2013–2014 Survey of Chars Using Raman Spectroscopy
Abstract
:1. Introduction
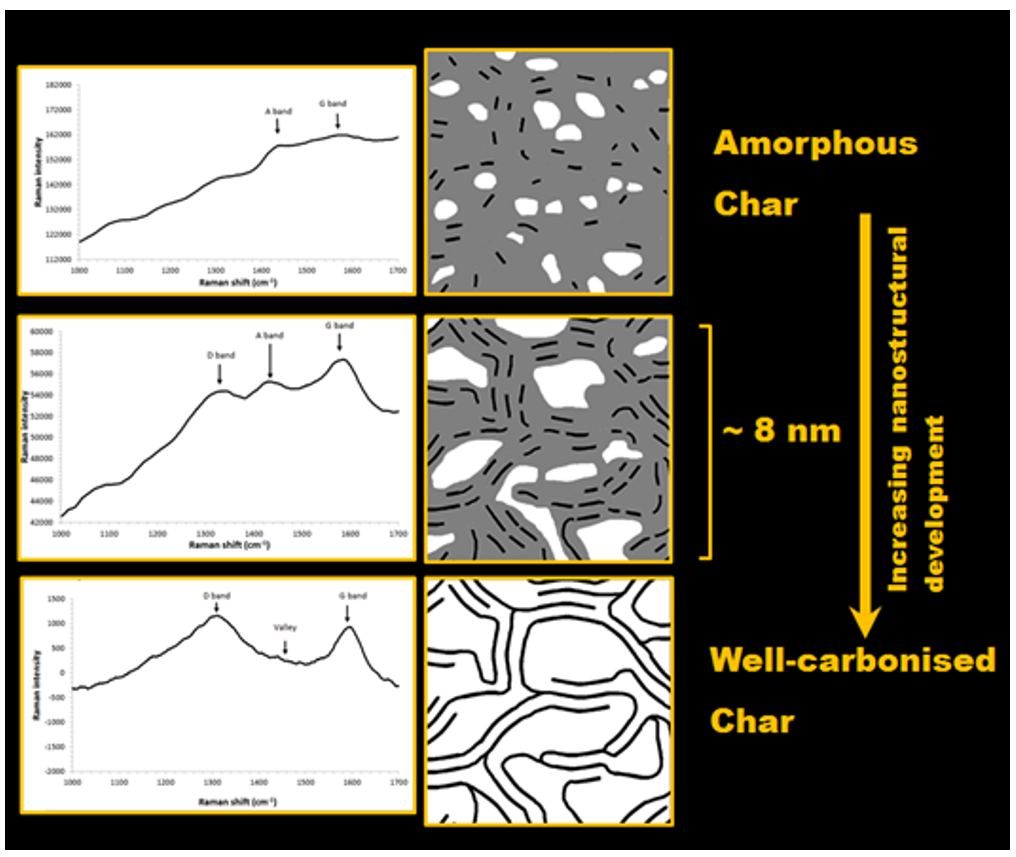
- Decreases in the Valley/G band height ratio (interpreted as the removal of amorphous carbon).
- Decreases in the A band/G band height ratio (interpreted as the removal of amorphous carbon).
- Decreases in the slope/G band height ratio (interpreted as the removal of hydrogen-rich amorphous carbon which causes this intense photoluminescence slope).
- Increases in the D band/G band height ratio (interpreted as the lateral extension of graphene-like polyaromatic domains as they organise and grow towards being a few nanometres across).
- Increases in the apparent position of the G band from around 1500 cm−1 to 1600 cm−1 (interpreted as organising of the carbon into larger clusters/domains of aromatic carbon, changes in level of strain on carbon–carbon bonds, and/or the effect of overlapping bands).
2. Materials and Methods
3. Results
4. Discussion
4.1. Raman Analysis
4.2. Recently-Produced Biochar Samples
4.3. Non-Biochar Samples
4.4. High-Ash Biochars
4.5. Potential Future Research
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Jehlička, J.; Culka, A.; Bersani, D.; Vandenabeele, P. Comparison of seven portable Raman spectrometers: Beryl as a case study. J. Raman Spectrosc. 2017, 48, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinezalonso, A.; Tascon, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. Carbonisation of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy. Carbon 2013, 59, 383–405. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro-Soares, J.; Cançado, L.G.; Falcão, N.P.S.; Martins Ferreira, E.H.; Achete, C.A.; Jorio, A. The use of Raman spectroscopy to characterize the carbon materials found in Amazonian anthrosoils. J. Raman Spectrosc. 2012, 44, 283–289. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Yamauchi, S.; Kurimoto, Y. Raman spectroscopic study on pyrolyzed wood and bark of Japanese cedar: Temperature dependence of Raman parameters. J. Wood Sci. 2003, 49, 235–240. [Google Scholar] [CrossRef]
- Rhim, Y.R.; Zhang, D.J.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. Understanding and visualising the nanostructural development of chars during carbonisation. In Proceedings of the 3rd Asia Pacific Biochar Conference, Chuncheon, Korea, 19–23 October 2016. [Google Scholar]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1951, 209, 196–218. [Google Scholar]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M. Predicting C aromaticity of biochars based on their elemental composition. Org. Geochem. 2013, 62, 1–6. [Google Scholar] [CrossRef]
- Zickler, G.A.; Schoberl, T.; Paris, O. Mechanical properties of pyrolysed wood: A nanoindentation study. Philos. Mag. 2006, 86, 1373–1386. [Google Scholar] [CrossRef]
- Mochidzuki, K.; Soutric, F.; Tadokoro, K.; Antal, M.J., Jr.; Toth, M.; Zelei, B.; Varhegyi, G. Electrical and physical properties of carbonized charcoals. Ind. Eng. Chem. Res. 2003, 42, 5140–5151. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.P.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2013, 5, 122–131. [Google Scholar] [CrossRef] [Green Version]
- McDonald-Wharry, J.S.; Manley-Harris, M.; Pickering, K.L. Reviewing, combining, and updating the models for the nanostructure of non-graphitizing carbons produced from oxygen-containing precursors. Energy Fuels 2016, 30, 7811–7826. [Google Scholar] [CrossRef]
- Bachmann, H.J.; Bucheli, T.D.; Dieguez-Alonso, A.; Fabbri, D.; Knicker, H.; Schmidt, H.-P.; Ulbricht, A.; Becker, R.; Buscaroli, A.; Buerge, D.; et al. Toward the standardization of biochar analysis: The COST action TD1107 interlaboratory comparison. J. Agric. Food Chem. 2016, 64, 513–527. [Google Scholar] [CrossRef]
- Berryman, K.; Villamor, P.; Nairn, I.; van Dissen, R.; Begg, J.; Lee, J. Late Pleistocene surface rupture history of the Paeroa Fault, Taupo Rift, New Zealand. N. Z. J. Geol. Geophys. 2008, 51, 135–158. [Google Scholar] [CrossRef]
- Ascough, P.L.; Brock, F.; Collinson, M.E.; Painter, J.D.; Lane, D.W.; Bird, M.I. Chemical characteristics of macroscopic pyrogenic carbon following millennial-scale environmental exposure. Front. Environ. Sci. 2020, 7, 203. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Veres, M.; Tóth, S.; Koós, M. New aspects of Raman scattering in carbon-based amorphous materials. Diam. Relat. Mat. 2008, 17, 1692–1696. [Google Scholar] [CrossRef]
- Wang, T. Development of Methodologies for the Characterisation of Biochars Produced from Human and Animal Waste. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2013. [Google Scholar]
- Bridges, R. Design and Characterisation of an “Open Source” Pyrolyser for Biochar Production. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2013. [Google Scholar]
- Wang, L.; Skreiberg, Ø.; Gronli, M.; Specht, G.P.; Antal, M.J. Is elevated pressure required to achieve a high fixed-carbon yield of charcoal from biomass? Part 2: The importance of particle size. Energy Fuels 2013, 27, 2146–2156. [Google Scholar] [CrossRef]
- Wang, L.; Trninic, M.; Skreiberg, Ø.; Gronli, M.; Considine, R.; Antal, M.J. Is elevated pressure required to achieve a high fixed-carbon yield of charcoal from biomass? Part 1: Round-robin results for three different corncob materials. Energy Fuels 2011, 25, 3251–3265. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Bachmann, H.J.; Bucheli, T.; Fabbri, D.; Knicker, H.; Ulbricht, A. Analytical Standards for Biochar Characterization and Certification: Lessons from the EU-COST Ring Trial. 2014. [Google Scholar]
- McDonald-Wharry, J.; Manley-Harris, M.; Pickering, K. A comparison of the charring and carbonisation of oxygen-rich precursors with the thermal reduction of graphene oxide. Philos. Mag. 2015, 95, 4054–4077. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Beyssac, O.; Brunet, F.; Petitet, J.-P.; Goffé, B.; Rouzaud, J.-N.l. Experimental study of the microtextural and structural transformations of carbonaceous materials under pressure and temperature. Eur. J. Min. 2004, 15, 937–951. [Google Scholar] [CrossRef]
- Scott, A.C.; Damblon, F. Charcoal: Taphonomy and significance in geology, botany and archaeology. Paleogeogr. Paleoclimatol. Paleoecol. 2010, 291, 1–10. [Google Scholar] [CrossRef]
- Sander, P.M.; Gee, C.T. Fossil charcoal: Techniques and applications. Rev. Palaeobot. Palynol. 1990, 63, 269–279. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quat. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- Alon, D.; Mintz, G.; Cohen, I.; Weiner, S.; Boaretto, E. The use of Raman spectroscopy to monitor the removal of humic substances from charcoal; quality control for 14C dating of charcoal. Radiocarbon 2002, 44, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ascough, P.L.; Bird, M.I.; Francis, S.M.; Thornton, B.; Midwood, A.J.; Scott, A.C.; Apperley, D. Variability in oxidative degradation of charcoal: Influence of production conditions and environmental exposure. Geochim. Cosmochim. Acta 2011, 75, 2361–2378. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.D.; Johnson, R.L.; Lehmann, J.; Olk, D.C.; Neves, E.G.; Thompson, M.L.; Schmidt-Rohr, K. Abundant and Stable Char Residues in Soils: Implications for Soil Fertility and Carbon Sequestration. Environ. Sci. Technol. 2012, 46, 9571–9576. [Google Scholar] [CrossRef] [PubMed]
- Haumaier, L.; Zech, W. Black carbon—Possible source of highly aromatic components of soil humic acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Inoue, J.; Yoshie, A.; Tanaka, T.; Onji, T.; Inoue, Y. Disappearance and alteration process of charcoal fragments in cumulative soils studied using Raman spectroscopy. Geoderma 2017, 285, 164–172. [Google Scholar] [CrossRef] [Green Version]
- McDonald-Wharry, J.; Ripberger, G.; Manley-Harris, M.; Pickering, K. Studying carbonisation with Raman spectroscopy. In Proceedings of the New Zealand Biochar Workshop, Palmerston North, New Zealand, 4–5 July 2013. [Google Scholar]

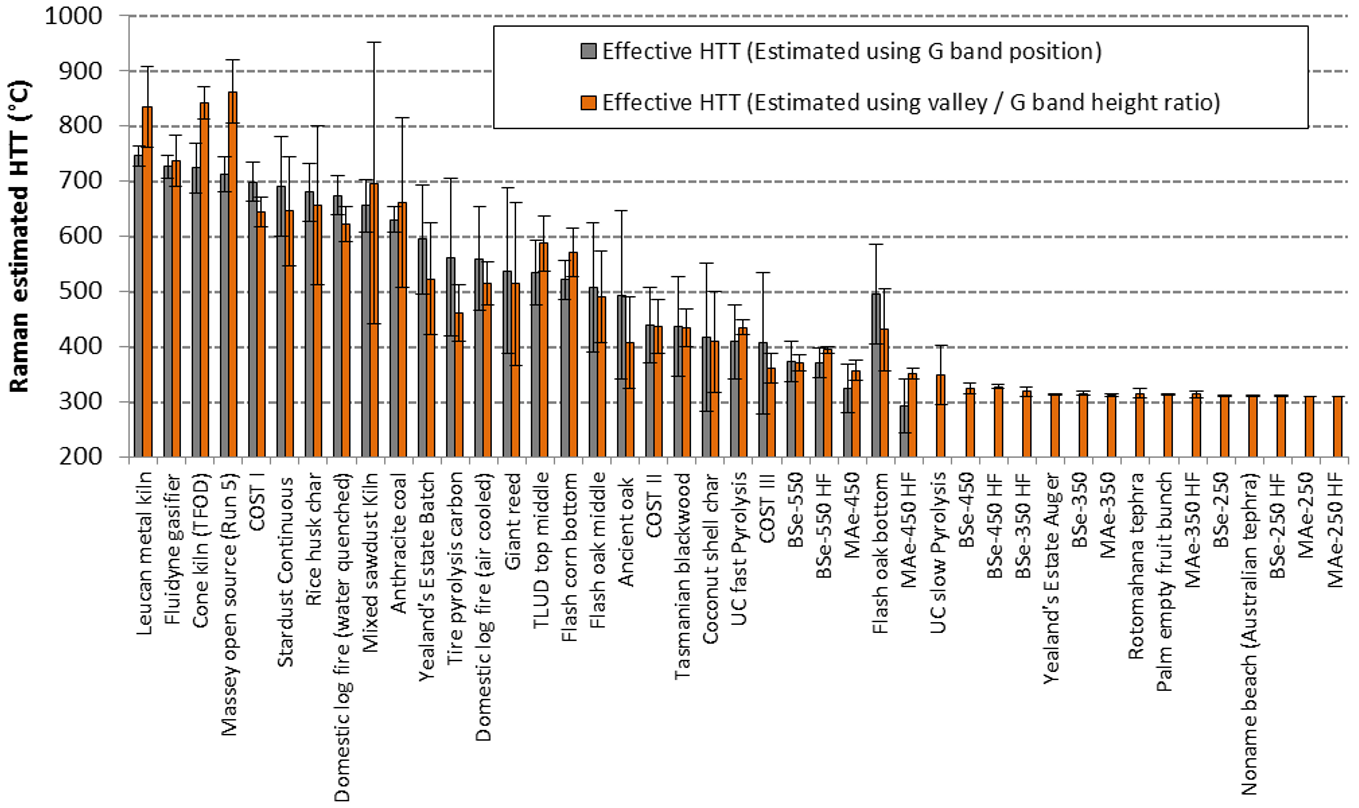

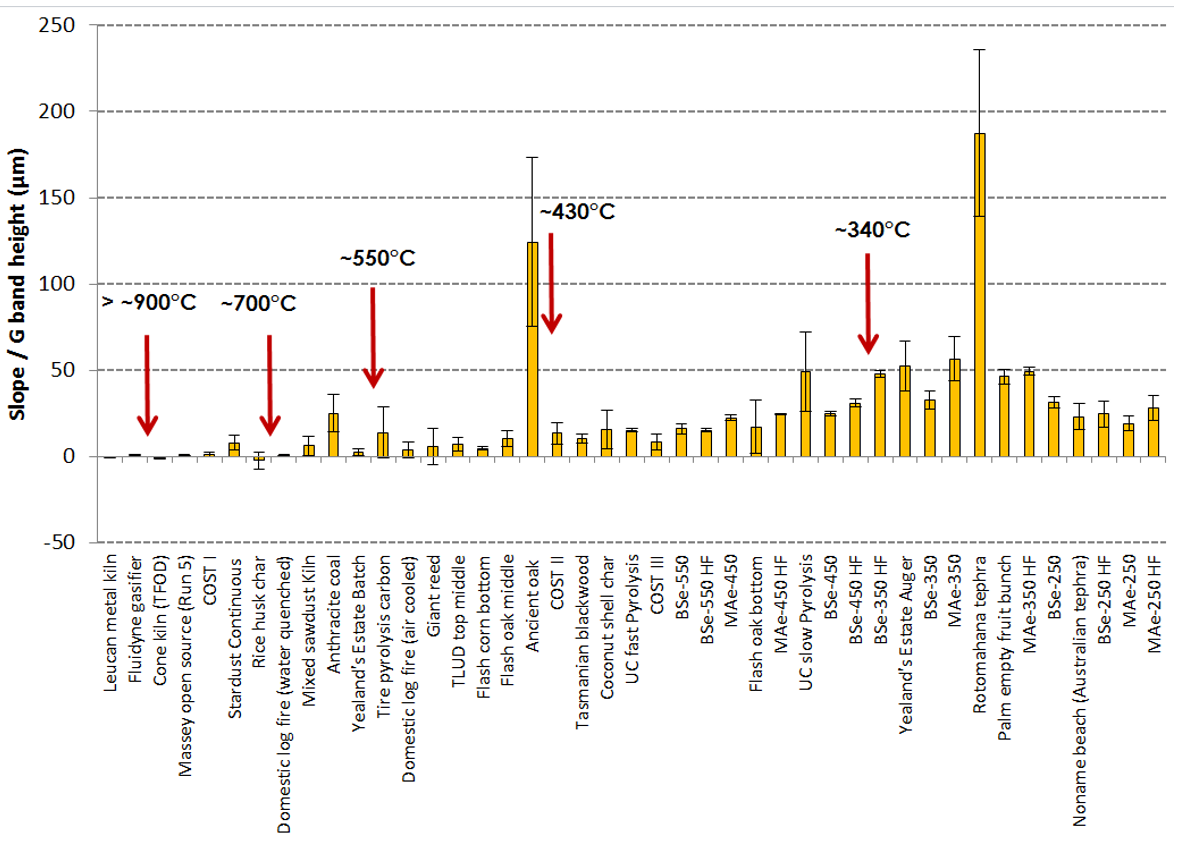
| Sample Short Name | Precursor and Preparation Details | Contributors and References to Other Research Involving These Samples |
|---|---|---|
| BSe-250 | Precursor: Biosolids and eucalyptus (1:1 by dry weight) Production: Slow pyrolysis in lab scale gas-fired drum Notes: Number indicates target processing temperature in °C | Tao Wang, New Zealand Biochar Research Centre, Massey University [16,26] |
| BSe-350 | ||
| BSe-450 | ||
| BSe-550 | ||
| MAe-250 | Precursor: Cattle manure and eucalyptus (1:1 by dry weight) Production: Slow pyrolysis in lab scale gas-fired drum Notes: Number indicates target processing temperature in °C | |
| MAe-350 | ||
| MAe-450 | ||
| BSe-250 HF | Same as the above samples, except these sub-samples labelled HF had been treated after pyrolysis with 10% Hydrofluoric acid 4 times each to remove approximately 70% of the ash content | |
| BSe-350 HF | ||
| BSe-450 HF | ||
| BSe-550 HF | ||
| MAe-250 HF | ||
| MAe-350 HF | ||
| MAe-450 HF | ||
| Massey open source (Run 5) | Precursor: Pinus Radiata woodchips Production: Open-source batch pyrolyser, HTT ≈ 705 °C | Rhonda Bridges, Jim Jones, Massey University [27] |
| UC slow Pyrolysis | Precursor: Pinus Radiata sawdust Production: Slow pyrolysis | Tansy Wigley, Shusheng Pang, Alex Yip, University of Canterbury |
| UC fast Pyrolysis | Precursor: Pinus Radiata sawdust Production: Fast pyrolysis | |
| Anthracite coal | Gronigen coal | Fiona Petchey, University of Waikato Radiocarbon Dating Laboratory The ‘Noname beach’ sample has featured in this recent study [23] |
| Rotomahana tephra | Char recovered from tephra, water washed | |
| Noname beach (Australian tephra) | Wood recovered from approximately 92-thousand-year-old tephra, HCl washed | |
| Ancient oak | Oak from N11 Rathnew–Arklow Archaeological site in Ireland dated to around 3700 years old | |
| Leucan metal kiln | Precursor: Leucan Insularum wood Production: Metal kiln | Frank Cushing and Marjorie Falanrum, Yap Institute of Natural Sciences |
| Fluidyne gasifier | Precursor: Pinus Radiata wood Production: Fluidyne Down Draught Pioneer engine gasifier. Process temperatures: 1200–1500 °C | Doug Williams |
| Domestic log fire (water quenched) | Precursor: Acacia wood Production: Log placed in domestic fire for 20 min and removed as red/orange embers which were either quenched in cold water or left to cool in air | John McDonald-Wharry |
| Domestic log fire (air cooled) | ||
| Yealand’s Estate Batch | Precursor: Grape prunings Production: Batch | Aaron Black, Yealand’s Estate |
| Yealand’s Estate Auger | Precursor: Grape prunings Production: Auger | |
| Giant reed | Precursor: Giant Reed (Arundo Donax) Production: Batch, double-drum retort | Jim Hunt |
| Tasmanian blackwood | Precursor: Tasmanian blackwood (Acacia Melanoxylon) Production: Batch, double-drum retort | |
| Cone kiln (TFOD) | Precursor: Douglas fir mill ends Production: Cone kiln, top-fed open draft device (TFOD) | Kelpie Wilson |
| Tyre pyrolysis carbon | Precursor: End-of-life tires Production: Destructive distillation | Trevor Bayley, Green Distillation Technologies Corporation Ltd. |
| Flash oak bottom | Precursor: Cowboy oak wood sawdust Production: Flash carbonization | Michael J Antal Jr. Hawaii Natural Energy Institute, University of Hawaii at Manoa [28,29] |
| Flash oak middle | Precursor: Cowboy oak wood sawdust Production: Flash carbonization | |
| Flash corn bottom | Precursor: Waimanalo corn cob Production: Flash carbonization | |
| Rice husk char | Precursor: Rick husk Production: Double cyclone furnace (direct heating/combustion system; 2000 kW/hr; rice husk fuel for rice drying in rice mill) | Trevor Richards, Biochar Systems Limited. Kilang Beras Saudara Ban Eng Hin SDn. Bhd. |
| Coconut shell char | Precursor: Coconut shell Production: Typical raw product for the activated carbon market. | Trevor Richards, Biochar Systems Limited. Various sources |
| Mixed sawdust Kiln | Precursor: Mixed forest sawdust Production: Typical ‘Japanese-type’ kilns operated at 700–800 °C | |
| Palm empty fruit bunch | Precursor: Empty fruit bunch from oil palm Production: Nasmech | |
| COST I | Precursor: Mixed wood shavings Production: Pyreg 500-III pyrolysis unit (620 °C) | EU-COST Action on Biochar WG1, [21,30] |
| COST II | Precursor: Paper sludge and wheat husks Production: Pyreg 500-III pyrolysis unit (500 °C) | |
| COST III | Precursor: Sewage sludge Production: Pyreg 500-III pyrolysis unit (600 °C) | |
| TLUD top middle | Precursor: Pine woodchips Production: Top-lit updraft gasifier (TLUD) Sample taken from middle of the top of the char bed. | Earl Mardle |
| Stardust Continuous | Precursor: Pine woodchips Production: Continuous auger pyrolyser | Simon Day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonald-Wharry, J. 2013–2014 Survey of Chars Using Raman Spectroscopy. C 2021, 7, 63. https://doi.org/10.3390/c7030063
McDonald-Wharry J. 2013–2014 Survey of Chars Using Raman Spectroscopy. C. 2021; 7(3):63. https://doi.org/10.3390/c7030063
Chicago/Turabian StyleMcDonald-Wharry, John. 2021. "2013–2014 Survey of Chars Using Raman Spectroscopy" C 7, no. 3: 63. https://doi.org/10.3390/c7030063
APA StyleMcDonald-Wharry, J. (2021). 2013–2014 Survey of Chars Using Raman Spectroscopy. C, 7(3), 63. https://doi.org/10.3390/c7030063





