Excitons in Carbonic Nanostructures
Abstract
1. Introduction
2. The Structures and Properties of Fluorescent Nanocarbon Materials
2.1. The Broad Family of Fluorescent Nanocarbons
2.2. Going Deeper into Nanocarbon Structures and Their Formation
2.3. Variability in Quantum Yield, Emission Color, and the Strong Stokes Shifts
3. The Key Questions to be Resolved
3.1. Why Are Carbon Nanoparticles so Emissive?
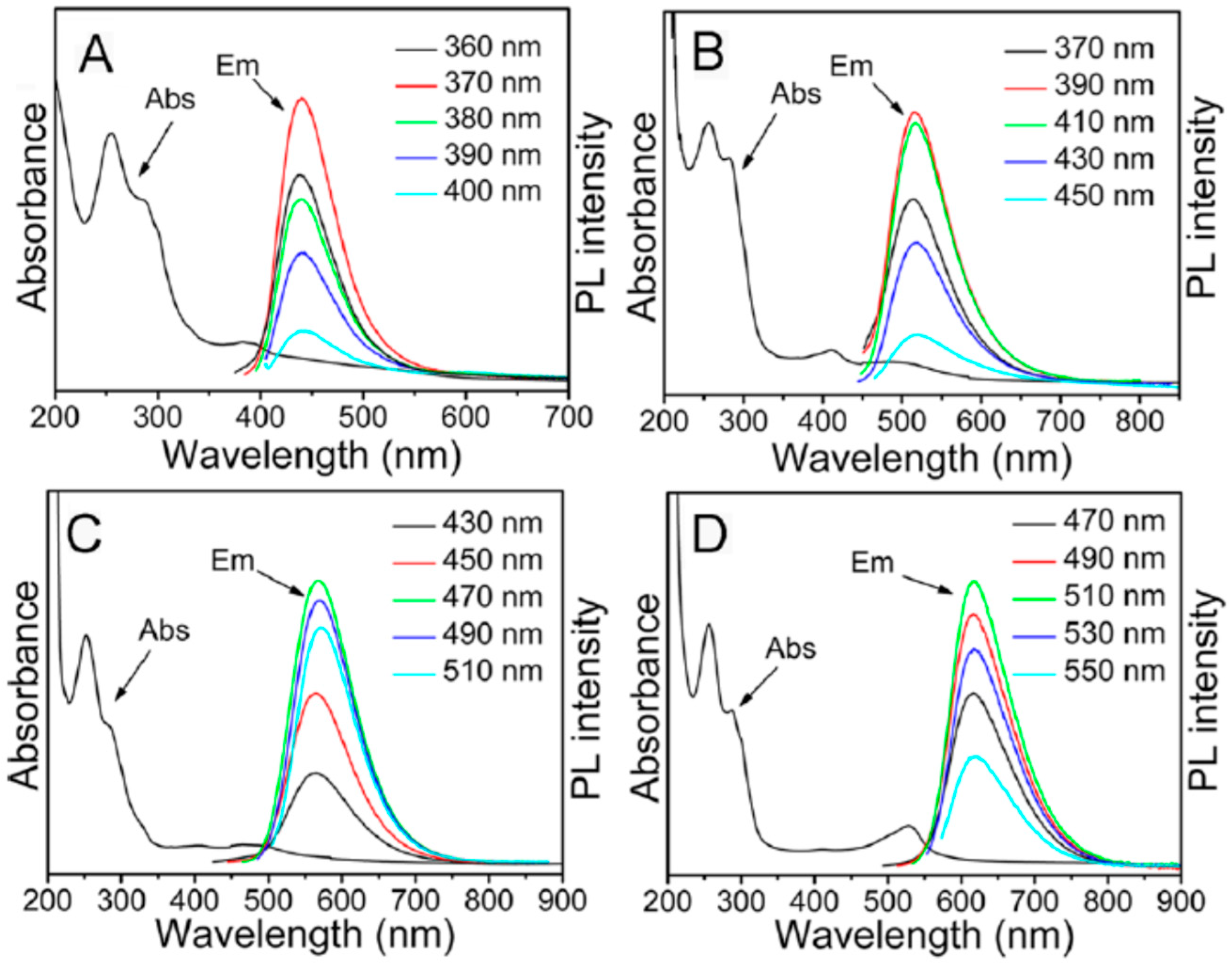
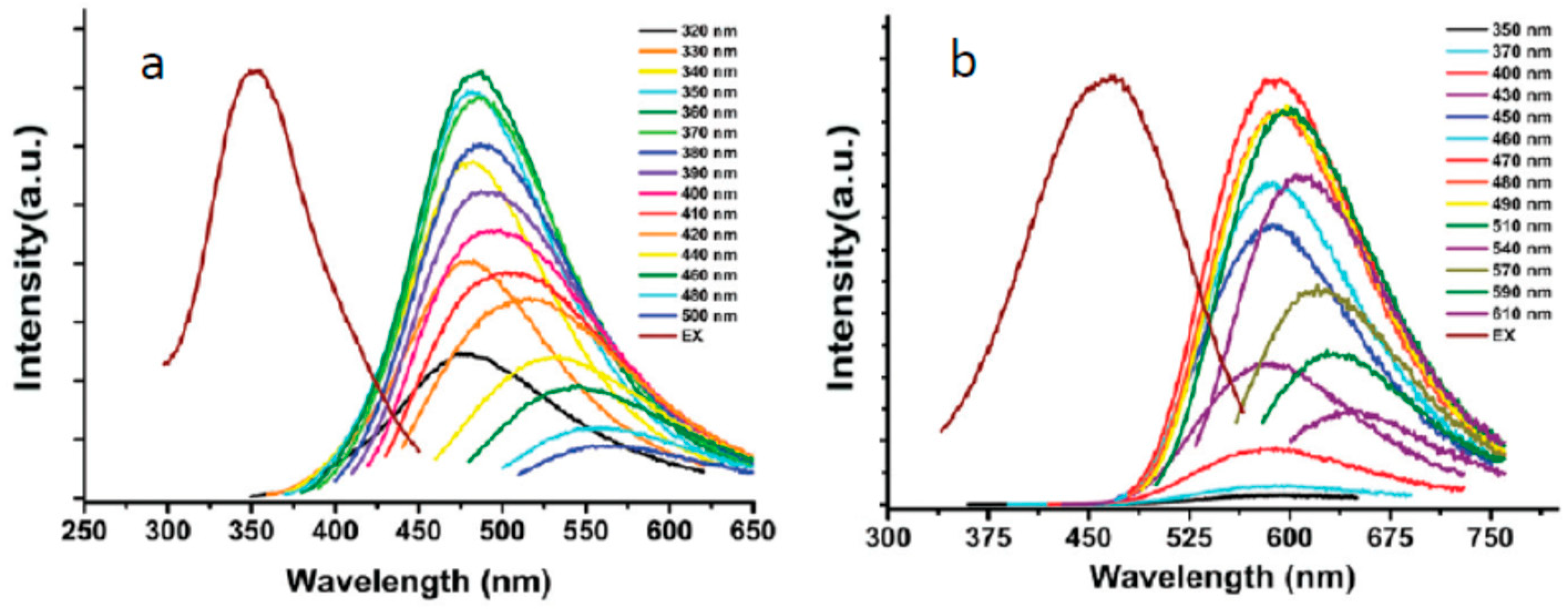
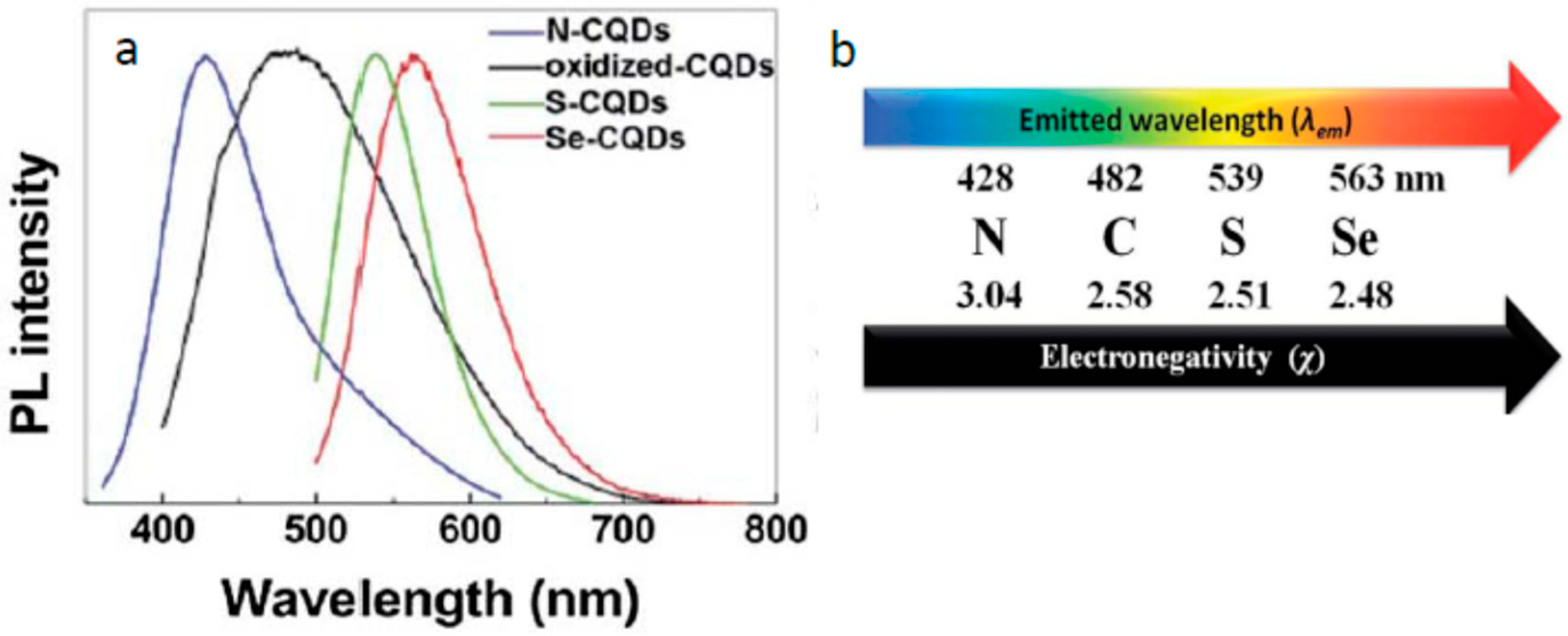
3.2. What Determines the Excitation and Emission Peak Positions?
3.3. Why Are the Strong Stokes Shifts Systematically Observed?
3.4. What Is the Origin of Spectral Heterogeneity and Tunability?
3.5. What Is the Photophysical Mechanism Governing the Emission?
4. Chromophore Behavior in Nanoscale Ensembles
4.1. On the Involvement of Wannier–Mott Loosely Bound Excitons
4.2. The Excitonic States in Aggregates of Organic Dyes
4.3. Coherent and Non-Coherent Exciton Transfer
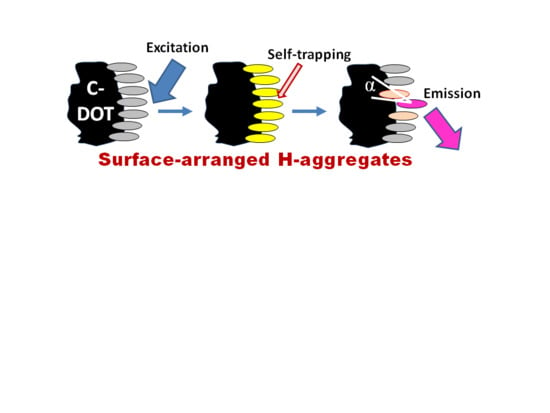
4.4. Exciton Self-Trapping
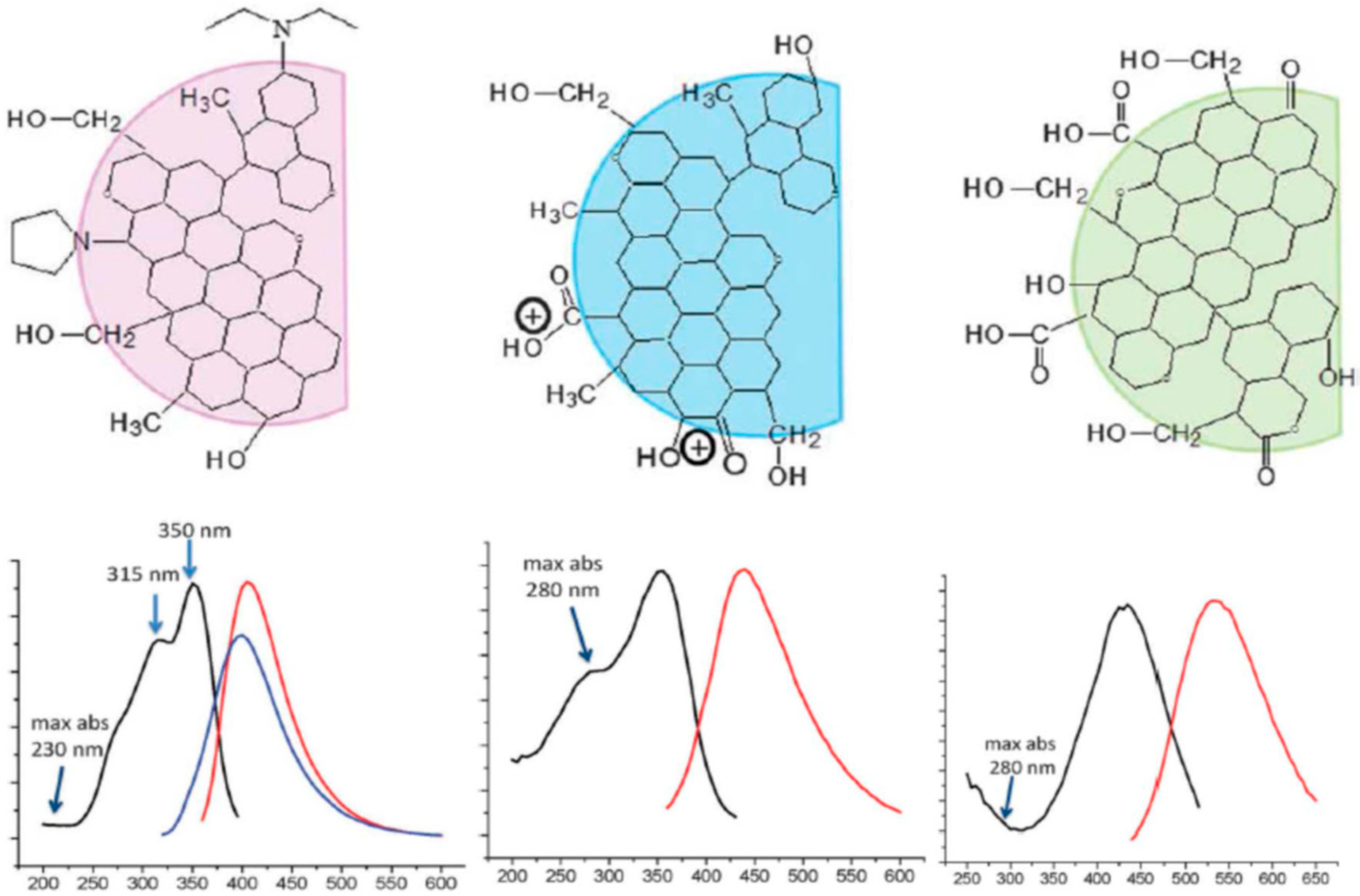
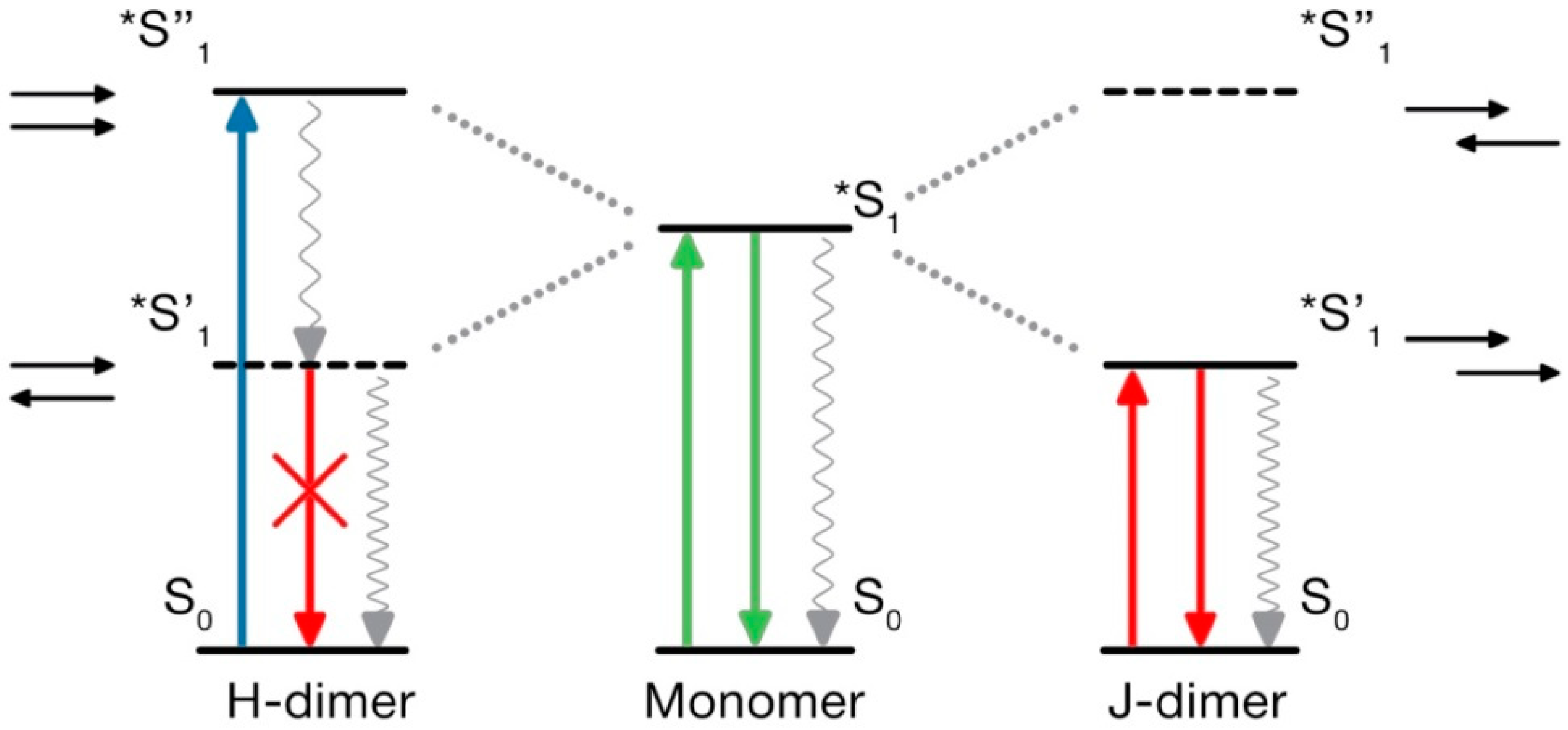
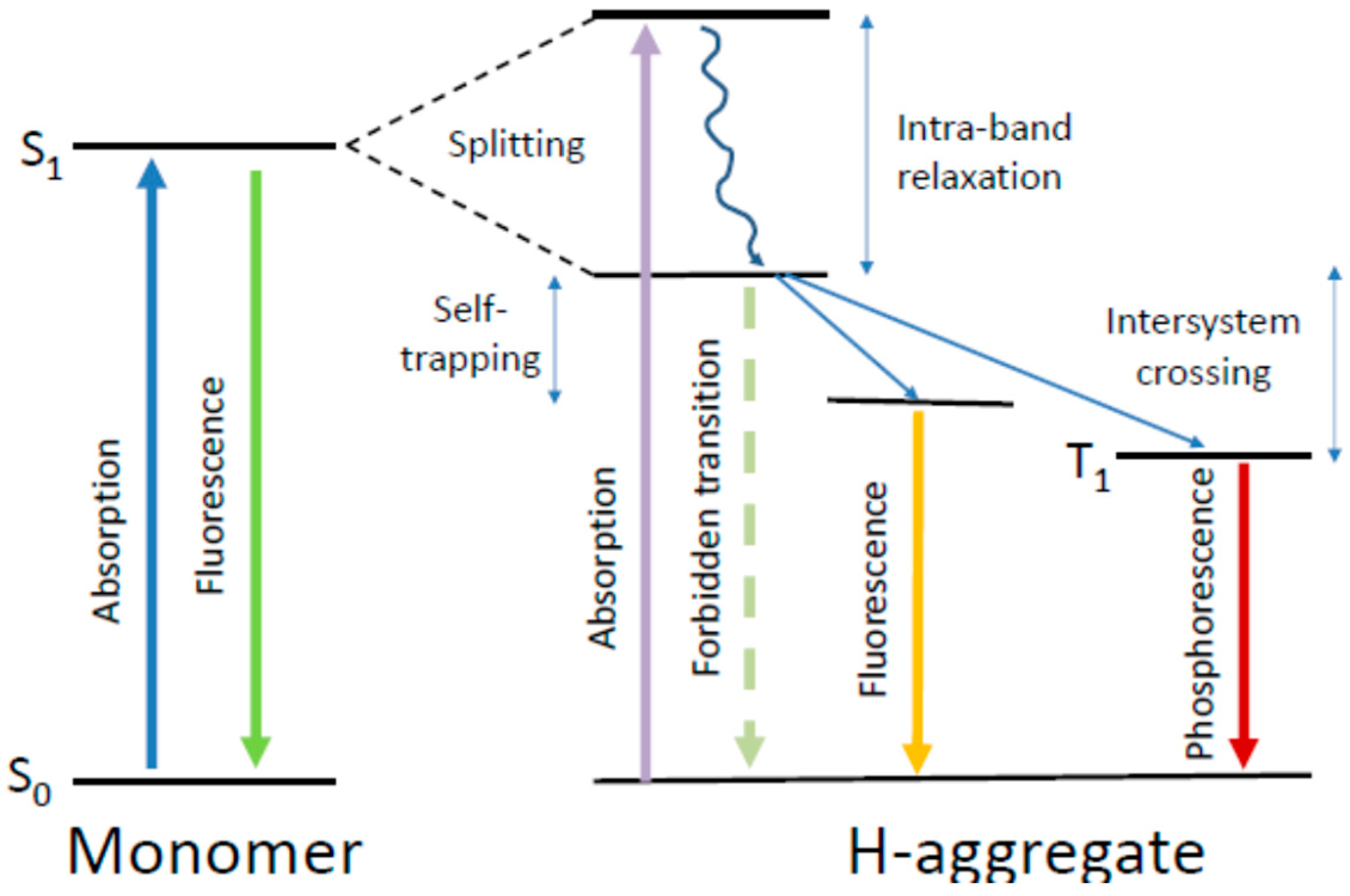
4.5. What Is Special in H-Aggregates of Organic Dyes?
5. The Novel Excitonic Concept
5.1. Carbonic Nanoparticles as Collective Emitters
5.2. Optical Anisotropy and Macrodipoles
5.3. Strong Two-Photon Absorbance
5.4. Molecular Disorder and Relaxations
5.5. Triplet State Generation
6. The Basic Model of Excitonic States
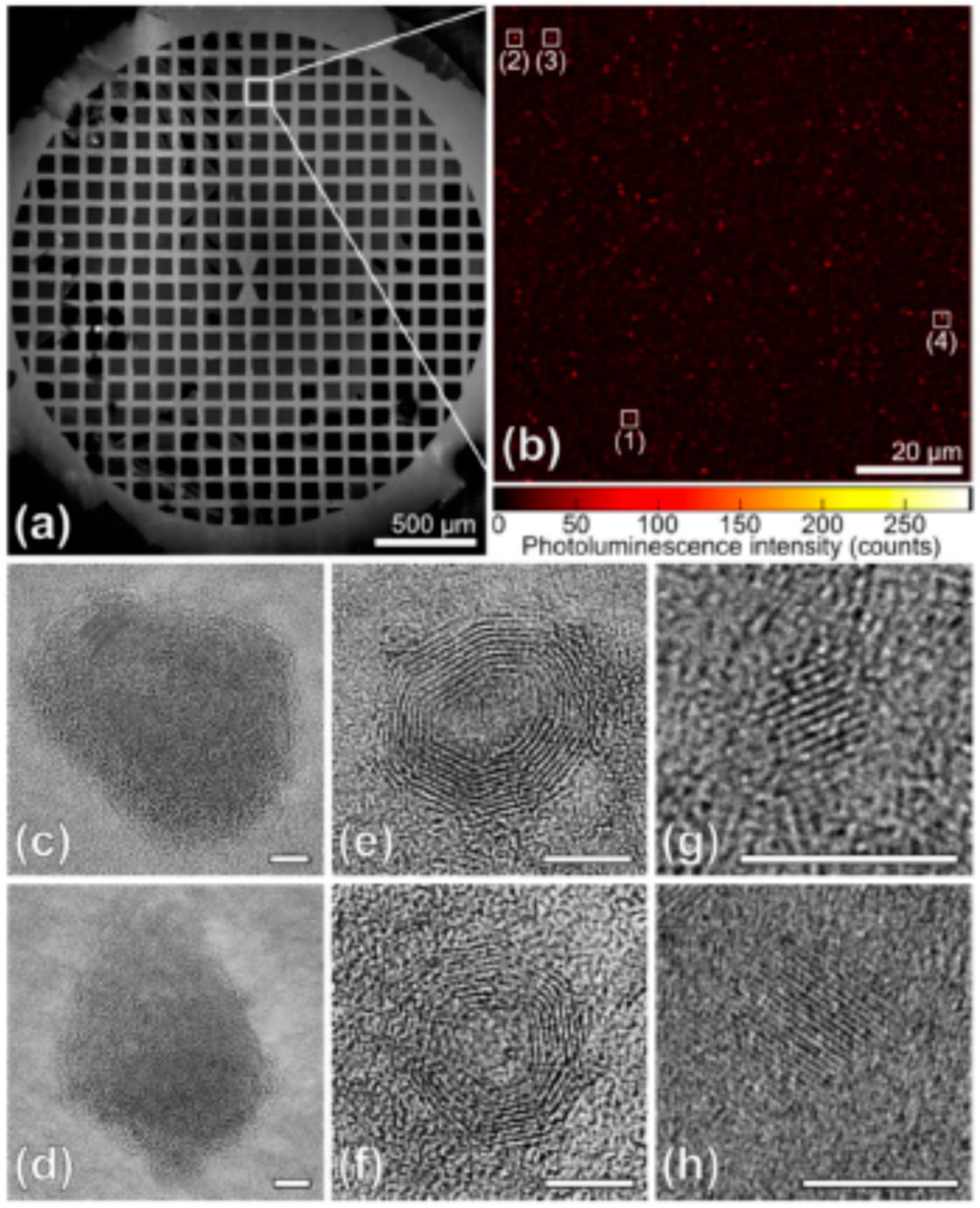
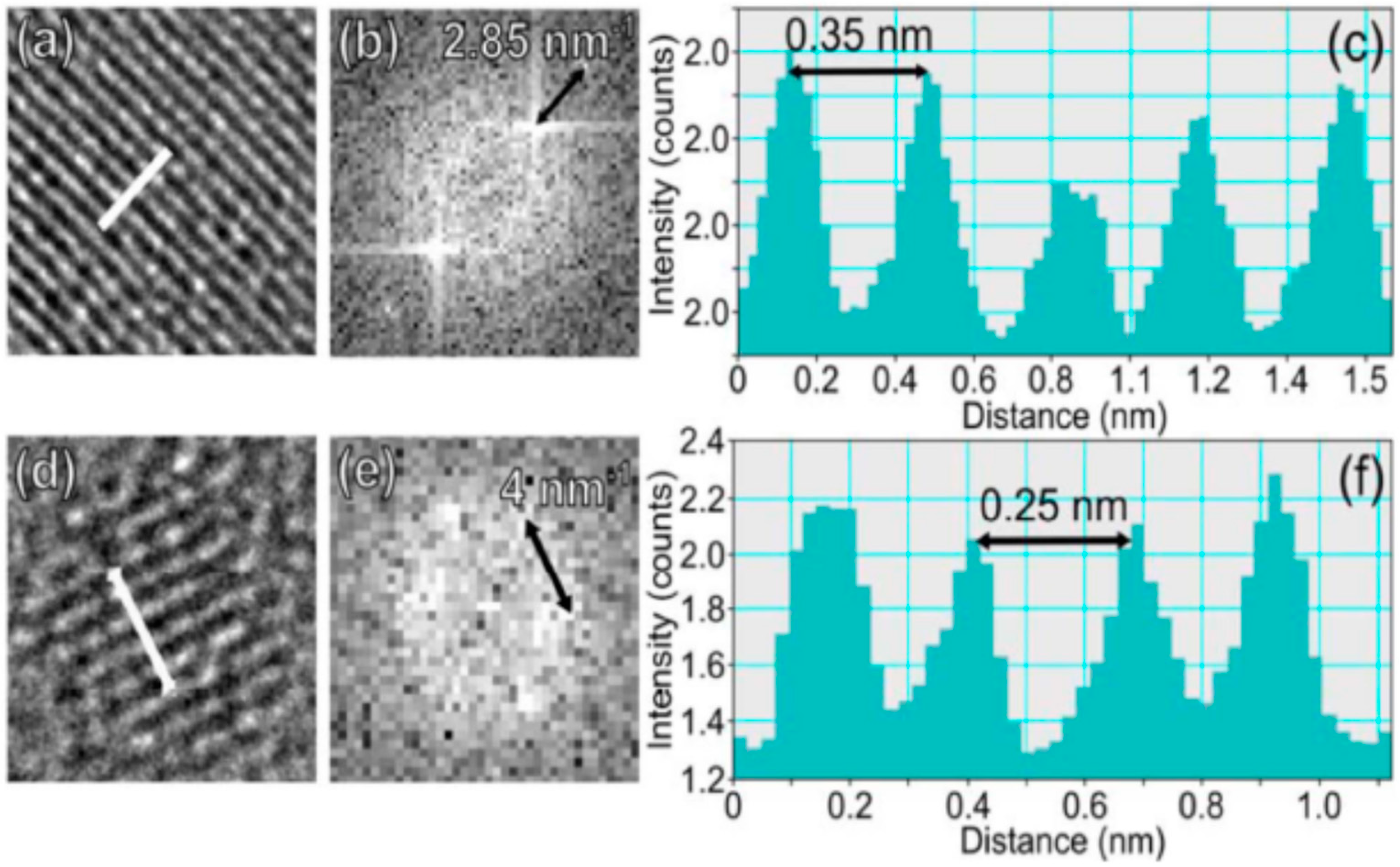
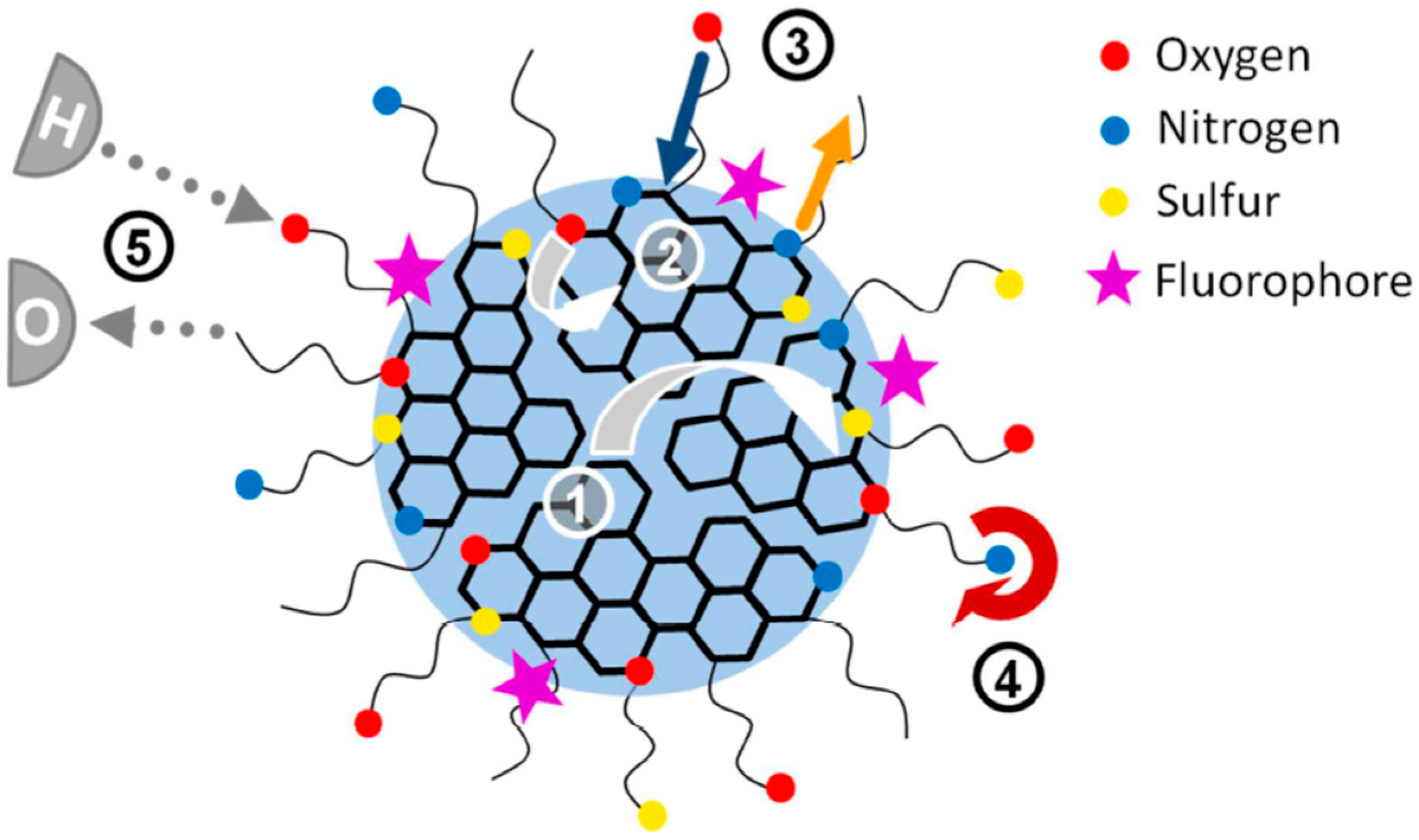
6.1. The Role of Particle Core
6.2. The Role of Particle Shell
6.3. The Self-Trapped Excitons and Their Emission
7. Concluding Thoughts and Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.C.; Bergstrom, R.W. Light absorption by carbonaceous particles: An investigative review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar] [CrossRef]
- Krueger, A. New Carbon Materials: Biological Applications of Functionalized Nanodiamond Materials. Chem. Eur. J. 2008, 14, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat. Mater. 2005, 4, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, Y. Photoluminescence studies on exciton photophysics in carbon nanotubes. J. Mater. Chem. C 2013, 1, 6499–6521. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yang, H.-H.; Zhu, C.-L.; Chen, X.; Chen, G.-N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2012, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lamola, A.A.; Turro, N.J. Energy Transfer and Organic Photochemistry; Interscience Publishers: Geneva, Switzerland; New York, NY, USA, 1969; Volume 14. [Google Scholar]
- Nozik, A.J. Multiple exciton generation in semiconductor quantum dots. Chem. Phys. Lett. 2008, 457, 3–11. [Google Scholar] [CrossRef]
- Reisch, A.; Klymchenko, A.S. Fluorescent polymer nanoparticles based on dyes: Seeking brighter tools for bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Dekaliuk, M.O. The origin of emissive states of carbon nanoparticles derived from ensemble-averaged and single-molecular studies. Nanoscale 2016, 8, 14057–14069. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular Fluorescence in Citric Acid-Based Carbon Dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Galande, C.; Mohite, A.D.; Naumov, A.V.; Gao, W.; Ci, L.; Ajayan, A.; Gao, H.; Srivastava, A.; Weisman, R.B.; Ajayan, P.M. Quasi-molecular fluorescence from graphene oxide. Sci. Rep. 2011, 1, 85. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, T.; Gurzadyan, G.G. The Origin of Fluorescence from Graphene Oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef] [PubMed]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Mol. Biol. 2000, 7, 730. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Zhu, C.; Liu, M.; Ji, Y.; Feng, L.; Tao, L.; Wei, Y. Carbon-dots derived from nanodiamond: Photoluminescence tunable nanoparticles for cell imaging. J. Colloid Interface Sci. 2013, 397, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-Dependent Fluorescence in Carbon Dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Huang, Y.; Wong, T.; Sun, H. Self-trapped exciton emission from carbon dots investigated by polarization anisotropy of photoluminescence and photoexcitation. Nanoscale 2017, 9, 12637–12646. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.W. Electrochemical tuning of luminescent carbon nanodots: From preparation to luminescence mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Lowe, K.; Tchounwou, P.B.; Kafoury, R. A Rev. on the respiratory system toxicity of carbon nanoparticles. Int. J. Environ. Res. Public Health 2016, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Malyukin, Y.; Viagin, O.; Maksimchuk, P.; Dekaliuk, M.; Demchenko, A. Insight into the mechanism of the photoluminescence of carbon nanoparticles derived from cryogenic studies. Nanoscale 2018, 10, 9320–9328. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chizhik, A.M.; Karedla, N.; Dekaliuk, M.O.; Gregor, I.; Schuhmann, H.; Seibt, M.; Bodensiek, K.; Schaap, I.A.; Schulz, O. Photoluminescence of carbon nanodots: Dipole emission centers and electron–phonon coupling. Nano Lett. 2014, 14, 5656–5661. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, B.; Cui, X.; Wei, Q.; Tajima, K.; Li, L.-S. Independent Tuning of the Band Gap and Redox Potential of Graphene Quantum Dots. J. Phys. Chem. Lett. 2011, 2, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhang, Z.; Zhang, J.; Chen, N. Graphene Quantum Dots: An Emerging Material for the Energy-Related Applications and Beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Dong, X.; Wei, L.; Su, Y.; Li, Z.; Geng, H.; Yang, C.; Zhang, Y. Efficient long lifetime room temperature phosphorescence of carbon dots in a potash alum matrix. J. Mater. Chem. C 2015, 3, 2798–2801. [Google Scholar] [CrossRef]
- Hirata, S. Recent Advances in Materials with Room Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Kargbo, O.; Jin, Y.; Ding, S.-N. Recent advances in luminescent carbon dots. Curr. Anal. Chem. 2015, 11, 4–21. [Google Scholar] [CrossRef]
- Reckmeier, C.; Schneider, J.; Susha, A.; Rogach, A. Luminescent colloidal carbon dots: Optical properties and effects of doping [Invited]. Opt. Express 2016, 24, A312–A340. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Pathan, S.H.; Mitra, S.; Modha, B.H.; Goswami, A.; Pramanik, P. Tuning of photoluminescence on different surface functionalized carbon quantum dots. RSC Adv. 2012, 2, 3602–3606. [Google Scholar] [CrossRef]
- Hsu, P.C.; Shih, Z.Y.; Lee, C.H.; Chang, H.T. Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chem. 2012, 14, 917–920. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Radovic, L.R. (Ed.) Chemistry and Physics of Carbon; CRC Press: Boca Raton, FL, USA, 2012; Volume 31. [Google Scholar]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011, 21, 2445–2450. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, D.; Chen, X.; Wang, F.; Song, H.; Shen, D. Long lifetime pure organic phosphorescence based on water soluble carbon dots. Chem. Commun. 2013, 49, 5751–5753. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.B.; Narayanan, R.; El-Sayed, M.A. Chemical and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2–sp3-Hybridized atomic domains determine optical features of carbon dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticle: Synthesis, Characterization and Bio-imaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Yang, Z.C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2009, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, S.; Dai, L.; Li, L. Nitrogen-doped colloidal graphene quantum dots and their size-dependent electrocatalytic activity for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 46, 18932–18935. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, Y.; Yang, Y.; Cui, J.; Huang, Z.; Wang, Y.; Yang, L.; Wang, H.; Xiao, Y.; Rong, J. One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J. Mater. Chem. B 2013, 1, 39–42. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef] [PubMed]
- Gokus, T.; Nair, R.; Bonetti, A.; Bohmler, M.; Lombardo, A.; Novoselov, K.; Geim, A.; Ferrari, A.; Hartschuh, A. Making graphene luminescent by oxygen plasma treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2011, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Dekaliuk, M.O.; Viagin, O.; Malyukin, Y.V.; Demchenko, A.P. Fluorescent carbon nanomaterials: “quantum dots” or nanoclusters? Phys. Chem. Chem. Phys. 2014, 16, 16075–16084. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, S.-J.; Wang, H.-Y.; Qu, S.-N.; Zhang, Y.-L.; Zhang, J.-H.; Chen, Q.-D.; Xu, H.-L.; Han, W.; Yang, B. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano 2014, 8, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Rondeau-Gagné, S.; Morin, J.-F. Preparation of carbon nanomaterials from molecular precursors. Chem. Soc. Rev. 2014, 43, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Dekaliuk, M.O. Novel fluorescent carbonic nanomaterials for sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 042001. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.B.; Gómez-Navarro, C.; Sundaram, R.S.; Burghard, M.; Kern, K. Electrical conduction mechanism in chemically derived graphene monolayers. Nano Lett. 2009, 9, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Lavin-Lopez, M.; Valverde, J.; Sanchez-Silva, L.; Romero, A. Solvent-based exfoliation via sonication of graphitic materials for graphene manufacture. Ind. Eng. Chem. Res. 2016, 55, 845–855. [Google Scholar] [CrossRef]
- Björk, J.; Hanke, F.; Palma, C.-A.; Samori, P.; Cecchini, M.; Persson, M. Adsorption of aromatic and anti-aromatic systems on graphene through π-π stacking. J. Phys. Chem. Lett. 2010, 1, 3407–3412. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat Nano 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.; Hwang, C.-C.; Ruan, G. Coal as an abundant source of graphene quantum dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhu, J.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Wang, H.; Song, H. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, Z.; Zhang, H.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Ehrat, F.; Wang, Y.; Milowska, K.Z.; Reckmeier, C.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Carbon dots: A unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 2015, 15, 6030–6035. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Sciortino, L.; Popescu, R.; Venezia, A.; Sciortino, A.; Buscarino, G.; Agnello, S.; Schneider, R.; Gerthsen, D.; Cannas, M. Fluorescent nitrogen-rich carbon nanodots with an unexpected β-C3N4 nanocrystalline structure. J. Mater. Chem. C 2016, 4, 2598–2605. [Google Scholar] [CrossRef]
- Sciortino, A.; Cayuela, A.; Soriano, M.; Gelardi, F.; Cannas, M.; Valcárcel, M.; Messina, F. Different natures of surface electronic transitions of carbon nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 22670–22677. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, F.J.; Jonkheijm, P.; Meijer, E.; Schenning, A.P. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Sao, S.; Mukherjee, I.; De, P.; Chaudhuri, D. Encapsulation induced aggregation: A self-assembly strategy for weakly pi-stacking chromophores. Chem. Commun. 2017, 53, 3994–3997. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F. Dipole–dipole interaction driven self-assembly of merocyanine dyes: From dimers to nanoscale objects and supramolecular materials. Acc. Chem. Res. 2016, 49, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Stolte, M.; Zitzler-Kunkel, A.; Bialas, D.; Steinbacher, A.; Brixner, T.; Würthner, F. Unraveling the structure and exciton coupling for multichromophoric merocyanine dye molecules. Phys. Chem. Chem. Phys. 2017, 19, 6368–6378. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Krysmann, M.J.; Kelarakis, A.; Dallas, P.; Giannelis, E.P. Formation Mechanism of Carbogenic Nanoparticles with Dual Photoluminescence Emission. J. Am. Chem. Soc. 2011, 134, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, W.; Bednarz, S.; Żmudzki, P.; Galica, M.; Bogdał, D. Novel efficient fluorophores synthesized from citric acid. RSC Adv. 2015, 5, 34795–34799. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, X.; Song, Y.; Lu, S.; Yang, B. Beyond bottom-up carbon nanodots: Citric-acid derived organic molecules. Nano Today 2016, 11, 128–132. [Google Scholar] [CrossRef]
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the Source of Carbon Dot Photoluminescence: Aromatic Domains versus Molecular Fluorophores. Nano Lett. 2017, 17, 7710–7716. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.; Das, A.; Chatterjee, T.; Mandal, P.K. Molecular origin of photoluminescence of carbon dots: Aggregation-induced orange-red emission. Phys. Chem. Chem. Phys. 2016, 18, 28274–28280. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, J.H.; Zeng, H.B.; Chen, Y.M.; Yang, S.C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q. Carbon dots with high fluorescence quantum yield: The fluorescence originates from organic fluorophores. Nanoscale 2016, 8, 14374–14378. [Google Scholar] [CrossRef] [PubMed]
- Reckmeier, C.J.; Schneider, J.; Xiong, Y.; Häusler, J.; Kasák, P.; Schnick, W.; Rogach, A.L. Aggregated Molecular Fluorophores in the Ammonothermal Synthesis of Carbon Dots. Chem. Mater. 2017, 29, 10352–10361. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe (III) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, X.; Ma, J.; Gu, Y.; Qian, Z.; Chen, J.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozak, O.; Zboril, R. Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, B.; Lee, J.; Kim, S.; Kim, G.T.; Kang, H.; Lee, B.R.; Kim, H.; Shim, S.-H.; Lee, G. Integrative approach toward uncovering the origin of photoluminescence in dual heteroatom-doped carbon nanodots. Chem. Mater. 2016, 28, 6840–6847. [Google Scholar] [CrossRef]
- Demchenko, A.P. Site-selective Red-Edge effects. Methods Enzymol. 2008, 450, 59–78. [Google Scholar] [PubMed]
- Van Dam, B.; Nie, H.; Ju, B.; Marino, E.; Paulusse, J.M.; Schall, P.; Li, M.; Dohnalová, K. Excitation-Dependent Photoluminescence from Single-Carbon Dots. Small 2017, 13, 1702098. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, Y.; Kwon, O.-H.; Kim, B.-S. Carbon Dots: Bottom-Up Syntheses, Properties, and Light-Harvesting Applications. Chem. Asian J. 2018, 13, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2015, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable photoluminescence across the entire visible spectrum from carbon dots excited by white light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly Efficient Red-Emitting Carbon Dots with Gram-Scale Yield for Bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhou, D.; Li, D.; Ji, W.; Jing, P.; Han, D.; Liu, L.; Zeng, H.; Shen, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated sp2-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ge, J.; Liu, W.; Niu, G.; Jia, Q.; Wang, H.; Wang, P. Tunable multicolor carbon dots prepared from well-defined polythiophene derivatives and their emission mechanism. Nanoscale 2016, 8, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, X.; Bai, J.; Pan, G.; Zhu, Y.; Zhai, Y.; Shao, H.; Chen, X.; Dong, B.; Zhang, H. Emitting color tunable carbon dots by adjusting solvent towards light-emitting devices. Nanotechnology 2018, 29, 085705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, Y.; Fang, Y.; Zhang, L.; Chen, J.; Yi, C. Tuning photoluminescence and surface properties of carbon nanodots for chemical sensing. Nanoscale 2016, 8, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Tetsuka, H.; Nagoya, A.; Asahi, R. Highly luminescent flexible amino-functionalized graphene quantum dots@cellulose nanofiber–clay hybrids for white-light emitting diodes. J. Mater. Chem. C 2015, 3, 3536–3541. [Google Scholar] [CrossRef]
- Righetto, M.; Privitera, A.; Fortunati, I.; Mosconi, D.; Zerbetto, M.; Curri, M.L.; Corricelli, M.; Moretto, A.; Agnoli, S.; Franco, L. Spectroscopic Insights into Carbon Dot Systems. J. Phys. Chem. Lett. 2017, 8, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-F.; Huang, W.-L.; Chung, C.-J.; Chiang, I.-T.; Chen, L.-C.; Chang, H.-Y.; Su, W.-C.; Cheng, C.; Chen, S.-J.; Teng, H. Elucidating quantum confinement in graphene oxide dots based on excitation-wavelength-independent photoluminescence. J. Phys. Chem. Lett. 2016, 7, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Liu, X.; Guo, X.; Chu, M.; Zhang, L.; Shen, D. Amplified spontaneous green emission and lasing emission from carbon nanoparticles. Adv. Funct. Mater. 2014, 24, 2689–2695. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Yu, S.; Yang, H. Observation of Lasing Emission from Carbon Nanodots in Organic Solvents. Adv. Mater. 2012, 24, 2263–2267. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.; Álvaro, M.; García, H. Graphene as a Quencher of Electronic Excited States of Photochemical Probes. Langmuir 2012, 28, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Matte, H.R.; Subrahmanyam, K.; Rao, K.V.; George, S.J.; Rao, C. Quenching of fluorescence of aromatic molecules by graphene due to electron transfer. Chem. Phys. Lett. 2011, 506, 260–264. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.-T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, K.; He, Z.; Zhang, Y.; Zhao, Y.; Zhang, H.; Miao, Z. Investigation on Fluorescence Quenching Mechanism of Perylene Diimide Dyes by Graphene Oxide. Molecules 2016, 21, 1642. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wen, X.; Toh, Y.-R.; Lee, Y.-C.; Huang, K.-Y.; Huang, S.; Shrestha, S.; Conibeer, G.; Tang, J. Efficient electron transfer in carbon nanodot–graphene oxide nanocomposites. J. Mater. Chem. C 2014, 2, 2894–2901. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Pal, A.; Rahman, M.A.; Ali, M.; Khan, J.A.; Sk, M.P. Insights on the solvatochromic effects in N-doped yellow-orange emissive carbon dots. New J. Chem. 2018, 42, 19837–19843. [Google Scholar] [CrossRef]
- Beddard, G.; Porter, G. Concentration quenching in chlorophyll. Nature 1976, 260, 366. [Google Scholar] [CrossRef]
- An, B.-K.; Kwon, S.-K.; Jung, S.-D.; Park, S.Y. Enhanced emission and its switching in fluorescent organic nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R. The chemistry of excited complexes: A survey of reactions. In Advances in Physical Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1983; Volume 19, pp. 1–130. [Google Scholar]
- Birks, J.B. Photophysics Aromatic Molecules; Wiley-Interscience: London, UK, 1970; p. 704. [Google Scholar]
- Zhao, Z.; Chen, S.; Lam, J.W.; Wang, Z.; Lu, P.; Mahtab, F.; Sung, H.H.; Williams, I.D.; Ma, Y.; Kwok, H.S. Pyrene-substituted ethenes: Aggregation-enhanced excimer emission and highly efficient electroluminescence. J. Mater. Chem. 2011, 21, 7210–7216. [Google Scholar] [CrossRef]
- Reisch, A.; Trofymchuk, K.; Runser, A.; Fleith, G.; Rawiso, M.; Klymchenko, A.S. Tailoring Fluorescence Brightness and Switching of Nanoparticles through Dye Organization in the Polymer Matrix. ACS Appl. Mater. Interfaces 2017, 9, 43030–43042. [Google Scholar] [CrossRef] [PubMed]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence quenching by photoinduced electron transfer: A reporter for conformational dynamics of macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yu, P.; Toh, Y.R.; Lee, Y.C.; Hsu, A.C.; Tang, J. Near-infrared enhanced carbon nanodots by thermally assisted growth. Appl. Phys. Lett. 2012, 101, 163107. [Google Scholar] [CrossRef]
- Shamsipur, M.; Barati, A.; Karami, S. Long-wavelength, multicolor, and white-light emitting carbon-based dots: Achievements made, challenges remaining, and applications. Carbon 2017, 124, 429–472. [Google Scholar] [CrossRef]
- Lu, W.; Jiao, Y.; Gao, Y.; Qiao, J.; Mozneb, M.; Shuang, S.; Dong, C.; Li, C.-Z. Bright Yellow Fluorescent Carbon Dots as a Multifunctional Sensing Platform for the Label-Free Detection of Fluoroquinolones and Histidine. ACS Appl. Mater. Interfaces 2018, 10, 42915–42924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chao, D.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Yellow emissive carbon dots with quantum yield up to 68.6% from the solvent and manganese ions. Carbon 2018, 135, 253–259. [Google Scholar] [CrossRef]
- Jia, J.; Lu, W.-J.; Li, L.; Jiao, Y.; Gao, Y.-F.; Shuang, S.-M. Orange Luminescent Carbon Dots as Fluorescent Probe for Detection of Nitrite. Chin. J. Anal. Chem. 2019, 47, 560–566. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence carbon dots with excitation independent emission for pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, H.; Lai, J.; Peng, X.; Hu, Y.; Gu, W.; Ye, L. Carbon dots with red-shifted photoluminescence by fluorine doping for optical bio-imaging. Carbon 2018, 128, 78–85. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhai, Y.; Li, Z.; Zhu, P.; Mao, S.; Zhu, C.; Du, D.; Belfiore, L.A.; Tang, J.; Lin, Y. Red carbon dots: Optical property regulations and applications. Mater. Today 2019. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y. Near-infrared excitation/emission and multiphoton-induced fluorescence of carbon dots. Adv. Mater. 2018, 30, 1705913. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhou, X.; Qin, B.; Zhou, Z.; Zhao, Y. Highly fluorescent near-infrared emitting carbon dots derived from lemon juice and its bioimaging application. J. Lumin. 2019, 211, 298–304. [Google Scholar] [CrossRef]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Li, B.; Song, Y.; Zhao, X.; Zhang, G.; Zhang, S.; Lu, S.; Zhang, J.; Wang, H. Investigation of photoluminescence mechanism of graphene quantum dots and evaluation of their assembly into polymer dots. Carbon 2014, 77, 462–472. [Google Scholar] [CrossRef]
- Wen, X.; Yu, P.; Toh, Y.R.; Hao, X.; Tang, J. Intrinsic and Extrinsic Fluorescence in Carbon Nanodots: Ultrafast Time-Resolved Fluorescence and Carrier Dynamics. Adv. Opt. Mater. 2013, 1, 173–178. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Karakassides, M.; Giannelis, E.P. Surface functionalized carbogenic quantum dots. Small 2008, 4, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.-W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Liu, C.; Bao, L.; Yang, M.; Zhang, S.; Zhou, M.; Tang, B.; Wang, B.; Liu, Y.; Zhang, Z.-L.; Zhang, B. Surface Sensitive Photoluminescence of Carbon Nanodots: Coupling between Carbonyl Group and π-Electron System. J. Phys. Chem. Lett. 2019, 10, 3621–3629. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hou, Z.; Zhang, C.; Zhang, G.; Zhang, Y.; Dong, C.; Shuang, S. Concentration-dependent multicolor fluorescent carbon dots for colorimetric and fluorescent bimodal detections of Fe3+ and l-ascorbic acid. Anal. Methods 2019, 11, 669–676. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, N.; Sun, Z.; Wang, J.; Li, J.; Yu, J. Carbogenic nanodots derived from organo-templated zeolites with modulated full-color luminescence. Chem. Sci. 2016, 7, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lian, H.; Wei, Y.; He, X.; Chen, Y.; Wang, B.; Zeng, Q.; Lin, J. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.-C.; Yang, M.; Jiang, P.; Zhao, J.; Jiang, F.-L.; Liu, Y. Concentration-tuned Multicolor Carbon Dots: Microwave-assisted Synthesis, Characterization, Mechanism and Applications. New J. Chem. 2019, 43, 8950–8957. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-colour carbon dots: From energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef] [PubMed]
- Fuyuno, N.; Kozawa, D.; Miyauchi, Y.; Mouri, S.; Kitaura, R.; Shinohara, H.; Yasuda, T.; Komatsu, N.; Matsuda, K. Drastic change in photoluminescence properties of graphene quantum dots by chromatographic separation. Adv. Opt. Mater. 2014, 2, 983–989. [Google Scholar] [CrossRef]
- Chen, D.; Gao, H.; Chen, X.; Fang, G.; Yuan, S.; Yuan, Y. Excitation-independent dual-color carbon dots: Surface-state controlling and solid-state lighting. ACS Photonics 2017, 4, 2352–2358. [Google Scholar] [CrossRef]
- Gao, D.; Liu, X.; Jiang, D.; Zhao, H.; Zhu, Y.; Chen, X.; Luo, H.; Fan, H.; Zhang, X. Exploring of multicolor emissive carbon dots with novel double emission mechanism. Sens. Actuators B Chem. 2018, 277, 373–380. [Google Scholar] [CrossRef]
- Wen, Z.-H.; Yin, X.-B. Excitation-independent carbon dots, from photoluminescence mechanism to single-color application. RSC Adv. 2016, 6, 27829–27835. [Google Scholar] [CrossRef]
- Chizhik, A.M.; Stein, S.; Dekaliuk, M.O.; Battle, C.; Li, W.; Huss, A.; Platen, M.; Schaap, I.; Gregor, I.; Demchenko, A.P. Super-resolution optical fluctuation bio-imaging with dual-color carbon nanodots. Nano Lett. 2016, 16, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Xu, H.; Fu, Y. Photon Reabsorption and Nonradiative Energy-Transfer-Induced Quenching of Blue Photoluminescence from Aggregated Graphene Quantum Dots. J. Phys. Chem. C 2016, 120, 29432–29438. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.-B.; Shi, L.; Meng, X.; Wang, Y.; Chen, X.; Xu, H.; Zhang, W.; Fang, X.; Ding, T. Structure and photoluminescence evolution of nanodots during pyrolysis of citric acid: From molecular nanoclusters to carbogenic nanoparticles. J. Mater. Chem. C 2017, 5, 10302–10312. [Google Scholar] [CrossRef]
- Wei, X.; Mei, S.; Yang, D.; Zhang, G.; Xie, F.; Zhang, W.; Guo, R. Surface States Induced Photoluminescence Enhancement of Nitrogen-Doped Carbon Dots via Post-Treatments. Nanoscale Res. Lett. 2019, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sun, J.; Sun, X.; Song, R.; Xie, Z.; Zhou, S. Precisely Controlled Up/Down-Conversion Liquid and Solid State Photoluminescence of Carbon Dots. Adv. Opt. Mater. 2018, 6, 1800115. [Google Scholar] [CrossRef]
- Reckmeier, C.J.; Wang, Y.; Zboril, R.; Rogach, A.L. Influence of Doping and Temperature on Solvatochromic Shifts in Optical Spectra of Carbon Dots. J. Phys. Chem. C 2016, 120, 10591–10604. [Google Scholar] [CrossRef]
- Sciortino, A.; Marino, E.; Dam, B.v.; Schall, P.; Cannas, M.; Messina, F. Solvatochromism unravels the emission mechanism of carbon nanodots. J. Phys. Chem. Lett. 2016, 7, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Han, D.; Li, D.; Zhou, D.; Zhang, L.; Zhang, H.; Shen, D.; Qu, S. Origin of Anisotropic Photoluminescence in Heteroatom-Doped Carbon Nanodots. Adv. Opt. Mater. 2017, 5, 1601049. [Google Scholar] [CrossRef]
- Sciortino, A.; Madonia, A.; Gazzetto, M.; Sciortino, L.; Rohwer, E.J.; Feurer, T.; Gelardi, F.; Cannas, M.; Cannizzo, A.; Messina, F. The interaction of photoexcited Carbon nanodots with metal ions disclosed down to the femtosecond scale. Nanoscale 2017, 9, 11902–11911. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Levy, D.H. Excimer/exciplex formation in van der Waals dimers of aromatic molecules. J. Phys. Chem. 1996, 100, 11539–11545. [Google Scholar] [CrossRef]
- Stevens, B. Some effects of molecular orientation on fluorescence emission and energy transfer in crystalline aromatic hydrocarbons. Spectrochim. Acta 1962, 18, 439–448. [Google Scholar] [CrossRef]
- Kim, Y.; Bouffard, J.; Kooi, S.E.; Swager, T.M. Highly emissive conjugated polymer excimers. J. Am. Chem. Soc. 2005, 127, 13726–13731. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Tang, K.C.; Chou, P.T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Collective effects influencing fluorescence emission. Adv. Fluoresc. Report. Chem. Biol. II 2010, 9, 107–132. [Google Scholar]
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Time-resolved emission reveals ensemble of emissive states as the origin of multicolor fluorescence in carbon dots. Nano Lett. 2015, 15, 8300–8305. [Google Scholar] [CrossRef] [PubMed]
- LeCroy, G.E.; Messina, F.; Sciortino, A.; Bunker, C.E.; Wang, P.; Fernando, K.S.; Sun, Y.-P. Characteristic Excitation Wavelength Dependence of Fluorescence Emissions in Carbon “Quantum” Dots. J. Phys. Chem. C 2017, 121, 28180–28186. [Google Scholar] [CrossRef]
- Mishra, K.; Koley, S.; Ghosh, S. Ground-State Heterogeneity along with Fluorescent Byproducts Causes Excitation-Dependent Fluorescence and Time-Dependent Spectral Migration in Citric Acid-Derived Carbon Dots. J. Phys. Chem. Lett. 2019, 10, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of excitation dependent fluorescence in carbon nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Li, M.; Huang, F.; Wu, N. Origin of strong excitation wavelength dependent fluorescence of graphene oxide. ACS Nano 2013, 8, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Weber’s Red-Edge Effect that Changed the Paradigm in Photophysics and Photochemistry. In Perspectives on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2016; pp. 95–141. [Google Scholar]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Colón, L.A. Hidden Properties of Carbon Dots Revealed After HPLC Fractionation. J. Phys. Chem. Lett. 2012, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, B.; Huang, J. Amphibious fluorescent carbon dots: One-step green synthesis and application for light-emitting polymer nanocomposites. Chem. Commun. 2013, 49, 8078–8080. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-color inorganic carbon dot phosphors for white-light-emitting diodes. Adv. Opt. Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Feng, T.; Zeng, Q.; Lu, S.; Yan, X.; Liu, J.; Tao, S.; Yang, M.; Yang, B. Color-tunable carbon dots possessing solid-state emission for full-color light-emitting diodes applications. ACS Photonics 2017, 5, 502–510. [Google Scholar] [CrossRef]
- Mei, S.; Wei, X.; Hu, Z.; Wei, C.; Su, D.; Yang, D.; Zhang, G.; Zhang, W.; Guo, R. Amphipathic carbon dots with solvent-dependent optical properties and sensing application. Opt. Mater. 2019, 89, 224–230. [Google Scholar] [CrossRef]
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mukherjee, S.; Prasad, E.; Chadha, A. H-Bonding controls the emission properties of functionalized carbon nano-dots. Phys. Chem. Chem. Phys. 2017, 19, 7288–7296. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P.; Yesylevskyy, S.O. Interfacial behavior of fluorescent dyes. In Advance Fluorescence Reporters in Chemical and Biology III; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–62. [Google Scholar]
- Basu, N.; Mandal, D. Solvatochromic Response of Carbon Dots: Evidence of Solvent Interaction with Different Types of Emission Centers. J. Phys. Chem. C 2018, 122, 18732–18741. [Google Scholar] [CrossRef]
- Das, A.; Roy, D.; De, C.K.; Mandal, P.K. “Where does the fluorescing moiety reside in a carbon dot?”–Investigations based on fluorescence anisotropy decay and resonance energy transfer dynamics. Phys. Chem. Chem. Phys. 2018, 20, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Nie, H.; Liu, Z.; Xu, H.; Li, M.; Wu, C.; Wang, H.; Zhang, S.X.-A. Full-colour carbon dots: Integration of multiple emission centres into single particles. Nanoscale 2017, 9, 13326–13333. [Google Scholar] [CrossRef] [PubMed]
- Gharat, P.M.; Chethodil, J.M.; Srivastava, A.P.; Praseetha, P.; Pal, H.; Choudhury, S.D. An insight into the molecular and surface state photoluminescence of carbon dots revealed through solvent-induced modulations in their excitation wavelength dependent emission properties. PhotoChem. Photobiol. Sci. 2019, 18, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; An, X.; Gong, J. Novel pH sensitive N-doped carbon dots with both long fluorescence lifetime and high quantum yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yields and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Wagner, W.; Stepanenko, V.; Ren, X.; Ogi, S.; Würthner, F. Near-IR Absorbing J-Aggregate of an Amphiphilic BF2-Azadipyrromethene Dye by Kinetic Cooperative Self-Assembly. Angew. Chem. Int. Ed. 2017, 56, 5729–5733. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.S. Theory Excitons; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Bardeen, C.J. The structure and dynamics of molecular excitons. Annu. Rev. Phys. Chem. 2014, 65, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Rumbles, G. Excitons in nanoscale systems. Nat. Mater. 2006, 5, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Takagahara, T.; Takeda, K. Theory of the quantum confinement effect on excitons in quantum dots of indirect-gap materials. Phys. Rev. B 1992, 46, 15578. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.; Pons, T.; Medintz, I.; Mattoussi, H. Biosensing with luminescent semiconductor quantum dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Ullah, N.; Wan, Q.; Zhang, R. Revealing the trap emission in graphene-based nanostructures. Carbon 2019, 5, 5984–5993. [Google Scholar] [CrossRef]
- Feng, J.; Dong, H.; Yu, L.; Dong, L. The optical and electronic properties of graphene quantum dots with oxygen-containing groups: A density functional theory study. J. Mater. Chem. C 2017, 5, 5984–5993. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.Y.; Wang, Y.; Zhu, S.J.; Zhang, Y.L.; Zhang, J.H.; Chen, Q.D.; Han, W.; Xu, H.L.; Yang, B. Direct Observation of Quantum-Confined Graphene-Like States and Novel Hybrid States in Graphene Oxide by Transient Spectroscopy. Adv. Mater. 2013, 25, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, A.; Gazzetto, M.; Buscarino, G.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Rohwer, E.J.; Mauro, N.; Feurer, T. Disentangling size effects and spectral inhomogeneity in carbon nanodots by ultrafast dynamical hole-burning. Nanoscale 2018, 10, 15317–15323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Umari, P.; Mosconi, E.; De Angelis, F. Infrared dielectric screening determines the low exciton binding energy of metal-halide perovskites. J. Phys. Chem. Lett. 2018, 9, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Soavi, G.; Scotognella, F.; Lanzani, G.; Cerullo, G. Ultrafast Photophysics of Single-Walled Carbon Nanotubes. Adv. Opt. Mater. 2016, 4, 1670–1688. [Google Scholar] [CrossRef]
- Chizhik, A.I.; Chizhik, A.M.; Khoptyar, D.; Bär, S.; Meixner, A.J. Excitation isotropy of single CdSe/ZnS nanocrystals. Nano Lett. 2011, 11, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Davydov, A.S. Theory Molecular Excitons; Plenum press: New York, NY, USA, 1971. [Google Scholar]
- Broude, V.L.V.; Rashba, E.I.; Sheka, E.F. Spectroscopy of Molecular Excitons; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Saikin, S.K.; Eisfeld, A.; Valleau, S.; Aspuru-Guzik, A. Photonics meets excitonics: Natural and artificial molecular aggregates. Nanophotonics 2013, 2, 21–38. [Google Scholar] [CrossRef]
- Kasha, M. Energy transfer mechanisms and the molecular exciton model for molecular aggregates. Radiat. Res. 1963, 20, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.; Ashraf El-Bayoumi, M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- McRae, E.G.; Kasha, M. Enhancement of phosphorescence ability upon aggregation of dye molecules. J. Chem. Phys. 1958, 28, 721–722. [Google Scholar] [CrossRef]
- Hochstrasser, R.M.; Kasha, M. Application of the exciton model to mono-molecular lamellar systems. PhotoChem. Photobiol. 1964, 3, 317–331. [Google Scholar] [CrossRef]
- Bricks, J.L.; Slominskii, Y.L.; Panas, I.D.; Demchenko, A.P. Fluorescent J-aggregates of cyanine dyes: Basic research and applications review. Methods Appl. Fluoresc. 2017, 6, 012001. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ashiba, K. Highly fluorescent organic nanoparticles of thiacyanine dye: A synergetic effect of intermolecular H-aggregation and restricted intramolecular rotation. RSC Adv. 2011, 1, 834–838. [Google Scholar] [CrossRef]
- Rösch, U.; Yao, S.; Wortmann, R.; Würthner, F. Fluorescent H-Aggregates of Merocyanine Dyes. Angew. Chem. 2006, 118, 7184–7188. [Google Scholar] [CrossRef]
- Spano, F.C. The spectral signatures of Frenkel polarons in H-and J-aggregates. Acc. Chem. Res. 2009, 43, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, S.B.; Yakunin, S.; Peng, C.; Vismara, M.V.G.; Graeff, C.F.; Nüesch, F.A.; Jenatsch, S.; Hany, R.; Kovalenko, M.V.; Heier, J. Strongly Red-Shifted Photoluminescence Band Induced by Molecular Twisting in Cyanine (Cy3) Dye Films. J. Phys. Chem. C 2017, 121, 9587–9593. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Neogy, S.; Ghosh, S.K.; Kumbhakar, M. Molecular Origin and Self-Assembly of Fluorescent Carbon Nanodots in Polar Solvents. J. Phys. Chem. Lett. 2017, 8, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.C.; Silva, C. H-and J-aggregate behavior in polymeric semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Hestand, N.J.; Spano, F.C. Expanded theory of H-and J-molecular aggregates: The effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef] [PubMed]
- Brixner, T.; Hildner, R.; Köhler, J.; Lambert, C.; Würthner, F. Exciton Transport in Molecular Aggregates–From Natural Antennas to Synthetic Chromophore Systems. Adv. Energy Mater. 2017, 7, 1700236. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Yang, M.; Zhang, X.; Xu, W.; Zhu, Q.; Wu, J.; Tian, Y.; Zhou, H. Novel highly emissive H-aggregates with aggregate fluorescence change in a phenylbenzoxazole-based system. Chem. Commun. 2014, 50, 8723–8726. [Google Scholar] [CrossRef] [PubMed]
- Najafov, H.; Lee, B.; Zhou, Q.; Feldman, L.C.; Podzorov, V. Observation of long-range exciton diffusion in highly ordered organic semiconductors. Nat. Mater. 2010, 9, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yuan, W.; Jia, Z.; Liu, G. H- and J-aggregation of fluorene-based chromophores. J. Phys. Chem. B 2014, 118, 14536–14545. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.; Heyne, B. Calix[4]arene sulfonate as a template for forming fluorescent thiazole orange H-aggregates. Chem. Commun. 2010, 46, 3595–3597. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.; Settels, V.; Liu, W.; Würthner, F.; Meier, C.; Fink, R.F.; Schindlbeck, S.; Lochbrunner, S.; Engels, B.; Engel, V. Ultrafast exciton self-trapping upon geometry deformation in perylene-based molecular aggregates. J. Phys. Chem. Lett. 2013, 4, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.; Silvestri, L. Multiple mode exciton-vibrational coupling in H-aggregates: Synergistic enhancement of the quantum yield. J. Chem. Phys. 2010, 132, 094704. [Google Scholar] [CrossRef] [PubMed]
- Chenu, A.; Scholes, G.D. Coherence in energy transfer and photosynthesis. Annu. Rev. Phys. Chem. 2015, 66, 69–96. [Google Scholar] [CrossRef] [PubMed]
- Scholes, G.D.; Fleming, G.R.; Chen, L.X.; Aspuru-Guzik, A.; Buchleitner, A.; Coker, D.F.; Engel, G.S.; Van Grondelle, R.; Ishizaki, A.; Jonas, D.M. Using coherence to enhance function in chemical and biophysical systems. Nature 2017, 543, 647. [Google Scholar] [CrossRef] [PubMed]
- Walschaers, M.; Schlawin, F.; Wellens, T.; Buchleitner, A. Quantum transport on disordered and noisy networks: An interplay of structural complexity and uncertainty. Annu. Rev. Condens. Matter Phys. 2016, 7, 223–248. [Google Scholar] [CrossRef]
- Lloyd, S.; Mohseni, M. Symmetry-enhanced supertransfer of delocalized quantum states. New J. Phys. 2010, 12, 075020. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; Van Grondelle, R.; Scholes, G.D. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2016, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Collini, E.; Scholes, G.D. Coherent intrachain energy migration in a conjugated polymer at room temperature. Science 2009, 323, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cheng, Y.-C.; Fleming, G.R. Coherence dynamics in photosynthesis: Protein protection of excitonic coherence. Science 2007, 316, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Abramavicius, D.; Palmieri, B.; Voronine, D.V.; Sanda, F.; Mukamel, S. Coherent multidimensional optical spectroscopy of excitons in molecular aggregates; quasiparticle versus supermolecule perspectives. Chem. Rev. 2009, 109, 2350–2408. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Cichos, F.; Huisken, F.; von Borczyskowski, C. Electron–phonon coupling and localization of excitons in single silicon nanocrystals. Nano Lett. 2008, 8, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Schmidt, A.; Valencia, V.; Fluegel, B.; Mazumdar, S.; Armstrong, N.; Peyghambarian, N. Evidence for exciton-exciton binding in a molecular aggregate. Phys. Rev. B 1998, 57, R4206. [Google Scholar] [CrossRef]
- D’Avino, G.; Terenziani, F.; Painelli, A. Aggregates of quadrupolar dyes: Giant two-photon absorption from biexciton states. J. Phys. Chem. B 2006, 110, 25590–25592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Yuan, Y.; Jiang, J.; Yu, S.-H. One-pot gram-scale synthesis of nitrogen and sulfur embedded organic dots with distinctive fluorescence behaviors in free and aggregated states. Chem. Mater. 2016, 28, 4367–4374. [Google Scholar] [CrossRef]
- Agranovich, V.M. Excitations in Organic Solids; OUP Oxford: Oxford, UK, 2009; Volume 142. [Google Scholar]
- Matsui, A.H. Excitonic processes in aromatic molecular crystals of strong exciton-phonon coupling. Pure Appl. Chem. 1995, 67, 429–436. [Google Scholar] [CrossRef]
- Jursenas, S.; Gruodis, A.; Kodis, G.; Chachisvilis, M.; Gulbinas, V.; Silinsh, E.; Valkunas, L. Free and self-trapped charge-transfer excitons in crystals of dipolar molecules of N, N-dimethylaminobenzylidene 1, 3-indandione. J. Phys. Chem. B 1998, 102, 1086–1094. [Google Scholar] [CrossRef]
- Rashba, E.; Sturge, M. (Eds.) Excitons, v. 2; Amsterdam, The Netherlands, 1982. [Google Scholar]
- Song, K.; Williams, R.T. Self-Trapped Excitons; Springer: Berlin/Heidelberg, Germany, 1996; Volume 105. [Google Scholar]
- Rashba, E.I. Theory of strong interactions of electron excitations with lattice vibrations in molecular crystals. 2 Opt. Spektrosk. 1957, 2, 88–98. [Google Scholar]
- Pekar, S.I.; Rashba, E.I.; Sheka, V.I. Free and self-localized Wannier-Mott excitons in ionic-crystals and the activation-energy of their mutual thermal conversion. J. Eksp. Theor. Phys. 1979, 49, 129. [Google Scholar]
- Baessler, H.; Schweitzer, B. Site-selective fluorescence spectroscopy of conjugated polymers and oligomers. Acc. Chem. Res. 1999, 32, 173–182. [Google Scholar] [CrossRef]
- Fidder, H.; Knoester, J.; Wiersma, D.A. Optical properties of disordered molecular aggregates: A numerical study. J. Chem. Phys. 1991, 95, 7880–7890. [Google Scholar] [CrossRef]
- Bialas, D.; Bruening, C.; Schlosser, F.; Fimmel, B.; Thein, J.; Engel, V.; Wuerthner, F. Exciton-Vibrational Couplings in Homo-and Heterodimer Stacks of Perylene Bisimide Dyes within Cyclophanes: Studies on Absorption Properties and Theoretical Analysis. Chem. Eur. J. 2016, 22, 15011–15018. [Google Scholar] [CrossRef] [PubMed]
- Bialas, D.; Zitzler-Kunkel, A.; Kirchner, E.; Schmidt, D.; Würthner, F. Structural and quantum chemical analysis of exciton coupling in homo-and heteroaggregate stacks of merocyanines. Nat. Commun. 2016, 7, 12949. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.; Hinderhofer, A.; Broch, K.; Heinemeyer, U.; Kowarik, S.; Vorobiev, A.; Gerlach, A.; Schreiber, F. Structural and Optical Properties of Mixed Diindenoperylene–Perfluoropentacene Thin Films. J. Phys. Chem. C 2012, 116, 10917–10923. [Google Scholar] [CrossRef]
- Winiger, C.B.; Langenegger, S.M.; Calzaferri, G.; Häner, R. Formation of Two Homo-chromophoric H-Aggregates in DNA-Assembled Alternating Dye Stacks. Angew. Chem. Int. Ed. 2015, 54, 3643–3647. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Tretiak, S. Electron-vibrational dynamics of photoexcited polyfluorenes. J. Am. Chem. Soc. 2004, 126, 12130–12140. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, M.; Liu, Y.; He, F.; Gao, F.; Su, Y.; Wei, H.; Zhang, Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Kalytchuk, S.; Poláková, K.i.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Gude, V.; Roy, D.; Chatterjee, T.; De, C.K.; Mandal, P.K. On the molecular origin of photoluminescence of nonblinking carbon dot. J. Phys. Chem. C 2017, 121, 9634–9641. [Google Scholar] [CrossRef]
- Guo, R.; Li, T.; Shi, S. Aggregation-induced emission enhancement of carbon quantum dots and applications in light emitting devices. J. Mater. Chem. C 2019, 7, 5148–5154. [Google Scholar] [CrossRef]
- Long, P.; Feng, Y.; Cao, C.; Li, Y.; Han, J.; Li, S.; Peng, C.; Li, Z.; Feng, W. Self-Protective Room-Temperature Phosphorescence of Fluorine and Nitrogen Codoped Carbon Dots. Adv. Funct. Mater. 2018, 28, 1800791. [Google Scholar] [CrossRef]
- Gao, Y.; Han, H.; Lu, W.; Jiao, Y.; Liu, Y.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Matrix-free and highly efficient room-temperature phosphorescence of Nitrogen-doped carbon dots. Langmuir 2018, 34, 12845–12852. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ye, Y.; Ren, X.; Zhao, W.; Yue, D. High pH-induced efficient room-temperature phosphorescence from carbon dots in hydrogen-bonded matrices. J. Mater. Chem. C 2018, 6, 7890–7895. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Tan, L. Recent advances in optical properties and applications of colloidal quantum dots under two-photon excitation. Coord. Chem. Rev. 2017, 338, 141–185. [Google Scholar] [CrossRef]
- Fassioli, F.; Dinshaw, R.; Arpin, P.C.; Scholes, G.D. Photosynthetic light harvesting: Excitons and coherence. J. R. Soc. Interface 2014, 11, 20130901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Snaider, J.M.; Yuan, L.; Huang, L. Ultrafast Dynamic Microscopy of Carrier and Exciton Transport. Annu. Rev. Phys. Chem. 2019, 70, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Helgeson, R.; Jones, R.M.; McBranch, D.; Whitten, D. Superquenching in cyanine pendant poly (L-lysine) dyes: Dependence on molecular weight, solvent, and aggregation. J. Am. Chem. Soc. 2002, 124, 483–488. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.; Wiersma, D.A. Optical dynamics of exciton and polaron formation in molecular aggregates. Chem. Phys. 1989, 131, 135–144. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Sadhukhan, M. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Dutta Chowdhury, A.; Doong, R.-A. Highly sensitive and selective detection of nanomolar ferric ions using dopamine functionalized graphene quantum dots. ACS Appl. Mater. Interfaces 2016, 8, 21002–21010. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.S.; Wang, P.; Guliants, E.A.; Tackett, K.N.; Sun, Y.-P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J. Am. Chem. Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sahu, S.; Cao, L.; Bunker, C.E.; Peng, G.; Liu, Y.; Fernando, K.S.; Wang, P.; Guliants, E.A.; Meziani, M.J. Efficient Fluorescence Quenching in Carbon Dots by Surface-Doped Metals-Disruption of Excited State Redox Processes and Mechanistic Implications. Langmuir 2012, 28, 16141–16147. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yu, J.; Wu, C.; Szymanski, C.; McNeill, J. Amplified energy transfer in conjugated polymer nanoparticle tags and sensors. Nanoscale 2010, 2, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; McNeill, J. Light-harvesting and amplified energy transfer in conjugated polymer nanoparticles. Chem. Rev. 2016, 117, 838–859. [Google Scholar] [CrossRef] [PubMed]
- Lebedenko, A.N.; Grynyov, R.S.; Guralchuk, G.Y.; Sorokin, A.V.; Yefimova, S.L.; Malyukin, Y.V. Coherent mechanism of exciton transport in disordered J-aggregates. J. Phys. Chem. C 2009, 113, 12883–12887. [Google Scholar] [CrossRef]
- Jones, R.M.; Bergstedt, T.S.; Buscher, C.T.; McBranch, D.; Whitten, D. Superquenching and its applications in J-aggregated cyanine polymers. Langmuir 2001, 17, 2568–2571. [Google Scholar] [CrossRef]
- Xie, X.S.; Trautman, J.K. Optical studies of single molecules at room temperature. Annu. Rev. Phys. Chem. 1998, 49, 441–480. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef] [PubMed]
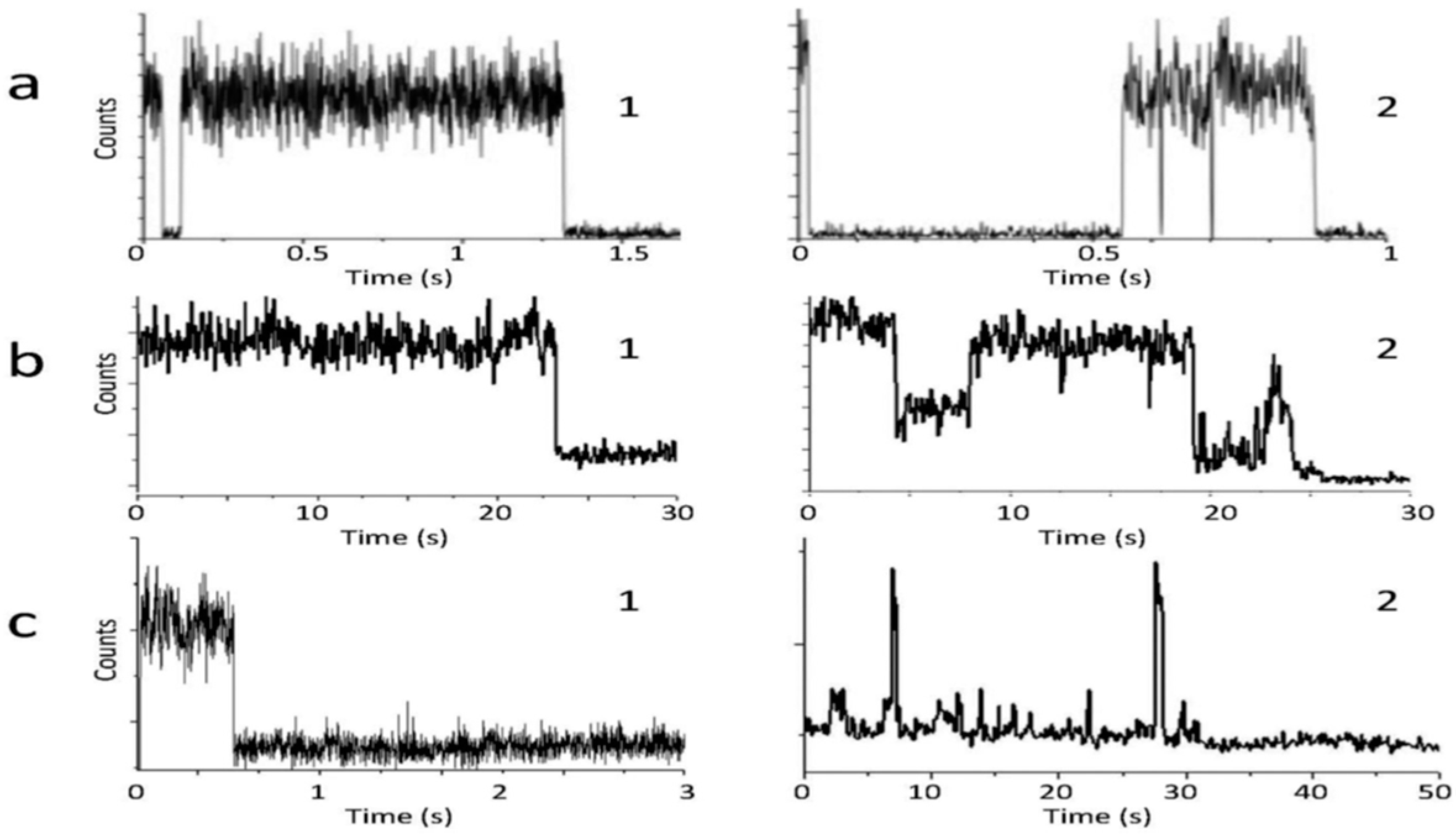
- Das, S.K.; Liu, Y.; Yeom, S.; Kim, D.Y.; Richards, C.I. Single-Particle Fluorescence Intensity Fluctuations of Carbon Nanodots. Nano Lett. 2014, 14, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Damm, C.; Walter, J.; Nacken, T.J.; Peukert, W. Photobleaching and stabilization of carbon nanodots produced by solvothermal synthesis. Phys. Chem. Chem. Phys. 2016, 18, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Verma, N.C.; Gupta, A.; Nandi, C.K. Reversible photoswitching of carbon dots. Sci. Rep. 2015, 5, 11423. [Google Scholar] [CrossRef] [PubMed]
- Whitten, D.; Chen, L.; Jones, R.; Bergstedt, T.; Heeger, P.; McBranch, D. From superquenching to biodetection: Building sensors based on fluorescent polyelectrolytes. Mol. Supramol. Photochem. 2001, 7, 189–208. [Google Scholar]
- Fathi, P.; Khamo, J.S.; Huang, X.; Srivastava, I.; Esch, M.B.; Zhang, K.; Pan, D. Bulk-state and single-particle imaging are central to understanding carbon dot photo-physics and elucidating the effects of precursor composition and reaction temperature. Carbon 2019, 145, 572–585. [Google Scholar] [CrossRef]
- Misra, S.K.; Srivastava, I.; Khamo, J.S.; Krishnamurthy, V.V.; Sar, D.; Schwartz-Duval, A.S.; Soares, J.A.; Zhang, K.; Pan, D. Carbon dots with induced surface oxidation permits imaging at single-particle level for intracellular studies. Nanoscale 2018, 10, 18510–18519. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.C.; Rao, C.; Nandi, C.K. Nitrogen-doped biocompatible carbon dot as a fluorescent probe for STORM nanoscopy. J. Phys. Chem. C 2018, 122, 4704–4709. [Google Scholar] [CrossRef]
- Khan, S.; Li, W.; Karedla, N.; Thiart, J.; Gregor, I.; Chizhik, A.M.; Enderlein, J.; Nandi, C.K.; Chizhik, A.I. Charge-driven fluorescence blinking in carbon nanodots. J. Phys. Chem. Lett. 2017, 8, 5751–5757. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Novotny, L. Robustness of quantum dot power-law blinking. Nano Lett. 2011, 11, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Frantsuzov, P.A.; Volkán-Kacsó, S.N.; Jankó, B.R. Universality of the fluorescence intermittency in nanoscale systems: Experiment and theory. Nano Lett. 2013, 13, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.; Sadkowski, P.; Tredwell, C. Picosecond rotational diffusion in kinetic and steady state fluorescence spectroscopy. Chem. Phys. Lett. 1977, 49, 416–420. [Google Scholar] [CrossRef]
- Chipem, F.A.; Krishnamoorthy, G. Temperature effect on dual fluorescence of 2-(2′-Hydroxyphenyl) benzimidazole and its nitrogen substituted analogues. J. Phys. Chem. B. 2013, 117, 14079–14088. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, T.; Kaiser, W. Vibrational and vibronic relaxation of large polyatomic molecules in liquids. Annu. Rev. Phys. Chem. 1991, 42, 83–107. [Google Scholar] [CrossRef]
- Pandey, A.; Guyot-Sionnest, P. Slow electron cooling in colloidal quantum dots. Science 2008, 322, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fu, J.; Xu, Q.; Sum, T.C. Slow Hot-Carrier Cooling in Halide Perovskites: Prospects for Hot-Carrier Solar Cells. Adv. Mater. 2019, 1802486. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Gregor, I.; Enderlein, J. Image analysis of defocused single-molecule images for three-dimensional molecule orientation studies. J. Phys. Chem. A 2004, 108, 6836–6841. [Google Scholar] [CrossRef]
- Agranovich, V.; Zakhidov, A. Interaction of charge transfer excitons with phonons in molecular crystals. Chem. Phys. Lett. 1977, 50, 278–281. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schmidt, K.; Fritz, T.; Hasche, T.; Agranovich, V.M.; Leo, K. The lowest energy Frenkel and charge-transfer excitons in quasi-one-dimensional structures: Application to MePTCDI and PTCDA crystals. Chem. Phys. 2000, 258, 73–96. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. Rsc Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Wang, J.; Wang, R.; Guo, X.; He, L.; Qiu, F.; Wang, G.; Zhu, B.; Zhu, X.; Liu, T. Amorphous carbon dots with high two-photon fluorescence for cellular imaging passivated by hyperbranched poly (amino amine). J. Mater. Chem. B 2015, 3, 700–706. [Google Scholar] [CrossRef]
- He, G.S.; Tan, L.-S.; Zheng, Q.; Prasad, P.N. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef] [PubMed]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-photon absorption standards in the 550–1600 nm excitation wavelength range. Opt. Express 2008, 16, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.C.; Mukamel, S. Excitons in confined geometries: Size scaling of nonlinear susceptibilities. J. Chem. Phys. 1991, 95, 7526–7540. [Google Scholar] [CrossRef][Green Version]
- Collini, E. Cooperative effects to enhance two-photon absorption efficiency: Intra-versus inter-molecular approach. Phys. Chem. Chem. Phys. 2012, 14, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Vurth, L.; Vial, J.-C.; Baldeck, P.; Marder, S.R.; Van der Sanden, B.; Stephan, O. Fluorescent Pluronic nanodots for in vivo two-photon imaging. Nanotechnology 2009, 20, 235102. [Google Scholar] [CrossRef] [PubMed]
- Cepraga, C.; Gallavardin, T.; Marotte, S.; Lanoë, P.-H.; Mulatier, J.-C.; Lerouge, F.; Parola, S.; Lindgren, M.; Baldeck, P.L.; Marvel, J. Biocompatible well-defined chromophore–polymer conjugates for photodynamic therapy and two-photon imaging. Polym. Chem. 2013, 4, 61–67. [Google Scholar] [CrossRef]
- Drobizhev, M.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P.N.; Anderson, H.L. Extremely strong near-IR two-photon absorption in conjugated porphyrin dimers: Quantitative description with three-essential-states model. J. Phys. Chem. B 2005, 109, 7223–7236. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yan, L.; Zhao, Z.; Ma, X.; Guo, Q.; Jia, M.; Lu, P.; Ramos-Ortiz, G.; Maldonado, J.L.; Rodriguez, M. Gigantic two-photon absorption cross sections and strong two-photon excited fluorescence in pyrene core dendrimers with fluorene/carbazole as dendrons and acetylene as linkages. J. Phys. Chem. B 2010, 114, 11737–11745. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.K.; Kim, K.S.; Kim, D.Y.; Noh, S.B.; Aratani, N.; Ikeda, C.; Osuka, A.; Kim, D. Relationship between two-photon absorption and the π-conjugation pathway in porphyrin arrays through dihedral angle control. J. Am. Chem. Soc. 2006, 128, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, S. Principles Nonlinear Optical Spectroscopy; Oxford University Press: New York, NY, USA, 1995; Volume 29. [Google Scholar]
- Ariese, F.; Bader, A.N.; Gooijer, C. Fluorescence line-narrowing spectroscopy for probing purposes in bioanalytical and environmental chemistry. TrAC Trends Anal. Chem. 2008, 27, 127–138. [Google Scholar] [CrossRef][Green Version]
- Sciortino, A.; Cannas, M.; Messina, F. Temperature-Dependence of Solvent-Induced Stokes Shift and Fluorescence Tunability in Carbon Nanodots. C 2019, 5, 20. [Google Scholar] [CrossRef]
- Oncul, S.; Demchenko, A.P. The effects of thermal quenching on the excited-state intramolecular proton transfer reaction in 3-hydroxyflavones. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2006, 65, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barragán, I.; Costa-Fernández, J.; Sanz-Medel, A.; Valledor, M.; Campo, J. Room-temperature phosphorescence (RTP) for optical sensing. TrAC Trends Anal. Chem. 2006, 25, 958–967. [Google Scholar] [CrossRef]
- Köhler, A.; Bässler, H. Triplet states in organic semiconductors. Mater. Sci. Eng. R Rep. 2009, 66, 71–109. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemical 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Lower, S.; El-Sayed, M. The triplet state and molecular electronic processes in organic molecules. Chem. Rev. 1966, 66, 199–241. [Google Scholar] [CrossRef]
- Tan, J.; Zou, R.; Zhang, J.; Li, W.; Zhang, L.; Yue, D. Large-scale synthesis of N-doped carbon quantum dots and their phosphorescence properties in a polyurethane matrix. Nanoscale 2016, 8, 4742–4747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Wang, Y.; Gao, X.; Cai, C.; Lin, H. Facile, Quick, and Gram-Scale Synthesis of Ultralong-Lifetime Room-Temperature-Phosphorescent Carbon Dots by Microwave Irradiation. Angew. Chem. Int. Ed. 2018, 57, 6216–6220. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, M.; Yang, M.; Yang, Q.; Zhang, Z.; Shi, J. Induction of long-lived room temperature phosphorescence of carbon dots by water in hydrogen-bonded matrices. Nat. Commun. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, S.; Ji, Y.; Chen, R.; Zhu, Q.; Wang, Y.; Zheng, C.; Tao, Y.; Fan, Q.; Huang, W. Invoking ultralong room temperature phosphorescence of purely organic compounds through H-aggregation engineering. Mater. Horiz. 2019. [Google Scholar] [CrossRef]
- Lanzani, G.; Stagira, S.; Cerullo, G.; De Silvestri, S.; Comoretto, D.; Moggio, I.; Cuniberti, C.; Musso, G.; Dellepiane, G. Triplet exciton generation and decay in a red polydiacetylene studied by femtosecond spectroscopy. Chem. Phys. Lett. 1999, 313, 525–532. [Google Scholar] [CrossRef]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lucenti, E.; Forni, A.; Botta, C.; Carlucci, L.; Giannini, C.; Marinotto, D.; Previtali, A.; Righetto, S.; Cariati, E. H-Aggregates Granting Crystallization-Induced Emissive Behavior and Ultralong Phosphorescence from a Pure Organic Molecule. J. Phys. Chem. Lett. 2017, 8, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Gonka, E.; Żyła, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: Synthetic routes, properties, and applications. Chem. Rev. 2016, 117, 3479–3716. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Zhang, X. Amphiphilic building blocks for self-assembly: From amphiphiles to supra-amphiphiles. Acc. Chem. Res. 2012, 45, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lohr, A.; Saha-Möller, C.R.; Würthner, F. Self-assembled π-stacks of functional dyes in solution: Structural and thermodynamic features. Chem. Soc. Rev. 2009, 38, 564–584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rao, K.V.; George, S.J. Supramolecular charge transfer nanostructures. Phys. Chem. Chem. Phys. 2014, 16, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Guldi, D.M.; Prato, M. Electrostatic interactions by design. Versatile methodology towards multifunctional assemblies/nanostructured electrodes. Chem. Commun. 2004, 2517–2525. [Google Scholar]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 2015, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.; da Silva, J.C.E. Fluorescent carbon dots capped with PEG 200 and mercaptosuccinic acid. J. Fluoresc. 2010, 20, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Strauss, V.; Kahnt, A.; Zolnhofer, E.M.; Meyer, K.; Maid, H.; Placht, C.; Bauer, W.; Nacken, T.J.; Peukert, W.; Etschel, S.H. Assigning electronic states in carbon nanodots. Adv. Funct. Mater. 2016, 26, 7975–7985. [Google Scholar] [CrossRef]
- Hutton, G.A.; Martindale, B.C.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Interference between Coulombic and CT-mediated couplings in molecular aggregates: H-to J-aggregate transformation in perylene-based π-stacks. J. Chem. Phys. 2015, 143, 244707. [Google Scholar] [CrossRef] [PubMed]
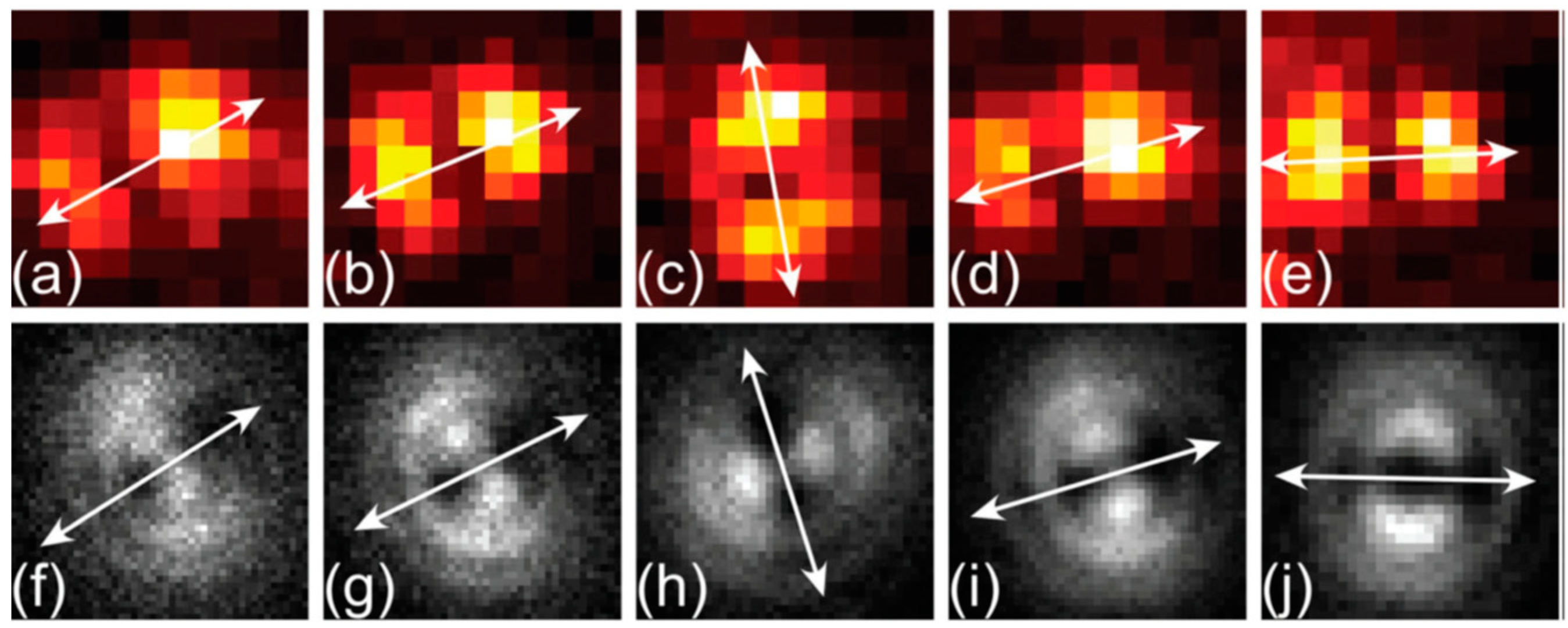
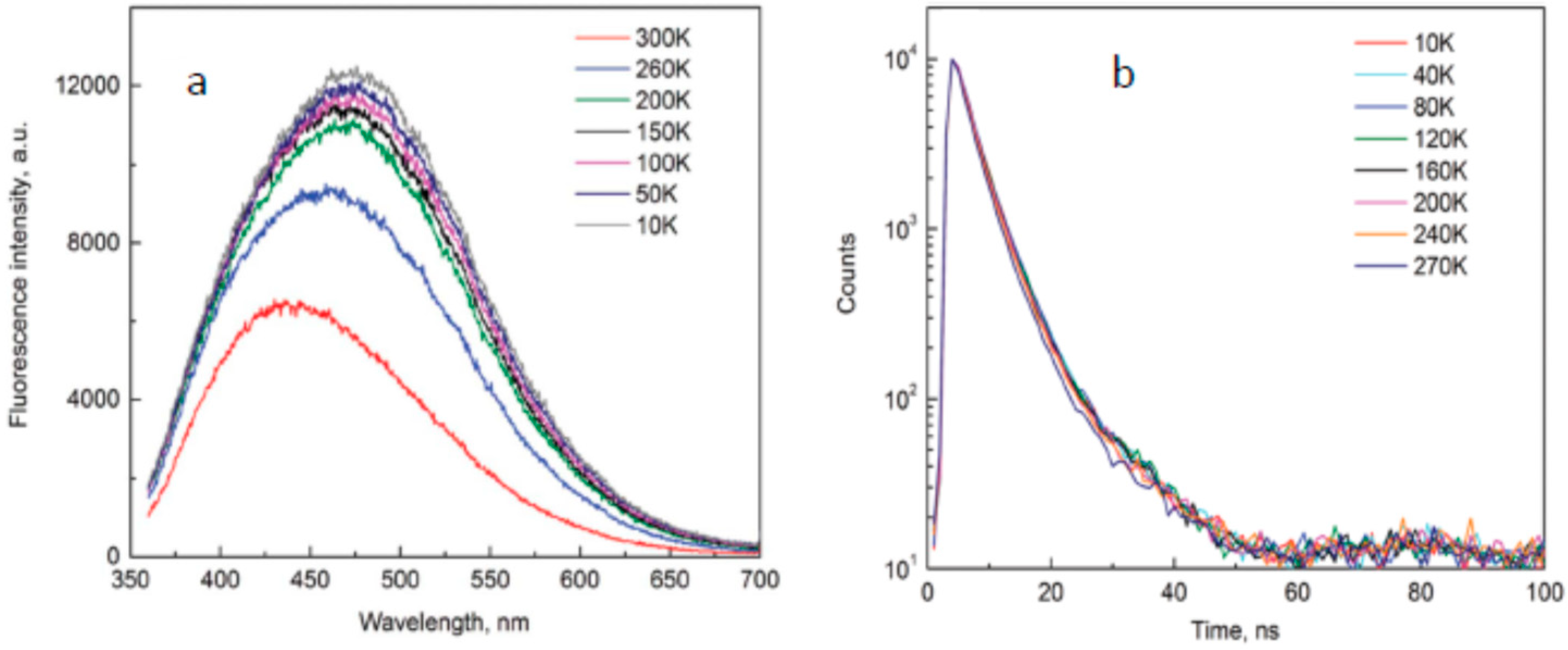
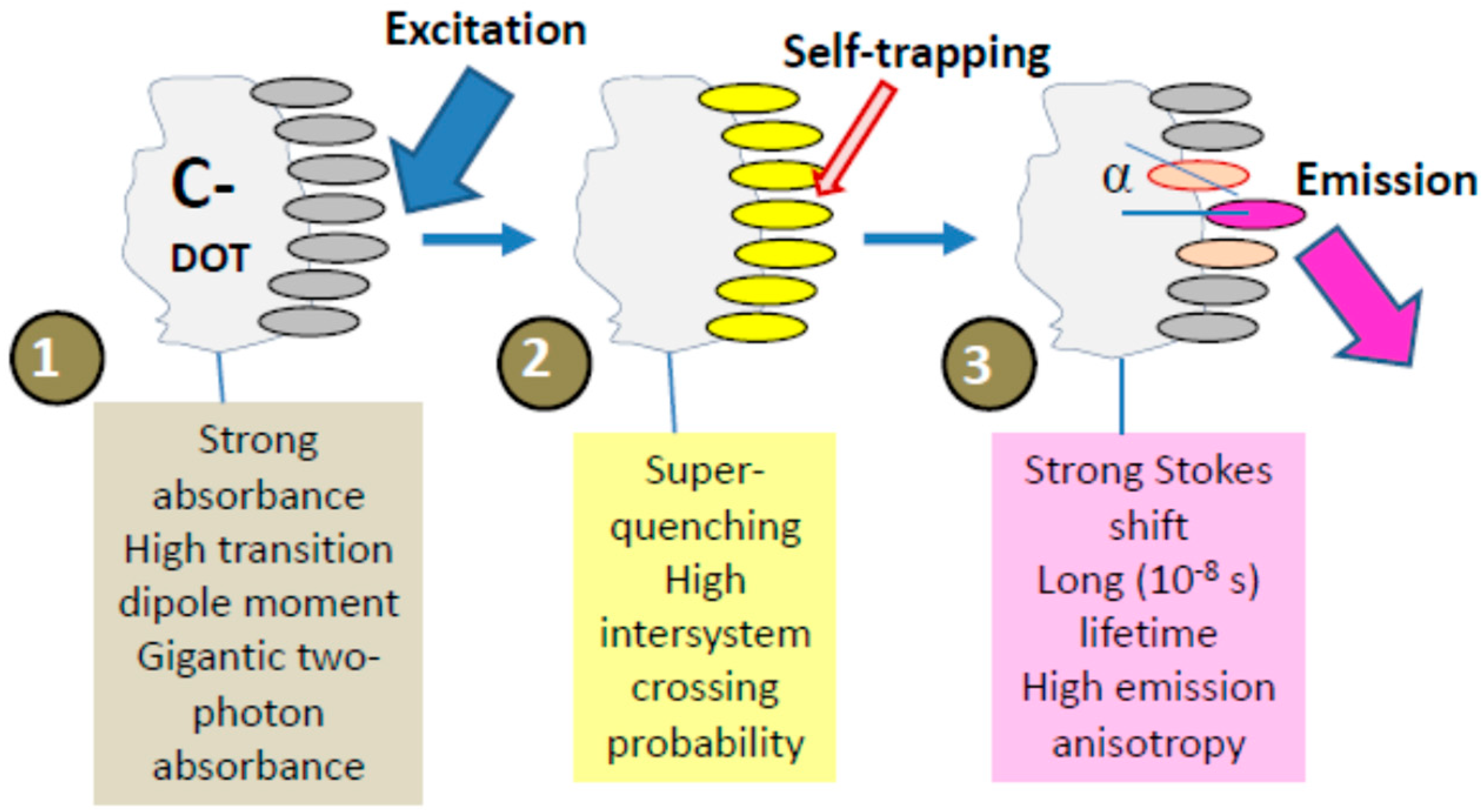
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demchenko, A.P. Excitons in Carbonic Nanostructures. C 2019, 5, 71. https://doi.org/10.3390/c5040071
Demchenko AP. Excitons in Carbonic Nanostructures. C. 2019; 5(4):71. https://doi.org/10.3390/c5040071
Chicago/Turabian StyleDemchenko, Alexander P. 2019. "Excitons in Carbonic Nanostructures" C 5, no. 4: 71. https://doi.org/10.3390/c5040071
APA StyleDemchenko, A. P. (2019). Excitons in Carbonic Nanostructures. C, 5(4), 71. https://doi.org/10.3390/c5040071