Functionalization of Carbon Nanomaterials for Biomedical Applications
Abstract
1. Introduction
2. Graphene
2.1. Synthesis
2.1.1. Bottom-Up Synthesis
2.1.2. Top-Down Synthesis
2.2. Functionalized Graphene-Based Materials
2.2.1. Non-Covalent Functionalized Graphene-Based Materials
2.2.2. Covalent Functionalized Graphene-Based Materials
2.2.3. Nanoparticle Functionalized Graphene-Based Materials
3. Carbon Nanotubes
3.1. Synthesis
3.2. Functionalized CNTs
3.2.1. Noncovalent Functionalization
3.2.2. Covalent Functionalization
4. NanoDiamonds and Diamond Films
4.1. Synthesis
4.2. Functionalized NanoDiamond
5. Carbon Fibers
5.1. Synthesis of Carbon Fibers
5.2. Functionalization of Carbon Fibers
6. Fullerenes
6.1. Synthesis of Fullerenes
6.2. Functionalization of Fullerenes
7. Conclusions
Funding
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets intoBlue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamonds and Fullerene, 1st ed.; Noyes Publications: Park Ridge, NJ, USA, 1994. [Google Scholar]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Perkins, B.L.; Naderi, N. Carbon Nanostructures in Bone Tissue Engineering. Open Orthop. J. 2016, 10, 877–899. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, S.I.; Song, G.Y.; Kim, I. Non-Covalently Functionalized Carbon Nanostructures for Synthesizing Carbon-Based Hybrid Nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Czerniak-Reczulska, M.; Niedzielski, P.; Balcerczyk, A.; Bartosz, G.; Karowicz-Bilinska, A.; Mitura, K. Biological properties of different type carbon particles. In vitro study on primary culture of endothelial cells. J. Nanosci. Nanotechnol. 2010, 10, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, Y.K.; Lee, J.Y.; Hong, J.H.; Khang, D. PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology 2017, 28, 465102. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, M. Target-directed functionalized ferrous phosphate-carbon dots fluorescent nanostructures as peroxidase mimetics for cancer cell detection and ROS-mediated therapy. Sens. Actuators B 2019, 297, 126739. [Google Scholar] [CrossRef]
- Gopi, K.; Murugan, V.; Gnanasekaran, J.; Krishnaswamy, B.; James, J. Amygdalin-Functionalized Carbon Quantum Dots for Probing β-Glucosidase Activity for Cancer Diagnosis and Therapeutics. ACS Biomater. Sci. Eng. 2019, 5, 3089–3099. [Google Scholar]
- Pirsaheb, M.; Mohammadi, S.; Salimi, A.; Payandeh, M. Functionalized fluorescent carbon nanostructures for targeted imaging of cancer cells: A review. Microchim. Acta 2019, 186, 231. [Google Scholar] [CrossRef]
- Meng, Z.; Song, Y.; Guo, C.; Cui, B.; Ji, H.; Ma, Z. Tailoring the dimensionality of carbon nanostructures as highly electrochemical supports for detection of carcinoembryonic antigens. RSC Adv. 2019, 9, 13431–13443. [Google Scholar] [CrossRef]
- Ibáñez-Redína, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Cui, L.; Wang, M.; Sun, B.; Ai, S.; Wang, S.; Zhang, C.Y. Substrate-free and label-free electrocatalysis-assisted biosensor for sensitive detection of microRNA in lung cancer cells. Chem. Commun. 2019, 55, 1172–1175. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, e1802368. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharm. Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jing, L.; Liu, Z.; Gao, D.; Chen, H.; Song, W.; Wang, T.; Fang, X.; Qin, W.; Zhen, Y.; et al. Mesoporous carbon nanospheres as a multifunctional carrier for cancer theranostics. Theranostics 2018, 8, 663–675. [Google Scholar] [CrossRef]
- Wang, H.; Bi, J.; Zhu, B.W.; Tan, M. Multicolorful Carbon Dots for Tumor Theranostics. Curr. Med. Chem. 2018, 25, 2894–2909. [Google Scholar] [CrossRef]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generationand quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013, 8, 2003–2014. [Google Scholar]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef]
- Ma, J.; Huang, J.; Song, S.; Chen, H.; Zhang, Z. Cancer-Targeted Nanotheranostics Recent Advances and Perspectives. Small 2016, 12, 4936–4954. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Yi, J.; Fu, J.; Di, J.; del Carmen Alonso, A.; Zhou, S. Fluorescent porous carbon nanocapsules for two-photon imaging, NIR/pH dual-responsive drug carrier, and photothermal therapy. Biomaterials 2015, 53, 117–126. [Google Scholar] [CrossRef]
- Li, M.; Teh, C.; Ang, C.Y.; Tan, S.Y.; Luo, Z.; Qu, Q.; Zhang, Y.; Korzh, V.; Zhao, Y. Near-Infrared Light-Absorptive Stealth Liposomes for Localized Photothermal Ablation of Tumors Combined with Chemotherapy. Adv. Funct. Mater. 2015, 25, 5602–5610. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Zhong, H.; Qin, X.; Wan, M.; Yang, B. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging. Phys. Chem. Chem. Phys. 2013, 15, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The Advances of Carbon Nanotubes in Cancer Diagnostics and Therapeutics. J. Nanomater. 2017, 2017, 3418932. [Google Scholar] [CrossRef]
- Bardhan, N.M. 30 years of advances in functionalization of carbon nanomaterials for biomedical applications: A practical review. J. Mater. Res. 2017, 32, 107–127. [Google Scholar] [CrossRef]
- Atta Editor, N.F. Designing Nanosensors for Chemical and Biological Applications; International Frequency Sensor Association (IFSA): Barcelona, Spain, 2017; ISBN 978-84-697-3290-8. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-5396-2. [Google Scholar]
- Aliofkhazraei, M.; Ali, N.; Milne, W.I.; Ozkan, C.S.; Mitura, S.; Gervasoni, J.L. Graphene Science Handbook: Nanostructure and Atomic Arrangement; CRC Press: Boca Raton, FL, USA, 2016; ISBN 0-429-16937-X. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Sudan, P.; Mauron, P.; Wenger, P. Model for the hydrogen adsorption on carbon nanostructures. Appl. Phys. A 2004, 78, 941–946. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Uz, M.; Jackson, K.; Donta, M.S.; Jung, J.; Lentner, M.T.; Hondred, J.A.; Claussen, J.C.; Mallapragada, S.K. Fabrication of High-resolution Graphene-based Flexible Electronics via Polymer Casting. Sci. Rep. 2019, 9, 10595. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Song, X.; Ran, Q.; Yu, C.; Yang, J.; Feng, H.; Yu, L.; Wei, D. Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology 2017, 28, 315501. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Chougale, A.D.; Kollu, P.; Patil, P.S.; Patil, P.B. Magnetic nanoparticle decorated graphene based electrochemical nanobiosensor for H2O2 sensing using HRP. Colloids Surf. B Biointerfaces 2018, 167, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Balamurugan, A.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J. Mater. Chem. B 2015, 3, 1360–1370. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Effective seed-assisted synthesis of gold nanoparticles anchored nitrogen-doped graphene for electrochemical detection of glucose and dopamine. Biosens. Bioelectron. 2016, 81, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B Chem. 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Wang, Y.; Sauriat-Dorizon, H.; Korri-Youssoufi, H. Direct electrochemical DNA biosensor based on reduced graphene oxide and metalloporphyrin nanocomposite. Sens. Actuators B Chem. 2017, 251, 40–48. [Google Scholar] [CrossRef]
- Tian, M.; Xu, S.; Zhang, J.; Wang, X.; Li, Z.; Liu, H.; Song, R.; Yu, Z.; Wang, J. RNA Detection Based on Graphene Field-Effect Transistor Biosensor. Adv. Condens. Matter Phys. 2018, 2018, 8146765. [Google Scholar] [CrossRef]
- Eksin, E.; Bikkarolla, S.K.; Erdem, A.; Papakonstantinou, P. Chitosan/Nitrogen Doped Reduced Graphene Oxide Modified Biosensor for Impedimetric Detection of microRNA. Electroanalysis 2018, 30, 551–560. [Google Scholar] [CrossRef]
- Holade, Y.; Tingry, S.; Servat, K.; Napporn, T.W.; Cornu, D.; Kokoh, K.B. Nanostructured Inorganic Materials at Work in Electrochemical Sensing and Biofuel Cells. Catalysts 2017, 7, 31. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose Oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of direct electron transfer and enzyme activity of glucose oxidase on graphene surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Chen, J.; Zheng, X.; Liu, C.; Xue, T.; Wang, H.; Jin, Z.; Qiao, L.; Zheng, W. Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv. 2012, 2, 6245–6249. [Google Scholar] [CrossRef]
- Wu, G.; Song, X.; Wu, Y.-F.; Chen, X.; Luo, F.; Chen, X. Non-enzymatic electrochemical glucose sensor based on platinum nanoflowers supported on graphene oxide. Talanta 2013, 105, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.T.; Aravind, S.S.J.; Arockiadoss, T.; Rakhi, R.B.; Ramaprabhu, S. Metal decorated graphene nanosheets as immobilization matrix for amperometric glucose biosensor. Sens. Actuators B Chem. 2010, 145, 71–77. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, D.; Justin, G.J.; Xue, Y.; Dai, L. Flexible fiber-shaped non-enzymatic sensors with a graphene-metal heterostructure based on graphene fibres decorated with gold nanosheets. Carbon 2018, 136, 329–336. [Google Scholar] [CrossRef]
- Janyasupab, M.; Promptmas, C. Development of Non-Enzymatic N-doped Graphene supported Cobalt/Iron Amperometric based Sensor for Glucose Detection in Urine. In Proceedings of the IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; pp. 577–582. [Google Scholar]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, X.; Xing, D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials 2014, 35, 4185–4194. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int. J. Biol. Macromol. 2018, 107, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, M.; Namazi, H.; Akbarzadeh, A.; Kabiri, R. Synthesis and characterization of PEG-functionalized graphene oxide as an effective pH-sensitive drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.; Lee, H.L.; Lee, S.; Choi, J.S.; Kim, S.J.; Jeong, Y.-I.; Kang, D.H. Redox-Responsive Nanocomposites Composed of Graphene Oxide and Chlorin e6 for Photodynamic Treatment of Cholangiocarcinoma. Bull. Korean Chem. Soc. 2018, 39, 1073–1082. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Deb, A.; Andrews, N.G.; Raghavan, V. Natural polymer functionalized graphene oxide for co-delivery of anticancer drugs: In-vitro and in-vivo. Int. J. Biol. Macromol. 2018, 113, 515–525. [Google Scholar] [CrossRef]
- Chai, D.; Hao, B.; Hu, R.; Zhang, F.; Yan, J.; Sun, Y.; Huang, X.; Zhang, Q.; Jiang, H. Delivery of Oridonin and Methotrexate via PEGylated Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 22915–22924. [Google Scholar] [CrossRef]
- Karimi Shervedani, R.; Mirhosseini, H.; Samiei Foroushani, M.; Torabi, M.; Rahsepar, F.R.; Norouzi-Barough, L. Immobilization of methotrexate anticancer drug onto the graphene surface and interaction with calf thymus DNA and 4T1 cancer cells. Bioelectrochemistry 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, B.; Li, C.; Kuang, W.; Zhang, J.; Xiong, Y.; Tan, S.; Cai, X.; Huang, L. Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery. Nanotechnol. Rev. 2018, 7, 291. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Curr. Appl. Phys. 2017, 17, 249–254. [Google Scholar] [CrossRef]
- Xie, M.; Lei, H.; Zhang, Y.; Xu, Y.; Shen, S.; Ge, Y.; Li, H.; Xie, J. Non-covalent modification of graphene oxide nanocomposites with chitosan/dextran and its application in drug delivery. RSC Adv. 2016, 6, 9328–9337. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Nandi, S.; Das, P.; Nandi, A.K. Fluorescent Graphene Oxide via Polymer Grafting: An Efficient Nanocarrier for Both Hydrophilic and Hydrophobic Drugs. ACS Appl. Mater. Interfaces 2015, 7, 3512–3523. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef]
- Lan, M.; Beghein, N.; Charlier, N.; Gallez, B. Carbon Blacks as EPR Sensors for Localized Measurements of Tissue Oxygenation. Magn. Reson. Med. 2004, 51, 1272–1278. [Google Scholar] [CrossRef]
- Rao, S.S.; Stesmans, A.; Keunen, K.; Kosynkin, D.V.; Higginbotham, A.; Tour, J.M. Unzipped graphene nanoribbons as sensitive O2 sensors: Electron spin resonance probing and dissociation kinetics. Appl. Phys. Lett. 2011, 98, 083116. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Choi, S.Y.; Baek, S.H.; Chang, S.-J.; Song, Y.; Rafique, R.; Lee, K.T.; Park, T.J. Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosens. Bioelectron. 2017, 93, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, L.; Wang, J.; Peng, F.; Luo, D.; Cui, R.; Niu, Y.; Qin, X.; Liu, Y.; Sun, H.; et al. Cell imaging by graphene oxide based on surface enhanced Raman scattering. Nanoscale 2012, 4, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X.-M. Gold Nanoclusters and Graphene Nanocomposites for Drug Delivery and Imaging of Cancer Cells. Angew. Chem. Int. Ed. 2011, 50, 11644–11648. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y.; Luo, Z.; Zhao, Y.; Ng, K.W.; Zhao, Y. Graphene oxide wrapped gold nanoparticles for intracellular Raman imaging and drug delivery. J. Mater. Chem. B 2013, 1, 6495–6500. [Google Scholar] [CrossRef]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, H.; Wang, M.; Huang, C.; Yang, D.; Jia, N. Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Mater. Sci. Eng. C 2017, 78, 817–825. [Google Scholar] [CrossRef]
- Patrick, C.W.; Mikos, A.G.; McIntire, L.V. Chapter I—Prospectus of Tissue Engineering. In Frontiers in Tissue Engineering; Patrick, C.W., Mikos, A.G., McIntire, L.V., Langer, R.S., Eds.; Pergamon: Oxford, UK, 1998; pp. 3–11. ISBN 978-0-08-042689-1. [Google Scholar]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Badami, D.V. X-ray studies of graphite formed by decomposing silicon carbide. Carbon 1965, 3, 53–57. [Google Scholar] [CrossRef]
- Van Bommel, A.J.; Crombeen, J.E.; Van Tooren, A. LEED and Auger electron observations of the SiC (0001) surface. Surf. Sci. 1975, 48, 463–472. [Google Scholar] [CrossRef]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Tromp, R.M.; Hannon, J.B. Thermodynamics and Kinetics of Graphene Growth on SiC (0001). Phys. Rev. Lett. 2009, 102, 106104. [Google Scholar] [CrossRef] [PubMed]
- Juang, Z.-Y.; Wu, C.-Y.; Lo, C.-W.; Chen, W.-Y.; Huang, C.-F.; Hwang, J.-C.; Chen, F.-R.; Leou, K.-C.; Tsai, C.-H. Synthesis of graphene on silicon carbide substrates at low temperature. Carbon 2009, 47, 2026–2031. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Yang, J.; Zeng, X.; Yuan, W. Preparation of single- and few-layer graphene sheets using co deposition on SiC substrate. J Nanomater. 2011, 2011, 44. [Google Scholar] [CrossRef]
- Osikoya, A.O.; Parlak, O.; Murugan, N.A.; Dikio, E.D.; Moloto, H.; Uzun, L.; Turner, A.P.F.; Tiwari, A. Acetylene-sourced CVD-synthesised catalytically active graphene for electrochemical biosensing. Biosens. Bioelectron. 2017, 89, 496–504. [Google Scholar] [CrossRef]
- Cushing, G.W.; Johánek, V.; Navin, J.K.; Harrison, I. Graphene Growth on Pt (111) by Ethylene Chemical Vapor Deposition at Surface Temperatures near 1000 K. J. Phys. Chem. C 2015, 119, 4759–4768. [Google Scholar] [CrossRef]
- Gotterbarm, K.; Zhao, W.; Höfert, O.; Gleichweit, C.; Papp, C.; Steinrück, H.-P. Growth and oxidation of graphene on Rh (111). Phys. Chem. Chem. Phys. 2013, 15, 19625–19631. [Google Scholar] [CrossRef]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Zhao, P.; Kumamoto, A.; Kim, S.; Chen, X.; Hou, B.; Chiashi, S.; Einarsson, E.; Ikuhara, Y.; Maruyama, S. Self-Limiting Chemical Vapor Deposition Growth of Monolayer Graphene from Ethanol. J. Phys. Chem. C 2013, 117, 10755–10763. [Google Scholar] [CrossRef]
- Bautista, C.; Mendoza, D. Multilayer graphene synthesized by CVD using liquid hexane as the carbon precursor. arXiv 2011, arXiv:1109.1318. [Google Scholar]
- Jang, J.; Son, M.; Chung, S.; Kim, K.; Cho, C.; Lee, B.H.; Ham, M.-H. Low-temperature-grown continuous graphene films from benzene by chemical vapor deposition at ambient pressure. Sci. Rep. 2015, 5, 17955. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hao, Y.; Ren, Y.; Charlton, M.; Lee, W.H.; Wu, Q.; Li, H.; Zhu, Y.; Wu, Y.; Piner, R.; et al. Graphene Growth Using a Solid Carbon Feedstock and Hydrogen. ACS Nano 2011, 5, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. Controllable Synthesis of Graphene on Rh. In Controlled Synthesis and Scanning Tunneling Microscopy Study of Graphene and Graphene-Based Heterostructures; Springer: Singapore, 2018; pp. 19–35. ISBN 978-981-10-5181-4. [Google Scholar]
- Wang, S.M.; Pei, Y.H.; Wang, X.; Wang, H.; Meng, Q.N.; Tian, H.W.; Zheng, X.L.; Zheng, W.T.; Liu, Y.C. Synthesis of graphene on a polycrystalline Co film by radio-frequency plasma-enhanced chemical vapour deposition. J. Phys. Appl. Phys. 2010, 43, 455402. [Google Scholar] [CrossRef]
- Dai, B.; Fu, L.; Zou, Z.; Wang, M.; Xu, H.; Wang, S.; Liu, Z. Rational design of a binary metal alloy for chemical vapour deposition growth of uniform single-layer graphene. Nat. Commun. 2011, 2, 522. [Google Scholar] [CrossRef]
- Choi, H.; Lim, Y.; Park, M.; Lee, S.; Kang, Y.; Kim, M.S.; Kim, J.; Jeon, M. Precise control of chemical vapor deposition graphene layer thickness using NixCu1−x alloys. J. Mater. Chem. C 2015, 3, 1463–1467. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Yuan, Q.; Xue, J.; Lu, G.; Liu, Z.; Wang, H.; Wang, H.; Ding, F.; Yu, Q.; et al. Fast growth of inch-sized single-crystalline graphene from a controlled single nucleus on Cu–Ni alloys. Nat. Mater. 2015, 15, 43. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Prakash, J.; Chen, Z.; Gauthier, M.; Sun, S. Chemical vapour deposition of graphene: Layer control, the transfer process, characterisation, and related applications. Int. Rev. Phys. Chem. 2019, 38, 149–199. [Google Scholar] [CrossRef]
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-enhanced chemical vapor deposition of graphene on copper substrates. AIP Adv. 2014, 4, 047128. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Knieke, C.; Berger, A.; Voigt, M.; Taylor, R.N.K.; Röhrl, J.; Peukert, W. Scalable production of graphene sheets by mechanical delamination. Carbon 2010, 48, 3196–3204. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, L.; Jiang, C.; Chen, S.; Nie, Z. Synthesis of graphene nanosheet powder with layer number control via a soluble salt-assisted route. RSC Adv. 2014, 4, 13350–13354. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Quezada-Renteria, J.A.; Ania, C.O.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Influence of protons on reduction degree and defect formation in electrochemically reduced graphene oxide. Carbon 2019, 149, 722–732. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Mei, X.; Meng, X.; Wu, F. Hydrothermal method for the production of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 68, 81–86. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Lee, H. Highly qualified reduced graphene oxides: The best chemical reduction. Chem. Commun. 2011, 47, 9681–9683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.; Lee, J.H. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In Vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Na, H.-K.; Kim, S.; Jang, H.; Chang, S.-J.; Min, D.-H. One-Pot Synthesis of Multifunctional Au@Graphene Oxide Nanocolloid Core@Shell Nanoparticles for Raman Bioimaging, Photothermal, and Photodynamic Therapy. Small 2015, 11, 2527–2535. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014, 32, 312–320. [Google Scholar] [CrossRef]
- Taha, A.A.; Mousa, A.; Al-ott, M.; Faroun, M.; Assali, M.; Gomez, P.R.; Thiab, S. Non-Covalent Functionalization of Graphene Sheets with Surfactants and their Antibacterial Activity. Palest. Med. Pharm. J. 2016, 1, 65–72. [Google Scholar]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Layek, R.K.; Uddin, M.E.; Kim, N.H.; Tak Lau, A.K.; Lee, J.H. Noncovalent functionalization of reduced graphene oxide with pluronic F127 and its nanocomposites with gum arabic. Compos. Part B Eng. 2017, 128, 155–163. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Liu, Z. Graphene based gene transfection. Nanoscale 2011, 3, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. pH-Responsive Chitosan-Mediated Graphene Dispersions. Langmuir 2010, 26, 16771–16774. [Google Scholar] [CrossRef] [PubMed]
- Husale, B.S.; Sahoo, S.; Radenovic, A.; Traversi, F.; Annibale, P.; Kis, A. ssDNA Binding Reveals the Atomic Structure of Graphene. Langmuir 2010, 26, 18078–18082. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, E.; Yang, Z.; Loh, K.P. Optimizing Label-Free DNA Electrical Detection on Graphene Platform. Anal. Chem. 2011, 83, 2452–2460. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Lee, D.-H.; Cho, H.-S.; Han, D.; Chand, R.; Yoon, T.-J.; Kim, Y.-S. Highly selective organic transistor biosensor with inkjet printed graphene oxide support system. J. Mater. Chem. B 2017, 5, 3580–3585. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Hage, C.-H.; Spadavecchia, J.; Serrano, A.Y.; Larroulet, I.; Pesquera, A.; Zurutuza, A.; Pisfil, M.G.; Héliot, L.; Boukaert, J.; et al. Plasmonic photothermal destruction of uropathogenic E. coli with reduced graphene oxide and core/shell nanocomposites of gold nanorods/reduced graphene oxide. J. Mater. Chem. B 2015, 3, 375–386. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, B.-S.; Park, S.; Yu, W.-R. A facile route to mechanically robust graphene oxide fibers. RSC Adv. 2019, 9, 20248–20255. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Sarkar, K.; Chatterjee, K. Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 2016, 8, 6820–6836. [Google Scholar] [CrossRef] [PubMed]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y.-P. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Gómez, M.A.; Martínez, G. Polymeric Modification of Graphene through Esterification of Graphite Oxide and Poly (vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G. Importance of Covalent Linkages in the Preparation of Effective Reduced Graphene Oxide−Poly (vinyl chloride) Nanocomposites. Macromolecules 2011, 44, 2685–2692. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-Soluble Graphene Covalently Functionalized by Biocompatible Poly-l-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
- Kasprzak, A.; Zuchowska, A.; Poplawska, M. Functionalization of graphene: Does the organic chemistry matter? Beilstein J. Org. Chem. 2018, 14, 2018–2026. [Google Scholar] [CrossRef]
- Wei, G.; Yan, M.; Dong, R.; Wang, D.; Zhou, X.; Chen, J.; Hao, J. Covalent Modification of Reduced Graphene Oxide by Means of Diazonium Chemistry and Use as a Drug-Delivery System. Chem. Eur. J. 2012, 18, 14708–14716. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold-graphene nanocomposites for sensing and biomedical applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.S.; Raj, C.R. Development of an Amperometric Cholesterol Biosensor Based on Graphene-Pt Nanoparticle Hybrid Material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Swain, A.K.; Pradhan, L.; Bahadur, D. Polymer Stabilized Fe3O4-Graphene as an Amphiphilic Drug Carrier for Thermo-Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2015, 7, 8013–8022. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, D.; Mei, L.; Li, Q.; Zhu, H.; Wang, T. Targeting Chemophotothermal Therapy of Hepatoma by Gold Nanorods/Graphene Oxide Core/Shell Nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef]
- Li, D.; Deng, M.; Yu, Z.; Liu, W.; Zhou, G.; Li, W.; Wang, X.; Yang, D.-P.; Zhang, W. Biocompatible and Stable GO-Coated Fe3O4 Nanocomposite: A Robust Drug Delivery Carrier for Simultaneous Tumor MR Imaging and Targeted Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2143–2154. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Yang, D.; Xu, L.; He, F.; Gai, S.; Yang, P. Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018, 47, 3931–3939. [Google Scholar] [CrossRef]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Li, Q.W.; Li, Y.; Zhang, X.F.; Chikkannanavar, S.B.; Zhao, Y.H.; Dangelewicz, A.M.; Zheng, L.X.; Doorn, S.K.; Jia, Q.X.; Peterson, D.E.; et al. Structure-Dependent Electrical Properties of Carbon Nanotube Fibers. Adv. Mater. 2007, 19, 3358–3363. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Chen, B.; Cassell, A.; Delzeit, L.; Han, J.; Meyyappan, M. Pore structure of raw and purified HiPco single-walled carbon nanotubes. Chem. Phys. Lett. 2002, 365, 69–74. [Google Scholar] [CrossRef]
- Priyanka, S.; Neelesh Kumar, M.; Keerti, J.; Jain, N.K. Biomedical Applications of Carbon Nanotubes: A Critical Review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar]
- Joselevich, E.; Lieber, C.M. Vectorial Growth of Metallic and Semiconducting Single-Wall Carbon Nanotubes. Nano Lett. 2002, 2, 1137–1141. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Cao, Y.; Cong, S.; Cao, X.; Wu, F.; Liu, Q.; Amer, M.R.; Zhou, C. Review of Electronics Based on Single-Walled Carbon Nanotubes. In Single-Walled Carbon Nanotubes: Preparation, Properties and Applications; Li, Y., Maruyama, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–224. ISBN 978-3-030-12700-8. [Google Scholar]
- Yan, Y.; Li, C.; Liu, C.; Mutlu, Z.; Dong, B.; Liu, J.; Ozkan, C.S.; Ozkan, M. Bundled and dispersed carbon nanotube assemblies on graphite superstructures as free-standing lithium-ion battery anodes. Carbon 2019, 142, 238–244. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Zeng, G.; Gong, J.; Zhang, P.; Feng, H.; Liu, Y.; Ren, X. Carbon nanotube-based environmental technologies: The adopted properties, primary mechanisms, and challenges. Rev. Environ. Sci. Biotechnol. 2018, 17, 571–590. [Google Scholar] [CrossRef]
- Slattery, A.D.; Shearer, C.J.; Shapter, J.G.; Blanch, A.J.; Quinton, J.S.; Gibson, C.T. Improved Application of Carbon Nanotube Atomic Force Microscopy Probes Using PeakForce Tapping Mode. Nanomaterials 2018, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes–Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, 1701213. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef]
- Rernglit, W.; Teanphonkrang, S.; Suginta, W.; Schulte, A. Amperometric enzymatic sensing of glucose using porous carbon nanotube films soaked with glucose oxidase. Microchim. Acta 2019, 186, 616. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, Y.J. Amperometric detection of dopamine based on tyrosinase–SWNTs–Ppy composite electrode. Talanta 2009, 80, 1007–1011. [Google Scholar] [CrossRef]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Antiochia, R.; Lavagnini, I.; Magno, F. Amperometric Mediated Carbon Nanotube Paste Biosensor for Fructose Determination. Anal. Lett. 2004, 37, 1657–1669. [Google Scholar] [CrossRef]
- Dias, A.C.M.S.; Gomes-Filho, S.L.R.; Silva, M.M.S.; Dutra, R.F. A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron. 2013, 44, 216–221. [Google Scholar] [CrossRef]
- Li, F.; Peng, J.; Wang, J.; Tang, H.; Tan, L.; Xie, Q.; Yao, S. Carbon nanotube-based label-free electrochemical biosensor for sensitive detection of miRNA-24. Biosens. Bioelectron. 2014, 54, 158–164. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Xiao, C.; Zhou, H.; Wang, X.; He, D. Carbon nanotube template synthesis of hierarchical NiCoO2 composite for non-enzyme glucose detection. Sens. Actuators B Chem. 2016, 222, 232–239. [Google Scholar] [CrossRef]
- Deb, A.K.; Das, S.C.; Saha, A.; Wayu, M.B.; Hensley Marksberry, M.; Baltz, R.J.; Chusuei, C.C. Ascorbic acid, acetaminophen, and hydrogen peroxide detection using a dendrimer-encapsulated Pt nanoparticle carbon nanotube composite. J. Appl. Electrochem. 2016, 46, 289–298. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the Functionalized Carbon Nanotubes as Smart Nanocarriers for Drug Delivery Applications. In Fullerens, Graphenes and Nanotubes; Elsevier BV: Amsterdam, The Netherlands, 2018; Chapter 4; pp. 105–133. [Google Scholar]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for In vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Karthika, V.; Kaleeswarran, P.; Gopinath, K.; Arumugam, A.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Biocompatible properties of nano-drug carriers using TiO2-Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater. Sci. Eng. C 2018, 90, 589–601. [Google Scholar] [CrossRef]
- Singh, R.; Mehra, N.K.; Jain, V.; Jain, N.K. Gemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cells. J. Drug Target. 2013, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Lavaee, P.; Ramezani, M.; Abnous, K. Reversible Targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur. J. Pharm. Biopharm. 2011, 77, 200–206. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Bian, X.; Zhu, Z.; Ding, D.; Li, X.; Jia, Z.; Jiang, X.; Hu, Y. Covalently Combining Carbon Nanotubes with Anticancer Agent: Preparation and Antitumor Activity. ACS Nano 2009, 3, 2740–2750. [Google Scholar] [CrossRef]
- Singh, N.; Sachdev, A.; Gopinath, P. Polysaccharide Functionalized Single Walled Carbon Nanotubes as Nanocarriers for Delivery of Curcumin in Lung Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 1534–1541. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, J.; Du, J.; Gao, Y.; Zhang, L.; Chen, L.; Yang, Y.; Liu, X. A targeted drug delivery system based on carbon nanotubes loaded with lobaplatin toward liver cancer cells. J. Mater. Res. 2018, 33, 2565–2575. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Liao, C.; Fang, Y.; Guo, B. Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes. J. Drug Deliv. Sci. Technol. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Daranciang, D.; Dai, H. Selective Probing and Imaging of Cells with Single Walled Carbon Nanotubes as Near-Infrared Fluorescent Molecules. Nano Lett. 2008, 8, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Yudasaka, M.; Ushijima, N.; Sakaguchi, N.; Maeda, Y.; Tanaka, T.; Kataura, H.; Yokoyama, A. Fate of Carbon Nanotubes Locally Implanted in Mice Evaluated by Near-Infrared Fluorescence Imaging: Implications for Tissue Regeneration. ACS Appl. Nano Mater. 2019, 2, 1382–1390. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, B.; Martin, R.B.; Henbest, K.B.; Harruff, B.A.; Riggs, J.E.; Guo, Z.-X.; Allard, L.F.; Sun, Y.-P. Visible Luminescence of Carbon Nanotubes and Dependence on Functionalization. J. Phys. Chem. B 2005, 109, 14779–14782. [Google Scholar] [CrossRef]
- Lin, C.-W.; Bachilo, S.M.; Zheng, Y.; Tsedev, U.; Huang, S.; Weisman, R.B.; Belcher, A.M. Creating fluorescent quantum defects in carbon nanotubes using hypochlorite and light. Nat. Commun. 2019, 10, 2874. [Google Scholar] [CrossRef]
- Heller, D.A.; Baik, S.; Eurell, T.E.; Strano, M.S. Single-Walled Carbon Nanotube Spectroscopy in Live Cells: Towards Long-Term Labels and Optical Sensors. Adv. Mater. 2005, 17, 2793–2799. [Google Scholar] [CrossRef]
- Ursu, E.-L.; Doroftei, F.; Peptanariu, D.; Pinteala, M.; Rotaru, A. DNA-assisted decoration of single-walled carbon nanotubes with gold nanoparticles for applications in surface-enhanced Raman scattering imaging of cells. J. Nanopart. Res. 2017, 19, 181. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, H. Microwave assisted fast fabrication of Fe3O4-MWCNTs nanocomposites and their application as MRI contrast agents. Mater. Lett. 2012, 67, 49–51. [Google Scholar] [CrossRef]
- Yan, C.; Chen, C.; Hou, L.; Zhang, H.; Che, Y.; Qi, Y.; Zhang, X.; Cheng, J.; Zhang, Z. Single-walled carbon nanotube-loaded doxorubicin and Gd-DTPA for targeted drug delivery and magnetic resonance imaging. J. Drug Target. 2017, 25, 163–171. [Google Scholar] [CrossRef]
- Kroustalli, A.; Zisimopoulou, A.E.; Koch, S.; Rongen, L.; Deligianni, D.; Diamantouros, S.; Athanassiou, G.; Kokozidou, M.; Mavrilas, D.; Jockenhoevel, S. Carbon nanotubes reinforced chitosan films: Mechanical properties and cell response of a novel biomaterial for cardiovascular tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Shrestha, B.K.; Kim, J.I.; Won Ko, S.; Park, C.H.; Kim, C.S. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering. Carbon 2018, 136, 430–443. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.U.H.A.; Mian, S.A.; Ali, G.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef]
- Lim, S.H.; Luo, Z.; Shen, Z.; Lin, J. Plasma-Assisted Synthesis of Carbon Nanotubes. Nanoscale Res. Lett. 2010, 5, 1377–1386. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Lodhi, N.; Raj, R.; Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Challenges in the Use of Carbon Nanotubes for Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 169–206. [Google Scholar] [CrossRef]
- Lamprecht, C.; Torin Huzil, J.; V Ivanova, M.; Foldvari, M. Non-Covalent Functionalization of Carbon Nanotubes with Surfactants for Pharmaceutical Applications—A Critical Mini-Review. Drug Deliv. Lett. 2011, 1, 45–57. [Google Scholar]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Pensabene, V.; Menciassi, A.; Dario, P. Dispersion of Multi-Walled Carbon Nanotubes in Aqueous Pluronic F127 Solutions for Biological Applications. Fuller. Nanotub. Carbon Nanostruct. 2009, 17, 11–25. [Google Scholar] [CrossRef]
- Angelikopoulos, P.; Gromov, A.; Leen, A.; Nerushev, O.; Bock, H.; Campbell, E.E.B. Dispersing Individual Single-Wall Carbon Nanotubes in Aqueous Surfactant Solutions below the cmc. J. Phys. Chem. C 2010, 114, 2–9. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Premkumar, T.; Geckeler, K.E. Dispersion of Single-Walled Carbon Nanotubes by Using Surfactants: Are the Type and Concentration Important? Chem. Eur. J. 2008, 14, 6044–6048. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Chang, D.W.; Nanjundan, A.K.; Baek, J.-B. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-953-307-498-6. [Google Scholar]
- Petrov, P.; Stassin, F.; Pagnoulle, C.; Jérôme, R. Noncovalent functionalization of multi-walled carbon nanotubes by pyrene containing polymers. Chem. Commun. 2003, 23, 2904–2905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.X.; Cheng, H.M. Water-Soluble Multiwalled Carbon Nanotubes Functionalized with Sulfonated Polyaniline. J. Phys. Chem. B 2006, 110, 9095–9099. [Google Scholar] [CrossRef]
- Ling, X.; Wei, Y.; Zou, L.; Xu, S. Functionalization and dispersion of multiwalled carbon nanotubes modified with poly-l-lysine. Colloids Surf. Physicochem. Eng. Asp. 2014, 443, 19–26. [Google Scholar] [CrossRef]
- Cheng, F.; Adronov, A. Noncovalent Functionalization and Solubilization of Carbon Nanotubes by Using a Conjugated Zn–Porphyrin Polymer. Chem. Eur. J. 2006, 12, 5053–5059. [Google Scholar] [CrossRef]
- Lee, J.U.; Huh, J.; Kim, K.H.; Park, C.; Jo, W.H. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly (ethylene glycol). Carbon 2007, 45, 1051–1057. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; et al. Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef]
- Sanz, V.; Borowiak, E.; Lukanov, P.; Galibert, A.M.; Flahaut, E.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Optimising DNA binding to carbon nanotubes by non-covalent methods. Carbon 2011, 49, 1775–1781. [Google Scholar] [CrossRef]
- Taeger, S.; Xuang, L.Y.; Günther, K.; Mertig, M. Noncovalent Sidewall Functionalization of Carbon Nanotubes by Biomolecules: Single-stranded DNA and Hydrophobin. AIP Conf. Proc. 2005, 786, 262–265. [Google Scholar]
- Barinov, A.; Gregoratti, L.; Dudin, P.; La Rosa, S.; Kiskinova, M. Imaging and Spectroscopy of Multiwalled Carbon Nanotubes during Oxidation: Defects and Oxygen Bonding. Adv. Mater. 2009, 21, 1916–1920. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Lin, W.-H.; Chang, Y.-C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Hiura, H.; Ebbesen, T.W.; Tanigaki, K. Opening and purification of carbon nanotubes in high yields. Adv. Mater. 1995, 7, 275–276. [Google Scholar] [CrossRef]
- Moradi, O.; Yari, M.; Zare, K.; Mirza, B.; Najafi, F. Carbon Nanotubes: A Review of Chemistry Principles and Reactions. Fuller. Nanotub. Carbon Nanostruct. 2012, 20, 138–151. [Google Scholar] [CrossRef]
- Hu, H.; Bhowmik, P.; Zhao, B.; Hamon, M.A.; Itkis, M.E.; Haddon, R.C. Determination of the acidic sites of purified single-walled carbon nanotubes by acid-base titration. Chem. Phys. Lett. 2001, 345, 25–28. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Lastella, S.; Kim, S.; Papadimitrakopoulos, F. Length Separation of Zwitterion-Functionalized Single Wall Carbon Nanotubes by GPC. J. Am. Chem. Soc. 2002, 124, 728–729. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Large-scale aligned carbon nanotubes films. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 235–239. [Google Scholar] [CrossRef]
- Dinan, N.M.; Atyabi, F.; Rouini, M.-R.; Amini, M.; Golabchifar, A.-A.; Dinarvand, R. Doxorubicin loaded folate-targeted carbon nanotubes: Preparation, cellular internalization, in vitro cytotoxicity and disposition kinetic study in the isolated perfused rat liver. Mater. Sci. Eng. C 2014, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.J.; Jalota-Badhwar, A.; Satavalekar, S.D.; Bhansali, S.G.; Aher, N.D.; Kharas, F.; Banerjee, S.S. PEG-conjugated highly dispersive multifunctional magnetic multi-walled carbon nanotubes for cellular imaging. Nanoscale 2012, 4, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Negra, F.D.; Meneghetti, M.; Menna, E. Microwave-Assisted Synthesis of a Soluble Single Wall Carbon Nanotube Derivative. Fuller. Nanotub. Carbon Nanostruct. 2003, 11, 25–34. [Google Scholar] [CrossRef]
- Li, X.F.; Yan, H.; Peng, S.X. Tribological Behavior of Poly (ethylene Glycol)-Carbon Nanotubes. Adv. Mater. Res. 2011, 217–218, 688–691. [Google Scholar] [CrossRef]
- D’Arlas, B.F.; Goyanes, S.; Rubiolo, G.H.; Mondragon, I.; Corcuera, M.A.; Eceiza, A. Surface Modification of Multiwalled Carbon Nanotubes via Esterification Using a Biodegradable Polyol. J. Nanosci. Nanotechnol. 2009, 9, 6064–6071. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Chen, Y.; Haddon, R.C.; Fang, S.; Rao, A.M.; Eklund, P.C.; Lee, W.H.; Dickey, E.C.; Grulke, E.A.; Pendergrass, J.C.; Chavan, A.; et al. Chemical Attachment of Organic Functional Groups to Single-walled Carbon Nanotube Material. J. Mater. Res. 1998, 13, 2423–2431. [Google Scholar] [CrossRef]
- Bettinger, H.F. Experimental and Computational Investigations of the Properties of Fluorinated Single-Walled Carbon Nanotubes. ChemPhysChem 2003, 4, 1283–1289. [Google Scholar] [CrossRef]
- Lipińska, M.E.; Rebelo, S.L.H.; Pereira, M.F.R.; Gomes, J.A.N.F.; Freire, C.; Figueiredo, J.L. New insights into the functionalization of multi-walled carbon nanotubes with aniline derivatives. Carbon 2012, 50, 3280–3294. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef]
- Samorì, C.; Ali-Boucetta, H.; Sainz, R.; Guo, C.; Toma, F.M.; Fabbro, C.; da Ros, T.; Prato, M.; Kostarelos, K.; Bianco, A. Enhanced anticancer activity of multi-walled carbon nanotube—Methotrexate conjugates using cleavable linkers. Chem. Commun. 2010, 46, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Abraham, J.; Whelan, P.; Graupner, R.; Ley, L.; Hennrich, F.; Kappes, M.; Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes with (R-)Oxycarbonyl Nitrenes. J. Am. Chem. Soc. 2003, 125, 8566–8580. [Google Scholar] [CrossRef] [PubMed]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.-D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise Quenching of Exciton Fluorescence in Carbon Nanotubes by Single-Molecule Reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Setaro, A.; Adeli, M.; Glaeske, M.; Przyrembel, D.; Bisswanger, T.; Gordeev, G.; Maschietto, F.; Faghani, A.; Paulus, B.; Weinelt, M.; et al. Preserving π-conjugation in covalently functionalized carbon nanotubes for optoelectronic applications. Nat. Commun. 2017, 8, 14281. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. Radical addition of perfluorinated alkyl iodides to multi-layered graphene and single-walled carbon nanotubes. Nano Res. 2010, 3, 138–145. [Google Scholar] [CrossRef]
- Nakamura, T.; Ishihara, M.; Ohana, T.; Tanaka, A.; Koga, Y. Sidewall modification of single-walled carbon nanotubes using photolysis of perfluoroazooctane. Diam. Relat. Mater. 2004, 13, 1971–1974. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall Functionalization of Carbon Nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Borondics, F.; Bokor, M.; Matus, P.; Tompa, K.; Pekker, S.; Jakab, E. Reductive Functionalization of Carbon Nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 375–382. [Google Scholar] [CrossRef]
- Viswanathan, G.; Chakrapani, N.; Yang, H.; Wei, B.; Chung, H.; Cho, K.; Ryu, C.Y.; Ajayan, P.M. Single-Step in Situ Synthesis of Polymer-Grafted Single-Wall Nanotube Composites. J. Am. Chem. Soc. 2003, 125, 9258–9259. [Google Scholar] [CrossRef]
- Shahin, D.I.; Anderson, T.J.; Feygelson, T.I.; Pate, B.B.; Wheeler, V.D.; Greenlee, J.D.; Hite, J.K.; Tadjer, M.J.; Christou, A.; Hobart, K.D. Thermal etching of nanocrystalline diamond films. Diam. Relat. Mater. 2015, 59, 116–121. [Google Scholar] [CrossRef]
- Lu, H.C.; Lin, M.Y.; Chou, S.L.; Peng, Y.C.; Lo, J.I.; Cheng, B.M. Identification of Nitrogen Defects in Diamond with Photoluminescence Excited in the 160−240 nm Region. Anal. Chem. 2012, 84, 9596–9600. [Google Scholar] [CrossRef] [PubMed]
- Walker, J. Optical absorption and luminescence in diamond. Rep. Prog. Phys. 1979, 42, 1606–1659. [Google Scholar] [CrossRef]
- Dischler, B. Volume 1: Tables and Interpretations. In Handbook of Spectral Lines in Diamond; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Liu, H.; Reilly, S.; Herrnsdorf, J.; Xie, E.; Savitski, V.G.; Kemp, A.J.; Gu, E.; Dawson, M.D. Large radius of curvature micro-lenses on single crystal diamond for application in monolithic diamond Raman lasers. Diam. Relat. Mater. 2016, 65, 37–41. [Google Scholar] [CrossRef]
- Woerner, E.; Wild, C.; Mueller-Sebert, W.; Koidl, P. CVD-diamond optical lenses. Diam. Relat. Mater. 2001, 10, 557–560. [Google Scholar] [CrossRef]
- Koizumi, S.; Umezawa, H.; Pernot, J.; Suzuki, M. Power Electronics Device Applications of Diamond Semiconductors, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018; ISBN 978-0-08-102183-5. [Google Scholar]
- Umezawa, H.; Nagase, M.; Kato, Y.; Shikata, S. High temperature application of diamond power device. Diam. Relat. Mater. 2012, 24, 201–205. [Google Scholar] [CrossRef]
- Shikata, S. Single crystal diamond wafers for high power electronics. Diam. Relat. Mater. 2016, 65, 168–175. [Google Scholar] [CrossRef]
- Blank, V.; Popov, M.; Pivovarov, G.; Lvova, N.; Gogolinsky, K.; Reshetov, V. Ultrahard and superhard phases of fullerite C60: Comparison with diamond on hardness and wear. Diam. Relat. Mater. 1998, 7, 427–431. [Google Scholar] [CrossRef]
- Wei, L.; Kuo, P.K.; Thomas, L.R.; Anthony, T.R.; Banholzer, W.F. Thermal conductivity of isotopically modified single crystal diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef]
- Inspektor, A.; Oles, E.J.; Bauer, C.E. Theory and practice in diamond coated metal-cutting tools. Int. J. Refract. Met. Hard Mater 1997, 15, 49–56. [Google Scholar] [CrossRef]
- Vajpayee, R.B.; Maharana, P.K.; Sharma, N.; Agarwal, T.; Jhanji, V. Diamond knife–assisted deep anterior lamellar keratoplasty to manage keratoconus. J. Cataract Refract. Surg. 2014, 40, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, K.T.; Perevedentseva, E.; Karmenyan, A.; Lin, M.D.; Cheng, C.L. Nanodiamond for biolabelling and toxicity evaluation in the zebrafish embryo in vivo. J. Biophotonics 2016, 9, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics 2012, 2, 302–312. [Google Scholar]
- Shergold, H.L.; Hartley, C.J. The surface chemistry of diamond. Int. J. Miner. Process. 1982, 9, 219–233. [Google Scholar] [CrossRef]
- Szunerits, S.; Nebel, C.E.; Hamers, R.J. Surface functionalization and biological applications of CVD diamond. MRS Bull. 2014, 39, 517–524. [Google Scholar] [CrossRef]
- Stavis, C.; Lasseter Clare, T.; Butler, J.E.; Radadia, A.D.; Carr, R.; Zeng, H.; King, W.P.; Carlisle, J.A.; Aksimentiev, A.; Bashir, R.; et al. Surface functionalization of thin-film diamond for highly stable and selective biological interfaces. Proc. Natl. Acad. Sci. USA 2011, 18, 983–988. [Google Scholar] [CrossRef]
- Krueger, A. Nanodiamond. In Carbon Materials and Nanotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 978-3-527-31803-2. [Google Scholar]
- Awschalom, D.D.; Hanson, R.; Wrachtrup, J.; Zhou, B.B. Quantum technologies with optically interfaced solid-state spins. Nat. Photonics 2018, 12, 516–527. [Google Scholar] [CrossRef]
- Degen, C.L.; Reinhard, F.; Cappellaro, P. Quantum sensing. Rev. Mod. Phys. 2017, 89, 035002. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Hsiao, W.W.; Haziza, S.; Simonneau, M.; Treussart, F.; Chang, H.C. Single particle tracking of fluorescent nanodiamonds in cells and organisms. Curr. Opin. Solid State Mater. Sci. 2017, 21, 35–42. [Google Scholar] [CrossRef]
- Alkahtani, M.H.; Alghannam, F.; Jiang, L.; Almethen, A.; Rampersaud, A.A.; Brick, R.; Gomes, C.L.; Scull, M.O.; Hemmer, P.R. Fluorescent nanodiamonds: Past, present, and future. Nanophotonics 2018, 7, 1423–1453. [Google Scholar] [CrossRef]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Coll. Interfaces Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Torelli, M.D.; Nunn, N.A.; Shenderova, O.A. A Perspective on Fluorescent Nanodiamond Bioimaging. Small 2019, 21, 1902151. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Drozd, V.; Durygin, A.; Rodriguez, J.; Barber, P.; Atluri, V.; Liu, X.; Voss, T.G.; Saxena, S.; Nair, M. Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, A.E.; Youan, B.-B.C. Nanodiamonds for Drug Delivery Systems. In Diamond-Based Materials for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Chen, X.; Zhang, W. Diamond nanostructures for drug delivery, bioimaging, and biosensing. Chem. Soc. Rev. 2017, 46, 734–760. [Google Scholar] [CrossRef]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and Their Applications in Cells. Small 2018, 14, e1704263. [Google Scholar] [CrossRef]
- Dale, M.W.; Morley, G.W. Medical applications of diamondmagnetometry: Commercial viability. arXiv 2017, arXiv:17050.1994. [Google Scholar]
- Kucsko, G.; Maurer, P.C.; Yao, N.Y.; Kubo, M.; Noh, H.J.; Lo, P.K.; Park, H.; Lukin, M.D. Nanometre-scale thermometry in a living cell. Nature 2013, 500, 54–58. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Cheng, C.-L.; Chang, H.-C. Nanodiamonds for optical bioimaging. J. Phys. D 2010, 43, 374021. [Google Scholar] [CrossRef]
- Aharonovich, I.; Neu, E. Diamond Nanophotonics. Adv. Opt. Mater. 2014, 2, 911–928. [Google Scholar] [CrossRef]
- Prabhakar, N.; Peurla, M.; Koho, S.; Deguchi, T.; Näreoja, T.; Chang, H.C.; Rosenholm, J.M.; Hänninen, P.E. STED-TEM correlative microscopy leveraging nanodiamonds as intracellular dual-contrast markers. Small 2017, 14, 1701807. [Google Scholar] [CrossRef] [PubMed]
- Morales-Zavala, F.; Casanova-Morales, N.; Gonzalez, B.R.; Chandía-Cristi, A.; Estrada, L.D.; Alvizú, I.; Waselowski, V.; Guzman, F.; Guerrero, S.; Oyarzún-Olave, M.; et al. Functionalization of stable fluorescent nanodiamonds towards reliable detection of biomarkers for Alzheimer’s disease. J. Nanobiotechnol. 2018, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.I.; Perevedentseva, E.; Chung, P.H.; Liu, K.K.; Cheng, C.Y.; Chang, C.C.; Cheng, C.L. Nanometer-Sized Diamond Particle as a Probe for Biolabeling. Biophys. J. 2007, 93, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.C.; Shenderova, O. Nanodiamond-mediated drug delivery and imaging: Challenges and opportunities. Exp. Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Chang, H.-C.; Dong, H.; Zhang, X. Carbon Nanomaterials for Bioimaging, Bioanalysis, and Therapy, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Martin, R.; Alvaro, M.; Herance, J.R.; Garcia, H. Fenton-treated functionalized diamond nanoparticles as gene delivery system. ACS Nano 2010, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.; Spillane, K.M.; Papageorgiou, G.; Wray, W.; Ishiwata, H.; Labarca, M.; Leal-Ortiz, S.; Reid, G.; Webb, M.; Foord, J.; et al. Functionalisation of Detonation Nanodiamond for Monodispersed, Soluble DNA-Nanodiamond Conjugates Using Mixed Silane Bead-Assisted Sonication Disintegration. Sci. Rep. 2018, 8, 728. [Google Scholar] [CrossRef]
- Grall, R.; Girard, H.; Saad, L.; Petit, T.; Gesset, C.; Combis-Schlumberger, M.; Paget, V.; Delic, J.; Arnault, J.C.; Chevillard, S. Impairing the radioresistance of cancer cells by hydrogenated nanodiamonds. Biomaterials 2015, 61, 290–298. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Bacakova, L.; Broz, A.; Liskova, J.; Stankova, L.; Potocky, S.; Kromka, A. The Application of Nanodiamond in Biotechnology and Tissue Engineering; IntechOpen: London, UK, 2016. [Google Scholar]
- Williams, O.A. Nanocrystalline diamond. Diam. Relat. Mater. 2011, 20, 621–640. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Detonation-synthesis nanodiamonds: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2007, 76, 339–360. [Google Scholar] [CrossRef]
- Angus, J.C. Diamond synthesis by chemical vapor deposition: The early years. Diam. Relat. Mater. 2014, 49, 77–96. [Google Scholar] [CrossRef]
- Schwander, M.; Partes, K. A review of diamond synthesis by CVD processes. Diam. Relat. Mater. 2011, 20, 1287–1301. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, J.B.; Liu, Q.X. Preparation of nancrystalline diamond using pulsed laser induced reactive quencing. J. Phys. Condens. Matter 1988, 10, 7923–7927. [Google Scholar] [CrossRef]
- Amans, D.; Chenus, A.C.; Ledoux, G.; Dujardin, C.; Reynaud, C.; Sublemontier, O.; Masenelli-Varlot, K.; Guillois, O. Nanodiamond synthesis by pulsed laser ablation in liquids. Diam. Relat. Mater. 2009, 18, 177–180. [Google Scholar] [CrossRef]
- Abbaschian, R.; Zhu, H.; Clarke, C. High pressure–high temperature growth of diamond crystals using split sphere apparatus. Diam. Relat. Mater. 2005, 14, 1916–1919. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Zaiser, M.; Lyutovich, Y.; Banhart, F. Irradiation-induced transformation of graphite to diamond: A quantitative study. Phys. Rev. B 2000, 62, 3058–3064. [Google Scholar] [CrossRef]
- Daulton, T.L.; Kirk, M.A.; Lewis, R.S.; Rehn, L.E. Production of nanodiamonds by high-energy ion irradiation of graphite at room temperature. Nucl. Instrum. Methods Phys. Res. B 2001, 175, 12–20. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Carbon onions as nanoscopic pressure cells for diamond formation. Nature 1996, 382, 433–435. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Iakoubovskii, K.; Baidakova, M.V.; Wouters, B.H.; Stesmans, A.; Adriaenssens, G.J.; Vul, A.Y.; Grobet, P.J. Structure and defects of detonation synthesis nanodiamond. Diam. Relat. Mater. 2000, 9, 861–865. [Google Scholar] [CrossRef]
- Railkar, T.A.; Kang, W.P.; Windischmann, H.; Malshe, A.P.; Neseem, H.A.; Devidson, J.L.; Brown, W.D. A Critical Review of Chemical Vapor-Deposited (CVD) Diamond for Electronic Applications. Crit. Rev. Solid State Mater. Sci. 2000, 25, 163–277. [Google Scholar] [CrossRef]
- Baitinger, E.M.; Belenkov, E.A.; Brzhezinskaya, M.M.; Greshnyakov, V.A. Specific Features of the Structure of Detonation Nanodiamonds from Results of Electron Microscopy Investigations. Phys. Solid State 2012, 54, 1715–1722. [Google Scholar] [CrossRef]
- Kumar, A.; Lin, P.A.; Xue, A.; Hao, B.; Yap, Y.K.; Sankaran, M. Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour. Nat. Commun. 2013, 4, 2618. [Google Scholar] [CrossRef]
- Nee, C.-N.; Yap, S.-L.; Tou, T.-Y.; Chang, H.-C.; Yap, S.-S. Direct synthesis of nanodiamonds by femtosecond laser irradiation of ethanol. Sci. Rep. 2016, 6, 33966. [Google Scholar] [CrossRef]
- Pearce, S.R.J.; Henley, S.J.; Claeyssens, F.; May, P.W.; Hallam, K.R.; Smith, J.A.; Rosser, K.N. Production of nanocrystalline diamond by laser ablation at the solid-liquid interface. Diam. Relat. Mater. 2004, 13, 661–665. [Google Scholar] [CrossRef]
- Basso, L.; Gorrini, F.; Bazzanella, N.; Cazzanelli, M.; Dorigoni, C.; Bifone, A.; Miotello, A. The modeling and synthesis of nanodiamonds by laser ablation of graphite and diamond-like carbon in liquid-confined ambient. Appl. Phys. A 2018, 124, 72. [Google Scholar] [CrossRef]
- Zaiser, M. Microstructural Processes in Irradiated Materials; Zinkle, S.J., Lucas, G.E., Ewing, R.C., Williams, J.S., Eds.; Materials Research Society: Warrendale, PA, USA, 1999. [Google Scholar]
- Lyutovich, Y.; Banhart, F. Low-pressure transformation of graphite to diamond under irradiation. Appl. Phys. Lett. 1999, 74, 659–660. [Google Scholar] [CrossRef]
- Ginés, L.; Mandal, S.; Ahmed, A.I.; Cheng, C.L.; Sow, M.; Williams, A. Positive zeta potential of nanodiamonds. Nanoscale 2017, 9, 12549–12555. [Google Scholar] [CrossRef]
- Gibson, N.M.; Luo, T.-J.M.; Shenderova, O.; Koscheev, P.A.; Brenner, D.W. Electrostatically mediated adsorption by nanodiamond and nanocarbon particles. J. Nanopart. Res. 2012, 14, 700. [Google Scholar] [CrossRef]
- Petrova, N.; Zhukov, A.; Gareeva, F.; Koscheev, A.; Petrov, I.; Shenderova, O. Interpretation of electrokinetic measurements of nanodiamond particles. Diam. Relat. Mater. 2012, 30, 62–69. [Google Scholar] [CrossRef]
- Mitev, D.; Dimitrova, R.; Spassova, M.; Minchev, R.; Stavrev, S. Surface peculiarities of detonation nanodiamonds in dependence of fabrication and purification methods. Diam. Relat. Mater. 2007, 16, 776–780. [Google Scholar] [CrossRef]
- Anikeev, V.I.; Zaikovskii, V.I. Treatment of detonation carbon in supercritical water. Russ. J Appl. Chem. 2010, 83, 1202–1208. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Shenderova, O.; Koscheev, A.; Zaripov, N.; Petrov, I.; Skryabin, Y.; Detkov, P.; Turner, S. Surface Chemistry and Properties of Ozone-Purified Detonation Nanodiamonds. J. Phys. Chem. C 2011, 115, 9827–9837. [Google Scholar] [CrossRef]
- Martín, R.; Heydorn, P.C.; Heydorn, M.; Garcia, H. General strategy for high-density covalent functionalization of diamond nanoparticles using Fenton chemistry. Chem. Mater. 2009, 21, 4505–4514. [Google Scholar] [CrossRef]
- Krueger, A.; Stegk, J.; Liang, Y.; Lu, L.; Jarre, G. Biotinylated nanodiamond: Simple and efficient functionalization of detonation diamond. Langmuir 2008, 24, 4200–4204. [Google Scholar] [CrossRef]
- Liang, Y.; Ozawa, M.; Krueger, A. A general procedure to functionalize agglomerating nanoparticles demonstrated on nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Arghya, P. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Yeap, W.S.; Chen, S.; Loh, K.P. Detonation nanodiamond: An organic platform for the Suzuki coupling of organic molecules. Langmuir 2009, 25, 185–191. [Google Scholar] [CrossRef]
- Kuo, T.-C.; McCreery, R.L.; Swain, G.M. Electrochemical Modifi cation of boron doped chemical vapor deposited surfaces with covalent bonded monolayers. Electrochem. Solid State Lett. 1999, 2, 288–290. [Google Scholar] [CrossRef]
- Uetsuka, H.; Shin, D.; Tokuda, N.; Saeki, K.; Nebel, C.E. Electrochemical Grafting of Boron-Doped Single-Crystalline Chemical Vapor Deposition Diamond with Nitrophenyl Molecules. Langmuir 2007, 23, 3466–3472. [Google Scholar] [CrossRef]
- Wang, J.; Firestone, M.A.; Auciello, O.; Carlisle, J.A. Surface Functionalization of Ultrananocrystalline Diamond Films by Electrochemical Reduction of Aryldiazonium Salts. Langmuir 2004, 20, 11450–11456. [Google Scholar] [CrossRef]
- Lud, S.Q.; Steenackers, M.; Jordan, R.; Bruno, P.; Gruen, D.M.; Feulner, P.; Garrido, J.A.; Stutzmann, M. Chemical Grafting of Biphenyl Self-Assembled Monolayers on Ultrananocrystalline Diamond. J. Am. Chem. Soc. 2006, 128, 16884–16891. [Google Scholar] [CrossRef]
- Strother, T.; Knickerbocker, T.; Russell, J.N.; Butler, J.E.; Smith, L.M.; Hamers, R.J. Photochemical Functionalisation of Diamond Films. Langmuir 2002, 18, 968–971. [Google Scholar] [CrossRef]
- Hoeb, M.; Auernhammer, M.; Schoell, S.J.; Brandt, M.S.; Garrido, J.A.; Stutzmann, M.; Sharp, I.D. Thermally Induced Alkylation of Diamond. Langmuir 2010, 26, 18862–18867. [Google Scholar] [CrossRef]
- Miller, J.B.; Brown, D.W. Photochemical Modification of Diamond Surfaces. Langmuir 1996, 12, 5809–5817. [Google Scholar] [CrossRef]
- Song, K.-S.; Zhang, G.-J.; Nakamura, Y.; Furukawa, K.; Hiraki, T.; Yang, J.-H.; Funatsu, T.; Ohdomari, I.; Kawarada, H. Label-free DNA sensors using ultrasensitive diamond field-effect transistors in solution. Phys. Rev. E 2006, 74, 041919. [Google Scholar] [CrossRef]
- Szunerits, S.; Manesse, M.; Actis, P.; Marcus, B.; Denuault, G.; Jama, C.; Boukherroub, R. Investigation of the infl uence of the surface termination of boron-doped diamond electrodes on the oxygen reduction in basic medium. Electrochem. Solid State Lett. 2007, 10, 34–36. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent Incorporation of Aminated Nanodiamond into an Epoxy Polymer Network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef]
- Krueger, A.; Ozawa, M.; Jarre, G.; Liang, Y.; Stegk, J.; Lu, L. Deagglomeration and functionalisation of detonation diamond. Phys. State Solid A 2007, 204, 2881–2887. [Google Scholar] [CrossRef]
- Torrengo, S.; Miotello, A.; Minati, L.; Bernagozzi, I.; Ferrari, M.; Dipalo, M.; Kohn, E.; Speranza, G. The role of oxygen in the one step amination process of nanocrystalline diamond surface. Diam. Relat. Mater. 2011, 20, 990–994. [Google Scholar] [CrossRef]
- Chawla, K.K. Carbon Fibers in Composite Materials, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aeosp. Sci. 2005, 41, 143–451. [Google Scholar] [CrossRef]
- Pooja, B.; Alka, G. Carbon Fibres: Production, Properties and Potential Use. Mater. Sci. India 2017, 14, 52–57. [Google Scholar]
- Balasubramanian, M. Composite Materials and Processing; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef]
- Sugawara, K.; Yugami, A.; Kojima, A. Voltammetric detection of biological molecules using chopped carbon fiber. Anal. Sci. 2010, 26, 1059–1063. [Google Scholar] [CrossRef]
- Guitchounts, G.; Markowitz, J.E.; Liberti, W.A.; Gardner, T.J. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng. 2010, 10, 046016. [Google Scholar] [CrossRef]
- Gillis, W.F.; Lissandrello, C.A.; Shen, J.; Pearre, B.W.; Mertiri, A.; Deku, F.; Cogan, S.; Holinski, B.J.; Chew, D.J.; White, A.E. Carbon fiber on polyimide ultra-microelectrodes. J. Neural Eng. 2018, 15, 016010. [Google Scholar] [CrossRef]
- Stout, D.A.; Raimondo, E.; Webster, T.J. Improved cardiomyocyte functions of carbon nanofiber cardiac patches. Mater. Res. Soc. Symp. Proc. 2012, 6, 1417. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Fan, Y.; Wang, X.; Watari, F.; Li, X. Fiber-reinforced scaffolds in soft tissue engineering. Regen. Biomater. 2017, 4, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.J.; Rider, A.E.; Ishaq, M.; Kumar, S.; Kondyurin, A.; Bilek, M.M.M.; Levchenko, I.; Ostrikov, K. Carbon nanostructures for hard tissue engineering. RSC Adv. 2013, 3, 11058–11072. [Google Scholar] [CrossRef]
- Huey, D.; Hu, J.; Athanasiou, K. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- García-Ruíz, J.P.; Lantada, A.D. 3D Printed Structures Filled with Carbon Fibers and Functionalized with Mesenchymal Stem Cell Conditioned Media as In Vitro Cell Niches for Promoting Chondrogenesis. Materials 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Sahu, S.K.; Sinha, A.; Chakraborty, J.; Garai, S. Facile synthesis of carbon fiber reinforced polymer-hydroxyapatite ternary composite: A mechanically strong bioactive bone graft. Mater. Sci. Eng. C 2019, 97, 388–396. [Google Scholar] [CrossRef]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef]
- Chen, M.C.; Sun, Y.C.; Chen, Y.H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef]
- Patel, A.; Mukundan, S.; Wang, W.; Karumuri, A.; Sant, V.; Mukhopadhyay, S.M.; Sant, S. Carbon-based hierarchical scaffolds for myoblast differentiation: Synergy between nano-functionalization and alignment. Acta Biomater. 2016, 32, 77–88. [Google Scholar] [CrossRef]
- Amezcua, R.; Shirolkar, A.; Fraze, C.; Stout, D.A. Nanomaterials for Cardiac Myocyte Tissue Engineering. Nanomaterials 2016, 6, 133. [Google Scholar] [CrossRef]
- Asiri, A.M.; Marwani, H.M.; Khan, S.B.; Webster, T.J. Understanding greater cardiomyocyte functions on aligned compared to random carbon nanofibers in PLGA. Int. J. Nanomed. 2015, 10, 89–96. [Google Scholar]
- Lin, Y.-H.; Lin, J.-H.; Wang, S.-H.; Ko, T.-H.; Tseng, G.-C. Evaluation of silver-containing activated carbon fiber for wound healing study: In vitro and in vivo. J. Biomed. Mater. Res. B 2012, 100, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science, 2nd ed.; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Mendes, D.G.; Angel, D.; Grishkan, A.; Boss, J. Histological response to carbon fibre. J. Bone Jt. Surg. 1985, 67, 645–649. [Google Scholar] [CrossRef]
- Houtz, R.C. Orlon acrylic fibre: Chemistry and properties. J. Text Res. 1950, 20, 786–801. [Google Scholar] [CrossRef]
- Shamsuddin, S.R.; Ho, K.K.C.; Lee, K.-Y.; Hodgkinson, J.M.; Bismarck, A. Carbon Fiber: Properties, Testing, and Analysis. In Wiley Encyclopedia of Composites; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Johnson, D.J. Structure-property relationships in carbon fibres. J. Phys. Appl. Phys. 1987, 20, 286–291. [Google Scholar] [CrossRef]
- Bajaj, P.; Chavan, R.B.; Manjeet, B. Thermal Studies on the Saponification Products of Acryionitrile Terpolymer. J. Macromol. Sci. Chem. A 1986, 23, 335–347. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis of electrospun polyacrylonitrile derived carbon fibers and comparison of properties with bulk form. PLoS ONE 2018, 13, e0201345. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Exfoliation of carbon fibers. J. Phys. Chem. Sol. 2004, 65, 109–117. [Google Scholar] [CrossRef]
- Dai, Z.; Shi, X.; Liu, H.; Li, H.; Han, Y.; Zhou, J. High-strength lignin-based carbon fibers via a lowenergy method. RSC Adv. 2018, 8, 1218–1224. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Singer, L.S.; Lewis, I.C. ESR study of the kinetics of carbonization. Carbon 1978, 16, 417–423. [Google Scholar] [CrossRef]
- Edie, D.D.; Dunham, M.G. Melt spinning pitch-based carbon fibers. Carbon 1989, 27, 647–655. [Google Scholar] [CrossRef]
- Barr, J.B.; Chwastiak, S.; Didchenko, R.; Lewis, I.C.; Lewis, R.T.; Singer, L.S. High Modulus Carbon Fibers from Pitch Precursor. Appl. Polym. Symp. 1975, 29, 161–173. [Google Scholar]
- Park, S.J. (Ed.) Carbon Fibers, 2nd ed.; Springer: Singapore, 2018. [Google Scholar]
- Pape, D.; Adam, F.; Fritsch, E.; Mueller, K.; Kohn, D. Primary lumbosacral stability after open posterior and endoscopic anterior fusion with interbody implants: A roentgen stereophotogrammetric analysis. Spine 2000, 25, 2514–2518. [Google Scholar] [CrossRef]
- Naveen, K.; Kumar, G.A.; Sangeeta, D.K. Carbon Fibers in Biomedical Applications. In Recent Development Field Carbon Fibers; IntechOpen: London, UK, 2018; pp. 83–102. [Google Scholar]
- Stout, D.A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr. Pharm. Des. 2015, 21, 2037–2044. [Google Scholar] [CrossRef]
- Yang, C.; Deng, G.; Chen, W.; Ye, X.; Mo, X. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloids Surf. B 2014, 122, 270–276. [Google Scholar] [CrossRef]
- Chand, S. Review Carbon Fibers for Composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Tiwari, S.; Bijwe, J. Surface Treatment of Carbon Fibers—A Review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef]
- Zhang, J. Different Surface Treatments of Carbon Fibers and Their Influence on the Interfacial Prop-Erties of Carbon Fiber/Epoxy Composites. Ph.D. Thesis, Ecole Centrale Paris, Paris, France, 2012. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites, 1st ed.; CRC Press: Boca Raton, NJ, USA, 2005; ISBN 978-0-429-11682-7. [Google Scholar]
- Langston, T.A. Wettability of Nitric Acid OxidizedCarbon Fibers. J. Reinf. Plast. Comp. 2010, 29, 2156–2169. [Google Scholar] [CrossRef]
- Feng, A.; Wu, G.; Pan, G.; Pan, C.; Wang, Y. The Behavior of Acid Treating Carbon Fiber and the Mechanical Properties and Thermal Conductivity of Phenolic Resin Matrix Composites. J. Nanosci. Nanotechnol. 2017, 17, 3786–3791. [Google Scholar] [CrossRef]
- Burakowski Nohara, L.; Petraconi Filho, G.; Nohara, E.L.; Urban Kleinke, M.; Cerqueira Rezende, M. Evaluation of carbon fiber surface treated by chemical and cold plasma processes. Mater. Res. 2005, 8, 281–286. [Google Scholar] [CrossRef]
- Ahmed, J.K.; Hamzah, A.F.; Hamed, A. Thermal and Chemical Etching of Carbon Fiber. Int. J. Eng. Technol. 2017, 7, 519–526. [Google Scholar]
- Park, S.-J.; Oh, J.-S.; Rhee, K.-Y. Effect of Atmospheric Plasma Treatment of Carbon Fibers on Crack Resistance of Carbon Fibers-reinforced Epoxy Composites. Carbon Sci. 2005, 6, 106–110. [Google Scholar]
- Santos Lima, A.; Botelho Cocchieri, E.; Kostov, K.G.; Nascente, P.A.P.; da Silva, L.L.G. Atmospheric Plasma Treatment of Carbon Fibers forEnhancement of Their Adhesion Properties. IEEE Trans. Plasma Sci. 2013, 41, 319–324. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Chen, G. Influence of rare earth treatment on interfacial properties of CFs/epoxy composites. Mater. Sci. Eng. A 2007, 444, 170–177. [Google Scholar] [CrossRef]
- Osbeck, S.; Bradley, R.H.; Liu, C.; Idriss, H.; Warde, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Castro, E.; Hernandez Garcia, A.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar]
- Fukuzumi, S. Nanocarbons as Electron Donors and Acceptors in Photoinduced Electron-Transfer Reactions. ECS J. Solid State Sci. Technol. 2017, 6, 3055–3061. [Google Scholar] [CrossRef]
- Mikami, K.; Matsumoto, S.; Tonoi, T.; Okubo, Y.; Suenobu, T.; Fukuzumi, S. Solid state photochemistry for fullerene functionalization: Solid state photoinduced electron transfer in the Diels-Alder reaction with anthracenes. Tetrahedron Lett. 1998, 39, 3733–3736. [Google Scholar] [CrossRef]
- Tkachenko, N.V.; Rantala, L.; Tauber, A.Y.; Helaja, J.; Hynninen, P.H.; Lemmetyinen, H. Photoinduced Electron Transfer in Phytochlorin—[60] Fullerene Dyads. J. Am. Chem. Soc. 1999, 121, 9378–9387. [Google Scholar] [CrossRef]
- Koeppe, R.; Sariciftci, N.S. Photoinduced charge and energy transfer involving fullerene derivatives. Photochem. Photobiol. Sci. 2005, 5, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.M.; Srdjenovic, B. Biological Aspects of Fullerenes. In Handbook on Fullerene: Synthesis, Properties and Applications; Kadish, K.M., Ruoff, R.S., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2000; pp. 431–436. [Google Scholar]
- Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar]
- Sivaraman, N.; Dhamodaran, R.; Kaliappan, I.; Srinivassan, T.G.; Rao, P.R.V.; Mathews, C.K. Solubility of C60 in organic solvents. J. Org. Chem. 1992, 57, 6077–6079. [Google Scholar] [CrossRef]
- Wilson, S.R. Biological Aspects of Fullerenes. In Fullerenes: Chemistry, physics and technology; John Wiley and Sons, Inc.: New York, NY, USA, 2000; pp. 431–436. [Google Scholar]
- Da Ros, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 8, 663–669. [Google Scholar] [CrossRef]
- Roberts, R.A.; Smith, R.A.; Safe, S.; Szabo, C.; Tjalkens, R.B.; Robertson, F.M. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology 2010, 276, 85–94. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z. Radical scavenging activities of alpha-alanine C60 adduct. Bioorg. Med. Chem. Lett. 2006, 16, 3731–3734. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Bolskar, R.D. Fullerenes for Drug Delivery. In Encyclopedia of Nanotechnology; Chemistry and Materials Science; Springer: Dordrecht, The Netherlands, 2016; pp. 1267–1281. [Google Scholar]
- Ashcroft, J.M.; Tsyboulski, D.A.; Hartman, K.B.; Zakharian, T.Y.; Marks, J.W.; Weisman, R.B.; Rosenblum, M.G.; Wilson, L.J. Fullerene (C60) immunoconjugates: Interaction of water-soluble C60 derivatives with the murine anti-gp240 melanoma antibody. Chem. Commun. 2006, 28, 3004–3006. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef]
- Dietel, E.; Hirsch, A.; Pietzak, B.; Waiblinger, M.; Lips, K.; Weidinger, A.; Gruss, A.; Dinse, K.P. Atomic Nitrogen Encapsulated in Fullerenes: Effects of Cage Variations. J. Am. Chem. Soc. 1999, 121, 2432–2437. [Google Scholar] [CrossRef]
- Gonzalez, K.A.; Wilson, L.J.; Wu, W.; Nancollas, G.H. Synthesis and in vitro characterization of a tissue-selective fullerene: Vectoring C (60) (OH) (16) AMBP to mineralized bone. Bioorg. Med. Chem. 2002, 10, 1991–1996. [Google Scholar] [CrossRef]
- Mashino, T.; Shimotohno, K.; Ikegami, N.; Nishikawa, D.; Okuda, K.; Takahashi, K.; Nakamura, S.; Mochizuki, M. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1107–1109. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Park. Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Podolski, I.Y.; Podlubnaya, Z.A.; Godukhin, O.V. Fullerenes C60, Antiamyloid Action, the Brain, and Cognitive Processes. Biophysics 2010, 55, 71–76. [Google Scholar] [CrossRef]
- Murakami, M.; Hyodo, S.; Fujikawa, Y.; Fujimoto, T.; Maeda, K. Photoprotective effects of inclusion complexes of fullerenes with polyvinylpyrrolidone. Photodermatol. Photoimmunol. Photomed. 2013, 29, 196–203. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Perez-Cordero, E.; Echegoyen, L. Electrochemical detection of C60 6- and C70 6-: enhanced stability of fullerides in solution. J. Am. Chem. Soc. 1992, 114, 3978–3980. [Google Scholar] [CrossRef]
- Weibo, Y.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar]
- Diederich, F.; Gomez-Lopez, M. Supramolecular fullerene chemistry. Chem. Soc. Rev. 1999, 28, 263–277. [Google Scholar] [CrossRef]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale 2011, 3, 4035–4041. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Swirczewski, J.W.; Hsu, C.S.; Chowdhury, S.K.; Cameron, S.; Creegan, K. Multi-hydroxy additions onto C60 fullerenemolecules. J. Chem. Soc. Chem. Commun. 1992, 1791–1793. [Google Scholar] [CrossRef]
- Li, J.; Takeuchi, A.; Ozawa, M.; Li, X.; Saigo, K.; Kitazawa, K. C60 fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 1993, 1784–1785. [Google Scholar] [CrossRef]
- Wudl, F.; Hirsch, A.; Khemani, K.C.; Suzuki, T.; Allemand, P.M.; Koch, A.; Eckert, H.; Srdanov, G.; Webb, H.M. Survey of Chemical Reactivity of C60, Electrophile, and Dienopolarophile Par Excellence. In Synthesis, Properties, and Chemsitry of Large Carbon Clusters; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Milic, D.; Prato, M. Fullerene unsymmetrical bis-adducts as models for novel peptidomimetics. Eur. J. Org. Chem. 2010, 2010, 476–483. [Google Scholar] [CrossRef]
- Chhabra, N.; Aseri, M.L.; Padmanabhan, D. A review of drug isomerism and its significance. J. Appl. Basic Med. Res. 2013, 3, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Bingel, C. Cyclopropanierung von fullerenen. Chem. Ber. 1993, 126, 1957–1959. [Google Scholar] [CrossRef]
- Li, H.; Haque, S.A.; Kitaygorodskiy, A.; Meziani, M.J.; Torres-Castillo, M.; Sun, Y.P. Alternatively modified Bingel reaction for efficient syntheses of C60 hexakis-adducts. Org. Lett. 2006, 8, 5641–5643. [Google Scholar] [CrossRef] [PubMed]
- Brettreich, M.; Hirsch, A. A highly water-soluble dendro [60]-fullerene. Tetrahedron Lett. 1998, 39, 2731–2734. [Google Scholar] [CrossRef]
- Aroua, S.; Schweizer, W.B.; Yamakoshi, Y. C60 pyrrolidine biscarboxylic acid derivative as a versatile precursor for biocompatible fullerenes. Org. Lett. 2014, 16, 1688–1691. [Google Scholar] [CrossRef]
- Dallas, P.; Rasovic, I.; Rogers, G.; Porfyrakis, K. Endohedral Fullerenes: Optical Properties and Biomedical Applications. In Carbon Nanomaterials Sourcebook: Graphene, Fullerenes, Nanotubes and Nanodiamonds; Taylor & Francis: Boca Raton, FL, USA, 2016; pp. 255–270. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Vasella, A.; Uhlmann, P.; Waldraff, C.A.A.; Diederich, F.; Thilgen, C. Fullerene sugars: Preparation of enantiomerically pure, spiro-linked Cglycosides of C60. Angew. Chem. Int. Ed. 1992, 31, 1388–1390. [Google Scholar] [CrossRef]
- Yashiro, A.; Nishida, Y.; Ohno, M.; Eguchi, S.; Kobayashi, K. Fullerene glycoconjugates: A general synthetic approach via cycloaddition of per-Oacetyl glycosyl azides to [60] fullerene. Tetrahedron Lett. 1998, 39, 9031–9034. [Google Scholar] [CrossRef]
- Isobe, H.; Cho, K.; Solin, N.; Werz, D.B.; Seeberger, P.H.; Nakamura, E. Synthesis of fullerene glycoconjugates via a copper-catalyzed Huisgen cycloaddition reaction. Org. Lett. 2007, 9, 4611–4614. [Google Scholar] [CrossRef]
- Enes, R.F.; Tomé, A.C.; Cavaleiro, J.A.; El-Agamey, A.; McGarvey, D.J. Synthesis and solvent dependence of the photophysical properties of [60] fullerene–sugar conjugates. Tetrahedron 2005, 61, 11873–11881. [Google Scholar] [CrossRef]
- Tanimoto, S.; Sakai, S.; Matsumura, S.; Takahashi, D.; Toshima, K. Target selective photo-degradation of HIV-1 protease by a fullerene-sugar hybrid. Chem. Commun. 2008, 44, 5767–5769. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Atalick, S.; Guldi, D.M.; Lanig, H.; Brettreich, M.; Burghardt, S.; Hatzimarinaki, M.; Ravanelli, E.; Prato, M.; van Eldik, R.; et al. Electrostatic complexation and photoinduced electron transfer between Zn-cytochrome c and polyanionic fullerene dendrimers. Chem. Eur. J. 2003, 9, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.W.; Shih, J.S. Preparation and application of immobilized c60-glucose oxidase enzyme in fullerene C 60-coated piezoelectric quartz crystal glucose sensor. Sens. Actuators B 2001. Sens. Actuators B 2001, 81, 1–8. [Google Scholar] [CrossRef]
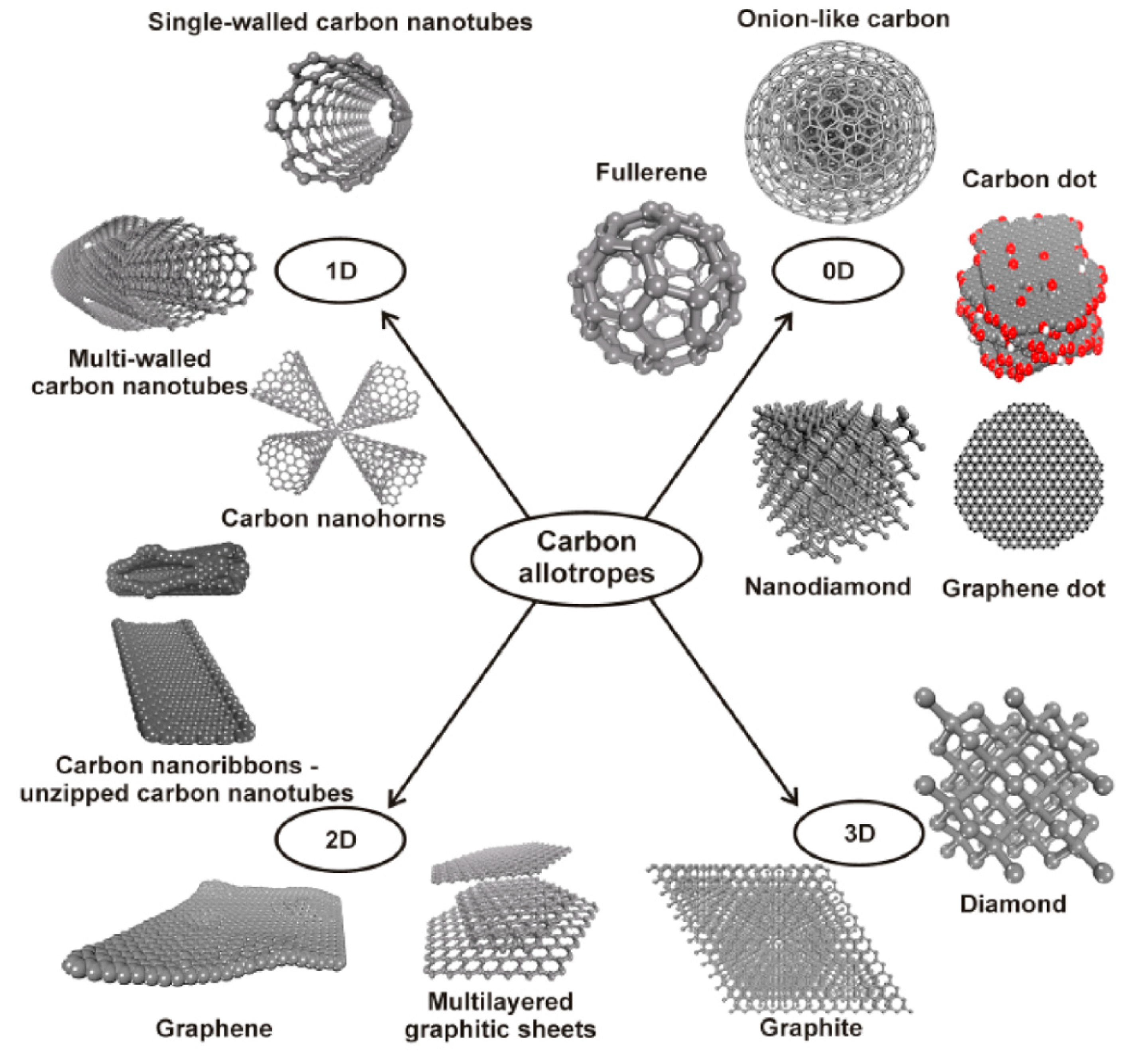
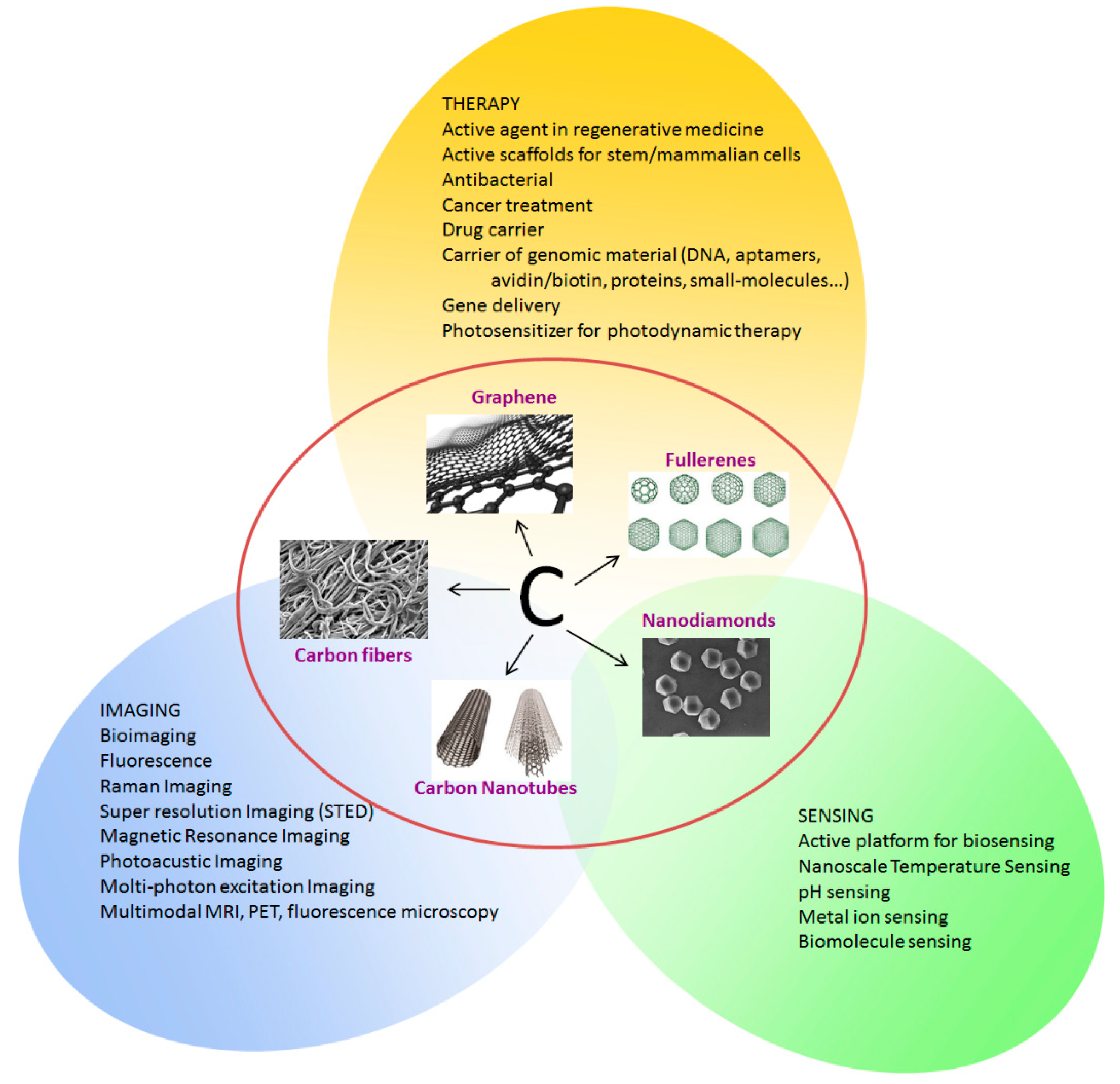
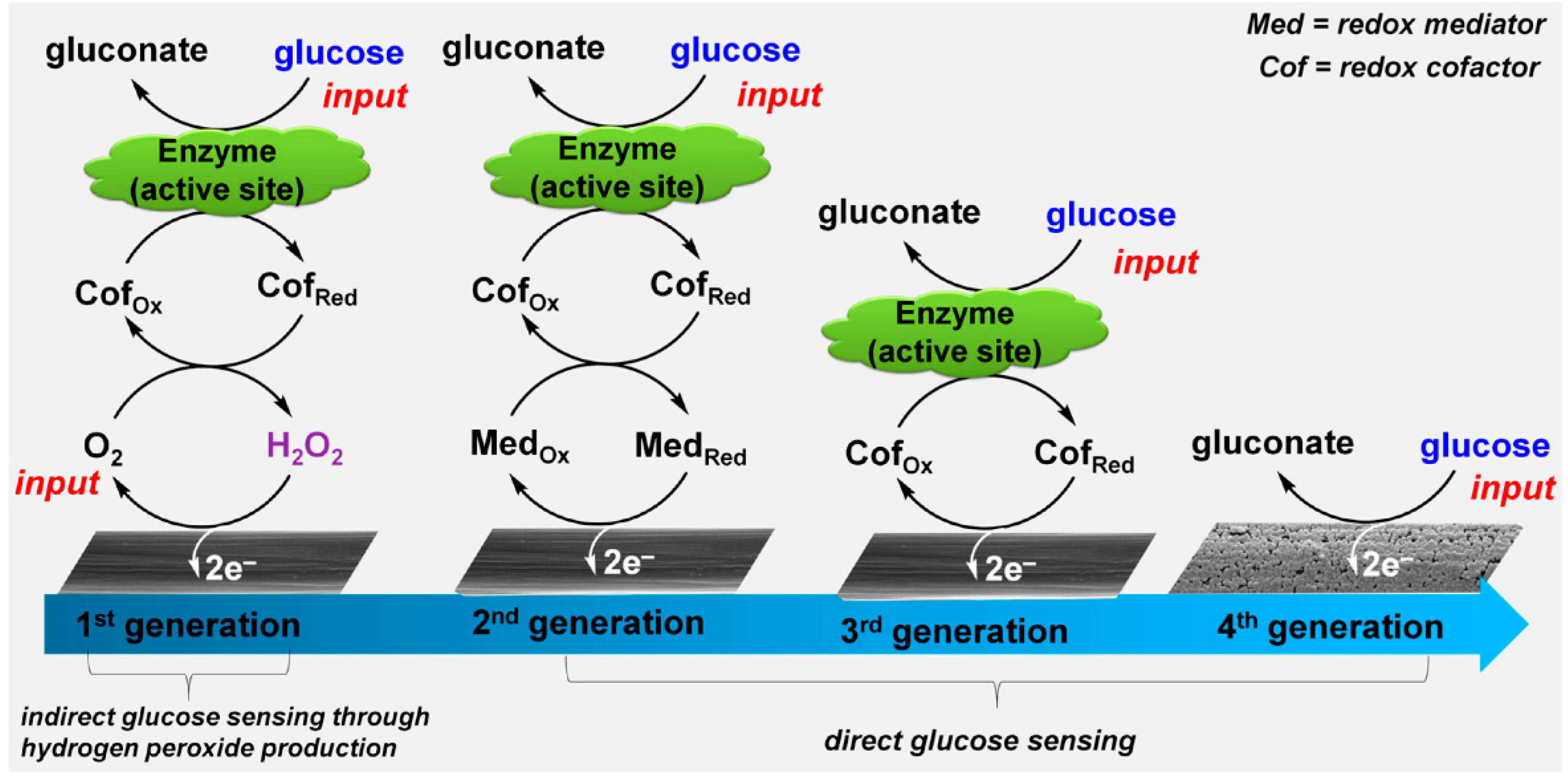
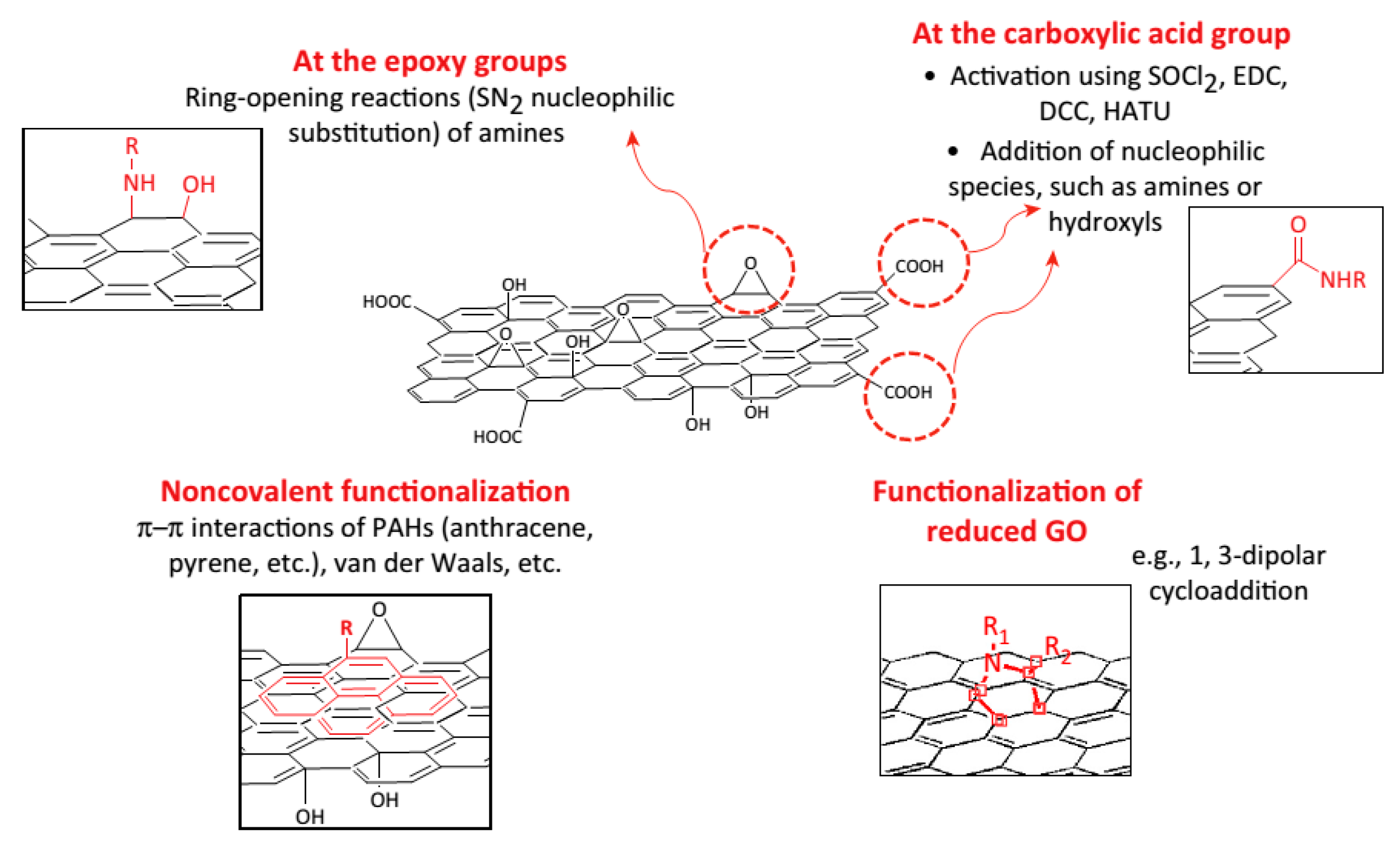
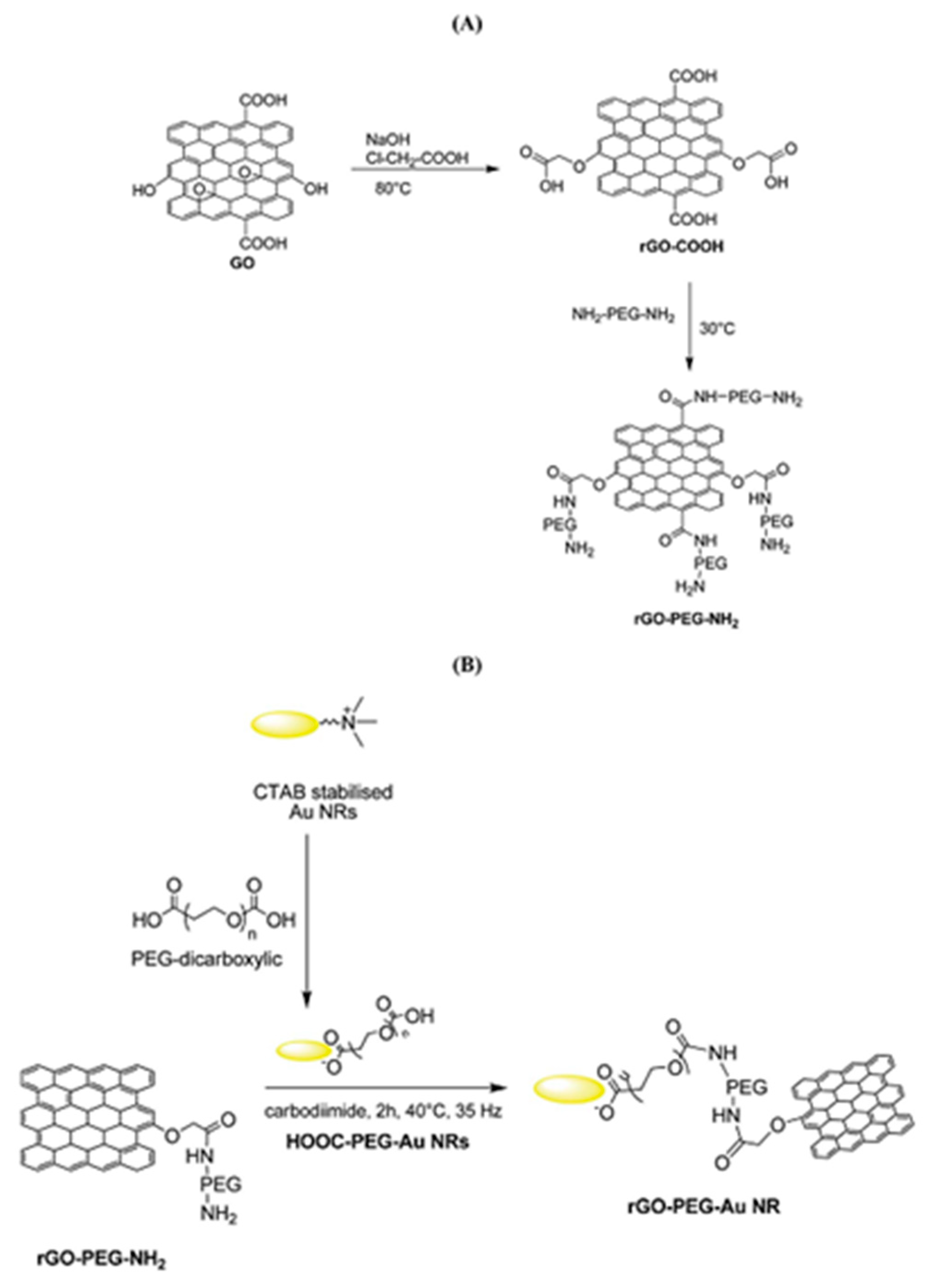
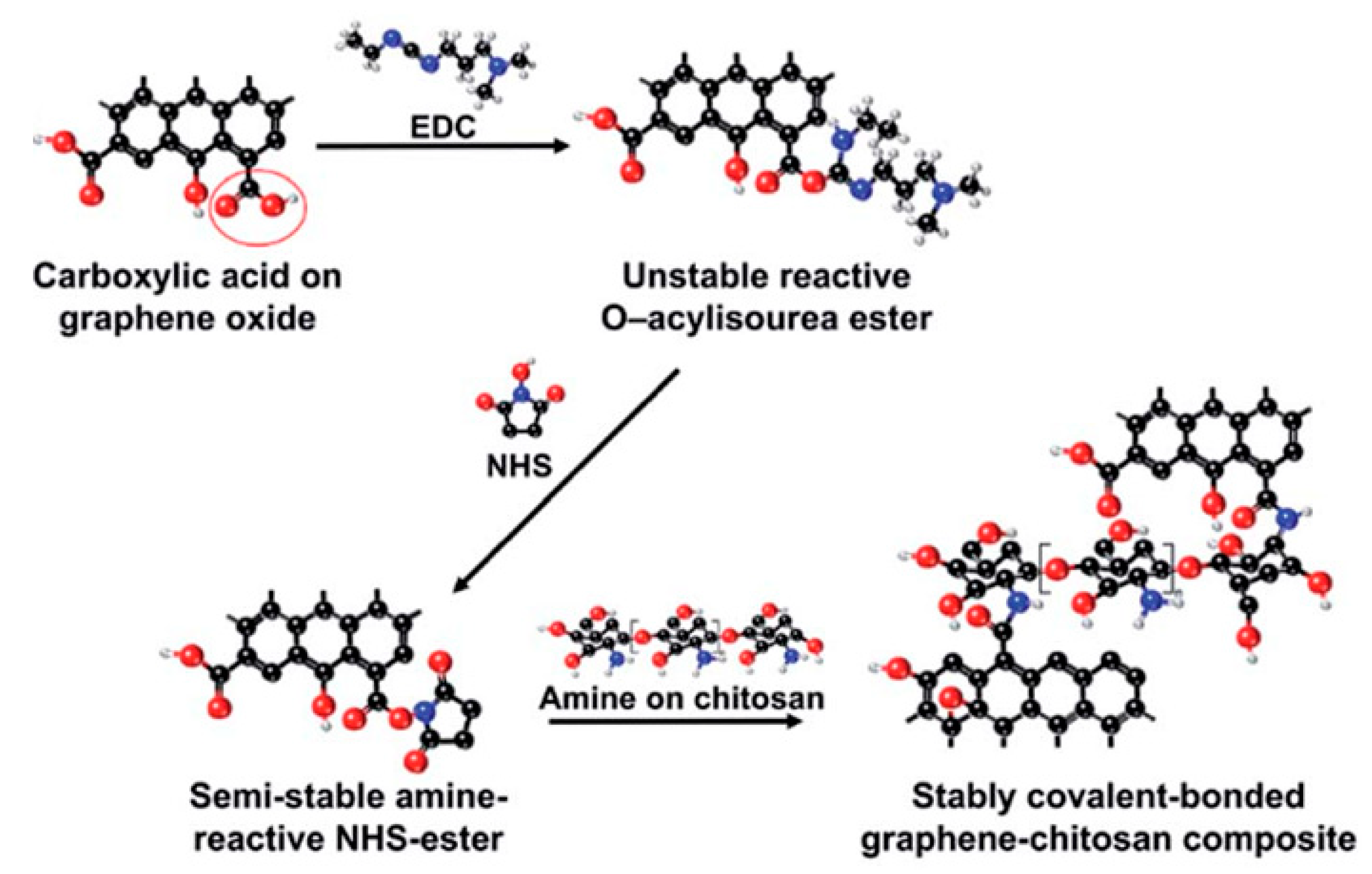
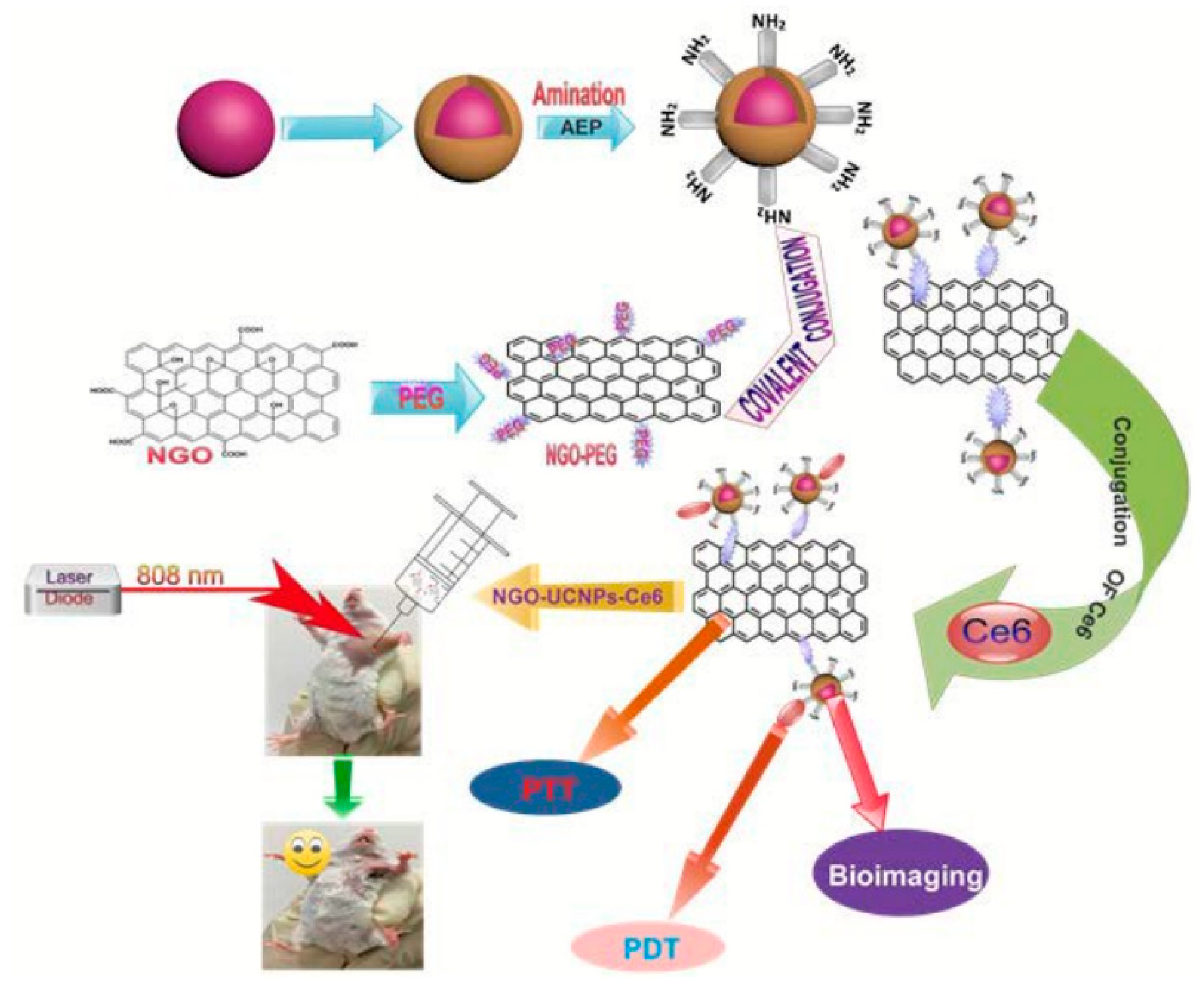
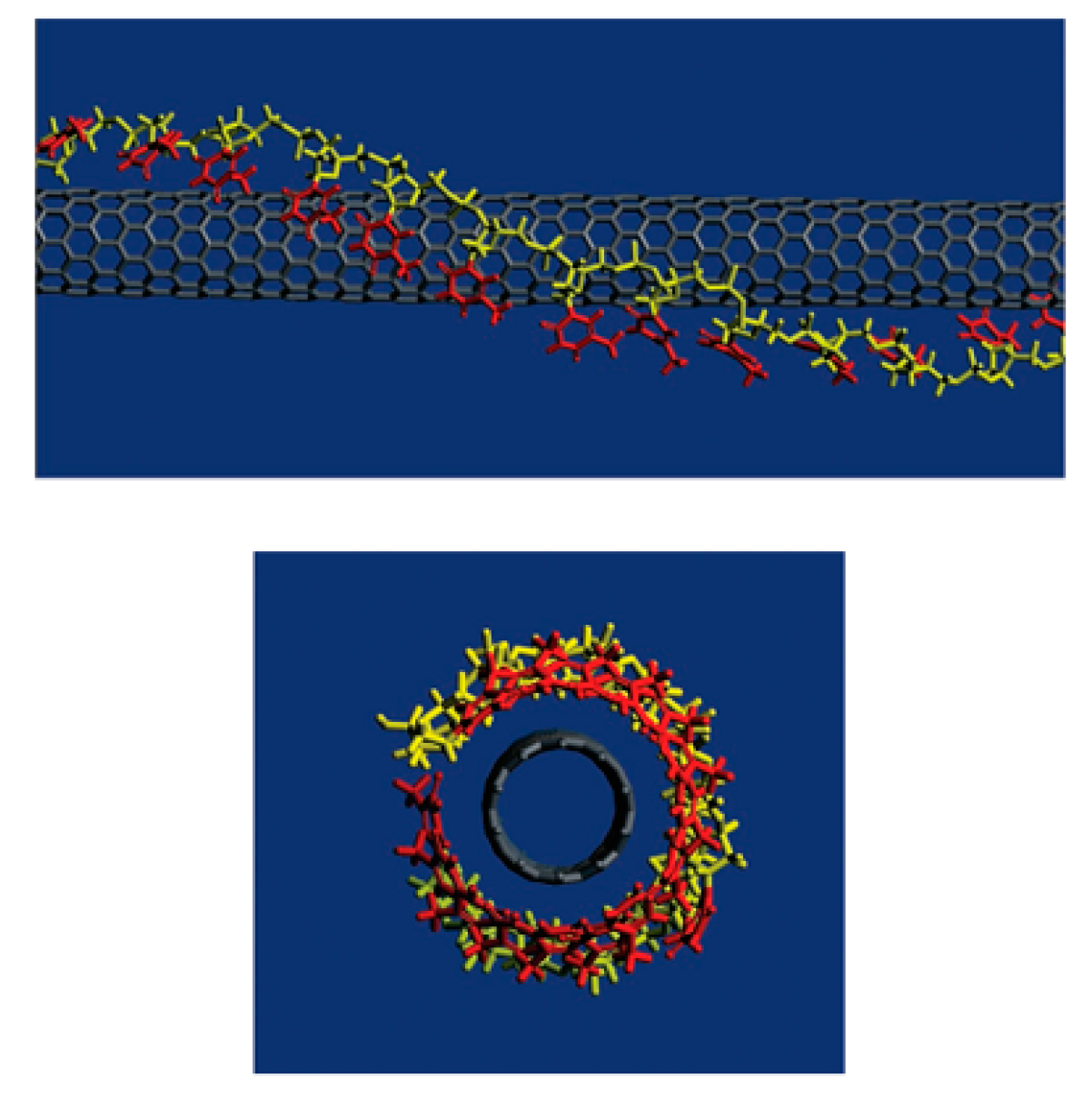
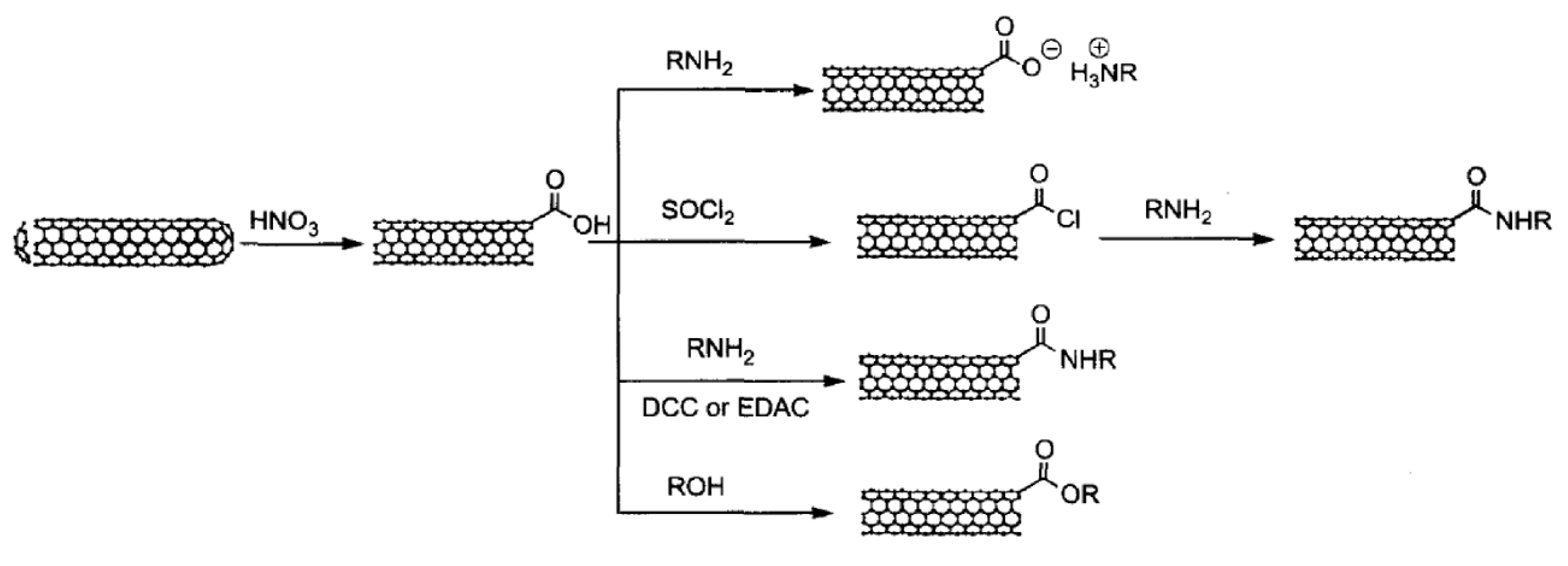
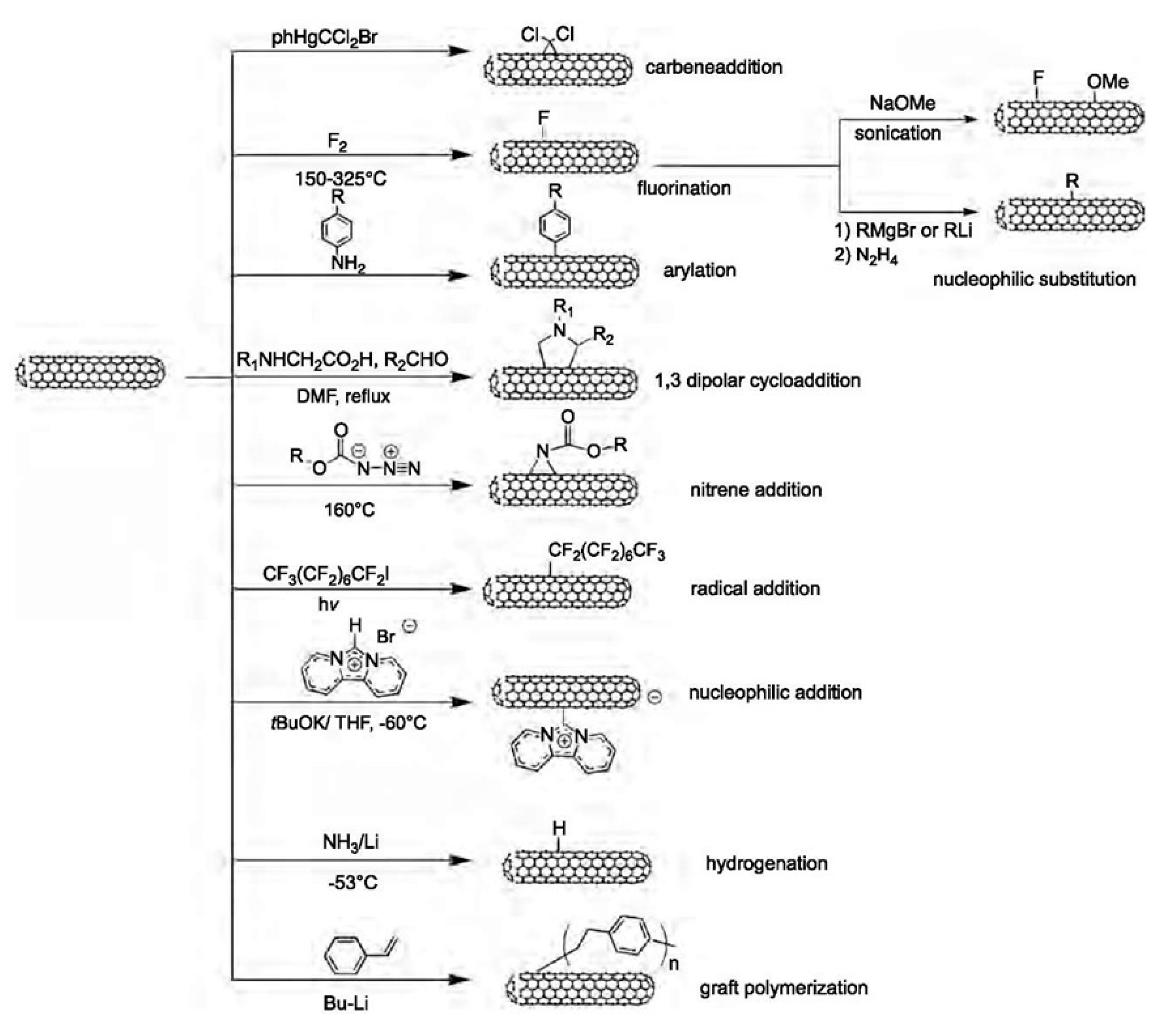
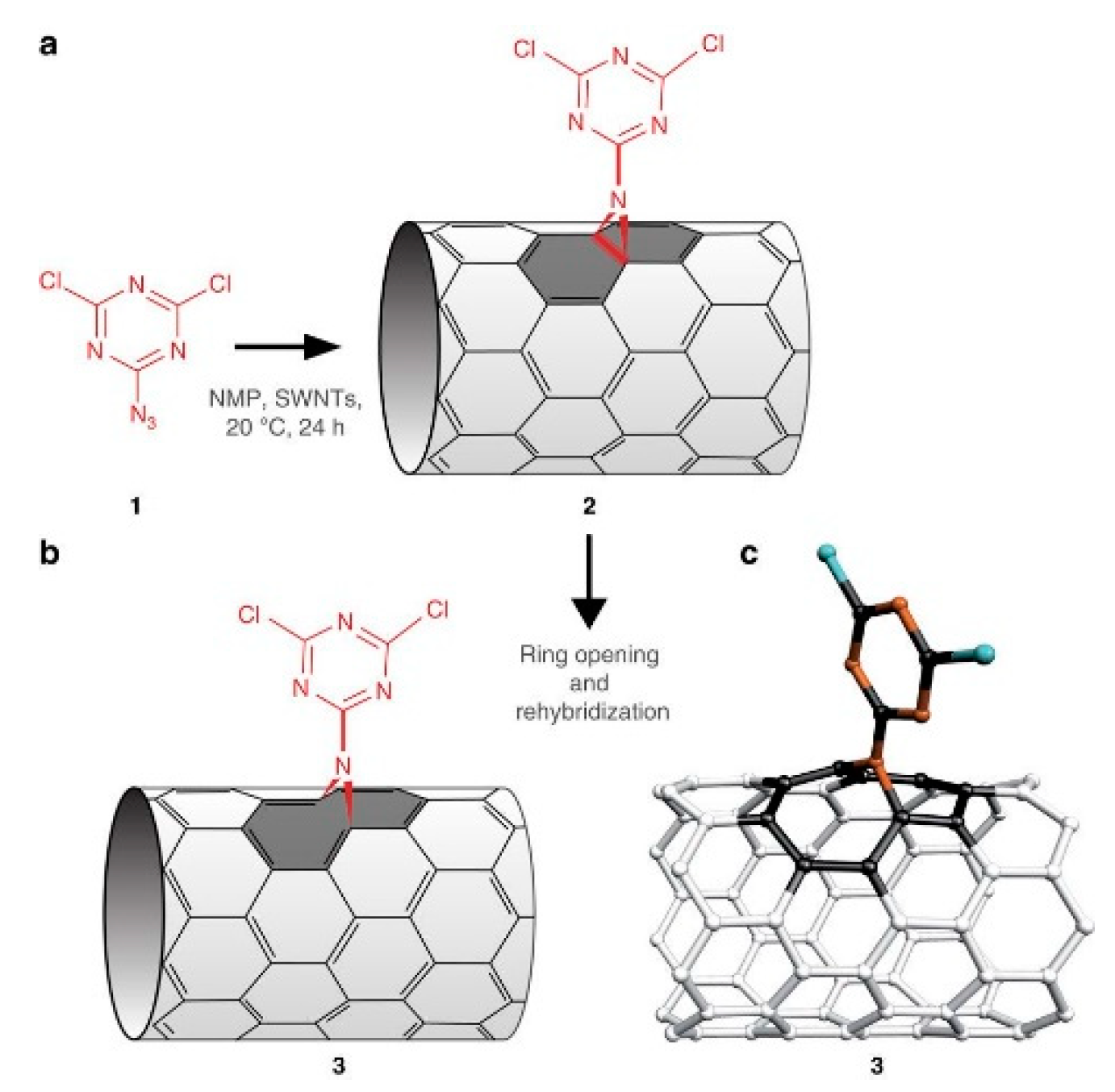
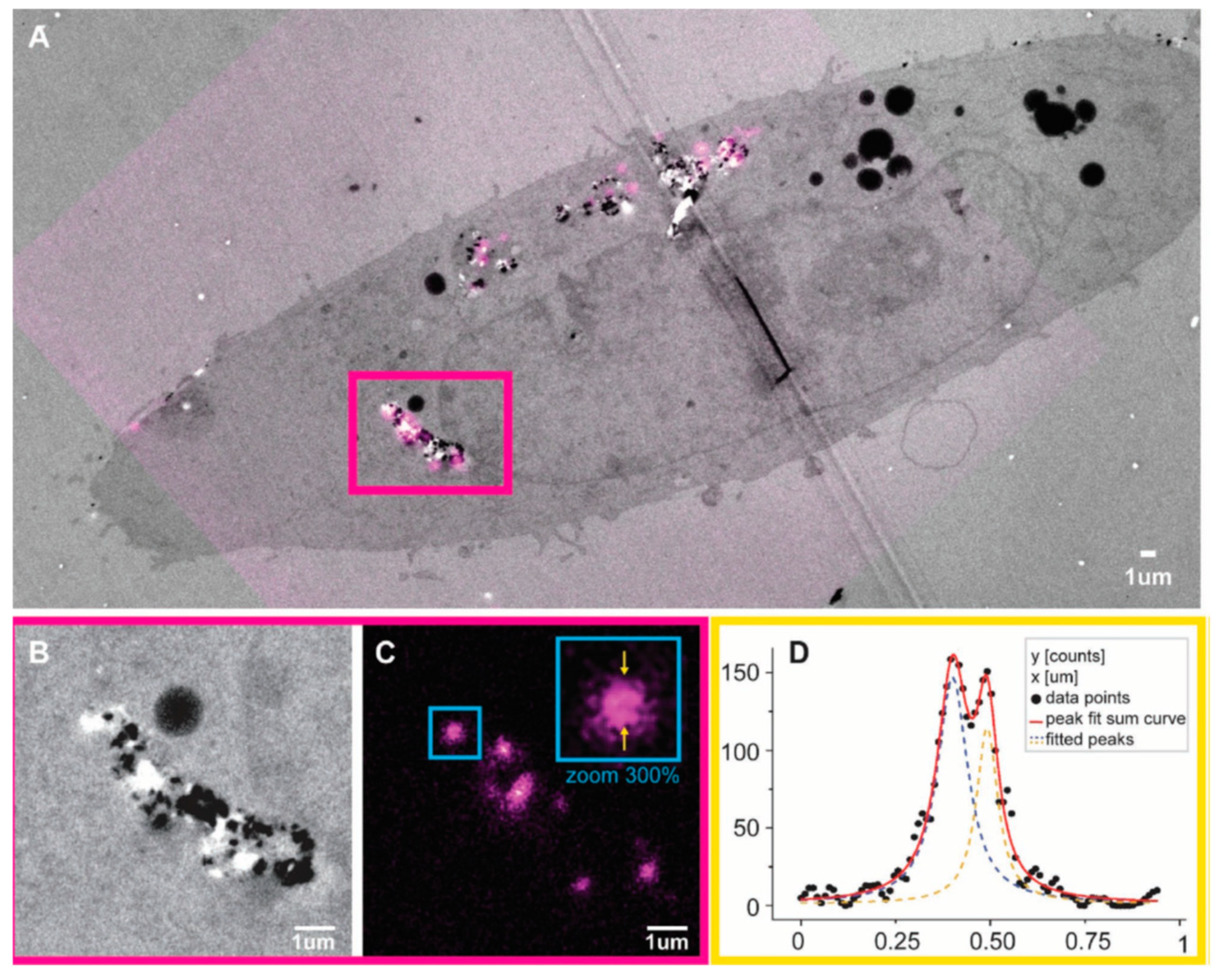
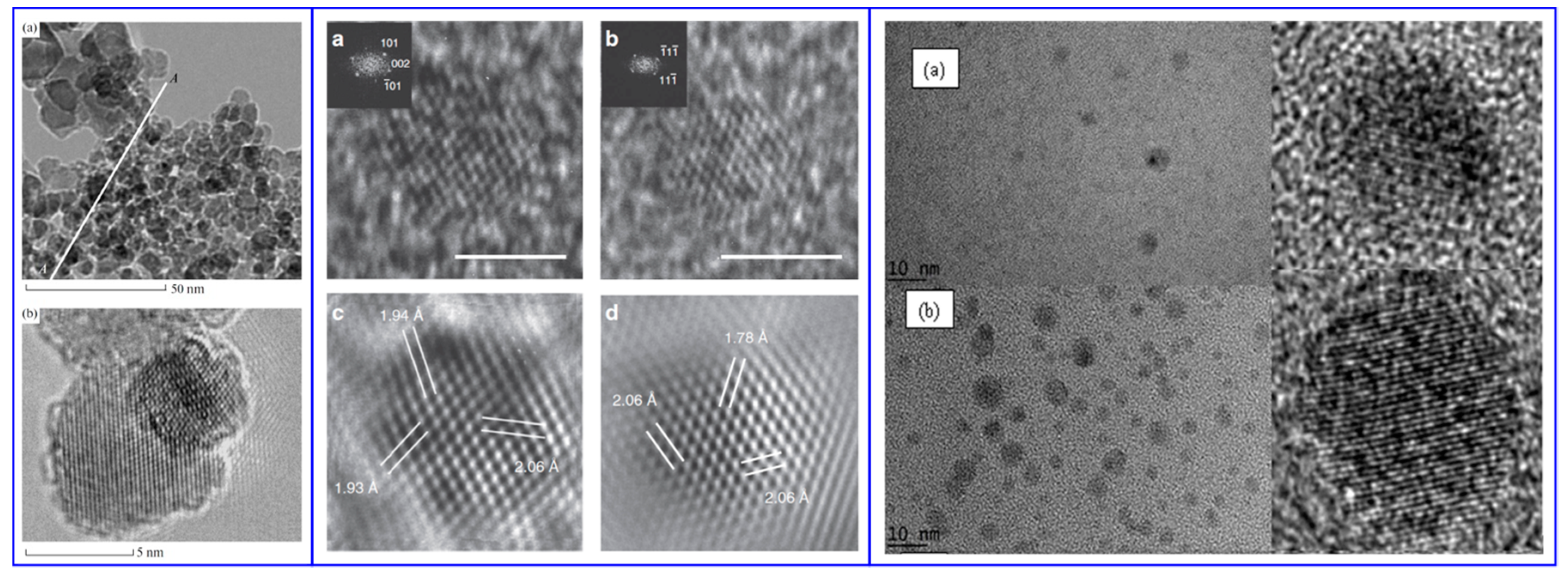
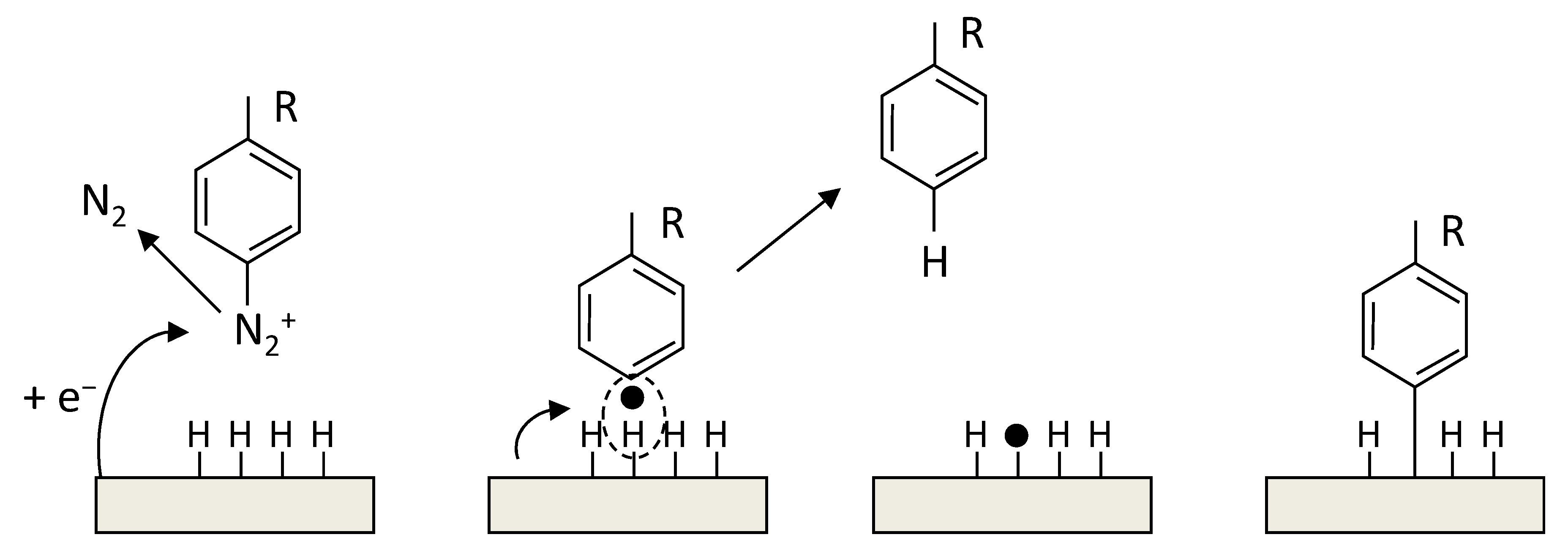
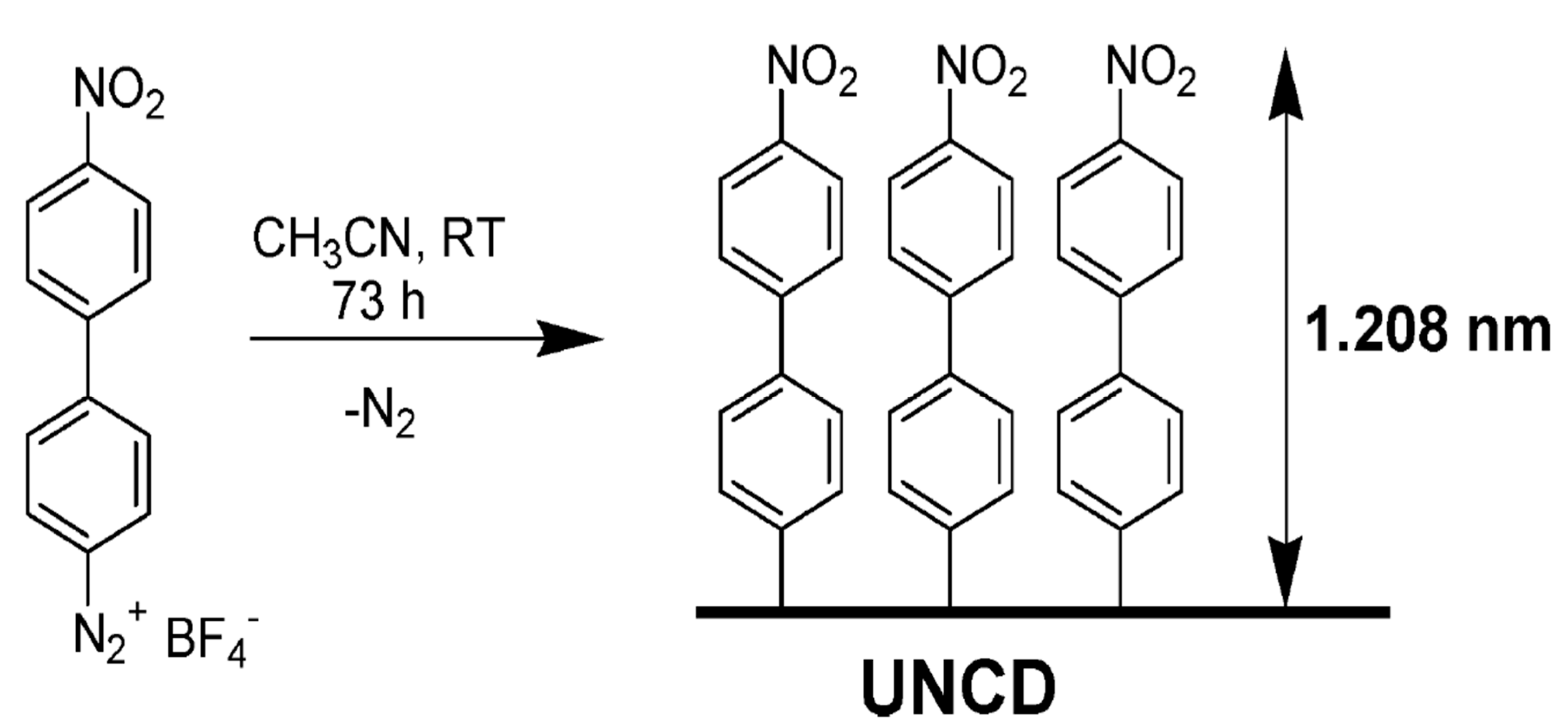
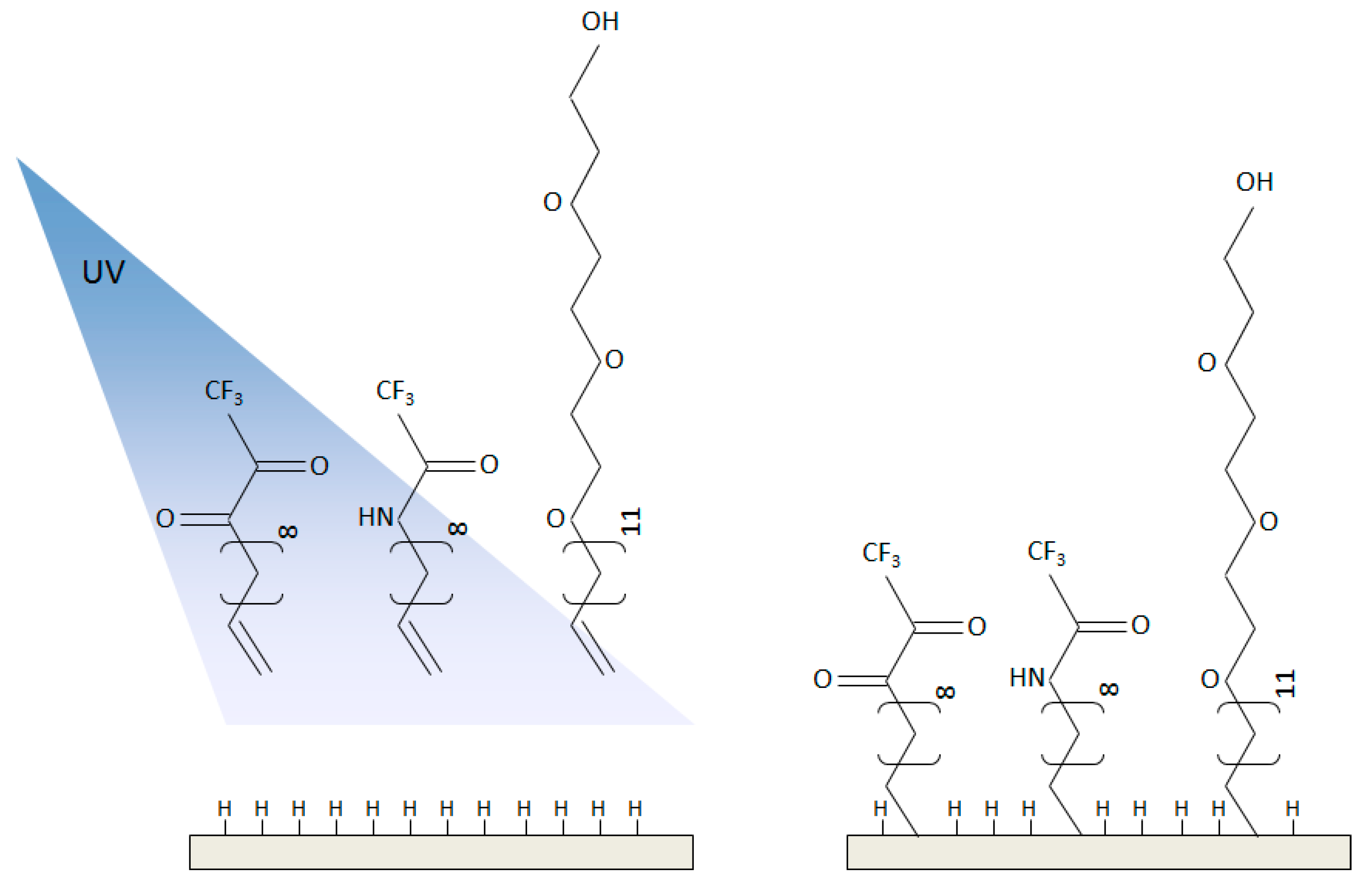

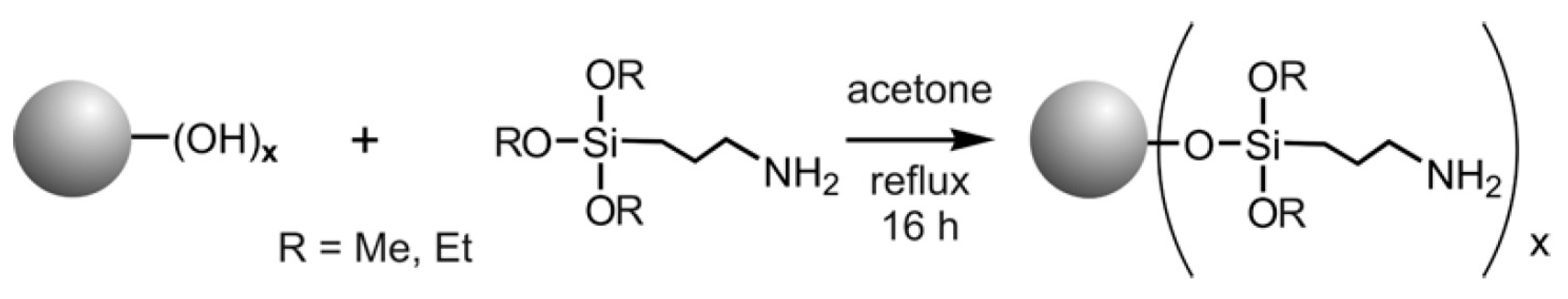
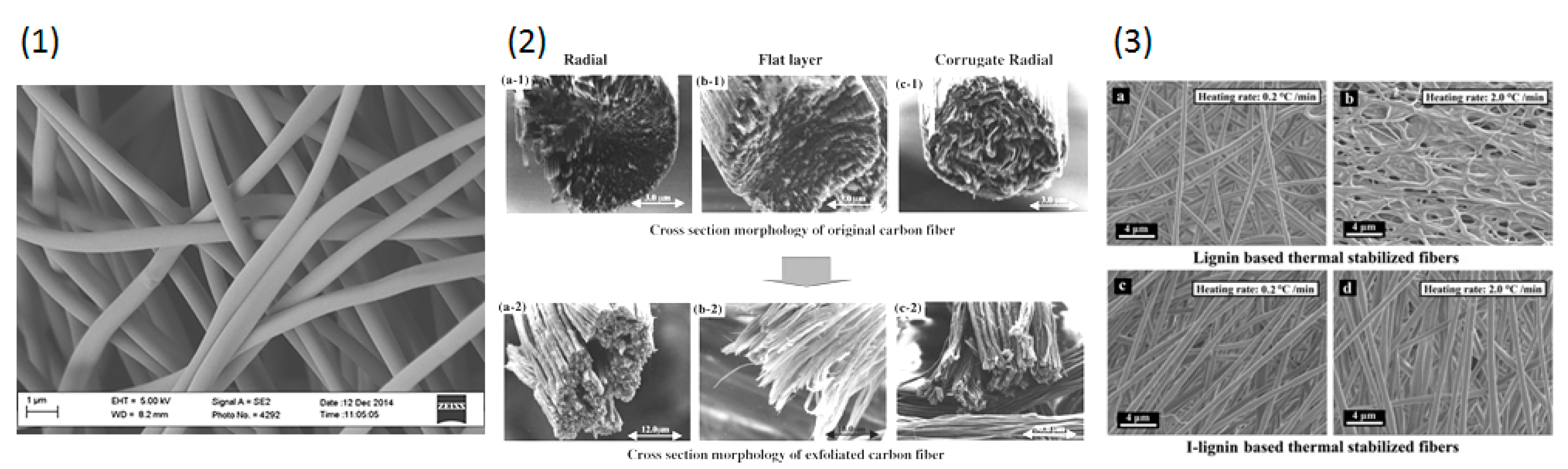
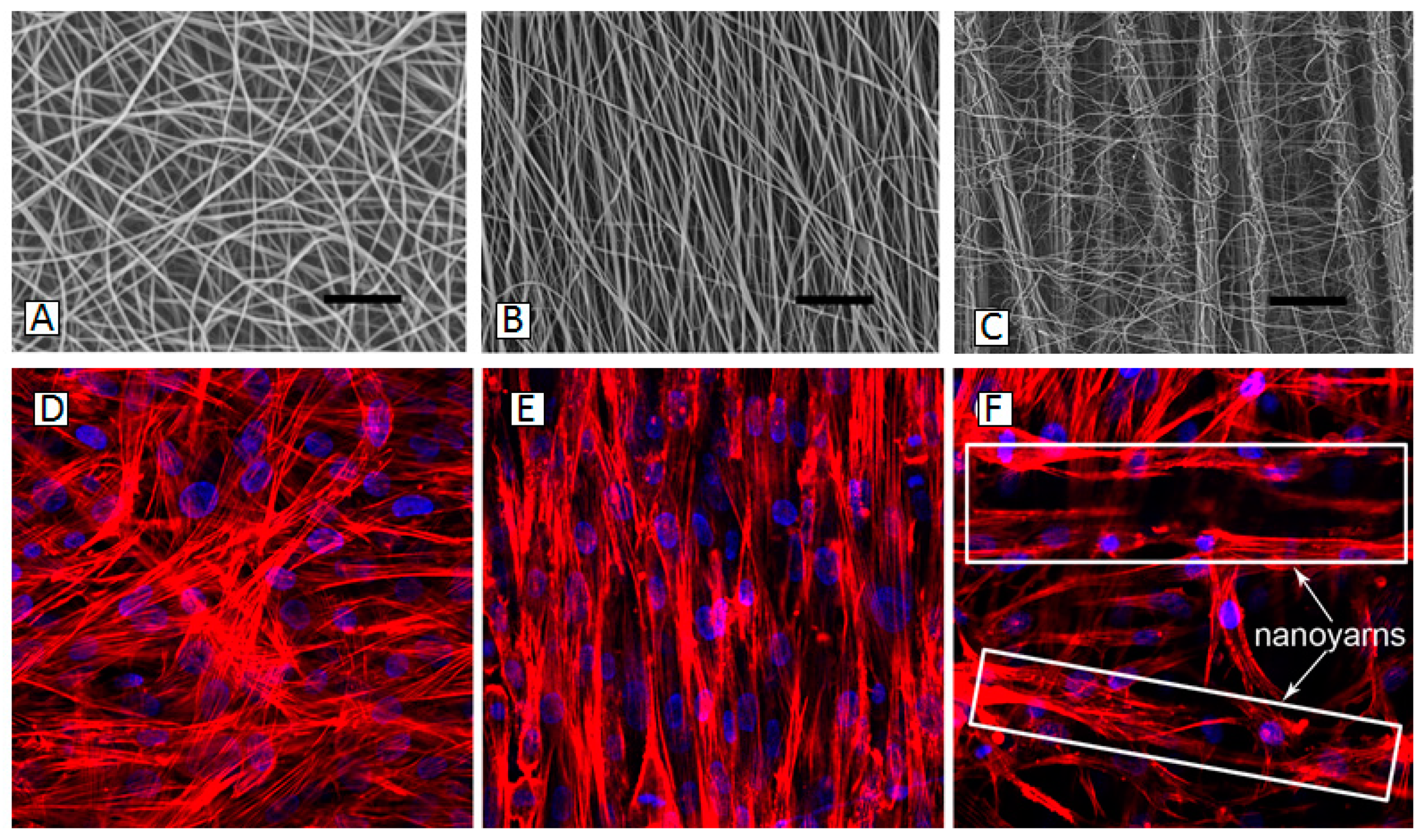
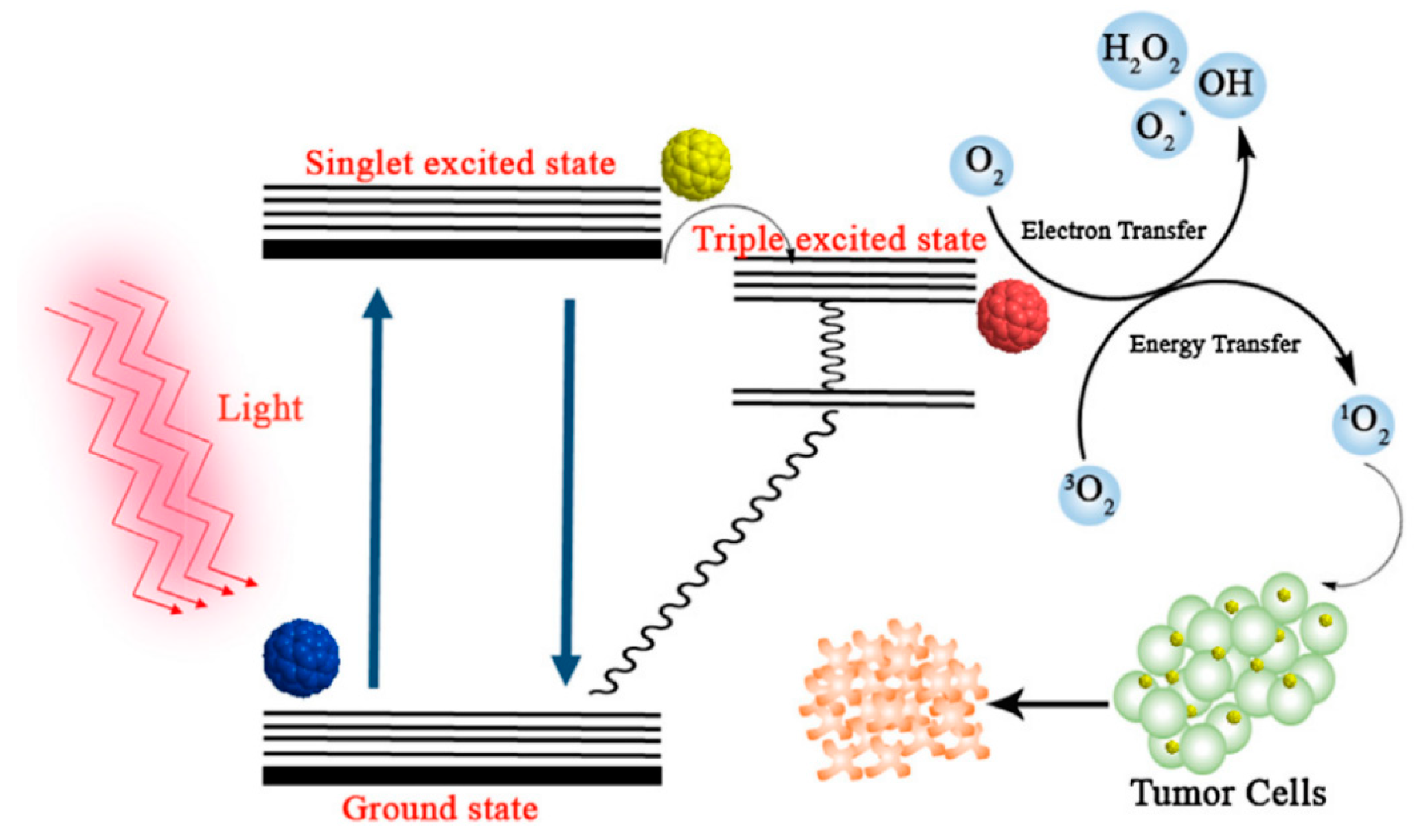
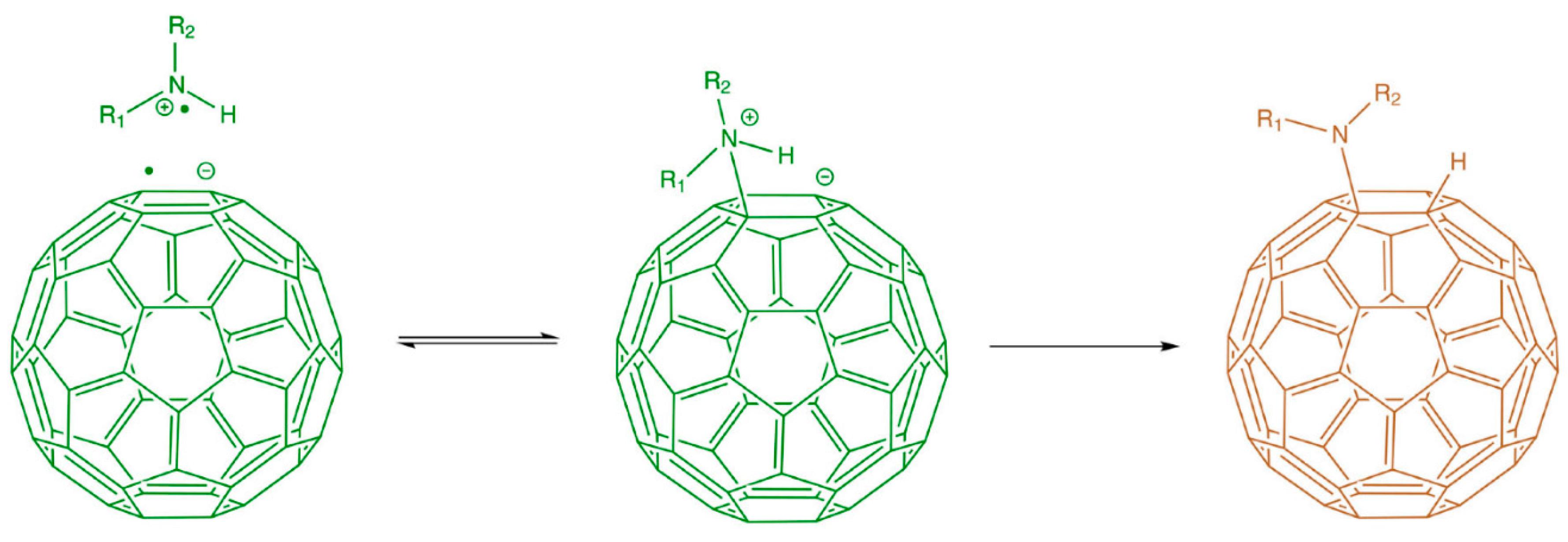
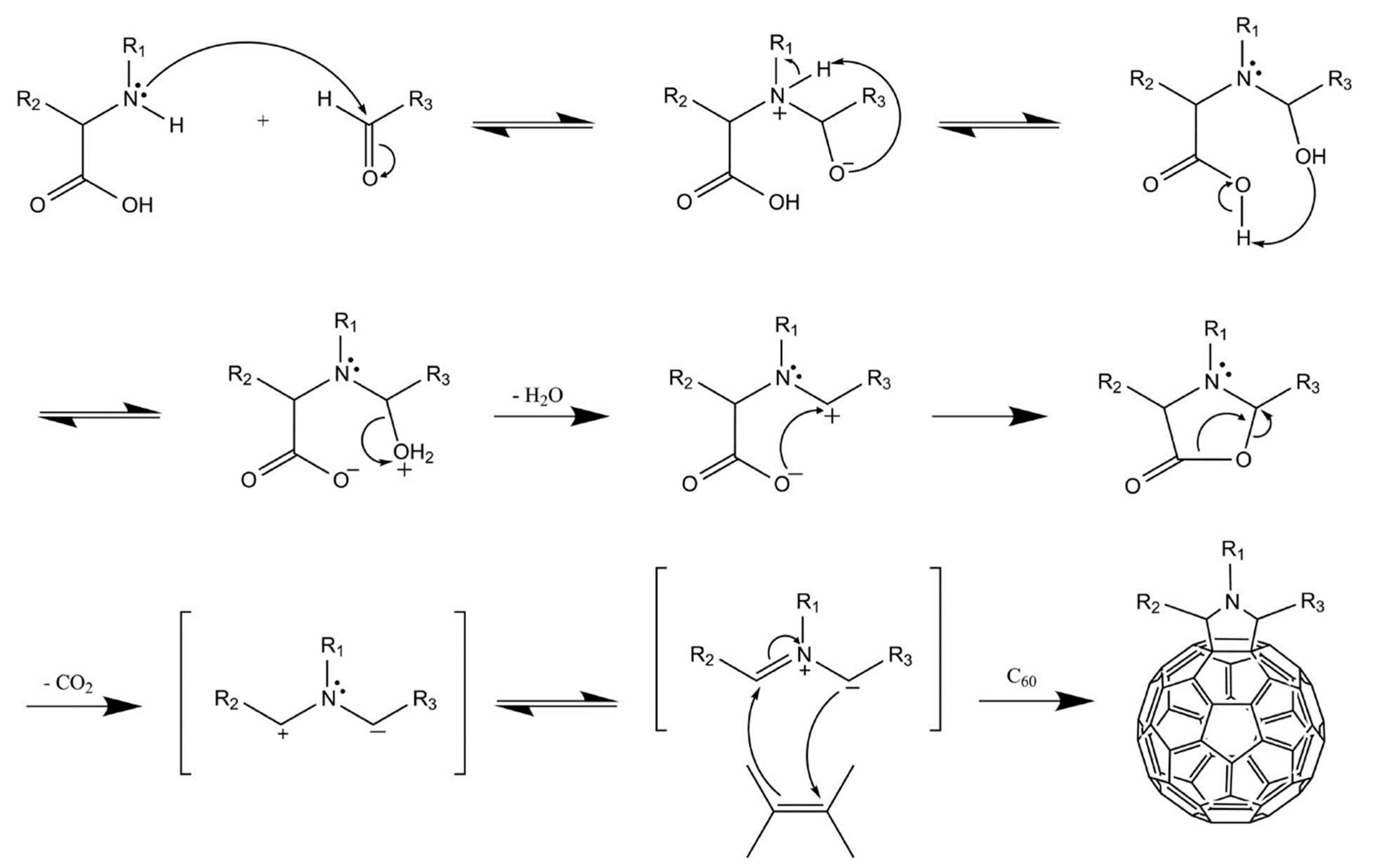
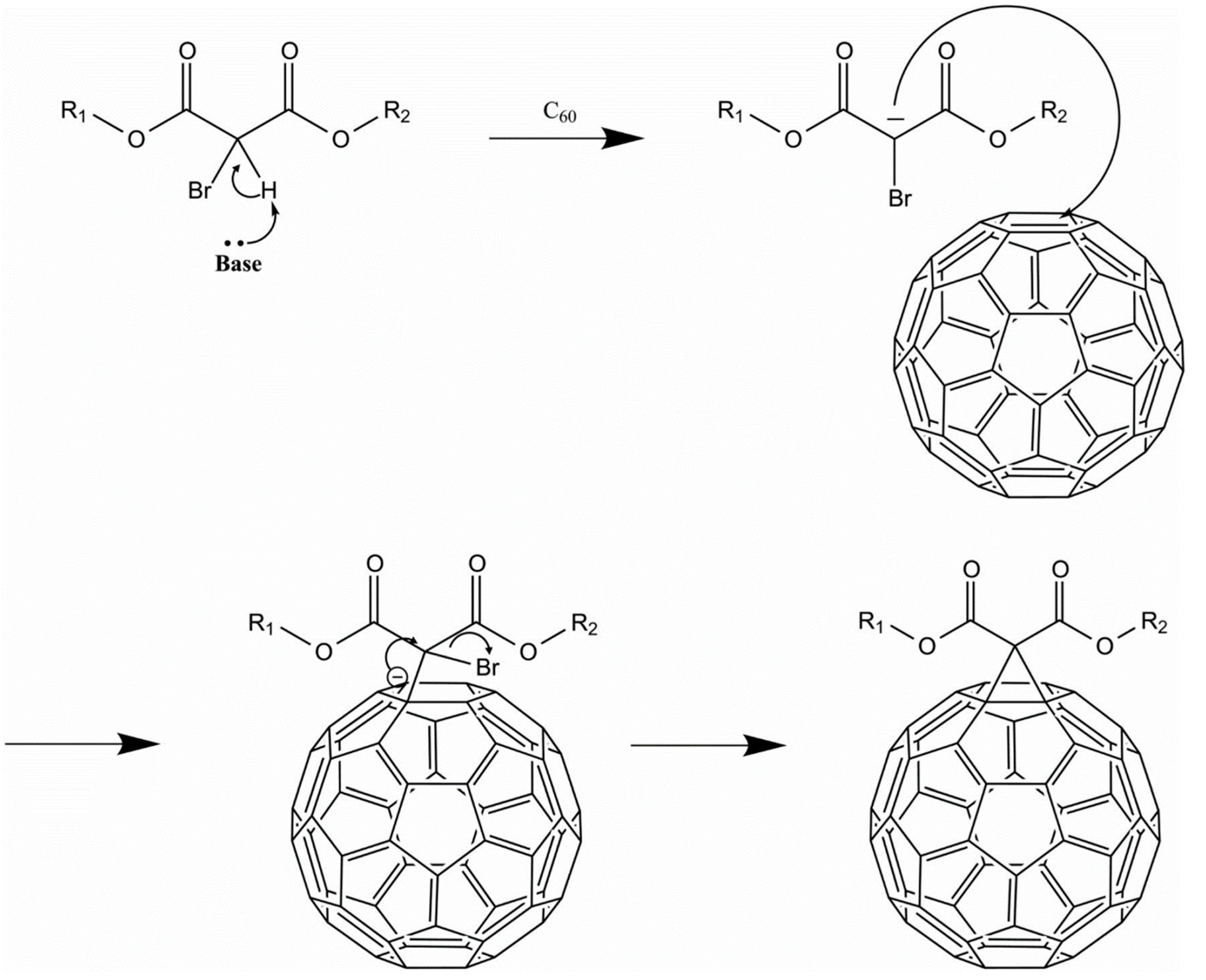
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C 2019, 5, 72. https://doi.org/10.3390/c5040072
Liu W, Speranza G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C. 2019; 5(4):72. https://doi.org/10.3390/c5040072
Chicago/Turabian StyleLiu, Wei, and Giorgio Speranza. 2019. "Functionalization of Carbon Nanomaterials for Biomedical Applications" C 5, no. 4: 72. https://doi.org/10.3390/c5040072
APA StyleLiu, W., & Speranza, G. (2019). Functionalization of Carbon Nanomaterials for Biomedical Applications. C, 5(4), 72. https://doi.org/10.3390/c5040072