Hemocompatibility of Carbon Nanostructures
Abstract
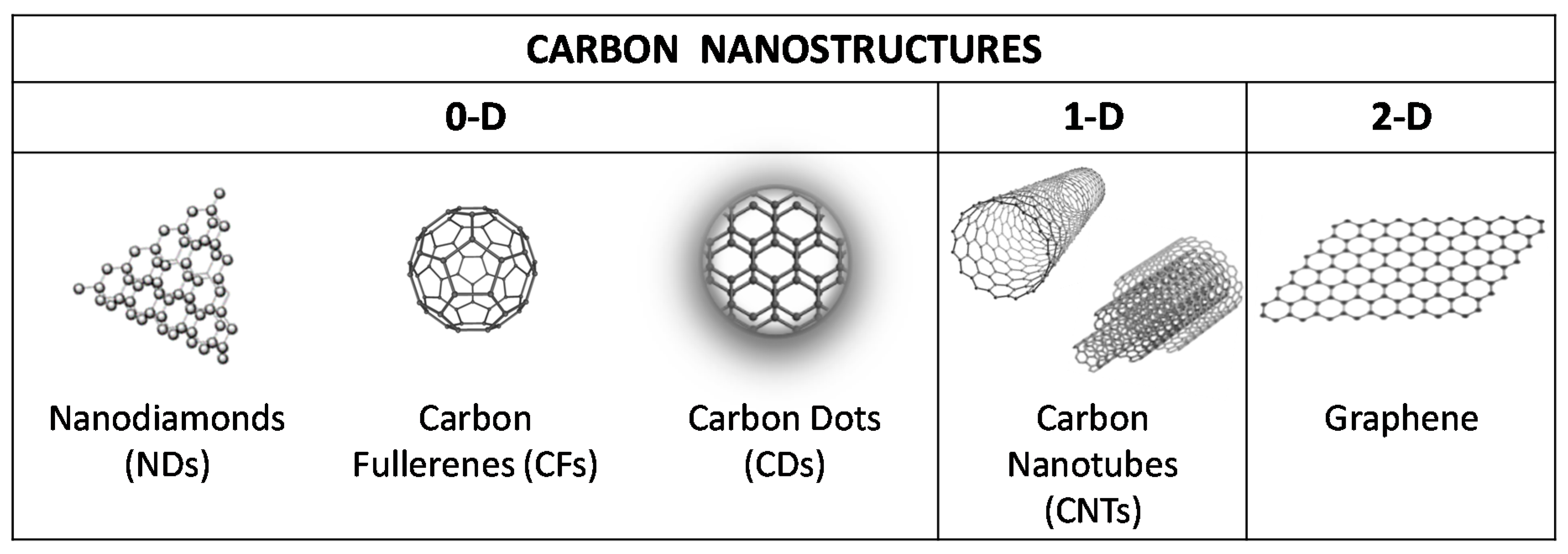
1. Introduction
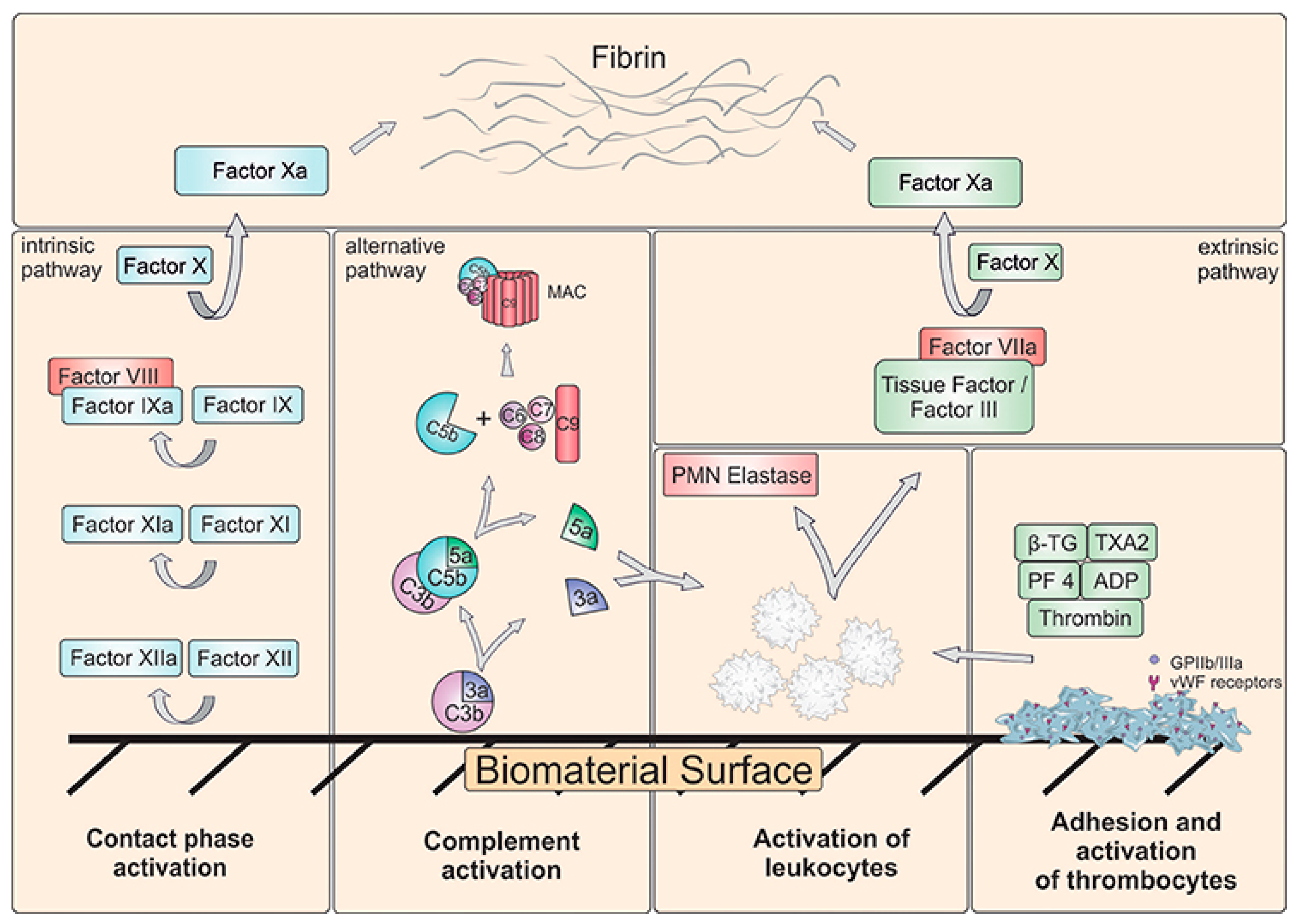
2. Hemocompatibility of Biomaterials—Short Overview
Blood–Material Interactions at the Nanoscale
Protein Corona
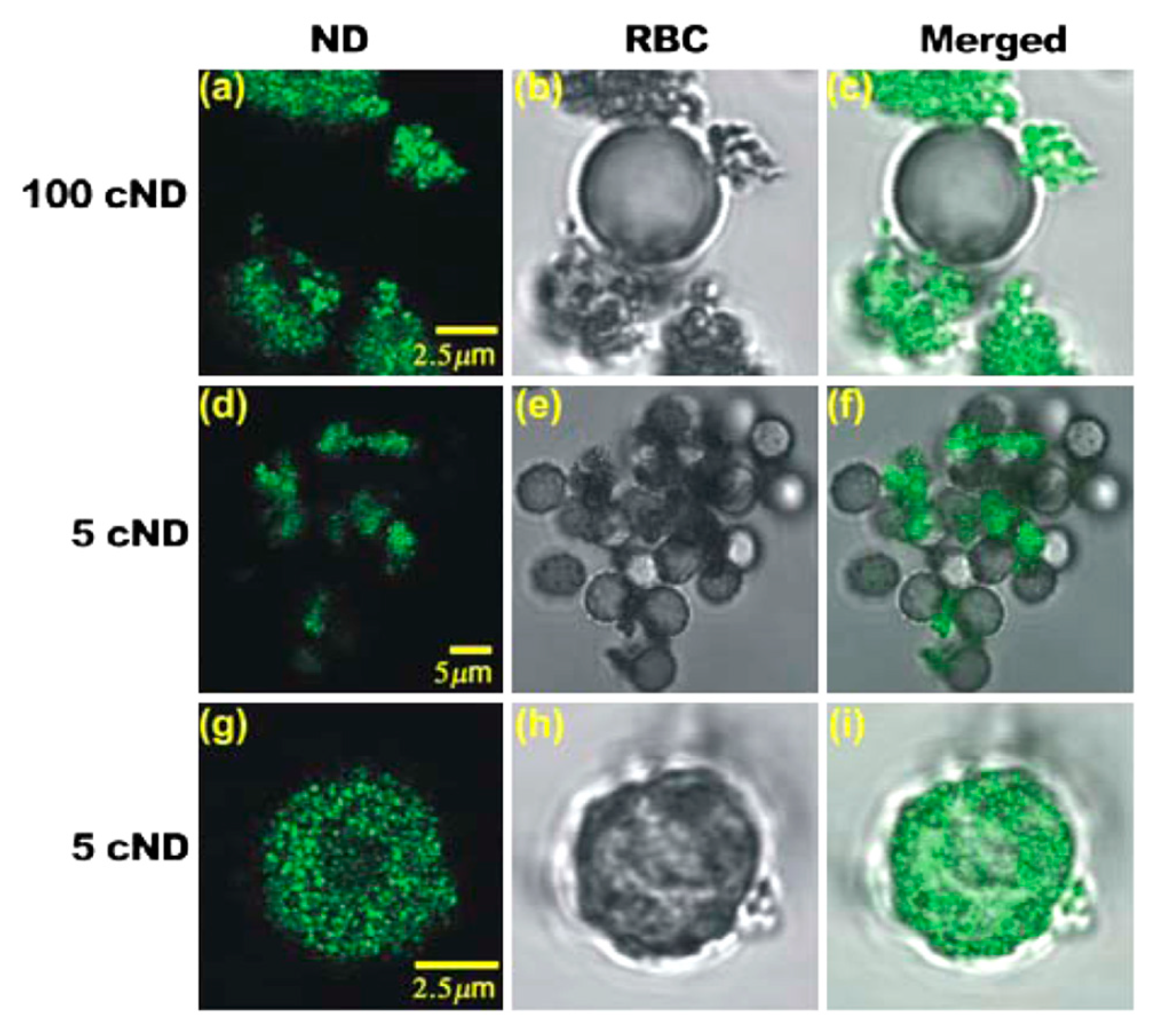
3. Carbon Nanodiamonds
4. Fullerenes
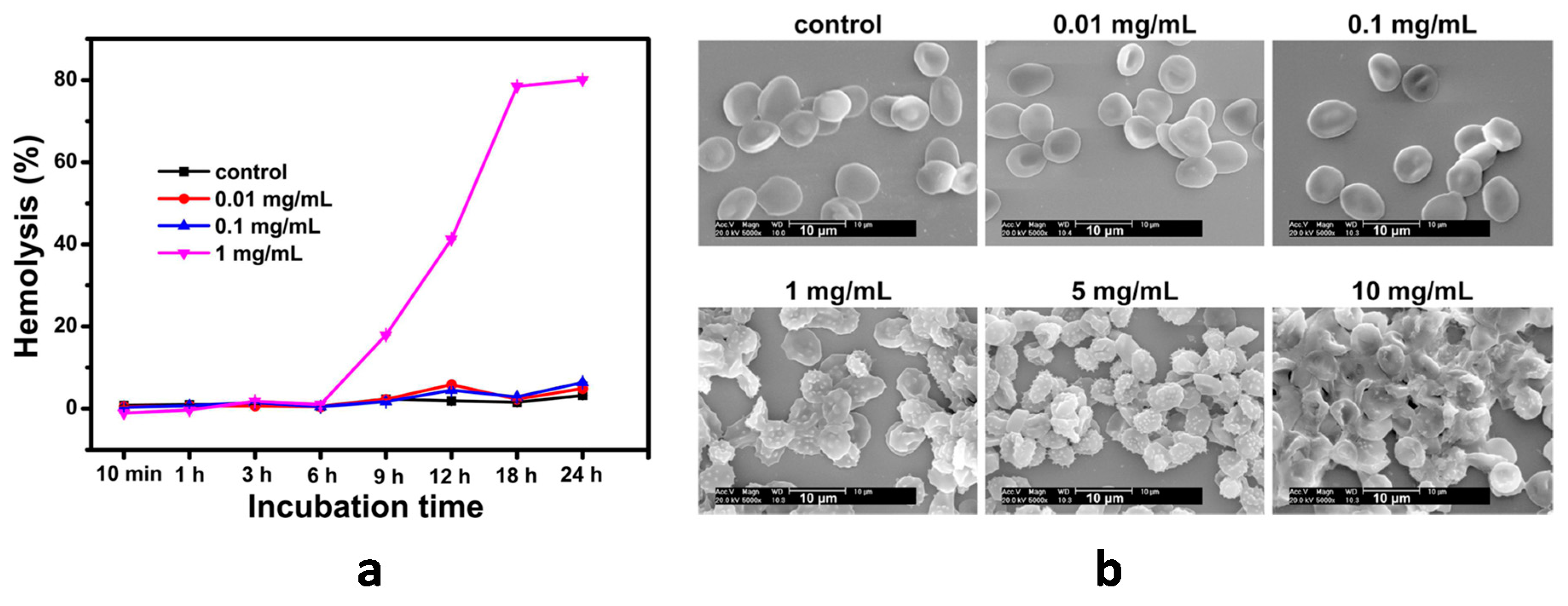
5. Carbon Dots
6. Carbon Nanotubes
7. Graphene-Based Nanostructures
8. Comparative Analysis
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Yang, N.; Jiang, X.; Pang, D.-W. Carbon Nanostructures. In Carbon Nanoparticles and Nanostructures; Yang, N., Jiang, X., Pang, D.-W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-28780-5. [Google Scholar]
- Notarianni, M.; Liu, J.; Vernon, K.; Motta, N. Synthesis and applications of carbon nanomaterials for energy generation and storage. Beilstein J. Nanotechnol. 2016, 7, 149–196. [Google Scholar] [CrossRef] [PubMed]
- Knupfer, M. Electronic properties of carbon nanostructures. Surf. Sci. Rep. 2001, 42, 1–74. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Fouad, H.; Alothman, O.Y. Nanocarbon: Preparation, properties, and applications. In Nanocarbon and its Composites; Elsevier: Duxford, UK, 2019; pp. 327–354. [Google Scholar]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Tinwala, H.; Wairkar, S. Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics. Mater. Sci. Eng. C 2019, 97, 913–931. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Luo, P.G.; Sahu, S.; Yang, S.T.; Sonkar, S.K.; Wang, J.; Wang, H.; Lecroy, G.E.; Cao, L.; Sun, Y.P. Carbon “quantum” dots for optical bioimaging. J. Mater. Chem. B 2013, 1, 2116–2127. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots - Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Botta, L. Nanocarbons in Electrospun Polymeric Nanomats for Tissue Engineering: A Review. Polymers (Basel) 2017, 9, 76. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Watari, F.; Liao, S.; Yokoyama, A.; Omori, M.; Ai, H.; Cui, F. Carbon nanotubes/hydroxyapatite nanocomposites fabricated by spark plasma sintering for bonegraft applications. Appl. Surf. Sci. 2012, 262, 194–199. [Google Scholar] [CrossRef]
- Shi, X.; Hudson, J.L.; Spicer, P.P.; Tour, J.M.; Krishnamoorti, R.; Mikos, A.G. Injectable Nanocomposites of Single-Walled Carbon Nanotubes and Biodegradable Polymers for Bone Tissue Engineering. Biomacromolecules 2006, 7, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, A.E.; Youan, B.-B.C. Nanodiamonds for drug delivery systems. Diamond Based Mater. Biomed. Appl. 2013, 186–205. [Google Scholar]
- Zhang, X.-Q.; Lam, R.; Xu, X.; Chow, E.K.; Kim, H.-J.; Ho, D. Multimodal Nanodiamond Drug Delivery Carriers for Selective Targeting, Imaging, and Enhanced Chemotherapeutic Efficacy. Adv. Mater. 2011, 23, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale 2011, 3, 4035. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Drozd, V.; Durygin, A.; Rodriguez, J.; Barber, P.; Atluri, V.; Liu, X.; Voss, T.G.; Saxena, S.; Nair, M. Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603. [Google Scholar] [CrossRef]
- Liu, K.-K.; Zheng, W.-W.; Wang, C.-C.; Chiu, Y.-C.; Cheng, C.-L.; Lo, Y.-S.; Chen, C.; Chao, J.-I. Covalent linkage of nanodiamond-paclitaxel for drug delivery and cancer therapy. Nanotechnology 2010, 21, 315106. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Li, W.; Zhang, X.; Peng, Y.; Huang, Q. Nanodiamonds as intracellular transporters of chemotherapeutic drug. Biomaterials 2010, 31, 8410–8418. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alfawaz, M.A.; Alshatwi, A.A. Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA and BCL2L1 genes. Chemosphere 2016, 144, 275–284. [Google Scholar] [CrossRef]
- Kurantowicz, N.; Sawosz, E.; Halik, G.; Strojny, B.; Hotowy, A.; Grodzik, M.; Piast, R.; Pasanphan, W.; Chwalibog, A. Toxicity studies of six types of carbon nanoparticles in a chicken-embryo model. Int. J. Nanomed. 2017, 12, 2887–2898. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity Studies of Fullerenes and Derivatives. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 620, pp. 168–180. ISBN 9780387767123. [Google Scholar]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Schrand, A.M.; Dai, L.; Schlager, J.J.; Hussain, S.M.; Osawa, E. Differential biocompatibility of carbon nanotubes and nanodiamonds. Diam. Relat. Mater. 2007, 16, 2118–2123. [Google Scholar] [CrossRef]
- Fisher, C.; Rider, A.E.; Jun Han, Z.; Kumar, S.; Levchenko, I.; Ostrikov, K. Applications and nanotoxicity of carbon nanotubes and graphene in biomedicine. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol. Sci. 2009, 114, 162–182. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef]
- Adach, K.; Fijalkowski, M.; Gajek, G.; Skolimowski, J.; Kontek, R.; Blaszczyk, A. Studies on the cytotoxicity of diamond nanoparticles against human cancer cells and lymphocytes. Chem. Biol. Interact. 2016, 254, 156–166. [Google Scholar] [CrossRef]
- Dönmez Güngüneş, Ç.; Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. A comparative study on the in vitro cytotoxic responses of two mammalian cell types to fullerenes, carbon nanotubes and iron oxide nanoparticles. Drug Chem. Toxicol. 2017, 40, 215–227. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biological response to carbon-family nanomaterials: Interactions at the nano-bio interface. Front. Bioeng. Biotechnol. 2019, 7, 1–22. [Google Scholar] [CrossRef]
- Uo, M.; Akasaka, T.; Watari, F.; Sato, Y.; Tohji, K. Toxicity evaluations of various carbon nanomaterials. Dent. Mater. J. 2011, 30, 245–263. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Vroman, L. When Blood Is Touched. Materials (Basel) 2009, 2, 1547–1557. [Google Scholar] [CrossRef]
- Brash, J.L. Studies of protein adsorption relevant to blood compatible materials. In Modern Aspects of Protein Adsorption on Biomaterials; Springer: Dordrecht, The Netherlands, 1991; pp. 39–47. [Google Scholar]
- Horbett, T.A. Protein Adsorption on Biomaterials. In Biomaterials: Interfacial Phenomena and Applications; American Chemical Society: Washington, DC, USA, 1982; pp. 233–244. [Google Scholar]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteomics 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Andrade, J.; Hlady, V. Plasma Protein Adsorption: The Big Twelve. Ann. N. Y. Acad. Sci. 1987, 516, 158–172. [Google Scholar] [CrossRef]
- Hoffman, A.S. Blood—Biomaterial Interactions: An Overview. In Biomaterials: Interfacial Phenomena and Applications; Cooper, Ed.; American Chemical Society: Washington, DC, USA, 1982; pp. 3–8. [Google Scholar]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Grunkemeier, J.M.; Horbett, T.A. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J. Biomed. Mater. Res. 1999, 44, 130–139. [Google Scholar] [CrossRef]
- Tzoneva, R.; Heuchel, M.; Groth, T.; Altankov, G.; Albrecht, W.; Paul, D. Fibrinogen adsorption and platelet interactions on polymer membranes. J. Biomater. Sci. Polym. Ed. 2002, 13, 1033–1050. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, L.C.; Vogler, E.A.; Siedlecki, C.A. Contact activation by the intrinsic pathway of blood plasma coagulation. In Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions; Elsevier Inc.: Duxford, UK, 2018; pp. 3–28. ISBN 9780081004999. [Google Scholar]
- Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef]
- Engberg, A.E.; Rosengren-Holmberg, J.P.; Chen, H.; Nilsson, B.; Lambris, J.D.; Nicholls, I.A.; Ekdahl, K.N. Blood protein-polymer adsorption: Implications for understanding complement-mediated hemoincompatibility. J. Biomed. Mater. Res. Part A 2011, 97A, 74–84. [Google Scholar] [CrossRef]
- Mödinger, Y.; Teixeira, G.Q.; Neidlinger-Wilke, C.; Ignatius, A. Role of the Complement System in the Response to Orthopedic Biomaterials. Int. J. Mol. Sci. 2018, 19, 3367. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Lambris, J.D.; Elwing, H.; Ricklin, D.; Nilsson, P.H.; Teramura, Y.; Nicholls, I.A.; Nilsson, B. Innate immunity activation on biomaterial surfaces: A Mechanistic Model and Coping Strategies. Adv. Drug Deliv. Rev. 2012, 63, 1042–1050. [Google Scholar] [CrossRef]
- Andersson, J.; Ekdahl, K.N.; Larsson, R.; Nilsson, U.R.; Nilsson, B. C3 Adsorbed to a Polymer Surface Can Form an Initiating Alternative Pathway Convertase. J. Immunol. 2002, 168, 5786–5791. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M. V Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Lundqvist, M.; Augustsson, C.; Lilja, M.; Lundkvist, K.; Dahlbäck, B.; Linse, S.; Cedervall, T. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE 2017, 12, e0175871. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Yang, Y.; Shen, J.; Moten, A.; Chen, C.; Shen, H.; Ferrari, M.; Zhao, Y. The nano-plasma interface: Implications of the protein corona. Colloids Surfaces B Biointerfaces 2014, 124, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Lim, C.T. Molecular interactions of graphene oxide with human blood plasma proteins. Nanoscale 2016, 8, 9425–9441. [Google Scholar]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2018, 1805740, 1–13. [Google Scholar] [CrossRef]
- Saha, K.; Moyano, D.F.; Rotello, V.M. Protein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticles. Mater. Horizons 2014, 1, 102–105. [Google Scholar] [CrossRef]
- Ge, C.; Tian, J.; Zhao, Y.; Chen, C.; Zhou, R.; Chai, Z. Towards understanding of nanoparticle–protein corona. Arch. Toxicol. 2015, 89, 519–539. [Google Scholar] [CrossRef]
- Nienhaus, K.; Nienhaus, G.U. Towards a molecular-level understanding of the protein corona around nanoparticles—Recent advances and persisting challenges. Curr. Opin. Biomed. Eng. 2019, 10, 11–22. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Hanada, K. Detonation nanodiamond: Perspective and Applications. Surf. Eng. 2009, 25, 487–489. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.-C.; Shenderova, O. Nanodiamond-mediated drug delivery and imaging: Challenges and opportunities. Expert Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.-Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-Mediated Delivery of Water-Insoluble Therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Pedersen, T.O.; Wu, X.; Xue, Y.; Sun, Y.; Finne-Wistrand, A.; Kloss, F.R.; Waag, T.; Krueger, A.; Steinmüller-Nethl, D.; et al. Biological Effects of Functionalizing Copolymer Scaffolds with Nanodiamond Particles. Tissue Eng. Part A 2013, 19, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Hopper, A.P.; Dugan, J.M.; Gill, A.A.; Fox, O.J.L.; May, P.W.; Haycock, J.W.; Claeyssens, F. Amine functionalized nanodiamond promotes cellular adhesion, proliferation and neurite outgrowth. Biomed. Mater. 2014, 9, 045009. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Paul, A. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Keremidarska, M.; Ganeva, A.; Mitev, D.; Hikov, T.; Presker, R.; Pramatarova, L.; Krasteva, N. Comparative study of cytotoxicity of detonation nanodiamond particles with an osteosarcoma cell line and primary mesenchymal stem cells. Biotechnol. Biotechnol. Equip. 2014, 28, 733–739. [Google Scholar] [CrossRef]
- Paget, V.; Sergent, J.A.; Grall, R.; Altmeyer-Morel, S.; Girard, H.A.; Petit, T.; Gesset, C.; Mermoux, M.; Bergonzo, P.; Arnault, J.C.; et al. Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 2014, 8, 46–56. [Google Scholar] [CrossRef]
- Chang, H.-C.; Hsiao, W.W.-W.; Su, M.-C. Biocompatibility of Nanodiamonds. In Fluorescent Nanodiamonds; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 73–89. [Google Scholar]
- Lin, C.-L.; Lin, C.-H.; Chang, H.-C.; Su, M.-C. Protein Attachment on Nanodiamonds. J. Phys. Chem. A 2015, 119, 7704–7711. [Google Scholar] [CrossRef]
- Aramesh, M.; Shimoni, O.; Ostrikov, K.; Prawer, S.; Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 2015, 7, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Niu, C.H.; Yang, Q.; Badea, I. Study on protein conformation and adsorption behaviors in nanodiamond particle-protein complexes. Nanotechnology 2011, 22, 145703. [Google Scholar] [CrossRef] [PubMed]
- Mona, J.; Kuo, C.J.; Perevedentseva, E.; Priezzhev, A.V.; Cheng, C.L. Adsorption of human blood plasma on nanodiamond and its influence on activated partial thromboplastin time. Diam. Relat. Mater. 2013, 39, 73–77. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, M.K.; Singh, S.K.; Grácio, J.J.; Dash, D. Nanodiamonds activate blood platelets and induce thromboembolism. Nanomedicine 2014, 9, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Hsieh, F.J.; Chen, C.P.; Chang, M.Y.; Hsieh, P.C.H.; Chen, C.C.; Hung, S.U.; Wu, C.C.; Chang, H.C. The hemocompatibility of oxidized diamond nanocrystals for biomedical applications. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, M.; Ficek, M.; Wróbel, M.S.; Chakraborty, R.; Fixler, D.; Wierzba, P.; Jȩdrzejewska-Szczerska, M. Haemocompatibility of modified nanodiamonds. Materials (Basel) 2017, 10, 352. [Google Scholar] [CrossRef]
- Puzyr, A.P.; Neshumaev, D.A.; Tarskikh, S.V.; Makarskaya, G.V.; Dolmatov, V.Y.; Bondar, V.S. Destruction of human blood cells in interaction with detonation nanodiamonds in experiments in vitro. Diam. Relat. Mater. 2004, 13, 2020–2023. [Google Scholar] [CrossRef]
- Lin, Y.-C. The influence of nanodiamond on the oxygenation states and micro rheological properties of human red blood cells in vitro. J. Biomed. Opt. 2012, 17, 101512. [Google Scholar] [CrossRef]
- Tsai, L.W.; Lin, Y.C.; Perevedentseva, E.; Lugovtsov, A.; Priezzhev, A.; Cheng, C.L. Nanodiamonds for medical applications: Interaction with blood in vitro and in vivo. Int. J. Mol. Sci. 2016, 17, 5. [Google Scholar] [CrossRef]
- Moore, L.; Yang, J.; Lan, T.T.H.; Osawa, E.; Lee, D.K.; Johnson, W.D.; Xi, J.; Chow, E.K.H.; Ho, D. Biocompatibility Assessment of Detonation Nanodiamond in Non-Human Primates and Rats Using Histological, Hematologic, and Urine Analysis. ACS Nano 2016, 10, 7385–7400. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]Fullerene is a Powerful Antioxidant in Vivo with No Acute or Subacute Toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Suzuki, T.; Ishii, H.; Nakae, D.; Ogata, A. Cytotoxic effects of hydroxylated fullerenes on isolated rat hepatocytes via mitochondrial dysfunction. Arch. Toxicol. 2011, 85, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kubota, R.; Kobayashi, N.; Tahara, M.; Sugimoto, N.; Nishimura, T.; Ikarashi, Y. Cytotoxic Effects of Hydroxylated Fullerenes in Three Types of Liver Cells. Materials (Basel) 2013, 6, 2713–2722. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Trpkovic, A.; Todorovic-Markovic, B.; Trajkovic, V. Toxicity of pristine versus functionalized fullerenes: Mechanisms of cell damage and the role of oxidative stress. Arch. Toxicol. 2012, 86, 1809–1827. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Hua, S.-Y.; Zhou, Z.-Q.; Wang, G.-C.; Jiang, F.-L.; Liu, Y. Characterization of fullerenol-protein interactions and an extended investigation on cytotoxicity. Colloids Surf. B Biointerfaces 2017, 157, 261–267. [Google Scholar] [CrossRef]
- Deguchi, S.; Yamazaki, T.; Mukai, S.; Usami, R.; Horikoshi, K. Stabilization of C60 Nanoparticles by Protein Adsorption and Its Implications for Toxicity Studies. Chem. Res. Toxicol. 2007, 20, 854–858. [Google Scholar] [CrossRef]
- Xia, S.; Li, J.; Zu, M.; Li, J.; Liu, J.; Bai, X.; Chang, Y.; Chen, K.; Gu, W.; Zeng, L.; et al. Small size fullerenol nanoparticles inhibit thrombosis and blood coagulation through inhibiting activities of thrombin and FXa. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Andrievsky, G.; Shakhnin, D.; Tronza, A.; Zhernosekov, D.; Tykhomyrov, A. The acceleration of blood plasma clot lysis in the presence of hydrated C60 fullerene nanostructures in super-small concentration. Fuller. Nanotub. Carbon Nanostruct. 2010, 18, 303–311. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zheng, Y.; Wang, B. Mechanical characteristics of human red blood cell membrane change due to C60 nanoparticle infiltration. Phys. Chem. Chem. Phys. 2013, 15, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Roberts, A.P.; Mount, A.S.; Klaine, S.J.; Ke, P.C. Translocation of C60 and Its Derivatives Across a Lipid Bilayer. Nano Lett. 2007, 7, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Todorovic-Markovic, B.; Kleut, D.; Misirkic, M.; Janjetovic, K.; Vucicevic, L.; Pantovic, A.; Jovanovic, S.; Dramicanin, M.; Markovic, Z.; et al. Oxidative stress-mediated hemolytic activity of solvent exchange-prepared fullerene (C60) nanoparticles. Nanotechnology 2010, 21, 375102. [Google Scholar] [CrossRef]
- Avilova, I.; Khakina, E.; Kraevaya, O.; Kotelnikov, A.; Kotelnikova, R.; Troshin, P.; Volkov, V. Self-diffusion of water-soluble fullerene derivatives in mouse erythrocytes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1537–1543. [Google Scholar] [CrossRef]
- Yang, X.L.; Huang, C.; Qiao, X.G.; Yao, L.; Zhao, D.X.; Tan, X. Photo-induced lipid peroxidation of erythrocyte membranes by a bis-methanophosphonate fullerene. Toxicol. Vitr. 2007, 21, 1493–1498. [Google Scholar] [CrossRef]
- Bosi, S.; Feruglio, L.; Da Ros, T.; Spalluto, G.; Gregoretti, B.; Terdoslavich, M.; Decorti, G.; Passamonti, S.; Moro, S.; Prato, M. Hemolytic effects of water-soluble fullerene derivatives. J. Med. Chem. 2004, 47, 6711–6715. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chemie Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef]
- Yang, S.T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Liu, J.-H.; Cao, L.; LeCroy, G.E.; Wang, P.; Meziani, M.J.; Dong, Y.; Liu, Y.; Luo, P.G.; Sun, Y.-P. Carbon “Quantum” Dots for Fluorescence Labeling of Cells. ACS Appl. Mater. Interfaces 2015, 7, 19439–19445. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Zhang, Y.; Xiao, Q. Nitrogen, Cobalt Co-doped Fluorescent Magnetic Carbon Dots as Ratiometric Fluorescent Probes for Cholesterol and Uric Acid in Human Blood Serum. ACS Omega 2019, 4, 9333–9342. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Zhang, Y.; Xue, W.; Liu, Z. Blood Compatibility Evaluations of Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2015, 7, 19153–19162. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Feng, R.; Zhang, Y.; Xue, W.; Liu, Z. Hyperbranched polyglycerol conjugated fluorescent carbon dots with improved: In vitro toxicity and red blood cell compatibility for bioimaging. RSC Adv. 2017, 7, 4975–4982. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Yu, L.; Li, Z.; Sun, S. Preparation of high-quality biocompatible carbon dots by extraction, with new thoughts on the luminescence mechanisms. Nanotechnology 2013, 24, 225601. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Deng, W.; Chen, J.; Wang, Y.; Zhou, J.; Du, P.; Xu, W.; Wang, Q.; Wang, Q.; et al. Photoluminescent Cationic Carbon Dots as efficient Non-Viral Delivery of Plasmid SOX9 and Chondrogenesis of Fibroblasts. Sci. Rep. 2018, 8, 7057. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iannazzo, D.; Piperno, A.; Pistone, A.; Grassi, G.; Galvagno, S. Recent Advances in Carbon Nanotubes as Delivery Systems for Anticancer Drugs. Curr. Med. Chem. 2013, 20, 1333–1354. [Google Scholar] [CrossRef]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef]
- Veetil, J.V.; Ye, K. Tailored carbon nanotubes for tissue engineering applications. Biotechnol. Prog. 2009, 25, 709–721. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon N. Y. 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Yao, Y.; Ma, Y.; Pu, Y. MWCNT interactions with protein: Surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity. Int. J. Nanomed. 2019, 14, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Prato, M. Under the lens: Carbon nanotube and protein interaction at the nanoscale. Chem. Commun. 2015, 51, 4347–4359. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, S.H.; Diduch, L.L.; Tegegn, T.Z.; Orecna, M.; Strader, M.B.; Karnaukhova, E.; Bonevich, J.E.; Holada, K.; Simak, J. The effect of protein corona composition on the interaction of carbon nanotubes with human blood platelets. Biomaterials 2014, 35, 6182–6194. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of Nanotube-Protein Corona Composition in Cell Culture Media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.L.; Li, D.J.; Yuan, L.; Yue, Y.C.; Liu, H.; Sun, X. Differences in cytocompatibility and hemocompatibility between carbon nanotubes and nitrogen-doped carbon nanotubes. Carbon N. Y. 2011, 49, 3125–3133. [Google Scholar] [CrossRef]
- Li, L.; Lin, R.; He, H.; Jiang, L.; Gao, M. Interaction of carboxylated single-walled carbon nanotubes with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 45–51. [Google Scholar] [CrossRef]
- Lou, K.; Zhu, Z.; Zhang, H.; Wang, Y.; Wang, X.; Cao, J. Comprehensive studies on the nature of interaction between carboxylated multi-walled carbon nanotubes and bovine serum albumin. Chem. Biol. Interact. 2016, 243, 54–61. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, H.; Wang, Y. New insight into the binding interaction of hydroxylated carbon nanotubes with bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 556–563. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Nikjoo, S.; Zare, N.; Delavar, D.; Beigoli, S.; Chamani, J. Characterization of the structural changes of human serum albumin upon interaction with single-walled and multi-walled carbon nanotubes: Spectroscopic and molecular modeling approaches. Res. Chem. Intermed. 2019, 45, 401–423. [Google Scholar] [CrossRef]
- Podila, R.; Vedantam, P.; Ke, P.C.; Brown, J.M.; Rao, A.M. Evidence for Charge-Transfer-Induced Conformational Changes in Carbon Nanostructure–Protein Corona. J. Phys. Chem. C 2012, 116, 22098–22103. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, D.; Hao, F.; Liu, R. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 2015, 292, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yang, Z.; Chong, Y.; Ge, C.; Weber, J.K.; Bell, D.R.; Zhou, R. Surface curvature relation to protein adsorption for carbon-based nanomaterials. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Capodanno, C.; Gambarotti, C.; Fasoli, E. Proteomic investigation on bio-corona of functionalized multi-walled carbon nanotubes. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Sopotnik, M.; Leonardi, A.; Križaj, I.; Dušak, P.; Makovec, D.; Mesarič, T.; Ulrih, N.P.; Junkar, I.; Sepčić, K.; Drobne, D. Comparative study of serum protein binding to three different carbon-based nanomaterials. Carbon N. Y. 2015, 95, 560–572. [Google Scholar] [CrossRef]
- Sacchetti, C.; Motamedchaboki, K.; Magrini, A.; Palmieri, G.; Mattei, M.; Bernardini, S.; Rosato, N.; Bottini, N.; Bottini, M. Surface Polyethylene Glycol Conformation Influences the Protein Corona of Polyethylene Glycol-Modified Single-Walled Carbon Nanotubes: Potential Implications on Biological Performance. ACS Nano 2013, 7, 1974–1989. [Google Scholar] [CrossRef]
- Morikawa, M.; Kuboki, Y.; Watari, F. Evaluation of protein adsorption to carbon nanotubes having different property, and identification of adsorbed proteins. Nano Biomed. 2012, 4, 66–75. [Google Scholar]
- Salvador-Morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green, M.L.H.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef]
- Pinals, R.L.; Yang, D.; Lui, A.; Cao, W.; Landry, M.P. Corona exchange dynamics on carbon nanotubes by multiplexed fluorescence monitoring. J. Am. Chem. Soc. 2020, 142, 1254–1264. [Google Scholar] [CrossRef]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed Proteins Influence the Biological Activity and Molecular Targeting of Nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Ding, Y.; Tian, R.; Li, L.; Liu, F. Adsorption of human serum albumin on functionalized single-walled carbon nanotubes reduced cytotoxicity. Chem. Biol. Interact. 2018, 295, 64–72. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, H.M.; Cao, J. Binding of hydroxylated single-walled carbon nanotubes to two hemoproteins, hemoglobin and myoglobin. J. Photochem. Photobiol. B Biol. 2014, 141, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef] [PubMed]
- Rybak-Smith, M.J.; Sim, R.B. Complement activation by carbon nanotubes. Adv. Drug Deliv. Rev. 2011, 63, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.J.; Wibroe, P.P.; Moghimi, S.M. Perspectives on carbon nanotube-mediated adverse immune effects. Adv. Drug Deliv. Rev. 2012, 64, 1700–1705. [Google Scholar] [CrossRef]
- Ling, W.L.; Biro, A.; Bally, I.; Tacnet, P.; Deniaud, A.; Doris, E.; Frachet, P.; Schoehn, G.; Pebay-Peyroula, E.; Arlaud, G.J. Proteins of the Innate Immune System Crystallize on Carbon Nanotubes but Are Not Activated. ACS Nano 2011, 5, 730–737. [Google Scholar] [CrossRef]
- Pondman, K.M.; Sobik, M.; Nayak, A.; Tsolaki, A.G.; Jäkel, A.; Flahaut, E.; Hampel, S.; ten Haken, B.; Sim, R.B.; Kishore, U. Complement activation by carbon nanotubes and its influence on the phagocytosis and cytokine response by macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1287–1299. [Google Scholar] [CrossRef]
- Pondman, K.M.; Salvador-Morales, C.; Paudyal, B.; Sim, R.B.; Kishore, U. Interactions of the innate immune system with carbon nanotubes. Nanoscale Horizons 2017, 2, 174–186. [Google Scholar] [CrossRef]
- Andersen, A.J.; Robinson, J.T.; Dai, H.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Single-Walled Carbon Nanotube Surface Control of Complement Recognition and Activation. ACS Nano 2013, 7, 1108–1119. [Google Scholar] [CrossRef]
- Andersen, A.J.; Windschiegl, B.; Ilbasmis-Tamer, S.; Degim, I.T.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Complement activation by PEG-functionalized multi-walled carbon nanotubes is independent of PEG molecular mass and surface density. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 469–473. [Google Scholar] [CrossRef]
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Owen, J.D.; Kock, N.D.; D’Agostino, R.; Torti, F.M.; Torti, S.V. Determinants of the thrombogenic potential of multiwalled carbon nanotubes. Biomaterials 2011, 32, 5970–5978. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Aseychev, A.V.; Kostevich, V.A.; Gusev, A.A.; Gusev, S.A.; Vlasova, I.I. Functionalization of single-walled carbon nanotubes regulates their effect on hemostasis. J. Phys. Conf. Ser. 2011, 291, 012054. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoet, P.H.M.; Vandervoort, P.; Dinsdale, D.; Nemery, B.; Hoylaerts, M.F. Enhanced peripheral thrombogenicity after lung inflammation is mediated by platelet-leukocyte activation: Role of P-selectin. J. Thromb. Haemost. 2007, 5, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Holzer, M.; Praetner, M.; Fent, J.; Lerchenberger, M.; Reichel, C.A.; Rehberg, M.; Lakatos, S.; Krombach, F. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology 2010, 269, 148–154. [Google Scholar] [CrossRef]
- Holzer, M.; Bihari, P.; Praetner, M.; Uhl, B.; Reichel, C.; Fent, J.; Vippola, M.; Lakatos, S.; Krombach, F. Carbon-based nanomaterials accelerate arteriolar thrombus formation in the murine microcirculation independently of their shape. J. Appl. Toxicol. 2014, 34, 1167–1176. [Google Scholar] [CrossRef]
- Semberova, J.; De Paoli Lacerda, S.H.; Simakova, O.; Holada, K.; Gelderman, M.P.; Simak, J. Carbon nanotubes activate blood platelets by inducing extracellular Ca 2+ influx sensitive to calcium entry inhibitors. Nano Lett. 2009, 9, 3312–3317. [Google Scholar] [CrossRef]
- De Paoli Lacerda, S.H.; Semberova, J.; Holada, K.; Simakova, O.; Hudson, S.D.; Simak, J. Carbon nanotubes activate store-operated calcium entry in human blood platelets. ACS Nano 2011, 5, 5808–5813. [Google Scholar] [CrossRef]
- Meng, J.; Cheng, X.; Liu, J.; Zhang, W.; Li, X.; Kong, H.; Xu, H. Effects of long and short carboxylated or aminated multiwalled carbon nanotubes on blood coagulation. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Fent, J.; Bihari, P.; Vippola, M.; Sarlin, E.; Lakatos, S. In vitro platelet activation, aggregation and platelet-granulocyte complex formation induced by surface modified single-walled carbon nanotubes. Toxicol. Vitr. 2015, 29, 1132–1139. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Gusev, A.A.; Gusev, S.A.; Vlasova, I.I. Albumin reduces thrombogenic potential of single-walled carbon nanotubes. Toxicol. Lett. 2013, 221, 137–145. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Mikhalchik, E.V.; Barinov, N.A.; Kostevich, V.A.; Smolina, N.V.; Klinov, D.V.; Sokolov, A.V. Adsorbed plasma proteins modulate the effects of single-walled carbon nanotubes on neutrophils in blood. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1615–1625. [Google Scholar] [CrossRef]
- Canapè, C.; Foillard, S.; Bonafè, R.; Maiocchi, A.; Doris, E. Comparative assessment of the in vitro toxicity of some functionalized carbon nanotubes and fullerenes. RSC Adv. 2015, 5, 68446–68453. [Google Scholar] [CrossRef]
- Donkor, A.D.; Su, Z.; Mandal, H.S.; Jin, X.; Tang, X.S. Carbon nanotubes inhibit the hemolytic activity of the pore-forming toxin pyolysin. Nano Res. 2009, 2, 517–525. [Google Scholar] [CrossRef]
- Sachar, S.; Saxena, R.K. Cytotoxic effect of poly-dispersed single walled carbon nanotubes on erythrocytes in vitro and in vivo. PLoS ONE 2011, 6, 20–25. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Saxena, R.K. Selective loss of younger erythrocytes from blood circulation and changes in erythropoietic patterns in bone marrow and spleen in mouse anemia induced by poly-dispersed single-walled carbon nanotubes. Nanotoxicology 2015, 9, 1032–1040. [Google Scholar] [CrossRef]
- Heo, Y.; Li, C.-A.; Kim, D.; Shin, S. Rheological alteration of erythrocytes exposed to carbon nanotubes. Clin. Hemorheol. Microcirc. 2017, 65, 49–56. [Google Scholar] [CrossRef]
- Geim, A.K. Nobel Lecture: Random walk to graphene. Rev. Mod. Phys. 2011, 83, 851–862. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon N. Y. 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Vijayaraghavan, A. Graphene—Properties and characterization. In Springer Handbook of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–82. ISBN 9783642205958. [Google Scholar]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.-G.; Park, J.Y.; Oh, Y.-K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016, 105, 205–227. [Google Scholar] [CrossRef]
- Jaleel, J.A.; Sruthi, S.; Pramod, K. Reinforcing nanomedicine using graphene family nanomaterials. J. Control. Release 2017, 255, 218–230. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Su, Y.-L.; Hu, S.-H.; Chen, S.-Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Gulzar, A.; Yang, P.; He, F.; Xu, J.; Yang, D.; Xu, L.; Jan, M.O. Bioapplications of graphene constructed functional nanomaterials. Chem. Biol. Interact. 2017, 262, 69–89. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Li, P.; Nie, Z.; Li, J. Applications of graphene and its derivatives in intracellular biosensing and bioimaging. Analyst 2016, 141, 4541–4553. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.; Li, W.J. Graphene-Based Glucose Sensors: A Brief Review. IEEE Trans. Nanobiosci. 2015, 14, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.-H.; Jiang, W.-J.; Zhao, J.-G.; Xiao, B.-G.; Zhang, G.-X.; Ma, C.-G. Graphene-Based Nanomaterials: Potential Tools for Neurorepair. Curr. Pharm. Des. 2018, 24, 56–61. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, H.; Chen, Y. Interaction of graphene oxide with human serum albumin and its mechanism. RSC Adv. 2014, 4, 55290–55295. [Google Scholar] [CrossRef]
- Li, S.; Aphale, A.N.; MacWan, I.G.; Patra, P.K.; Gonzalez, W.G.; Miksovska, J.; Leblanc, R.M. Graphene oxide as a quencher for fluorescent assay of amino acids, peptides, and proteins. ACS Appl. Mater. Interfaces 2012, 4, 7069–7075. [Google Scholar] [CrossRef]
- Wei, X.-Q.; Hao, L.-Y.; Shao, X.-R.; Zhang, Q.; Jia, X.-Q.; Zhang, Z.-R.; Lin, Y.-F.; Peng, Q. Insight into the Interaction of Graphene Oxide with Serum Proteins and the Impact of the Degree of Reduction and Concentration. ACS Appl. Mater. Interfaces 2015, 7, 13367–13374. [Google Scholar] [CrossRef]
- Loh, K.P.; Lim, C.T. Molecular Hemocompatibility of Graphene Oxide and Its Implication for Antithrombotic Applications. Small 2015, 11, 5105–5117. [Google Scholar]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced Cytotoxicity of Graphene Nanosheets Mediated by Blood-Protein Coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of graphene oxide on the structure and function of important multiple blood components by a dose-dependent pattern. J. Biomed. Mater. Res. Part A 2015, 103, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, V.; Zhao, W.; Boselli, L.; Lo Giudice, M.C.; Meder, F.; Polo, E.; Paton, K.R.; Backes, C.; Coleman, J.N.; Dawson, K.A. Biological recognition of graphene nanoflakes. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Chen, W.; Laurent, S.; Thirifays, C.; Burtea, C.; Rezaee, F.; Mahmoudi, M. Hard corona composition and cellular toxicities of the graphene sheets. Colloids Surfaces B Biointerfaces 2013, 109, 212–218. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, W.; Liu, F.; Liu, J.; Zhang, T.; Chen, W. Aggregation morphology is a key factor determining protein adsorption on graphene oxide and reduced graphene oxide nanomaterials. Environ. Sci. Nano 2019, 6, 1303–1309. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Petersen, S.V.; Bovet, N.; Laursen, B.W.; Moghimi, S.M. Soluble and immobilized graphene oxide activates complement system differently dependent on surface oxidation state. Biomaterials 2016, 78, 20–26. [Google Scholar] [CrossRef]
- Belling, J.N.; Jackman, J.A.; Yorulmaz Avsar, S.; Park, J.H.; Wang, Y.; Potroz, M.G.; Ferhan, A.R.; Weiss, P.S.; Cho, N.-J. Stealth Immune Properties of Graphene Oxide Enabled by Surface-Bound Complement Factor H. ACS Nano 2016, 10, 10161–10172. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ménard-Moyon, C.; Delogu, L.G.; Bianco, A. Graphene and the immune system: Challenges and potentiality. Adv. Drug Deliv. Rev. 2016, 105, 163–175. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Immunological effects of graphene family nanomaterials. NanoImpact 2017, 5, 109–118. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Bottini, M.; Fadeel, B. Graphene and the immune system: A romance of many dimensions. Front. Immunol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and Macrophage Response of Pristine and Functionalized Graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Shrivastava, S.; Grácio, J.J.A.; Dash, D. Thrombus Inducing Property of Atomically Thin Graphene Oxide Sheets. ACS Nano 2011, 5, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon N. Y. 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, B.G.; Alonso, B.; Sot, J.; García-Arribas, A.B.; Gil-Cartón, D.; Valle, M.; Zurutuza, A.; Goñi, F.M. Coating Graphene Oxide with Lipid Bilayers Greatly Decreases Its Hemolytic Properties. Langmuir 2017, 33, 8181–8191. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Mu, L. Biotransformation of graphene oxide nanosheets in blood plasma affects their interactions with cells. Environ. Sci. Nano 2017, 4, 1569–1578. [Google Scholar] [CrossRef]
- Papi, M.; Lauriola, M.C.; Palmieri, V.; Ciasca, G.; Maulucci, G.; De Spirito, M. Plasma protein corona reduces the haemolytic activity of graphene oxide nano and micro flakes. RSC Adv. 2015, 5, 81638–81641. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, N.; Yuan, J.; Wang, W.; Tang, Y.; Lu, C.; Zhang, J.; Shen, J. Antibacterial and anticoagulation properties of carboxylated graphene oxide–lanthanum complexes. J. Mater. Chem. 2012, 22, 1673–1678. [Google Scholar] [CrossRef]
- De Sousa, M.; Martins, C.H.Z.; Franqui, L.S.; Fonseca, L.C.; Delite, F.S.; Lanzoni, E.M.; Martinez, D.S.T.; Alves, O.L. Covalent functionalization of graphene oxide with d-mannose: Evaluating the hemolytic effect and protein corona formation. J. Mater. Chem. B 2018, 6, 2803–2812. [Google Scholar] [CrossRef]
- Geng, H.; Wang, T.; Cao, H.; Zhu, H.; Di, Z.; Liu, X. Antibacterial ability, cytocompatibility and hemocompatibility of fluorinated graphene. Colloids Surf. B Biointerfaces 2019, 173, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, Y.; Zhao, M.; Deng, J.; Li, X.; Li, D. The enhanced anticoagulation for graphene induced by COOH+ ion implantation. Nanoscale Res. Lett. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kanakia, S.; Toussaint, J.D.; Mullick Chowdhury, S.; Tembulkar, T.; Lee, S.; Jiang, Y.P.; Lin, R.Z.; Shroyer, K.R.; Moore, W.; Sitharaman, B. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 2014, 35, 7022–7031. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Ordooei, M.; Amiri, A.; Karimi-Zarchi, M. Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: In vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2018, 93, 70–78. [Google Scholar] [CrossRef]
- Geldert, A.; Liu, Y.; Loh, K.P.; Lim, C.T. Nano-bio interactions between carbon nanomaterials and blood plasma proteins: Why oxygen functionality matters. NPG Asia Mater. 2017, 9, e422. [Google Scholar]
- Mesarič, T.; Baweja, L.; Drašler, B.; Drobne, D.; Makovec, D.; Dušak, P.; Dhawan, A.; Sepčić, K. Effects of surface curvature and surface characteristics of carbon-based nanomaterials on the adsorption and activity of acetylcholinesterase. Carbon N. Y. 2013, 62, 222–232. [Google Scholar] [CrossRef]
- Mu, Q.; Liu, W.; Xing, Y.; Zhou, H.; Li, Z.; Zhang, Y.; Ji, L.; Wang, F.; Si, Z.; Zhang, B.; et al. Protein binding by functionalized multiwalled carbon nanotubes is governed by the surface chemistry of both parties and the nanotube diameter. J. Phys. Chem. C 2008, 112, 3300–3307. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Chen, M.; Yan, M.; Zeng, G.; Huang, D. How do proteins ‘response’ to common carbon nanomaterials? Adv. Colloid Interface Sci. 2019, 270, 101–107. [Google Scholar] [CrossRef]
- Belime, A.; Thielens, N.M.; Gravel, E.; Frachet, P.; Ancelet, S.; Tacnet, P.; Caneiro, C.; Chuprin, J.; Gaboriaud, C.; Schoehn, G.; et al. Recognition protein C1q of innate immunity agglutinates nanodiamonds without activating complement. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 292–302. [Google Scholar] [CrossRef]


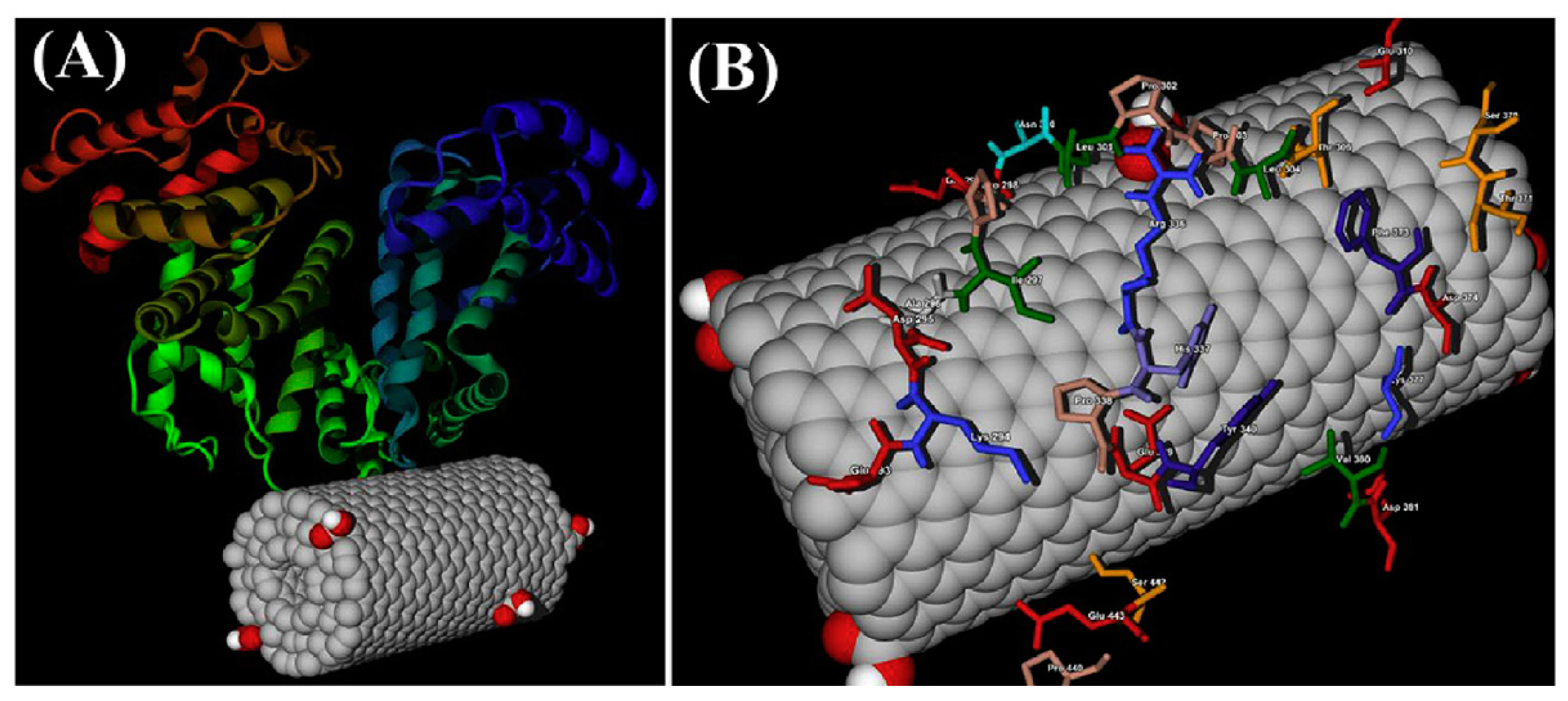
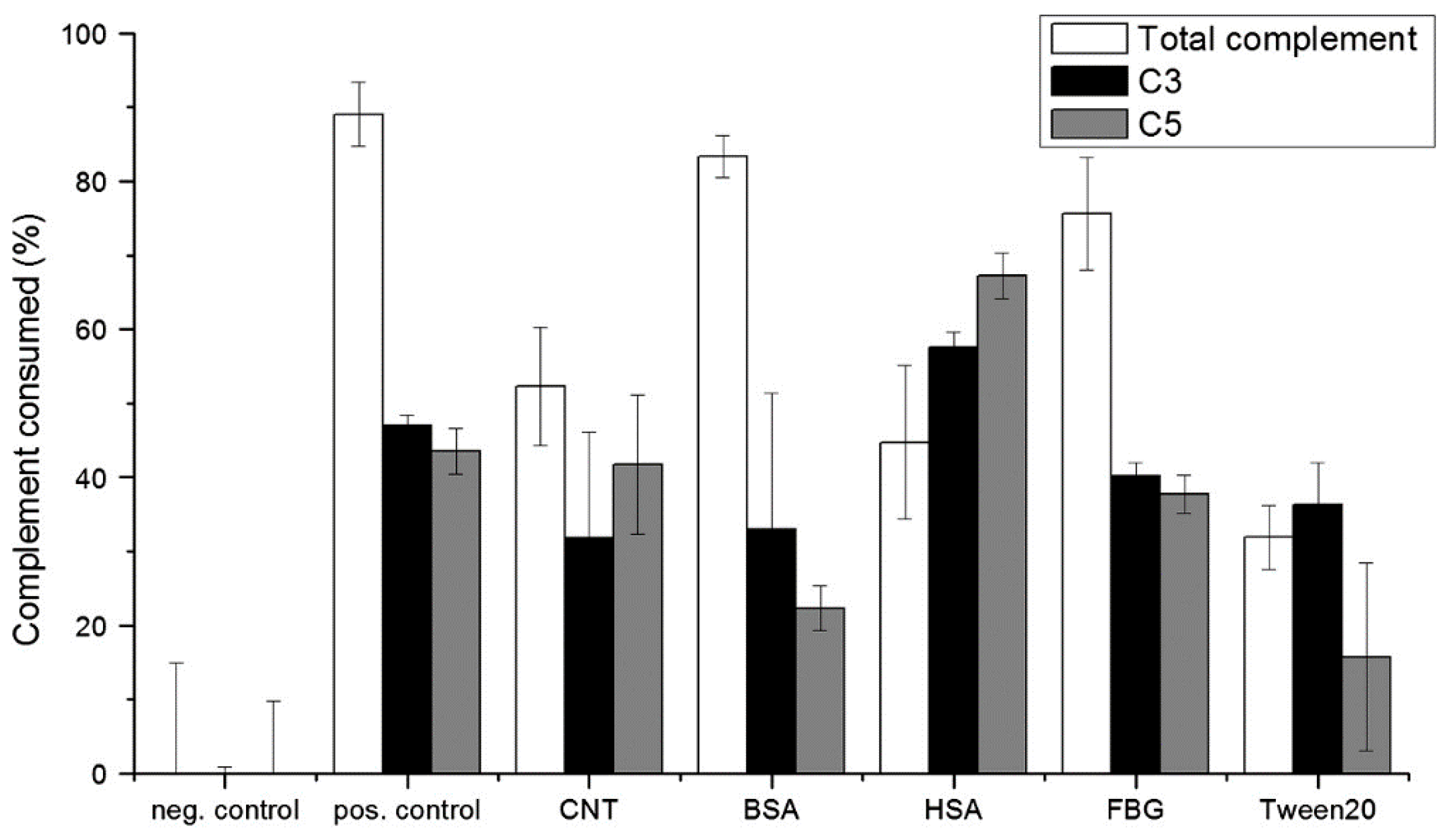
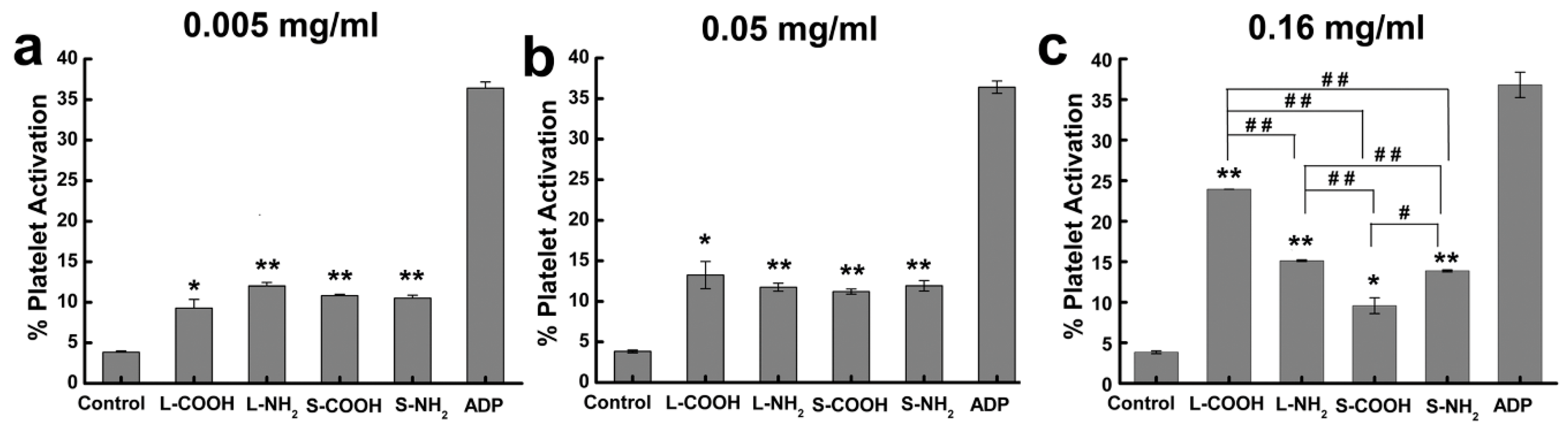
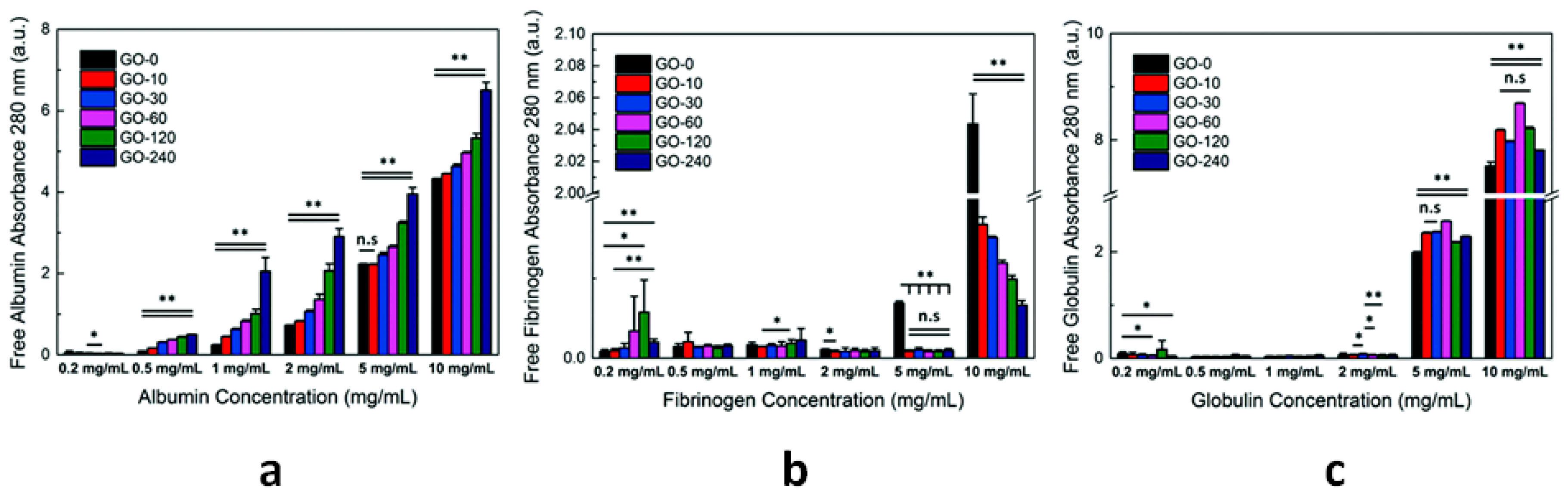
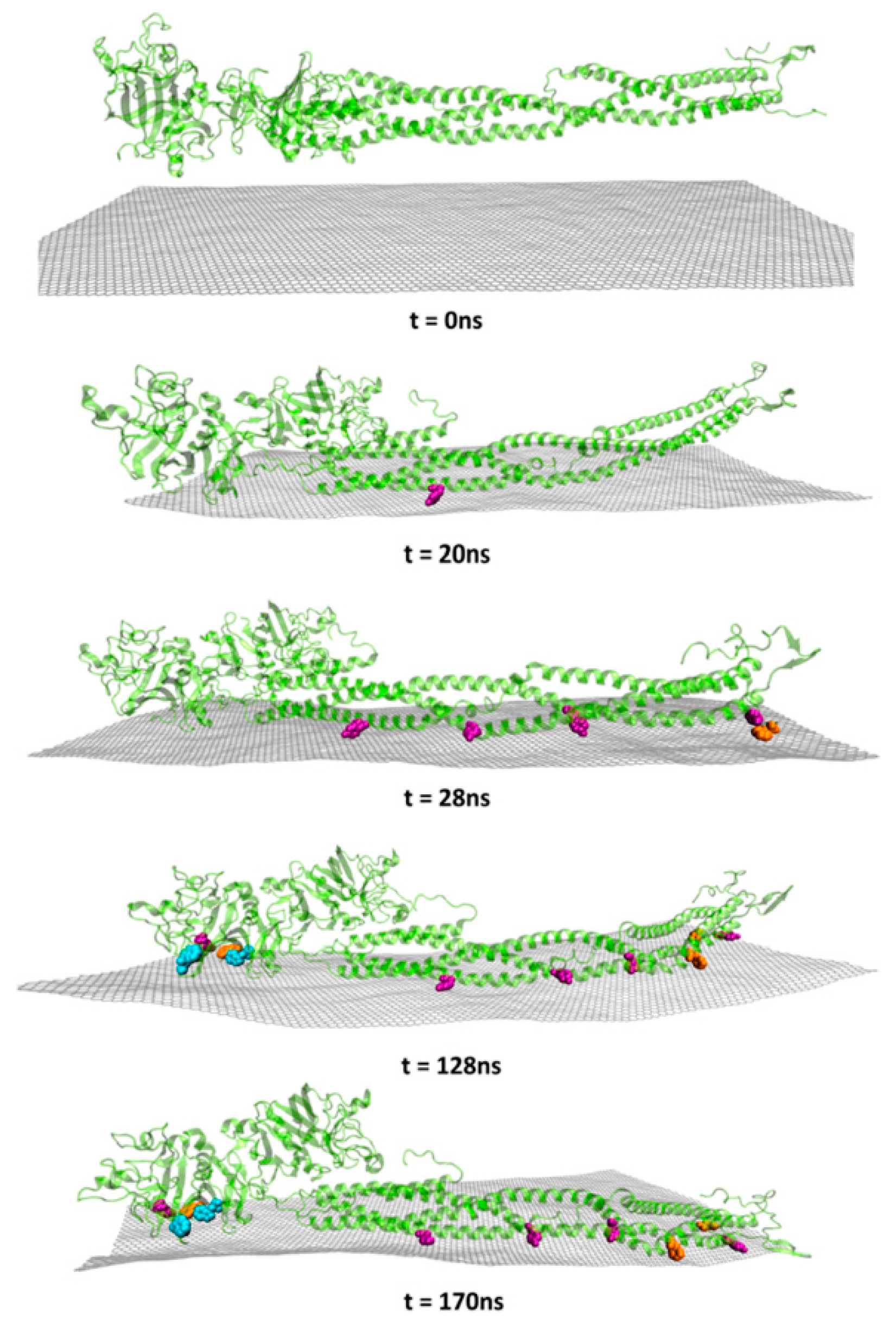
| CNs | Functionalization | PLATELETS | COAGULATION System | COMPLEMENT System | RBCs | In Vivo |
|---|---|---|---|---|---|---|
| NDs | -COOH | Activation and prothrombotic morphological alterations [82] | No effect on the intrinsic pathway (normal aPTT) [81,83] | C1q binding causes ND agglutination, phagocytosis and cytokine production [228] | Negligible hemolysis [83,84,86]. Hemolysis [85]. Erythrocyte deformation and aggregation [86] | Extensive thromboembolism in mice pulmonary vessels [82]. Absence of induced immune response [87] and inflammation [83] in mice. Well-tolerated in non-human primates and rats [88] |
| CFs | various | Minimal aggregation [164]. No aggregation [99] | Inhibition of fibrin polymerization [99] | - | Interaction [102,104,105] and modification of RBC membrane [101,103]. Hemolysis [103,106] | No significant effect on the development of rat carotid thrombosis [160]. Anticoagulant effects: increased tail bleeding time and inhibited thrombosis in rats [99]. Accelerated fibrinolysis [100] |
| CDs | various | Dose-dependent activation (P-selectin expression) [116] | Increased aPTT and PT at 1 mg/mL and 2 mg/mL [116] | Activation (increased C3a levels in blood plasma) at CD concentration ≥ 5 mg/mL [116] | Membrane deformation and hemolysis at concentrations ≥ 1 mg/mL [116]. Negligible hemolytic activity [118,119] | - |
| SW CNTs | Pristine | Activation and formation of platelet–granulocyte complexes [162,167]. Aggregation and activation [160] | - | Activation through classical pathway (C1q binding) [145] | No internalization, no toxicity [172]. Shape modification, fusion and hemolysis from bundled SWCNTs [175] | Accelerated thrombus formation in the microcirculation [162,163]. Amplification of vascular thrombosis in rats (carotid artery) [160] |
| -COOH | Activation and formation of platelet–granulocyte complexes [167] | Activation of the contact pathway [159] | - | Dose- and time-dependent hemolysis [173] | Toxic effect on erythrocytes and transient anemia in mice [173] | |
| MW CNTs | Pristine | Aggregation, activation [160,165] and formation of PMPs [164]. Minimal activation [158] | Activation of the intrinsic pathway [158] | Activation through classical and alternative pathway [145]. Binding of C1q and C1s-C1r-C1r-C1s but no C1q activation [153]. Consumption of C3 and C5 [154] | No significant hemolytic activity [171] | Pro-coagulant activity in a mouse model, formation of large intravascular aggregates [158]. Amplification of vascular thrombosis in rats (carotid artery) [160] |
| -COOH | Minimal activation [158]. Aggregation and release of PMPs [131]. Dose-dependent activation [166] | High activation of the intrinsic pathway [158]. Reduced fibrin clot formation times [166] | Activation through classical and alternative pathway (C1, C4a, C4b adsorption) [141] | - | Little pro-coagulant effect in mice [158] | |
| -NH2 | High activation and aggregation [158]. Dose-dependent activation [166] | Activation of the intrinsic pathway [158]. Reduced fibrin clot formation times [166] | Activation through classical and alternative pathway (C1, C4a, C4b adsorption) [141] | - | Modest effects on coagulation in mice—transient PLT depletion [158] | |
| GBNs | Pristine | Absence of activation and aggregation [210] | Normal values of PPT and aPTT [210] | - | No hemolytic effect up to 75 µg/mL [210] Hemolysis GS < GO [213] | - |
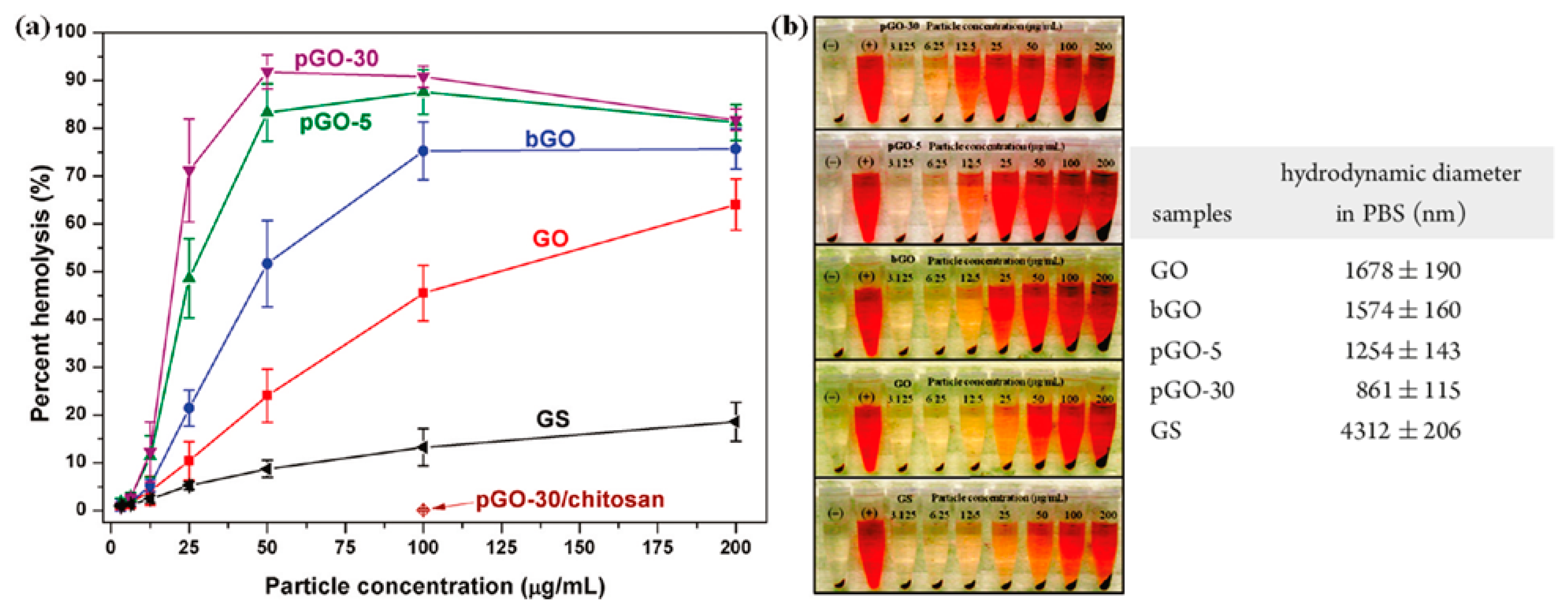
| GO | Variable content of oxygen-based groups | Strong aggregation and slight activation (GO > rGO) [211] | Prolonged aPTT [83] | Activation (increase of SC5b-9 and C4d levels) proportional to oxygen surface content [205] | Dose-dependent hemolytic activity [212,213]. Change of cell morphology and hemoglobin release [83] | Extensive pulmonary thromboembolism in mice [211]. Pathological effects when administered intravenously in mice [212] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedel, M. Hemocompatibility of Carbon Nanostructures. C 2020, 6, 12. https://doi.org/10.3390/c6010012
Fedel M. Hemocompatibility of Carbon Nanostructures. C. 2020; 6(1):12. https://doi.org/10.3390/c6010012
Chicago/Turabian StyleFedel, Mariangela. 2020. "Hemocompatibility of Carbon Nanostructures" C 6, no. 1: 12. https://doi.org/10.3390/c6010012
APA StyleFedel, M. (2020). Hemocompatibility of Carbon Nanostructures. C, 6(1), 12. https://doi.org/10.3390/c6010012





