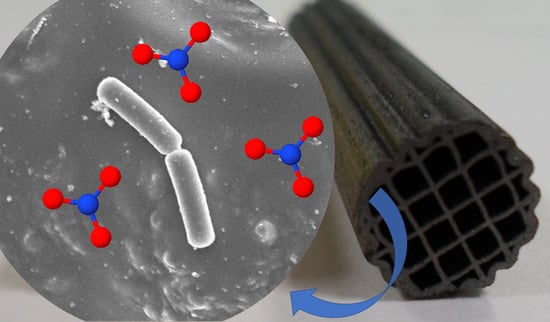
Bacteria Supported on Carbon-Coated Monoliths for Water Denitrification
Abstract
1. Introduction
2. Materials and Methods
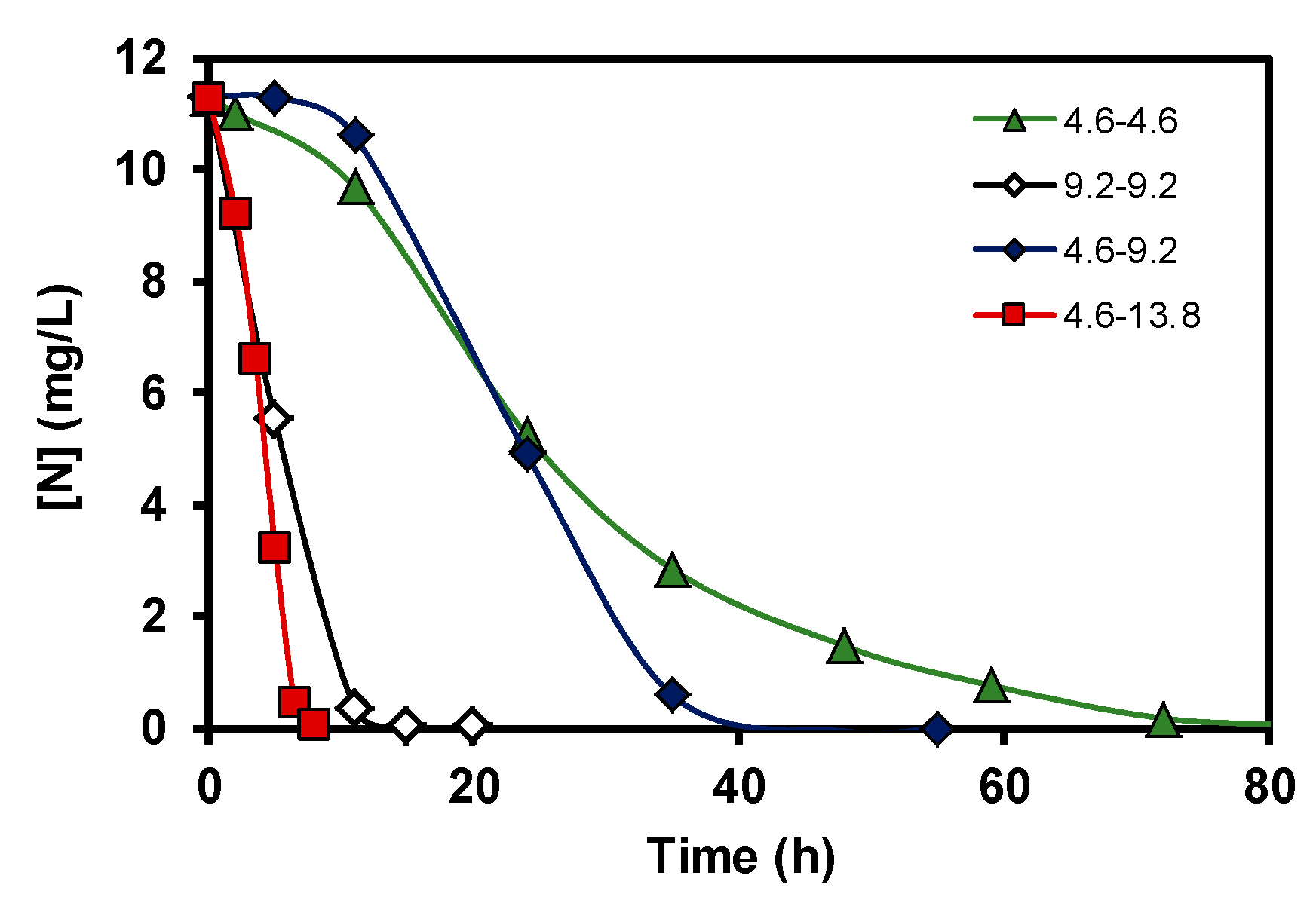
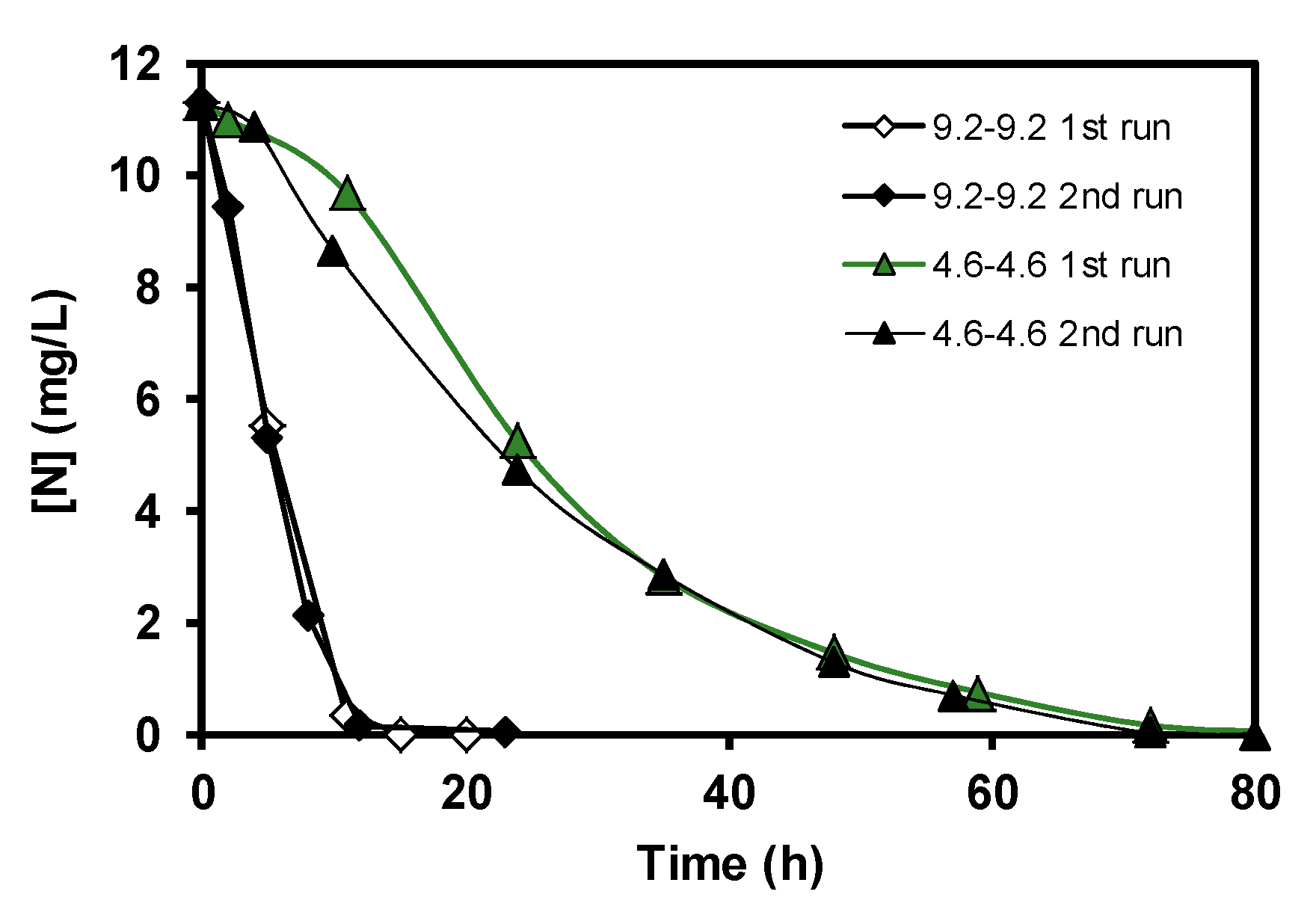
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sawayama, S.; Rao, K.K.; Hall, D.O. Nitrate and phosphate ion removal from water by Phormidium laminosum immobilized on hollow fibres in a photobioreactor. Appl. Microbiol. Biotechnol. 1998, 49, 463. [Google Scholar] [CrossRef]
- Zayed, G.; Winter, J. Removal of organic pollutants and of nitrate from wastewater from the dairy industry by denitrification. Appl. Microbiol. Biotechnol. 1998, 49, 469. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Bautista-Toledo, M.I.; Ferro-Garcia, M.A.; Rivera-Utrilla, J. Influence of support surface properties on activity of bacteria immobilised on activated carbons for water denitrification. Carbon 2003, 41, 1743. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hodar, F.J.; Perez-Cadenas, A.F.; Carrasco-Marin, F. Structural characterization of carbon xerogels: From film to monolith. Microporous Mesoporous Mater. 2012, 153, 24. [Google Scholar] [CrossRef]
- Avila, P.; Montes, M.; Miro, E.E. Monolithic reactors for environmental applications: A review on preparation technologies. Chem. Eng. J. 2005, 109, 11. [Google Scholar] [CrossRef]
- Kapteijn, F.; Heiszwolf, J.J.; Nijhuis, T.A.; Moulijn, J.A. Monoliths in multiphase catalytic processes—Aspects and prospects. Cattech 1999, 3, 24. [Google Scholar]
- Chaparro-Garnica, C.Y.; Davo-Quiñonero, A.; Bailon-Garcia, E.; Lozano-Castello, D.; Bueno-Lopez, A. Design of Monolithic Supports by 3D Printing for Its Application in the Preferential Oxidation of CO (CO-PrOx). ACS Appl. Mater. Interfaces 2019, 11, 36763. [Google Scholar] [CrossRef] [PubMed]
- Davo-Quiñonero, A.; Sorolla-Rosario, D.; Bailon-Garcia, E.; Lozano-Castello, D.; Bueno-Lopez, A. Improved asymmetrical honeycomb monolith catalyst prepared using a 3D printed template. J. Hazard. Mater. 2019, 368, 638. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadenas, A.F.; Zieverink, M.M.P.; Kapteijn, F.; Moulijn, J.A. Selective hydrogenation of fatty acid methyl esters on palladium catalysts supported on carbon-coated monoliths. Carbon 2006, 44, 173. [Google Scholar] [CrossRef]
- Valverde-Sarmiento, C.; Espinosa-Iglesias, D.; Bautista-Toledo, M.I.; Alvarez-Merino, M.A.; Maldonado-Hodar, F.J.; Carrasco-Marin, F.; Perez-Cadenas, A.F. Bacteria supported on carbon films for water denitrification. Chem. Eng. J. 2015, 259, 424. [Google Scholar] [CrossRef]
- Bautista-Toledo, M.I.; Espinosa-Iglesias, D.; Carrasco-Marin, F.; Perez-Cadenas, A.F.; Maldonado-Hodar, F.J. Influence of the physicochemical properties of inorganic supports on the activity of immobilized bacteria for water denitrification. J. Environ. Manag. 2015, 156, 81. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Kleerebezem, R.; Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Potential application of monolith packed columns as bioreactors, control of biofilm formation. Biotechnol. Bioeng. 2006, 93, 238. [Google Scholar] [CrossRef] [PubMed]
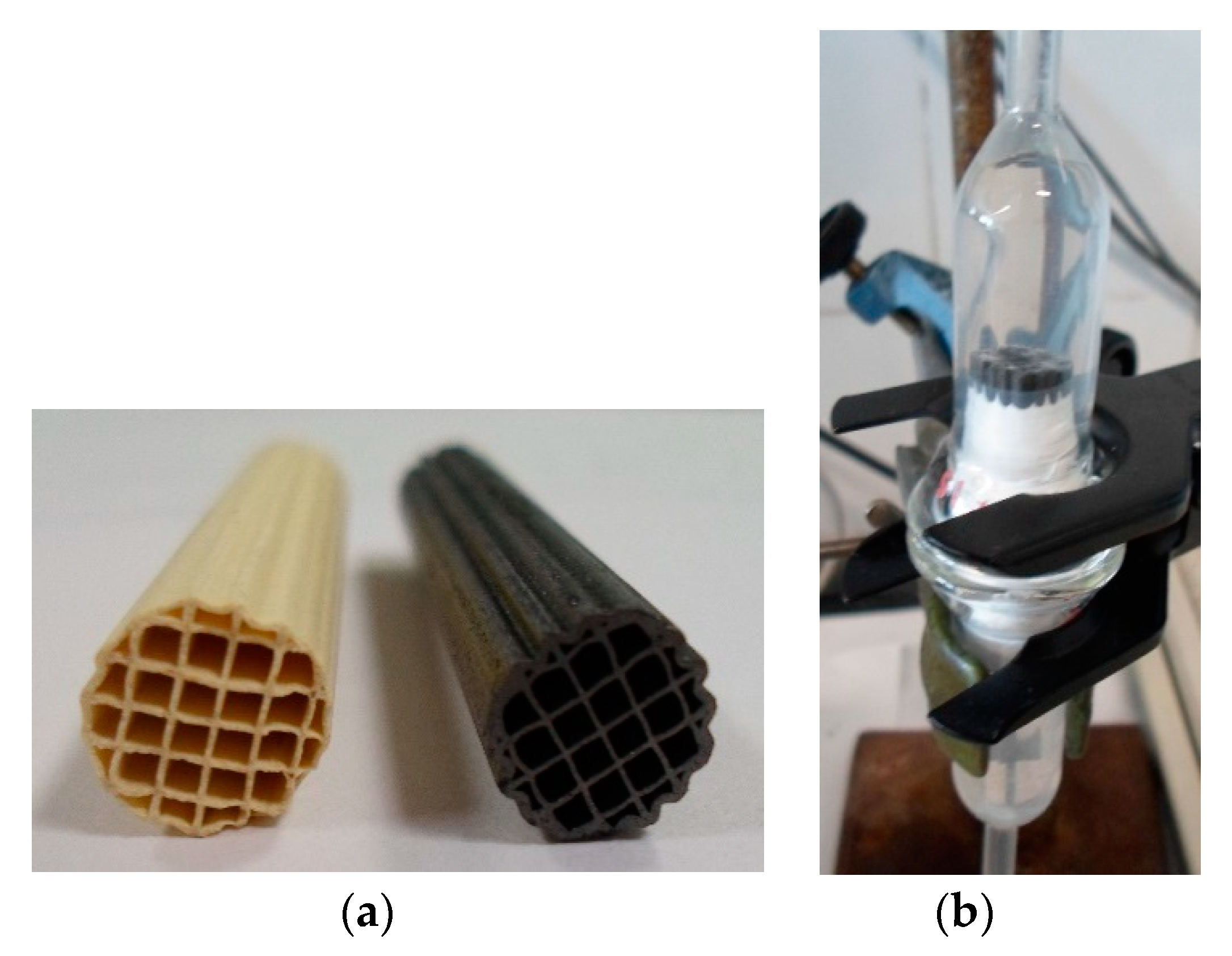
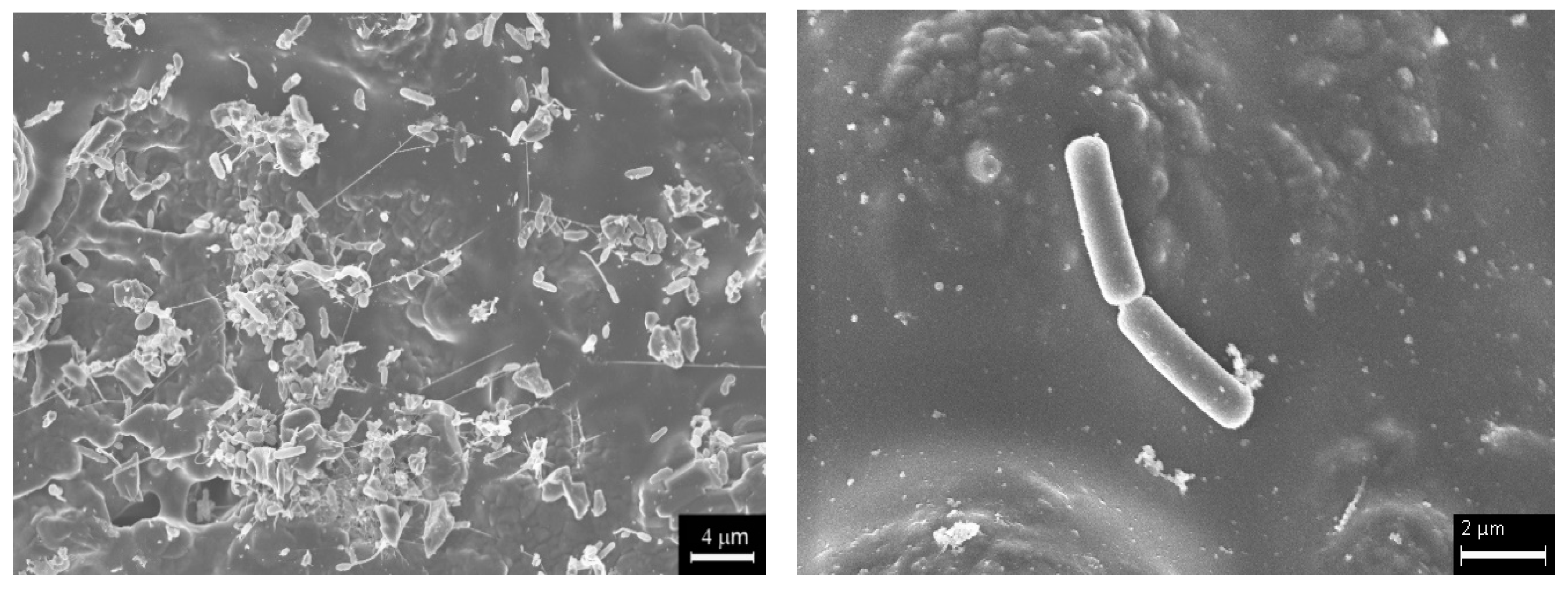


| Thickness (μm) | OTPD (wt.%) | SBET m2g−1 | VMICRO-N2 mm3g−1 |
|---|---|---|---|
| 7 | 9.0 | 64.4 | 30.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Iglesias, D.; Bailón-García, E.; Bautista-Toledo, M.I.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Bacteria Supported on Carbon-Coated Monoliths for Water Denitrification. C 2020, 6, 77. https://doi.org/10.3390/c6040077
Espinosa-Iglesias D, Bailón-García E, Bautista-Toledo MI, Carrasco-Marín F, Pérez-Cadenas AF. Bacteria Supported on Carbon-Coated Monoliths for Water Denitrification. C. 2020; 6(4):77. https://doi.org/10.3390/c6040077
Chicago/Turabian StyleEspinosa-Iglesias, David, Esther Bailón-García, Mª Isidora Bautista-Toledo, Francisco Carrasco-Marín, and Agustín F. Pérez-Cadenas. 2020. "Bacteria Supported on Carbon-Coated Monoliths for Water Denitrification" C 6, no. 4: 77. https://doi.org/10.3390/c6040077
APA StyleEspinosa-Iglesias, D., Bailón-García, E., Bautista-Toledo, M. I., Carrasco-Marín, F., & Pérez-Cadenas, A. F. (2020). Bacteria Supported on Carbon-Coated Monoliths for Water Denitrification. C, 6(4), 77. https://doi.org/10.3390/c6040077