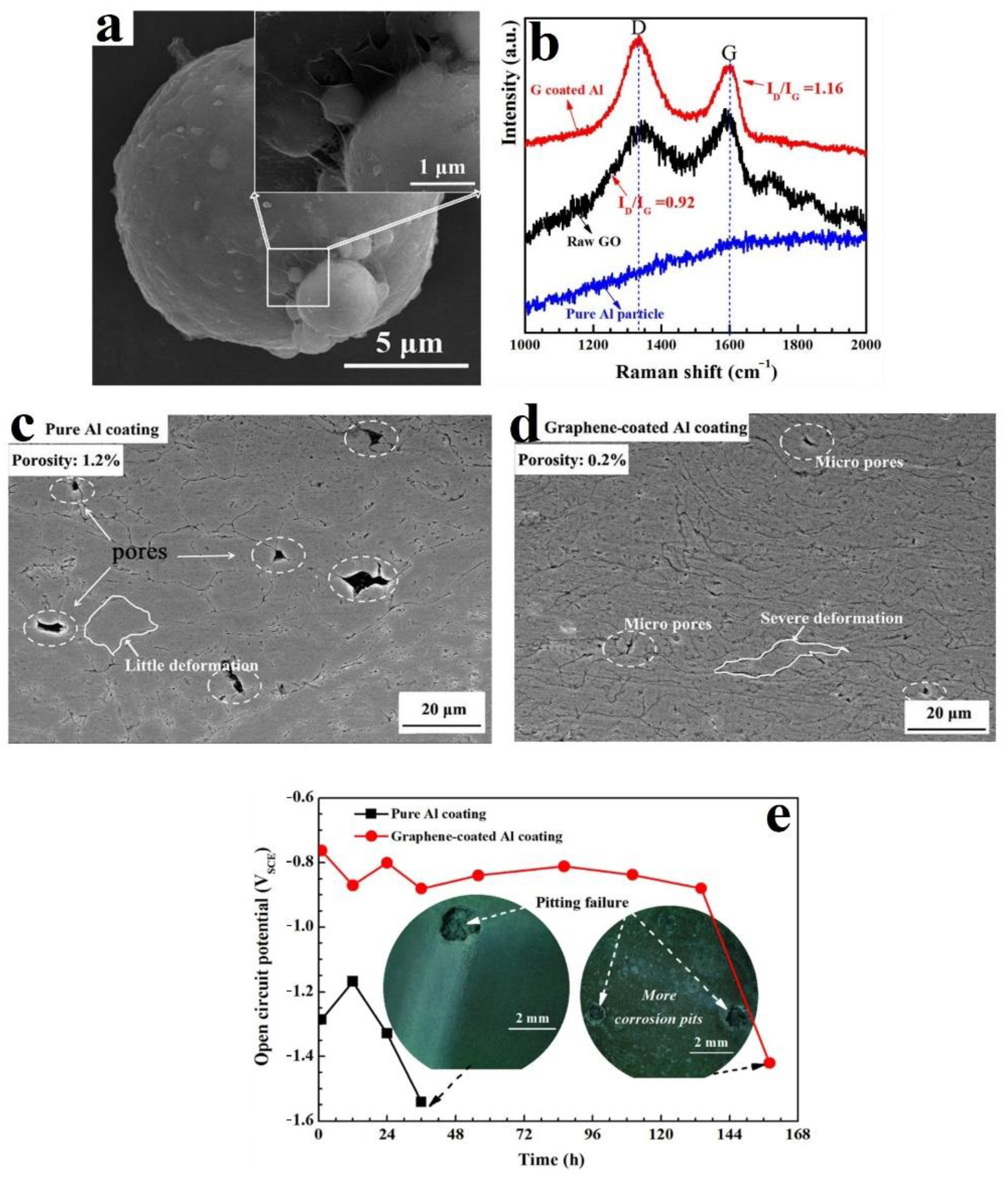
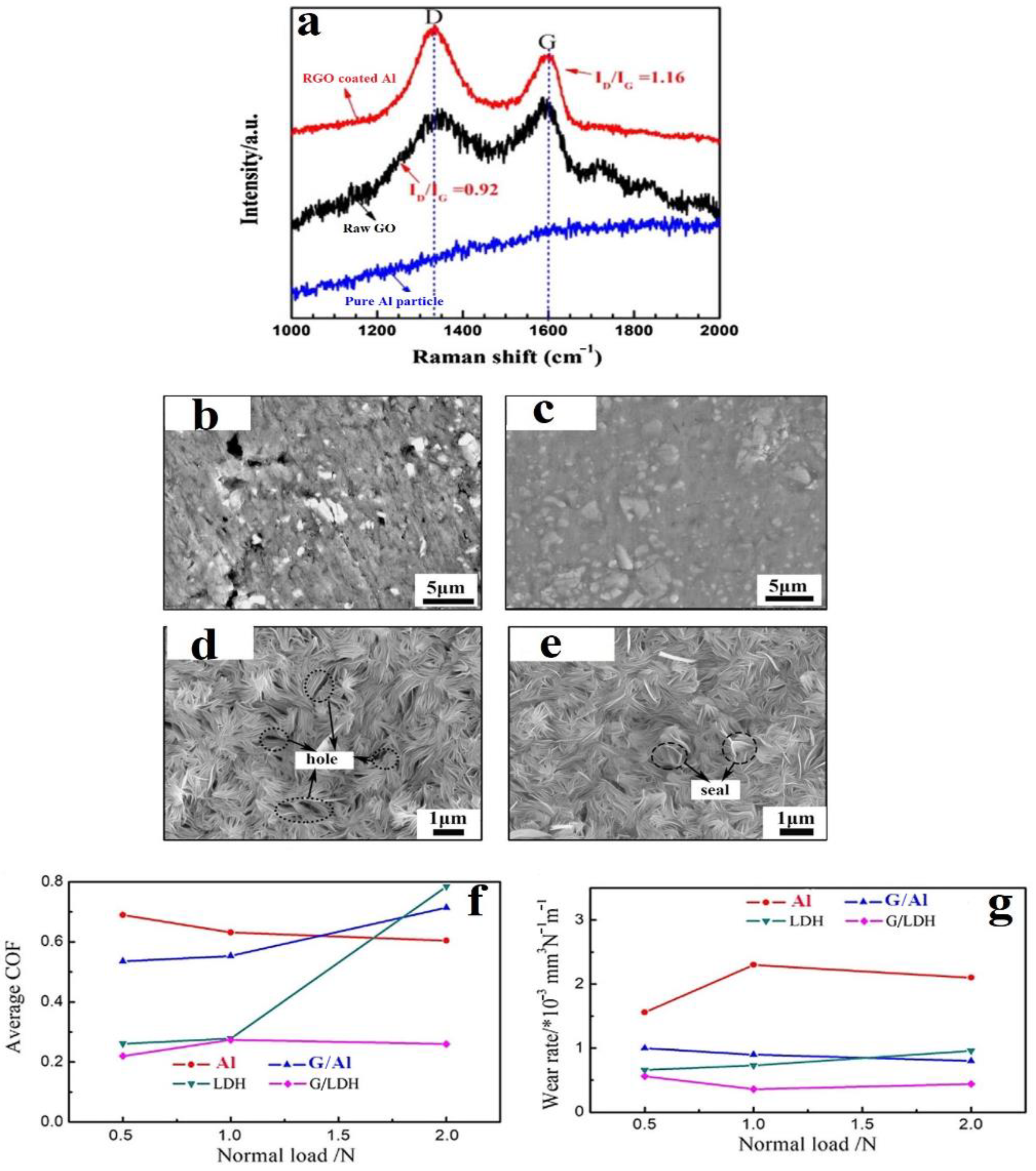
- Yin, S.; Zhang, Z.; Ekoi, E.J.; Wang, J.J.; Dowling, D.P.; Nicolosi, V.; Lupoi, R. Novel cold spray for fabricating graphene-reinforced metal matrix composites. Mater. Lett. 2017, 196, 172–175. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Rose, T.P.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Zhai, W.; Shi, X.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Wang, Y. Grain refinement: A mechanism for graphene nanoplatelets to reduce friction and wear of Ni3Al matrix self-lubricating composites. Wear 2014, 310, 33–40. [Google Scholar] [CrossRef]
- Sun, W.; Tan, A.W.Y.; Bhowmik, A.; Xue, F.; Marinescu, I.; Liu, E. Evaluation of cold sprayed graphene nanoplates–Inconel 718 composite coatings. Surf. Coat. Technol. 2019, 378, 125065. [Google Scholar] [CrossRef]
- Zhao, Z.; Meng, F.; Tang, J.; Liu, H.; Liu, H.; Yang, L.; Wang, J.; Xiong, T. A novel method of fabricating an antibacterial aluminum-matrix composite coating doped graphene/silver-nanoparticle. Mater. Lett. 2019, 245, 211–214. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Nanostructural Characteristics of Vacuum Cold-Sprayed Hydroxyapatite/Graphene-Nanosheet Coatings for Biomedical Applications. J. Therm. Spray Technol. 2014, 23, 1149–1156. [Google Scholar] [CrossRef]
- Samuel, E.; Lee, J.G.; Joshi, B.; Kim, T.G.; Kim, M.W.; Seong, I.W.; Yoon, W.Y.; Yoon, S.S. Supersonic cold spraying of titania nanoparticles on reduced graphene oxide for lithium ion battery anodes. J. Alloy. Compd. 2017, 715, 161–169. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Edmund, S.; Alotaibi, B.S.; El-Hamshary, H.; Yoon, S.S. Reduced graphene oxide supersonically sprayed on wearable fabric and decorated with iron oxide for supercapacitor applications. J. Mater. Sci. Technol. 2021, 82, 47–56. [Google Scholar] [CrossRef]
- Lee, J.G.; Joshi, B.N.; Lee, J.H.; Kim, T.G.; Kim, D.Y.; Al-Deyab, S.S.; Seong, I.W.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Stable High-Capacity Lithium Ion Battery Anodes Produced by Supersonic Spray Deposition of Hematite Nanoparticles and Self-Healing Reduced Graphene Oxide. Electrochim. Acta 2017, 228, 604–610. [Google Scholar] [CrossRef]
- Samuel, E.; Kim, T.G.; Park, C.W.; Joshi, B.; Swihart, M.T.; Yoon, S.S. Supersonically Sprayed Zn2SnO4/SnO2/CNT Nanocomposites for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 14031–14040. [Google Scholar] [CrossRef]
- Joshi, B.; Lee, J.G.; Samuel, E.; Jo, H.S.; Kim, T.G.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Supersonically blown reduced graphene oxide loaded Fe–Fe3C nanofibers for lithium ion battery anodes. J. Alloy. Compd. 2017, 726, 114–120. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Park, C.; Aldalbahi, A.; Rahaman, M.; Yoon, S.S. Supersonically sprayed rGO/ZIF8 on nickel nanocone substrate for highly stable supercapacitor electrodes. Electrochim. Acta 2020, 362, 137154. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, G.; Zhai, W.; Ai, Q.; Feng, J.; Zhang, L.; Si, P.; Ci, L. Aluminum/graphene composites with enhanced heat-dissipation properties by in-situ reduction of graphene oxide on aluminum particles. J. Alloy. Compd. 2018, 748, 854–860. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar]
- Tkachev, S.V.; Buslaeva, E.Y.; Naumkin, A.V.; Kotova, S.L.; Laure, I.V.; Gubin, S.P. Reduced graphene oxide. Inorg. Mater. 2012, 48, 796–802. [Google Scholar] [CrossRef]
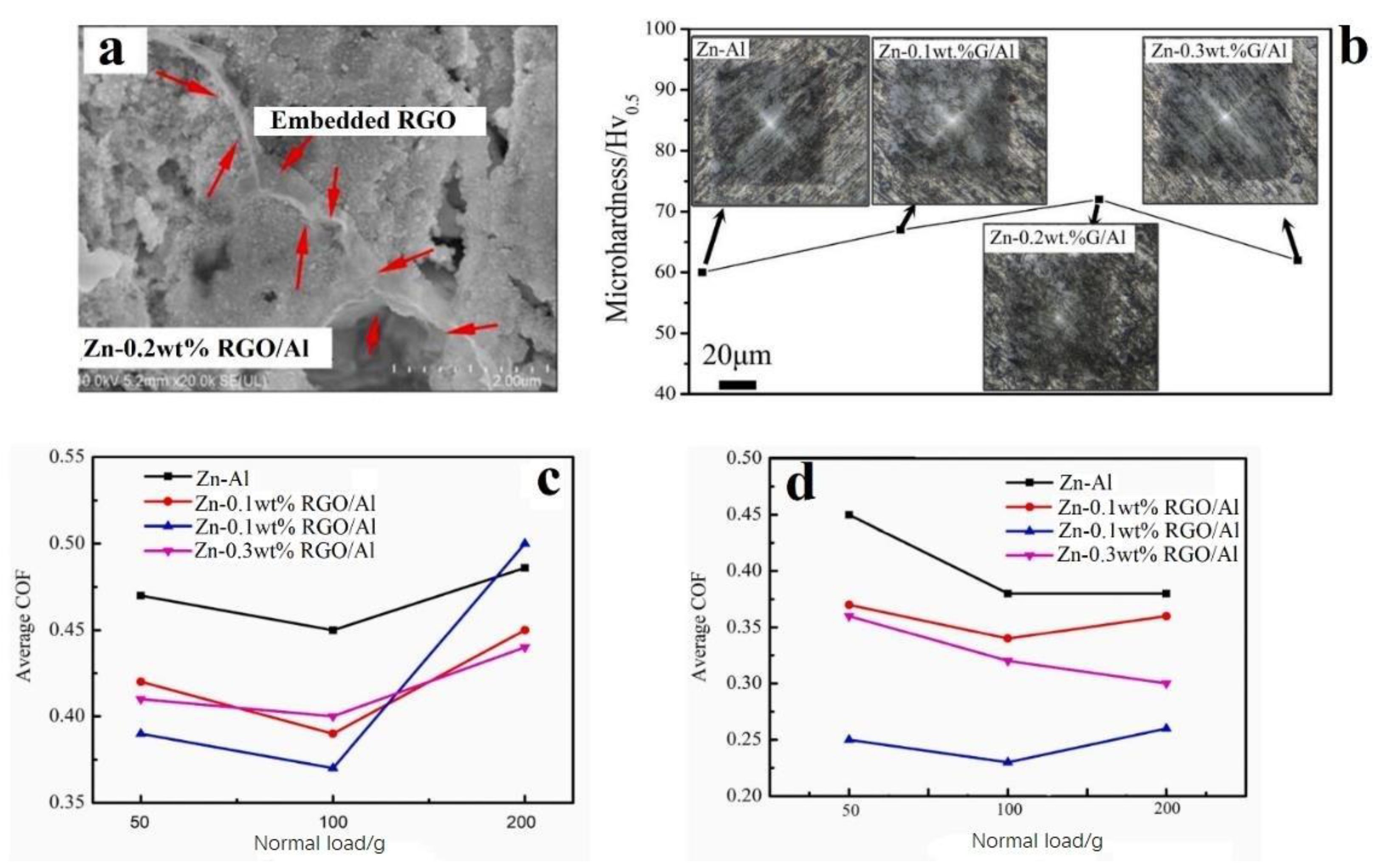
- Wu, H.; Zhang, L.; Liu, C.; Mai, Y.; Zhang, Y.; Jie, X. Deposition of Zn-G/Al composite coating with excellent cathodic protection on low-carbon steel by low-pressure cold spraying. J. Alloy. Compd. 2020, 821, 153483. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wu, H.; Mai, Y.; Liu, C.; Jie, X. High densification and anti-corrosion of graphene-coated aluminum coating deposited on AZ31B magnesium by low-pressure cold spray. Carbon Lett. 2020, 30, 581–584. [Google Scholar] [CrossRef]
- Wu, H.; Shen, G.; Li, R.; Zhang, L.; Jie, X.; Liu, G. Influence of embedded reduced graphene oxide on the corrosion-wear performance of cold sprayed Zn-rGO/Al coating in NaCl solution. Surf. Coat. Technol. 2022, 429, 127856. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Long, S.; Zhang, L.; Jie, X. Tribological behavior of graphene anchored Mg-Al layered double hydroxide film on Mg alloy pre-sprayed Al coating. Appl. Surf. Sci. 2020, 530, 146536. [Google Scholar] [CrossRef]
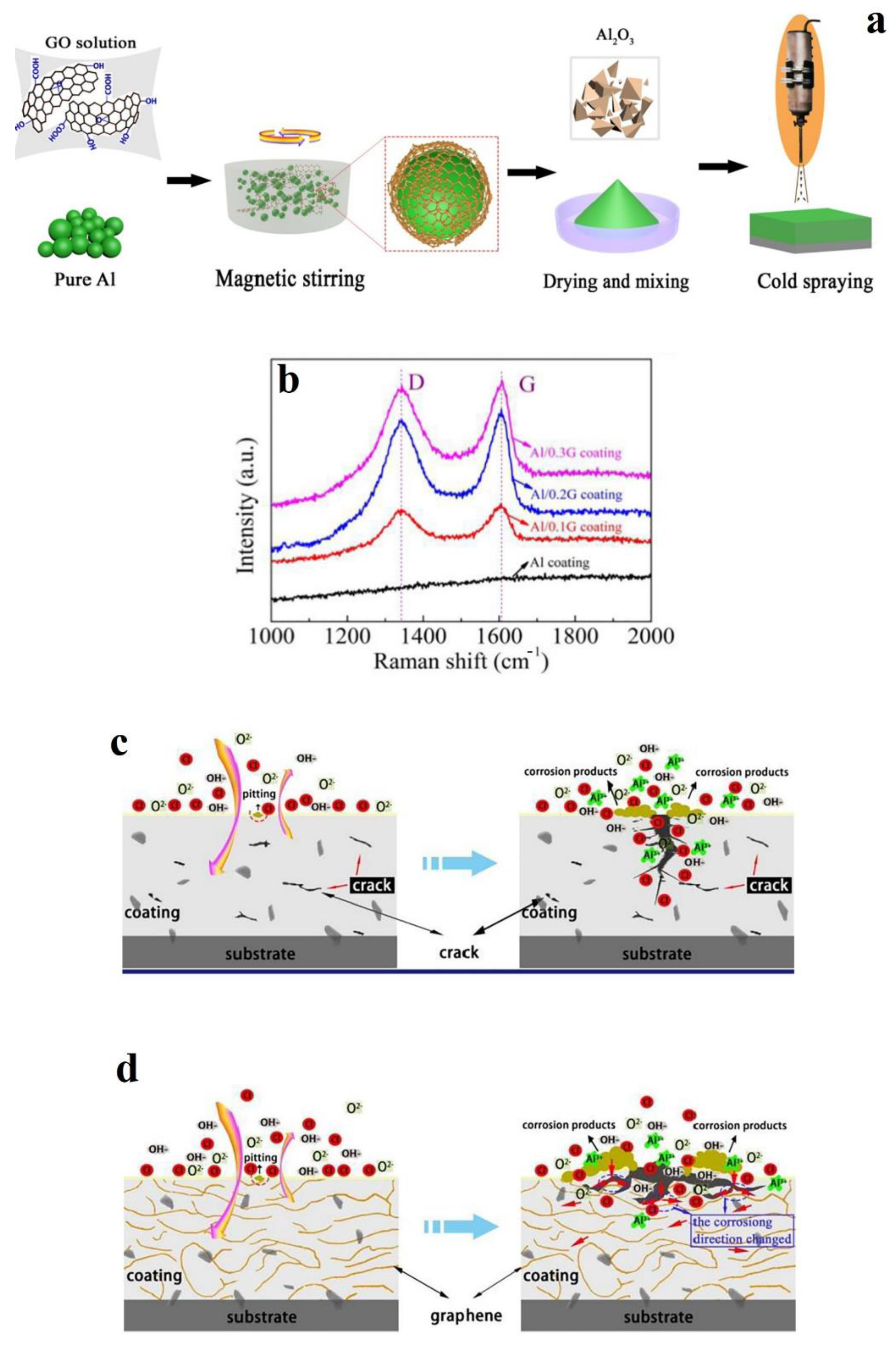
- Wang, X.; Zhang, L.; Zhou, X.; Wu, W.; Jie, X. Corrosion behavior of Al2O3-reinforced graphene encapsulated Al composite coating fabricated by low pressure cold spraying. Surf. Coat. Technol. 2020, 386, 125486. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Zhang, Y.; Long, S.; Jie, X. Corrosion behavior of Mg–Al LDH film in-situ assembled with graphene on Mg alloy pre-sprayed Al layer. J. Alloy. Compd. 2020, 834, 155107. [Google Scholar] [CrossRef]
- Prasad, K.; Nikzad, M.; Sbarski, I. Modeling Permeability in Multi-Phase Polymer Composites: A Critical Review of Semi-Empirical Approaches. Polym. Rev. 2021, 61, 194–237. [Google Scholar] [CrossRef]
- Kim, T.G.; Park, C.W.; Woo, D.Y.; Choi, J.; Yoon, S.S. Efficient heat spreader using supersonically sprayed graphene and silver nanowire. Appl. Therm. Eng. 2020, 165, 114572. [Google Scholar] [CrossRef]
- An, S.; Kim, D.-Y.; Lee, J.-G.; Jo, H.S.; Kim, M.-W.; Al-Deyab, S.S.; Choi, J.; Yoon, S.S. Supersonically sprayed reduced graphene oxide film to enhance critical heat flux in pool boiling. Int. J. Heat Mass Transf. 2016, 98, 124–130. [Google Scholar] [CrossRef]
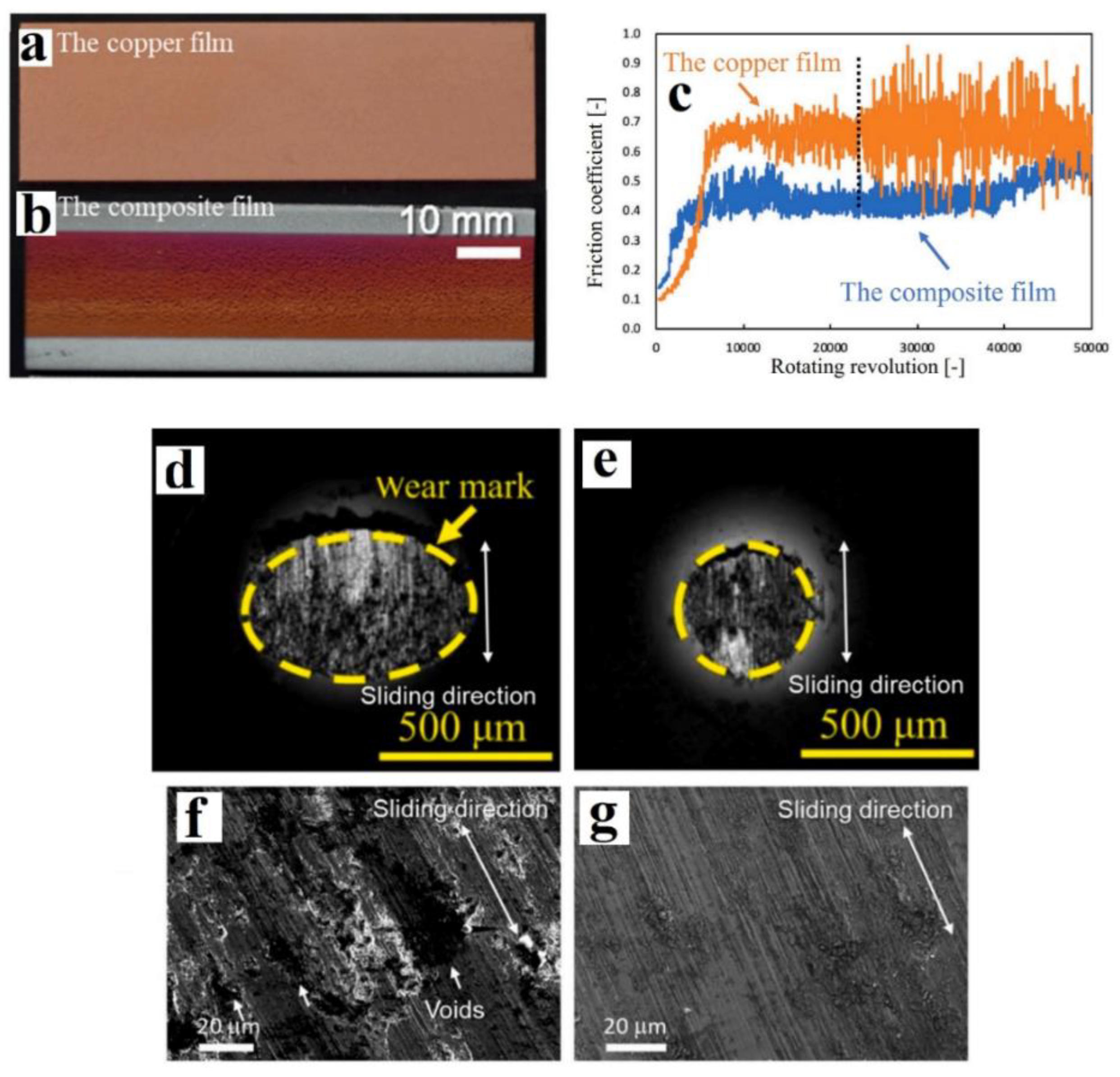
- Choi, J.; Okimura, N.; Yamada, T.; Hirata, Y.; Ohtake, N.; Akasaka, H. Deposition of graphene–copper composite film by cold spray from particles with graphene grown on copper particles. Diam. Relat. Mater. 2021, 116, 108384. [Google Scholar] [CrossRef]
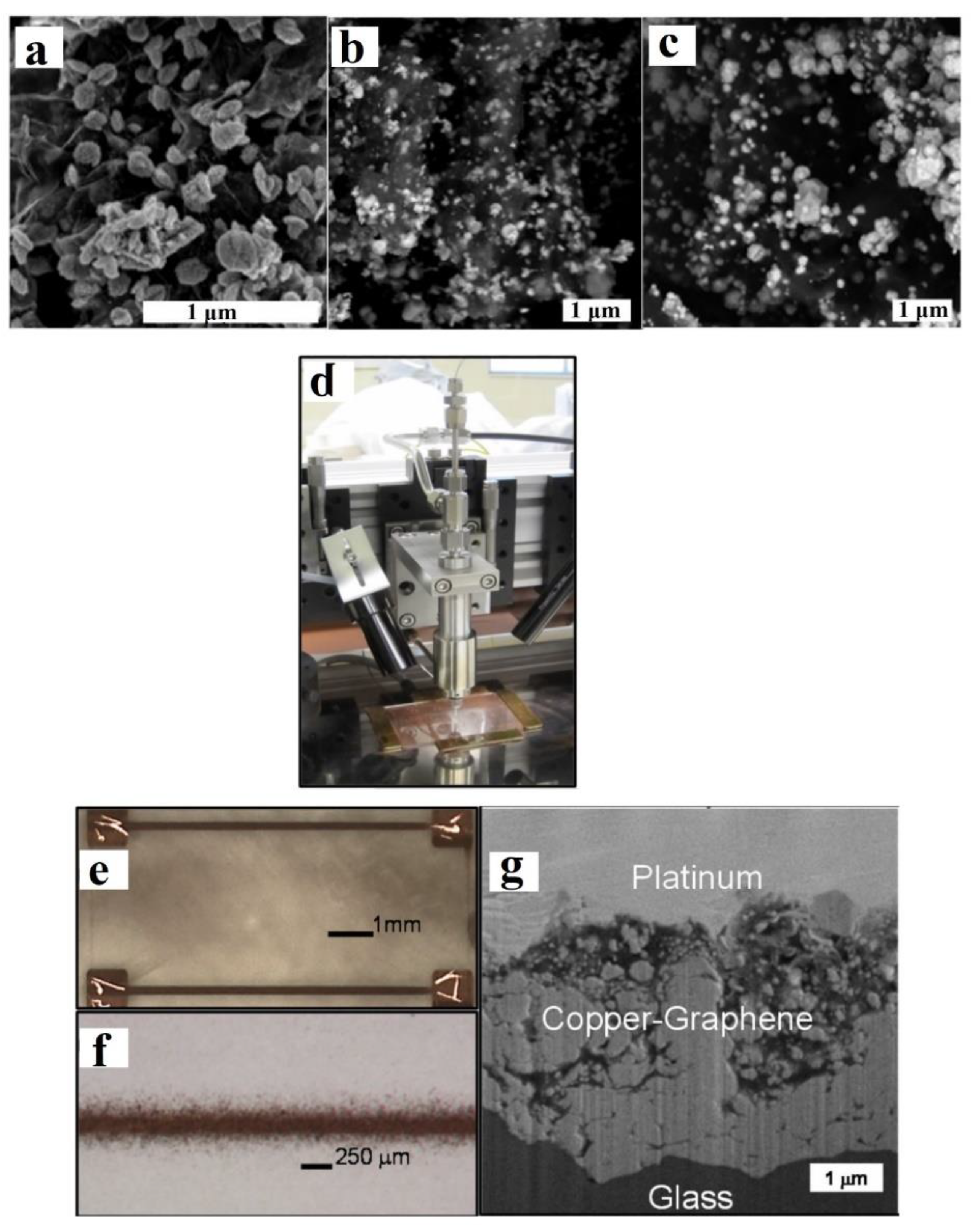
- Dardona, S.; Hoey, J.; She, Y.; Schmidt, W.R. Direct write of copper-graphene composite using micro-cold spray. AIP Adv. 2016, 6, 085013. [Google Scholar] [CrossRef]



| Sample | RS | Rfilm | Rct | Rfilm + Rct |
|---|---|---|---|---|
| Zn/Al | 5.387 | 163.0 | 5470 | 1215.6 |
| Zn/0.1% GNP/Al | 8.461 | 92.29 | 485.1 | 494.32 |
| Zn/0.2% GNP/Al | 2.89 | 1 | 183.7 | 184.7 |
| Zn/0.3% GNP/Al | 4.319 | 1.868 | 538 | 539.868 |
| Sample | Resistance (Ω) | Cross-Sectional Area (m2) | Resistivity (Ω-m) | Bulk Resistivity (Ω-m) |
|---|---|---|---|---|
| Line 1 | 33.1 | 3.28 × 10−10 | 1.09 × 10−6 | 65 |
| Line 2 | 28.8 | 5.65 × 10−10 | 1.63 × 10−6 | 96.9 |
| Line 3 | 51.4 | 4.88 × 10−10 | 2.51 × 10−6 | 149 |
| Line 4 | 39.2 | 4.90 × 10−10 | 1.92 × 10−6 | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, K.; Rahman Rashid, R.A.; Hutasoit, N.; Palanisamy, S.; Hameed, N. Fabrication of Metal/Graphene Composites via Cold Spray Process: State-of-the-Art and the Way Forward. C 2022, 8, 65. https://doi.org/10.3390/c8040065
Prasad K, Rahman Rashid RA, Hutasoit N, Palanisamy S, Hameed N. Fabrication of Metal/Graphene Composites via Cold Spray Process: State-of-the-Art and the Way Forward. C. 2022; 8(4):65. https://doi.org/10.3390/c8040065
Chicago/Turabian StylePrasad, Krishnamurthy, Rizwan Abdul Rahman Rashid, Novana Hutasoit, Suresh Palanisamy, and Nishar Hameed. 2022. "Fabrication of Metal/Graphene Composites via Cold Spray Process: State-of-the-Art and the Way Forward" C 8, no. 4: 65. https://doi.org/10.3390/c8040065
APA StylePrasad, K., Rahman Rashid, R. A., Hutasoit, N., Palanisamy, S., & Hameed, N. (2022). Fabrication of Metal/Graphene Composites via Cold Spray Process: State-of-the-Art and the Way Forward. C, 8(4), 65. https://doi.org/10.3390/c8040065








