Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Bimetallic Catalyst Synthesis
2.2. Catalyst Characterization
2.3. Nitrate Catalytic Reduction Experiments
3. Results and Discussion
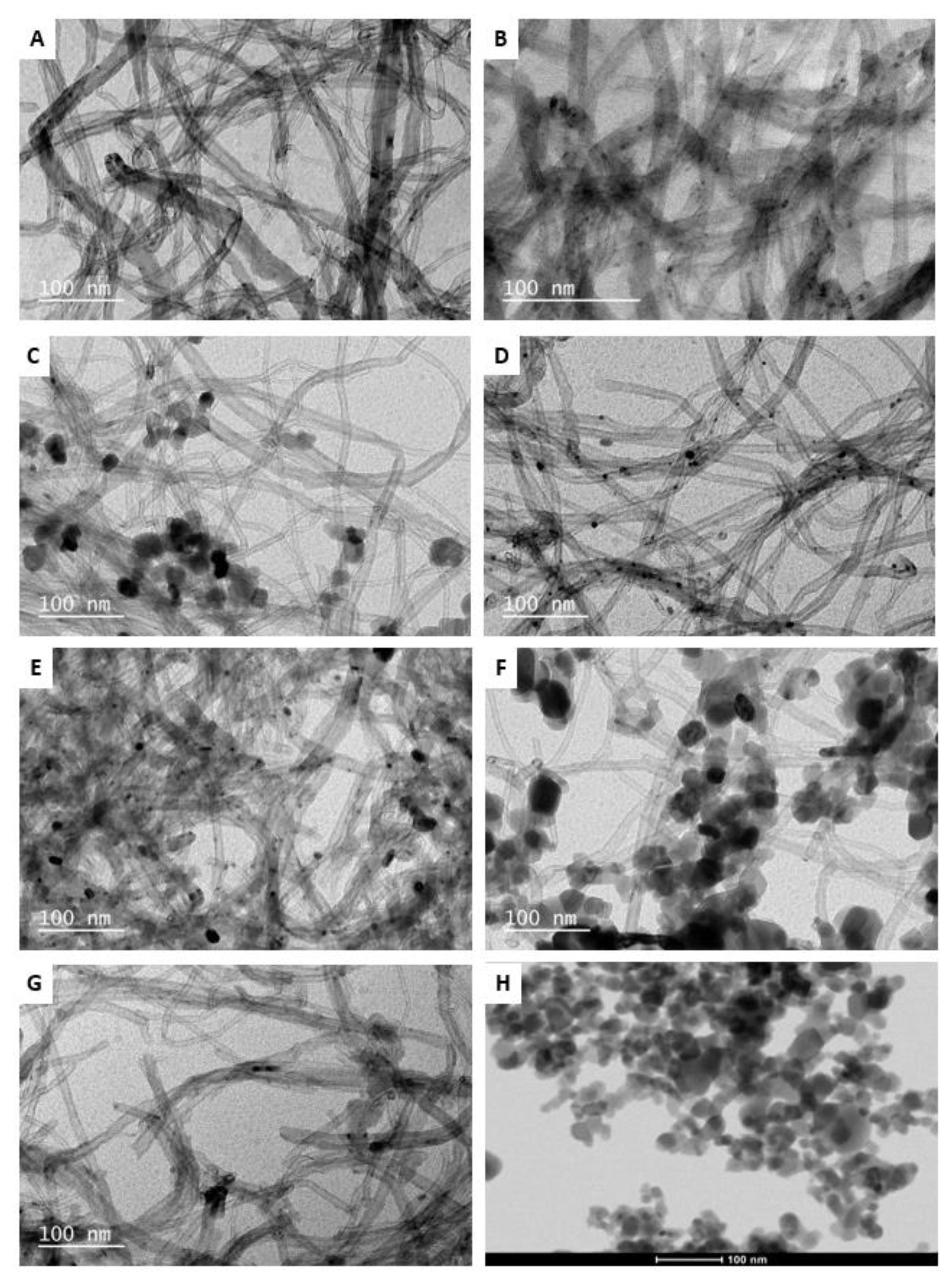
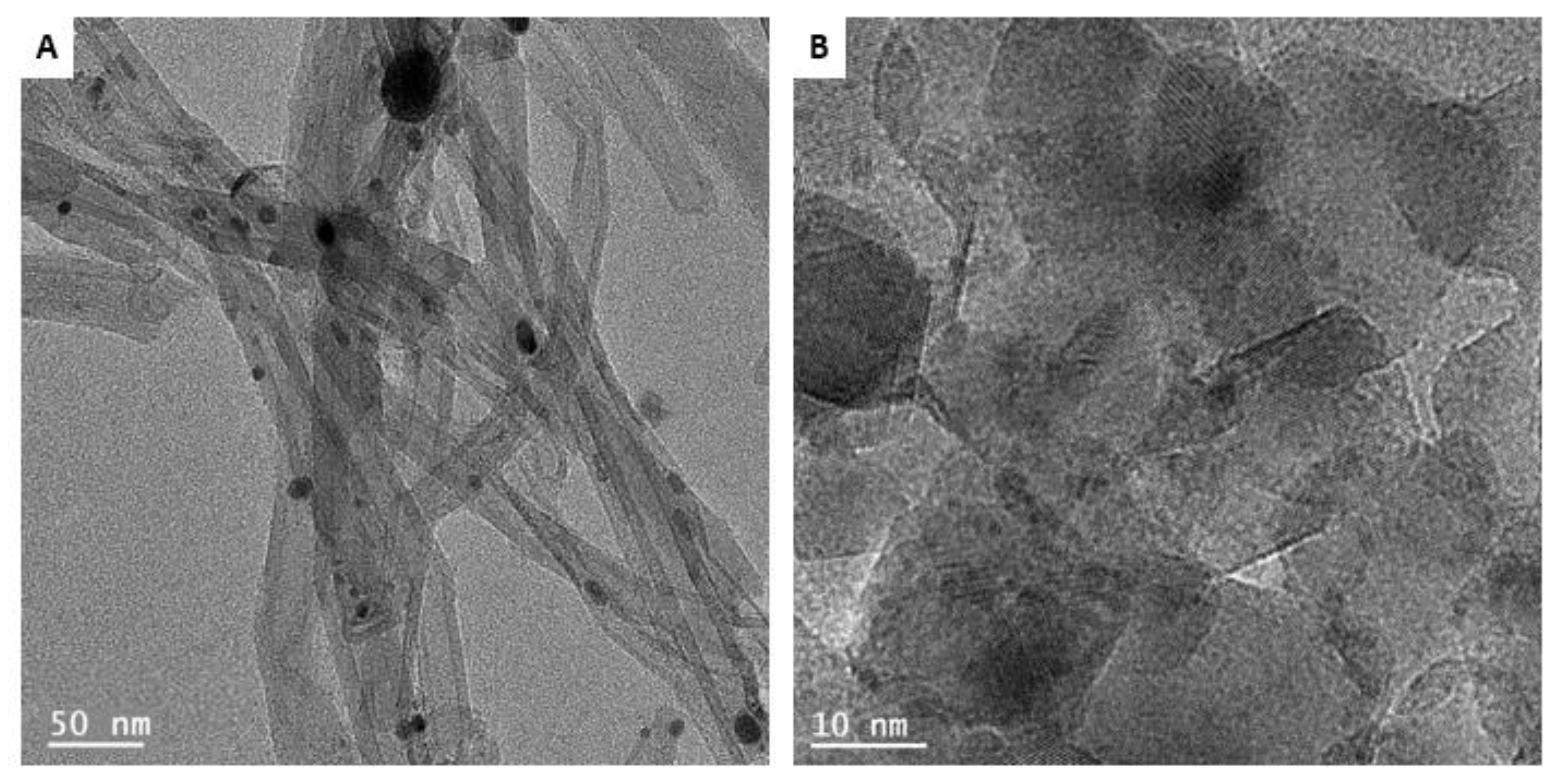
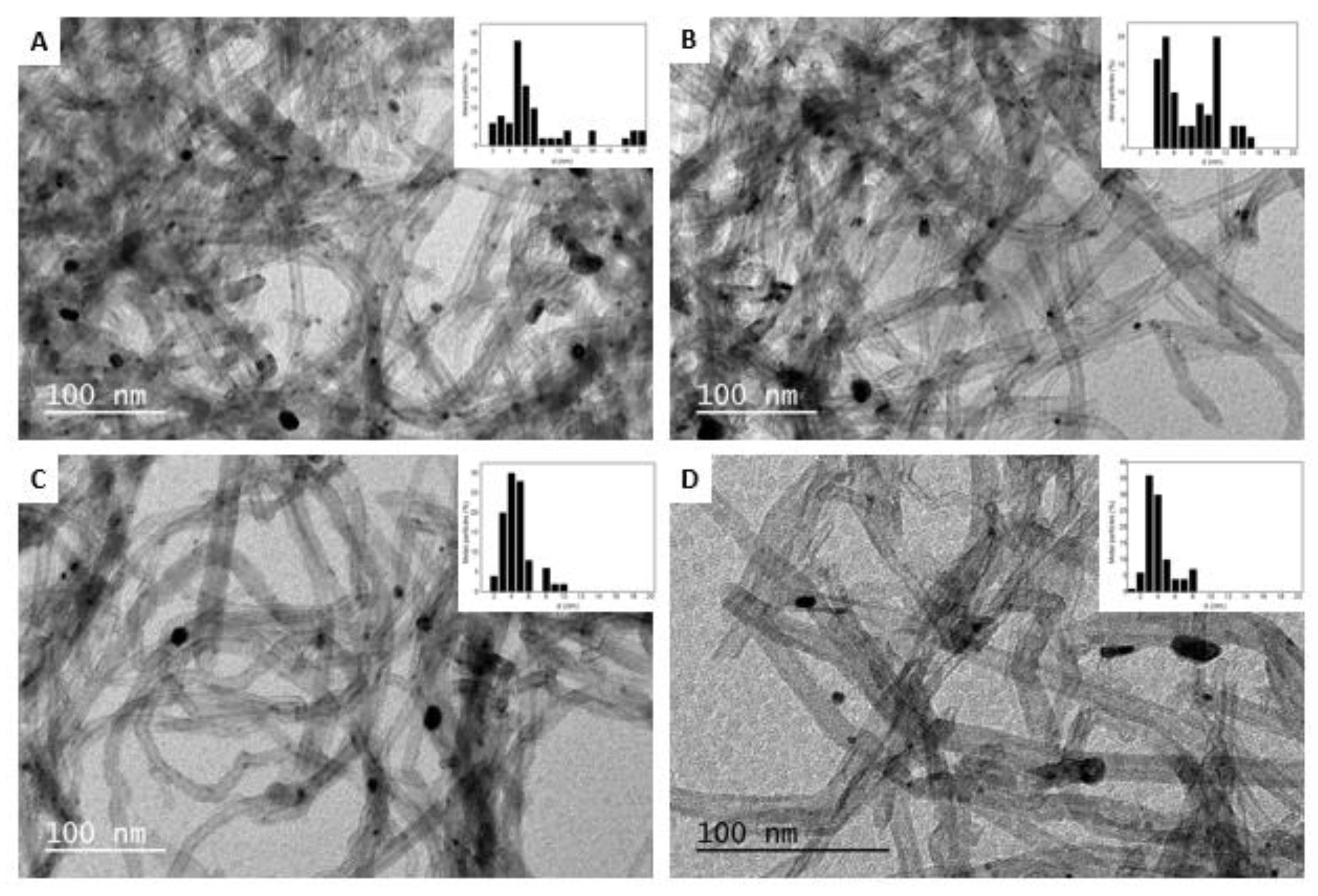
3.1. Catalyst Characterization
3.2. Catalytic Reduction Experiments
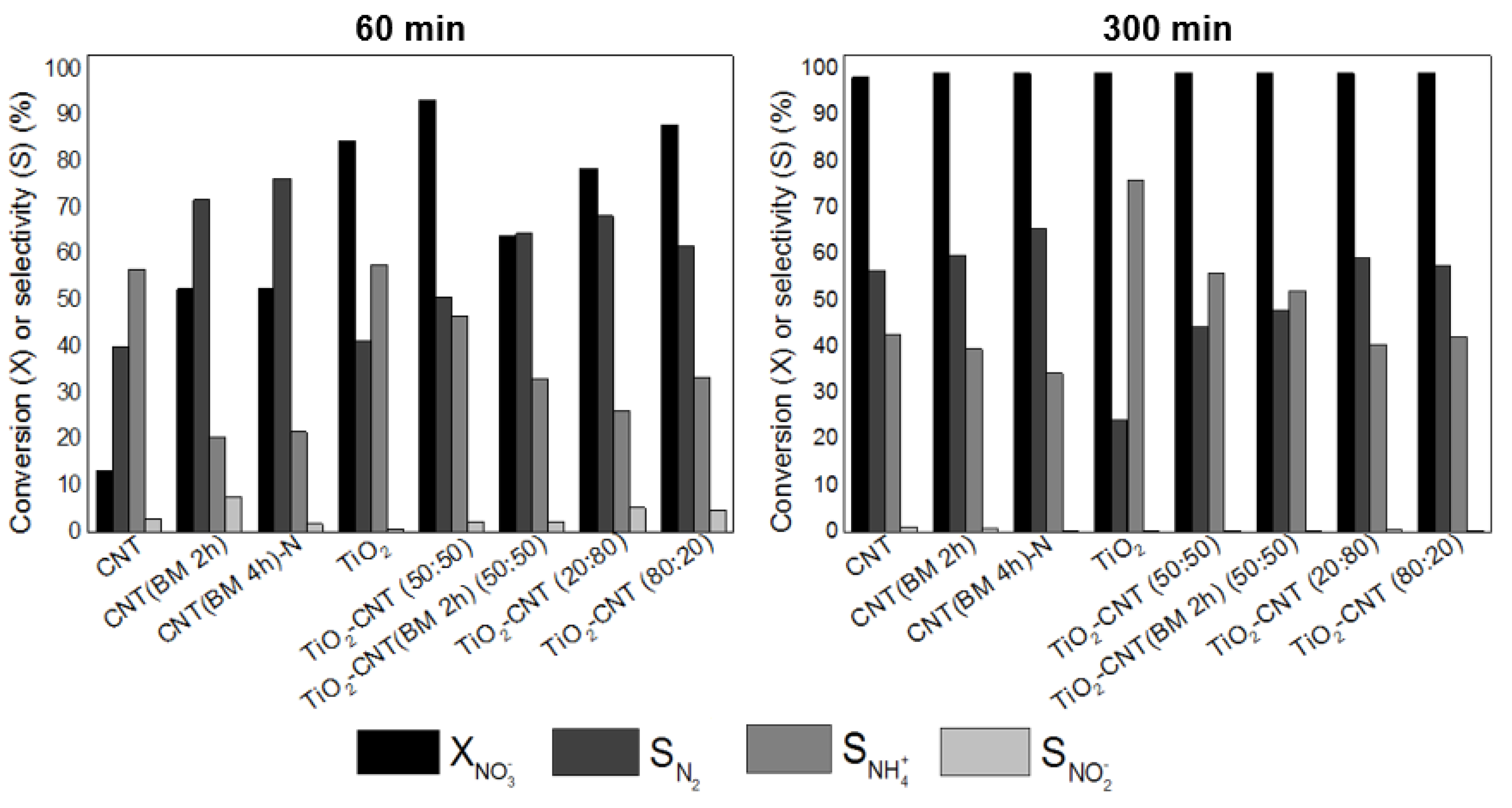
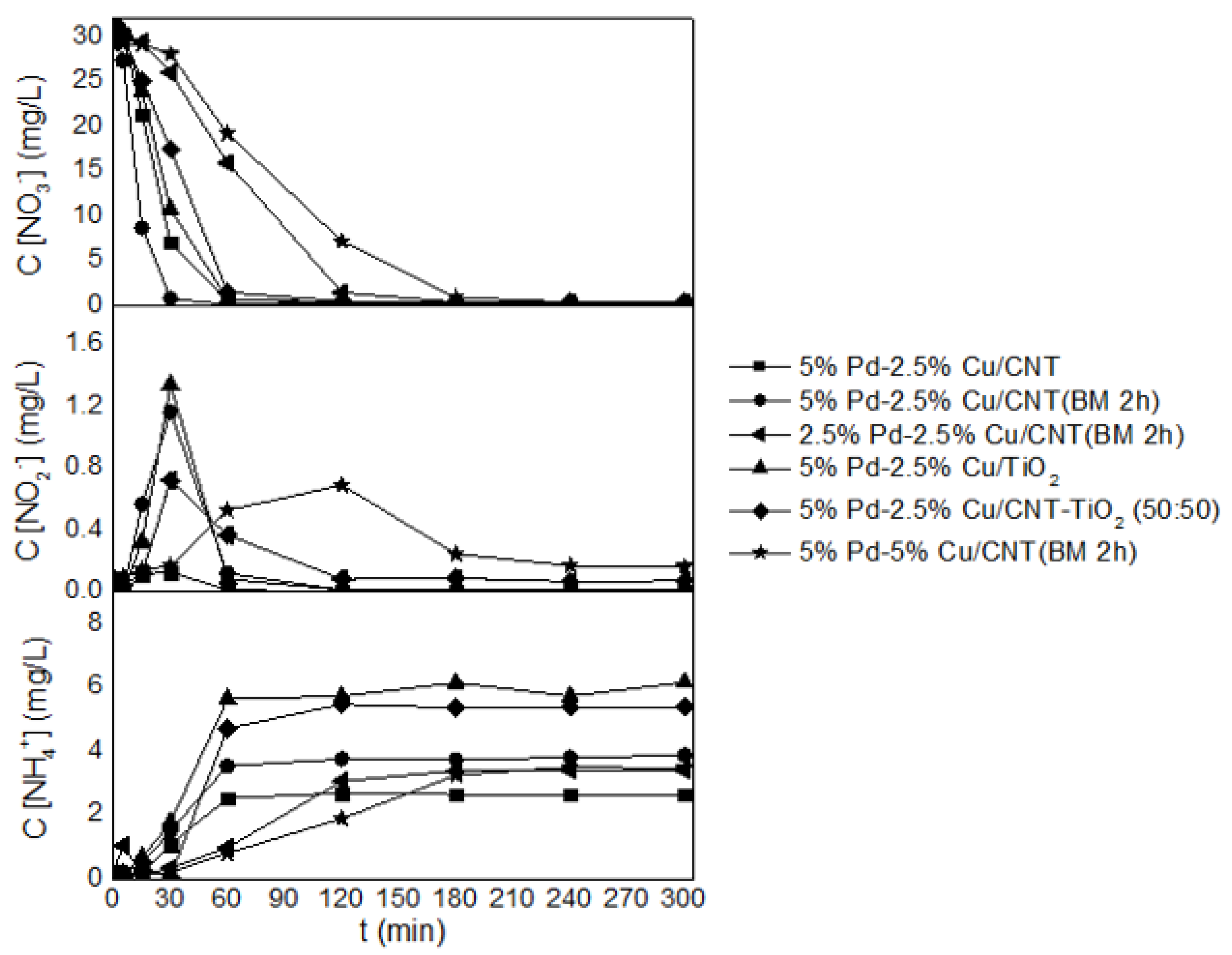
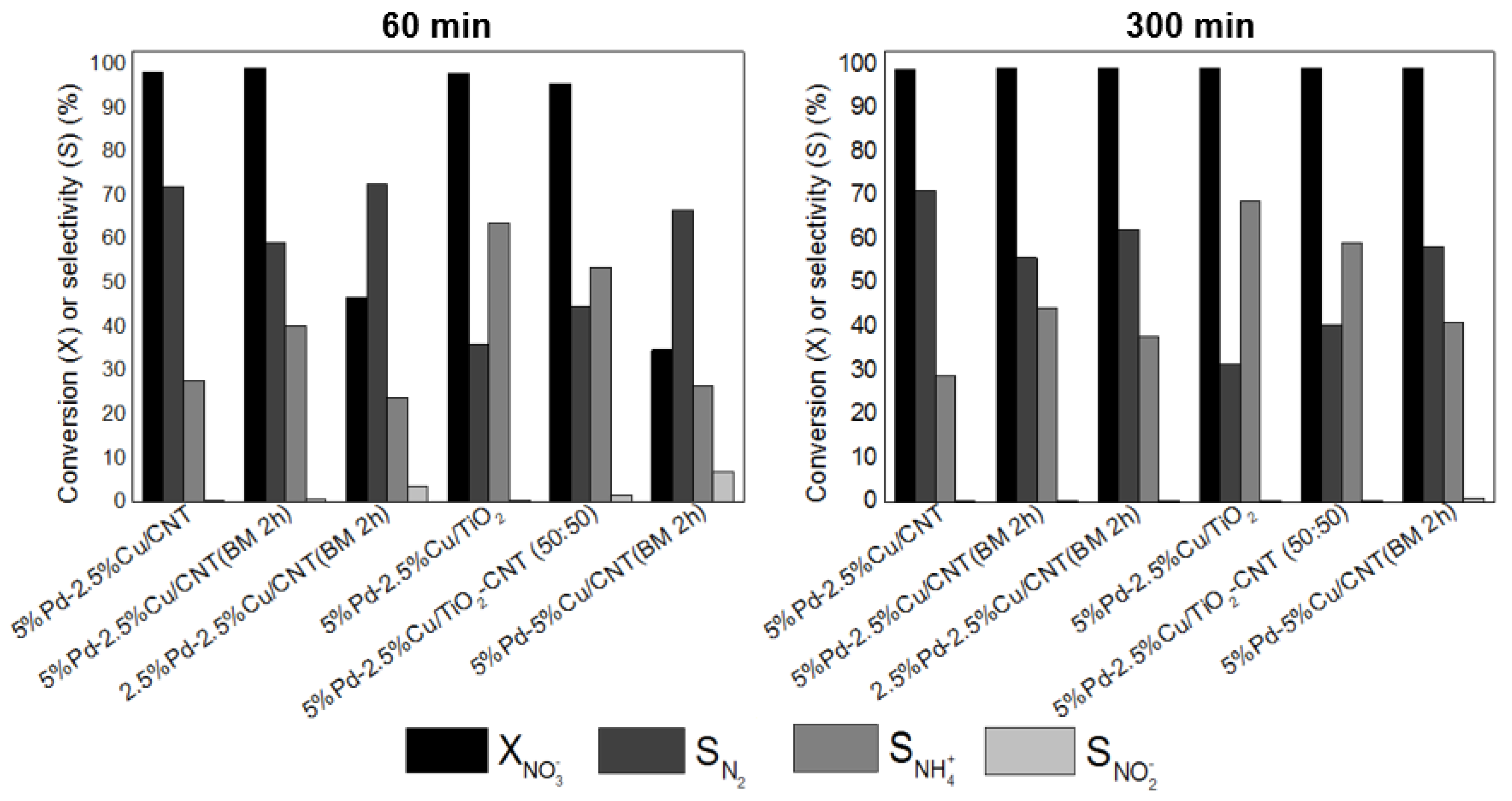
3.2.1. Effect of the Support
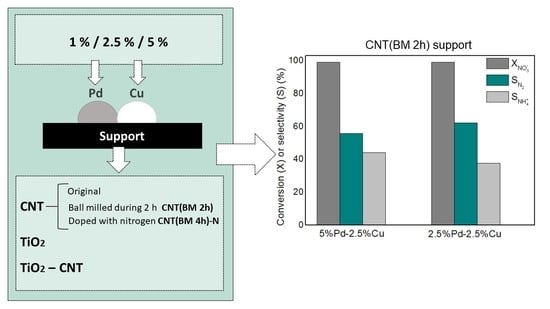
3.2.2. Optimization of Metal Loadings
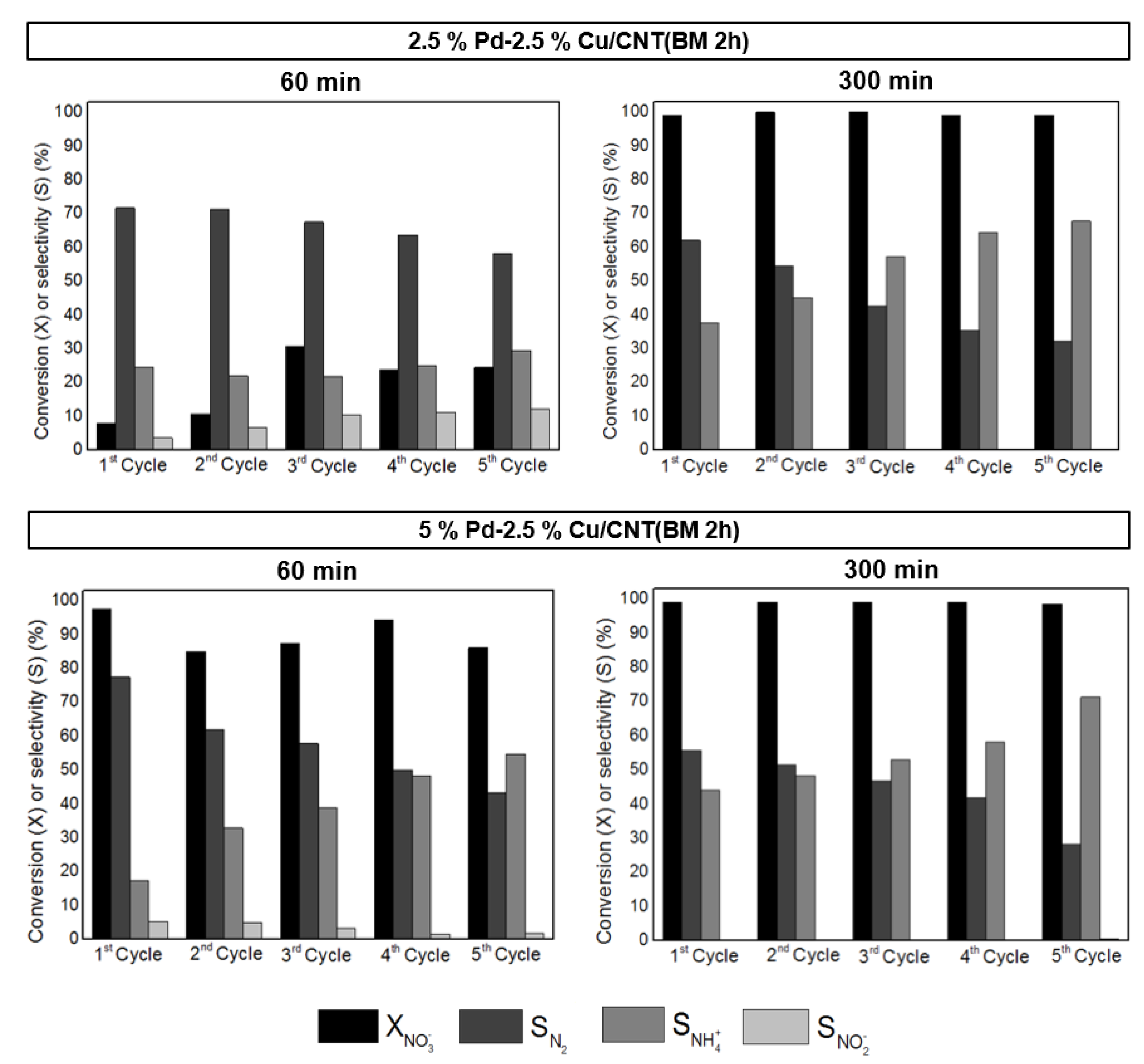
3.2.3. Reutilization Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prüsse, U.; Hähnlein, M.; Daum, J.; Vorlop, K.-D. Improving the catalytic nitrate reduction. Catal. Today 2000, 55, 79–90. [Google Scholar] [CrossRef]
- Barrabés, N.; Sá, J. Catalytic nitrate removal from water, past, present and future perspectives. Appl. Catal. B Environ. 2011, 104, 1–5. [Google Scholar] [CrossRef]
- Ruangchainikom, C.; Liao, C.-H.; Anotai, J.; Lee, M.-T. Effects of water characteristics on nitrate reduction by the Fe0/CO2 process. Chemosphere 2006, 63, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Gauthard, F.; Epron, F.; Barbier, J. Palladium and platinum-based catalysts in the catalytic reduction of nitrate in water: Effect of copper, silver, or gold addition. J. Catal. 2003, 220, 182–191. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Gallegos-Suarez, E.; Castillejos, E.; Rodríguez-Ramos, I.; Pereira, M.F.R. Nitrate reduction over a Pd-Cu/MWCNT catalyst: Application to a polluted groundwater. Environ. Technol. 2012, 33, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.B.; Darby, J.L.; Seidel, C.; Gorman, C.; Jensen, V.B.; Darby, J.L.; Seidel, C.; Gorman, C.; Gorman, C. Nitrate in Potable Water Supplies: Alternative Management Strategies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2203–2286. [Google Scholar] [CrossRef]
- Fern, M.J.; Ruiz-bevi, F. Effective catalytic removal of nitrates from drinking water: An unresolved problem? J. Clean. Product. 2019, 217, 398–408. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Nitrate removal from drinking water. J. Environ. Eng. 1997, 123, 371–380. [Google Scholar] [CrossRef]
- Sá, J.; Vinek, H. Catalytic hydrogenation of nitrates in water over a bimetallic catalyst. Appl.Catal. B Environ. 2005, 57, 247–256. [Google Scholar] [CrossRef]
- Prüsse, U.; Vorlop, K.-D. Supported bimetallic palladium catalysts for water-phase nitrate reduction. J. Mol. Catal. A Chem. 2001, 173, 313–328. [Google Scholar] [CrossRef]
- Barrabés, N.; Just, J.; Dafinov, A.; Medina, F.; Fierro, J.L.G.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Catalytic reduction of nitrate on Pt-Cu and Pd-Cu on active carbon using continuous reactor: The effect of copper nanoparticles. Appl. Catal. B Environ. 2006, 62, 77–85. [Google Scholar] [CrossRef]
- Reddy, K.J.; Lin, J. Nitrate removal from groundwater using catalytic reduction. Water Res. 2000, 34, 995–1001. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J.; Epron, F. Activated carbon supported metal catalysts for reduction of nitrate in water with high selectivity towards N2. Appl. Catal. B Environ. 2013, 138, 141–148. [Google Scholar] [CrossRef]
- Querini, C.A.; Neyertz, C.; Marchesini, F.A.; Boix, A.; Miro, E. Catalytic reduction of nitrate in water: Promoted palladium catalysts supported in resin. Appl. Catal. A Gen. 2010, 372, 40–47. [Google Scholar] [CrossRef]
- Wang, Y.; Sakamoto, Y.; Kamiya, Y. Remediation of actual groundwater polluted with nitrate by the catalytic reduction over copper—palladium supported on active carbon. Appl. Catal. A Gen. 2009, 361, 123–129. [Google Scholar] [CrossRef]
- Epron, F.; Gauthard, F.; Pinéda, C.; Barbier, J. Catalytic Reduction of Nitrate and Nitrite on Pt–Cu/Al2O3 Catalysts in Aqueous Solution: Role of the Interaction between Copper and Platinum in the Reaction. J. Catal. 2001, 198, 309–318. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Nitrate reduction in water catalysed by Pd—Cu on different supports. Desalination 2011, 279, 367–374. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Bimetallic catalysts supported on activated carbon for the nitrate reduction in water: Optimization of catalysts composition. Appl. Catal. B Environ. 2009, 91, 441–448. [Google Scholar] [CrossRef]
- Palomares, A.E.; Prato, J.G.; Rey, F.; Corma, A. Using the “memory effect” of hydrotalcites for improving the catalytic reduction of nitrates in water. J. Catal. 2004, 221, 62–66. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Nitrate Reduction Catalyzed by Pd–Cu and Pt–Cu Supported on Different Carbon Materials. Catal. Lett. 2010, 139, 97–104. [Google Scholar] [CrossRef]
- Tokazhanov, G.; Ramazanova, E.; Hamid, S.; Bae, S.; Lee, W. Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: A review. Chem. Eng. J. 2020, 384, 123252. [Google Scholar] [CrossRef]
- Hamid, S.; Niaz, Y.; Bae, S.; Lee, W. Support induced influence on the reactivity and selectivity of nitrate reduction by Sn-Pd bimetallic catalysts. J. Environ. Chem. Eng. 2020, 8, 103754. [Google Scholar] [CrossRef]
- Li, L.; Gong, L.; Wang, Y.; Liu, Q.; Zhang, J.; Mu, Y.; Yu, H. Removal of halogenated emerging contaminants from water by nitrogen-doped graphene decorated with palladium nanoparticles: Experimental investigation and theoretical analysis. Water Res. 2016, 98, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.G.P. Development of carbon materials as metal catalyst supports and metal-free catalysts for catalytic reduction of ions and advanced oxidation processes. Bol. Grupo Esp. Carbon 2016, 40, 20–23. [Google Scholar]
- Soares, O.S.G.P.; Orfao, J.J.M.; Pereira, M.F.R. Pd—Cu and Pt—Cu catalysts supported on carbon nanotubes for nitrate reduction in water. Ind. Eng. Chem. Res. 2010, 49, 7183–7192. [Google Scholar] [CrossRef]
- Sá, J.; Berger, T.; Föttinger, K.; Riss, A.; Anderson, J.A.; Vinek, H. Can TiO2 promote the reduction of nitrates in water? J. Catal. 2005, 234, 282–291. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Fernández-garcia, M. Metal Oxide Nanoparticles. Nanomater. Inorgan. Bioinorgan. Perspect. 2007, 60. [Google Scholar] [CrossRef]
- Silva, C.G.; Pereira, M.F.R.; Órfão, J.J.M.; Faria, J.L.; Soares, O.S.G.P. Catalytic and Photocatalytic Nitrate Reduction Over Pd-Cu Loaded Over Hybrid Materials of Multi-Walled Carbon Nanotubes and TiO2. Front. Chem. 2018, 6, 632. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Jardim, E.O.; Reyes-Carmona, Á.; Ruiz-Martínez, J.; Silvestre-Albero, J.; Rodríguez-Castellón, E.; Órfão, J.J.M.; Sepúlveda-Escribano, A.; Pereira, M.F.R. Effect of support and pre-treatment conditions on Pt–Sn catalysts: Application to nitrate reduction in water. J. Colloid Interface Sci. 2012, 369, 294–301. [Google Scholar] [CrossRef]
- Mendow, G.; Veizaga, N.S.; Querini, C.A.; Sánchez, B.S. A continuous process for the catalytic reduction of water nitrate. J. Environ. Chem. Eng. 2019, 7, 102808. [Google Scholar] [CrossRef]
- Bradu, C.; Constantin, C.; Papa, F.; Frunza, L.; Olaru, E.; Crini, G.; Morin-crini, N.; Euvrard, É.; Balint, I.; Zgura, I.; et al. Pd-Cu catalysts supported on anion exchange resin for the simultaneous catalytic reduction of nitrate ions and reductive dehalogenation of organochlorinated pollutants from water. Appl. Catal. A Gen. 2019, 570, 120–129. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Corma, A. A study of different supports for the catalytic reduction of nitrates from natural water with a continuous reactor. Catal. Today 2011, 172, 90–94. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Orge, C.A.; Pereira, M.F.R.; Faria, J.L. Photocatalytic ozonation of model aqueous solutions of oxalic and oxamic acids. Appl. Catal. B Environ. 2015, 174–175, 113–119. [Google Scholar] [CrossRef]
- Santos, A.S.G.G.; Orge, C.A.; Soares, O.S.G.P.; Pereira, M.F.R. 4-Nitrobenzaldehyde removal by catalytic ozonation in the presence of CNT. J. Water Proc. Eng. 2020, 38, 101573. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Fan, X.; Órfão, J.J.M.; Lapkin, A.A.; Pereira, M.F.R. Kinetic Modeling of Nitrate Reduction Catalyzed by Pd—Cu Supported on Carbon Nanotubes. Ind. Eng. Chem. Res. 2012, 51, 4854–4860. [Google Scholar] [CrossRef]
- Orge, C.A.; Soares, O.S.G.P.; Faria, J.L.; Pereira, M.F.R. Synthesis of TiO2-Carbon Nanotubes through ball-milling method for mineralization of oxamic acid (OMA) by photocatalytic ozonation. J. Environ. Chem. Eng. 2017, 5, 5599–5607. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 2013, 36, 159–175. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Ramalho, P.S.F.; Fernandes, A.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic bromate reduction in water: Influence of carbon support. J. Environ. Chem. Eng. 2019, 7, 103015. [Google Scholar] [CrossRef]
- Mabena, L.F.; Ray, S.S.; Mhlanga, S.D.; Coville, N.J. Nitrogen-doped carbon nanotubes as a metal catalyst support. Appl. Nanosci. 2011, 1, 67–77. [Google Scholar] [CrossRef]
- Lv, R.; Cui, T.; Jun, M.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.; Miyawaki, J.; Mochida, I. Open-Ended, N-Doped Carbon Nanotube–Graphene Hybrid Nanostructures as High-Performance Catalyst Support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. (International Ed. in English). Angew. Chem. 2016, 55. [Google Scholar] [CrossRef]
- Pattabiraman, R. Electrochemical investigations on carbon supported palladium catalysts. Appl. Catal. A Gen. 1997, 153, 9–20. [Google Scholar] [CrossRef]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N–Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Yan, B.; Shi, W.; Cui, F.; Wang, C. Well-dispersed Pd-Cu bimetals in TiO2 nanofiber matrix with enhanced activity and selectivity for nitrate catalytic reduction. Chem. Eng. J. 2017, 326, 182–191. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Hamid, S.B.A. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Yuranova, T.; Franch, C.; Palomares, A.E.; Garcia-Bordejé, E.; Kiwi-Minsker, L. Structured fibrous carbon-based catalysts for continuous nitrate removal from natural water. Appl. Catal. B Environ. 2012, 123, 221–228. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, X.; Wang, Y.; Ai, Z.; Zhao, D. Catalytic reduction of aqueous nitrates by metal supported catalysts on Al particles. Chem. Eng. J. 2014, 254, 410–417. [Google Scholar] [CrossRef]
- Yoshinaga, Y.; Akita, T.; Mikami, I.; Okuhara, T. Hydrogenation of Nitrate in Water to Nitrogen over Pd–Cu Supported on Active Carbon. J. Catal. 2002, 207, 37–45. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | VP p/p0=0.95 (cm3/g) | |
|---|---|---|---|
| Support | CNT | 197 | 0.421 |
| CNT (BM 2h) | 220 | 0.621 | |
| CNT (BM 4h)-N | 191 | 0.391 | |
| TiO2 | 50 | 0.130 | |
| TiO2-CNT (50:50) | 122 | 0.276 | |
| TiO2-CNT (BM 2h) (50:50) | 145 | 0.384 | |
| TiO2-CNT (80:20) | 79 | 0.186 | |
| TiO2-CNT (20:80) | 153 | 0.367 | |
| Catalyst | 1% Pd-1% Cu/CNT | 194 | 0.421 |
| 1% Pd-1% Cu/CNT (BM 2h) | 216 | 0.599 | |
| 1% Pd-1% Cu/CNT (BM 4h)-N | 185 | 0.480 | |
| 1% Pd-1% Cu/TiO2 | 46 | 0.143 | |
| 1% Pd-1% Cu/TiO2-CNT (50:50) | 118 | 0.283 | |
| 1% Pd-1% Cu/TiO2-CNT (BM 2h) (50:50) | 131 | 0.377 | |
| 1% Pd-1% Cu/TiO2-CNT (20:80) | 159 | 0.356 | |
| 1% Pd-1% Cu/TiO2-CNT (80:20) | 67 | 0.190 | |
| 5% Pd-2.5% Cu/CNT | 182 | 0.403 | |
| 5% Pd-2.5% Cu/CNT (BM 2h) | 203 | 0.574 | |
| 2.5% Pd-2.5% Cu/CNT (BM 2h) | 220 | 0.609 | |
| 5% Pd-2.5% Cu/TiO2 | 41 | 0.132 | |
| 5% Pd-2.5% Cu/TiO2-CNT(50:50) | 109 | 0.267 | |
| 5% Pd-5% Cu/CNT (BM 2h) | 209 | 0.556 |
| Catalyst | |
|---|---|
| 1% Pd-1% Cu/CNT | 2.2 |
| 5% Pd-2.5% Cu/CNT (BM 2h) | 86.7 |
| 2.5% Pd-2.5% Cu/CNT (BM 2h) | 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.S.G.G.; Restivo, J.; Orge, C.A.; Pereira, M.F.R.; Soares, O.S.G.P. Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization. C 2020, 6, 78. https://doi.org/10.3390/c6040078
Santos ASGG, Restivo J, Orge CA, Pereira MFR, Soares OSGP. Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization. C. 2020; 6(4):78. https://doi.org/10.3390/c6040078
Chicago/Turabian StyleSantos, A. Sofia G. G., João Restivo, Carla A. Orge, M. Fernando R. Pereira, and O. Salomé G. P. Soares. 2020. "Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization" C 6, no. 4: 78. https://doi.org/10.3390/c6040078
APA StyleSantos, A. S. G. G., Restivo, J., Orge, C. A., Pereira, M. F. R., & Soares, O. S. G. P. (2020). Nitrate Catalytic Reduction over Bimetallic Catalysts: Catalyst Optimization. C, 6(4), 78. https://doi.org/10.3390/c6040078