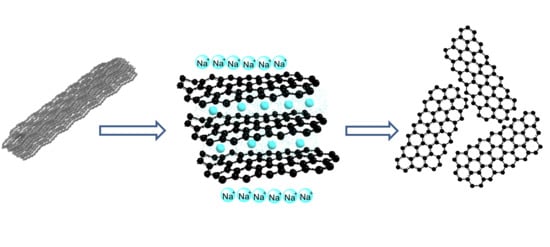
Synthesis of Graphene Nanosheets through Spontaneous Sodiation Process
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Electrolyte Preparation
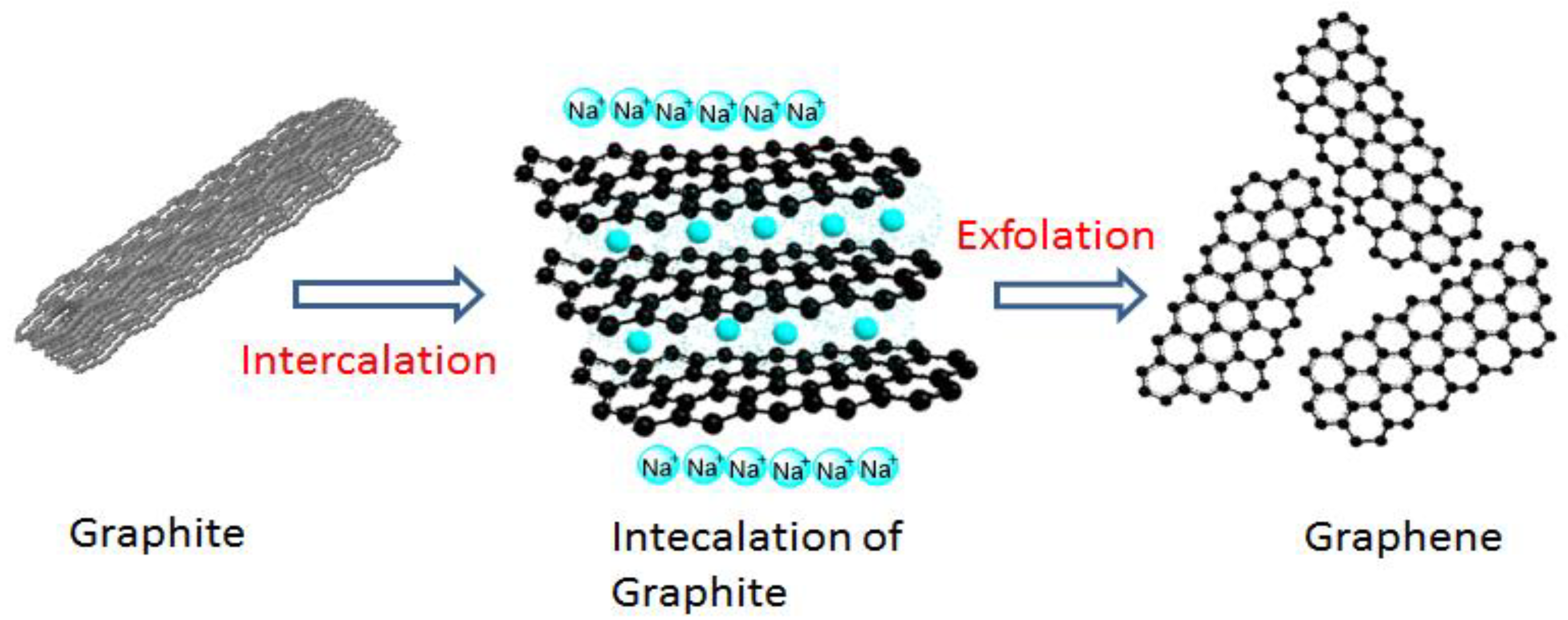
2.3. Fabrication of GIC
2.4. GNS Production
2.5. Characterization of Graphene Nanosheets
3. Results and Discussion
3.1. Intercalation of Sodium Ions into Graphite
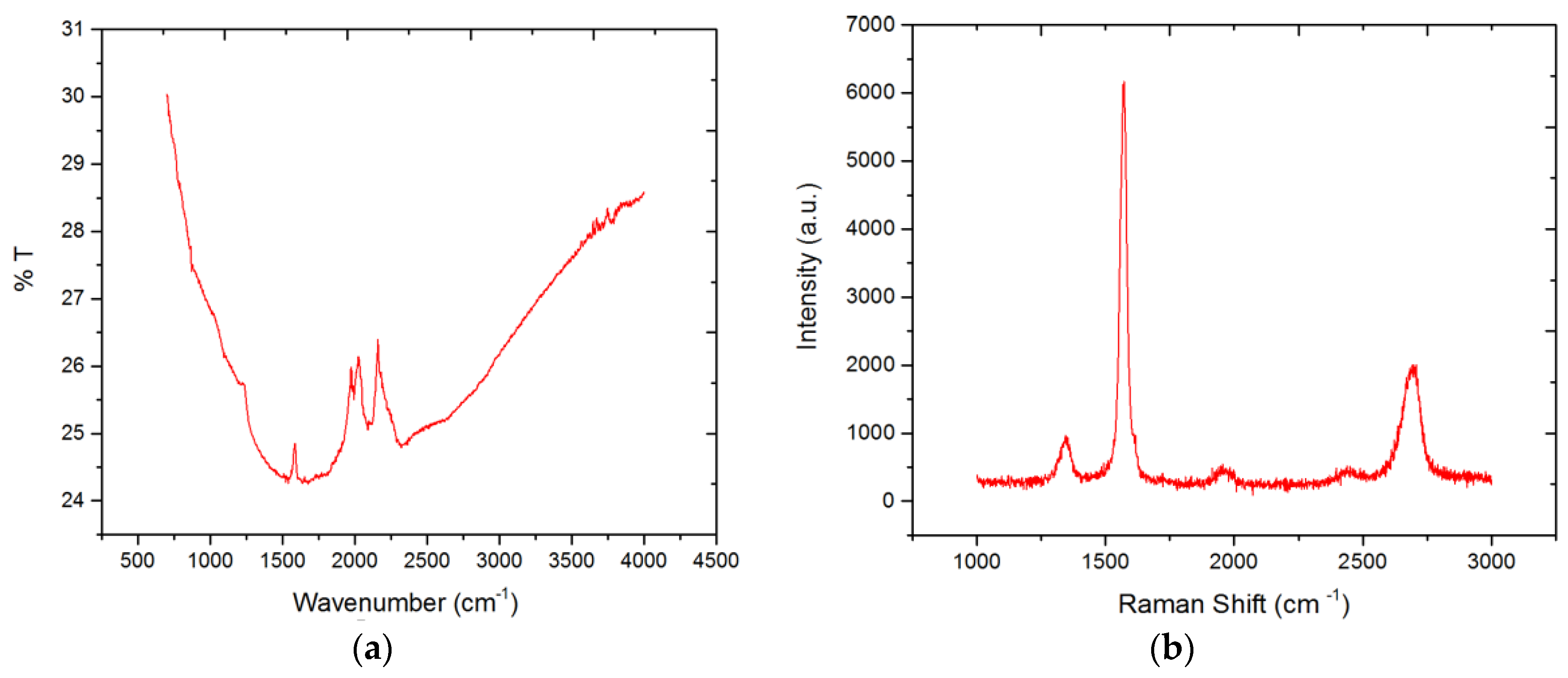
3.2. Raman Spectroscopy
3.3. Fourier Transform Infrared Spectroscopy
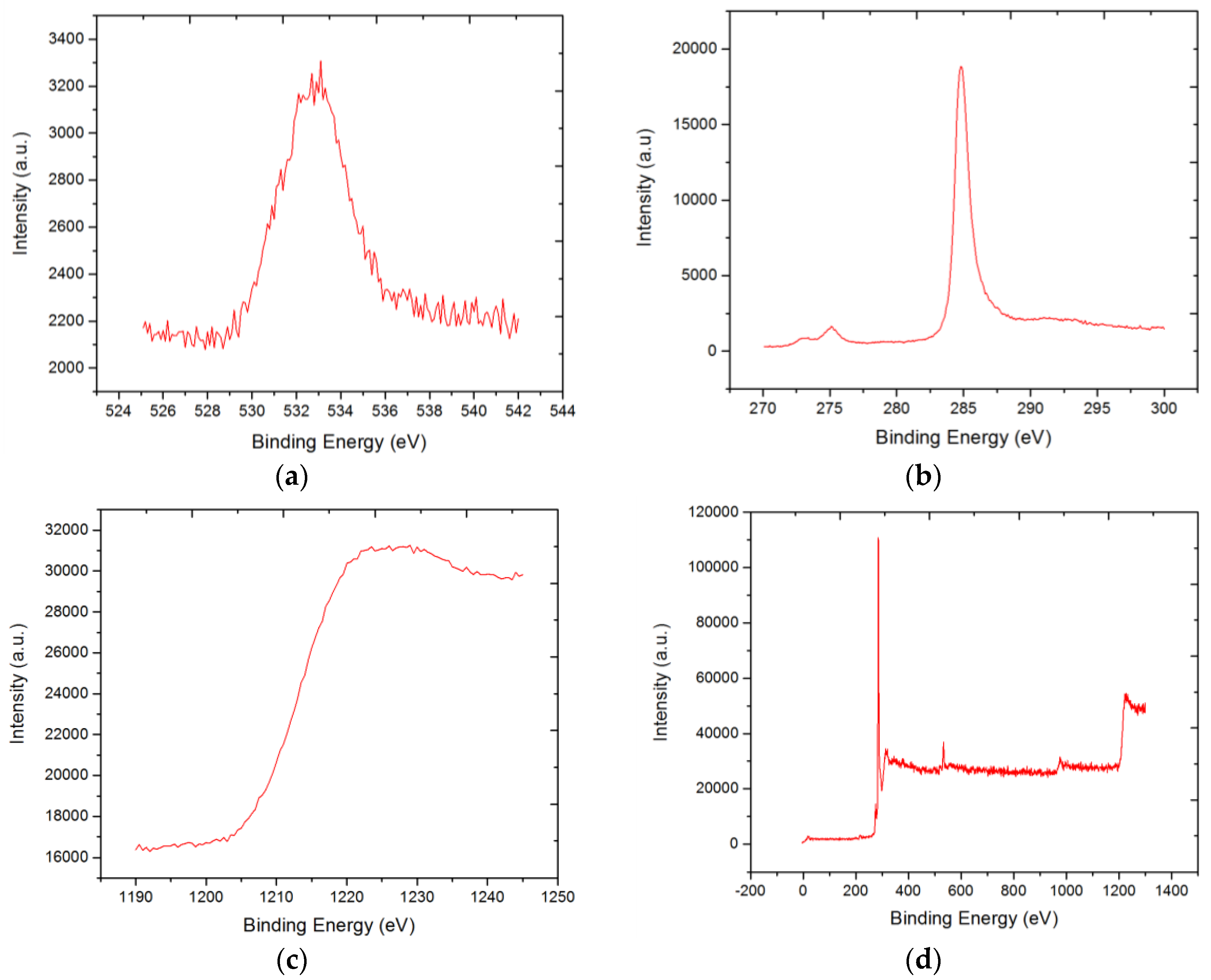
3.4. X-ray Photoelectron Spectroscopy
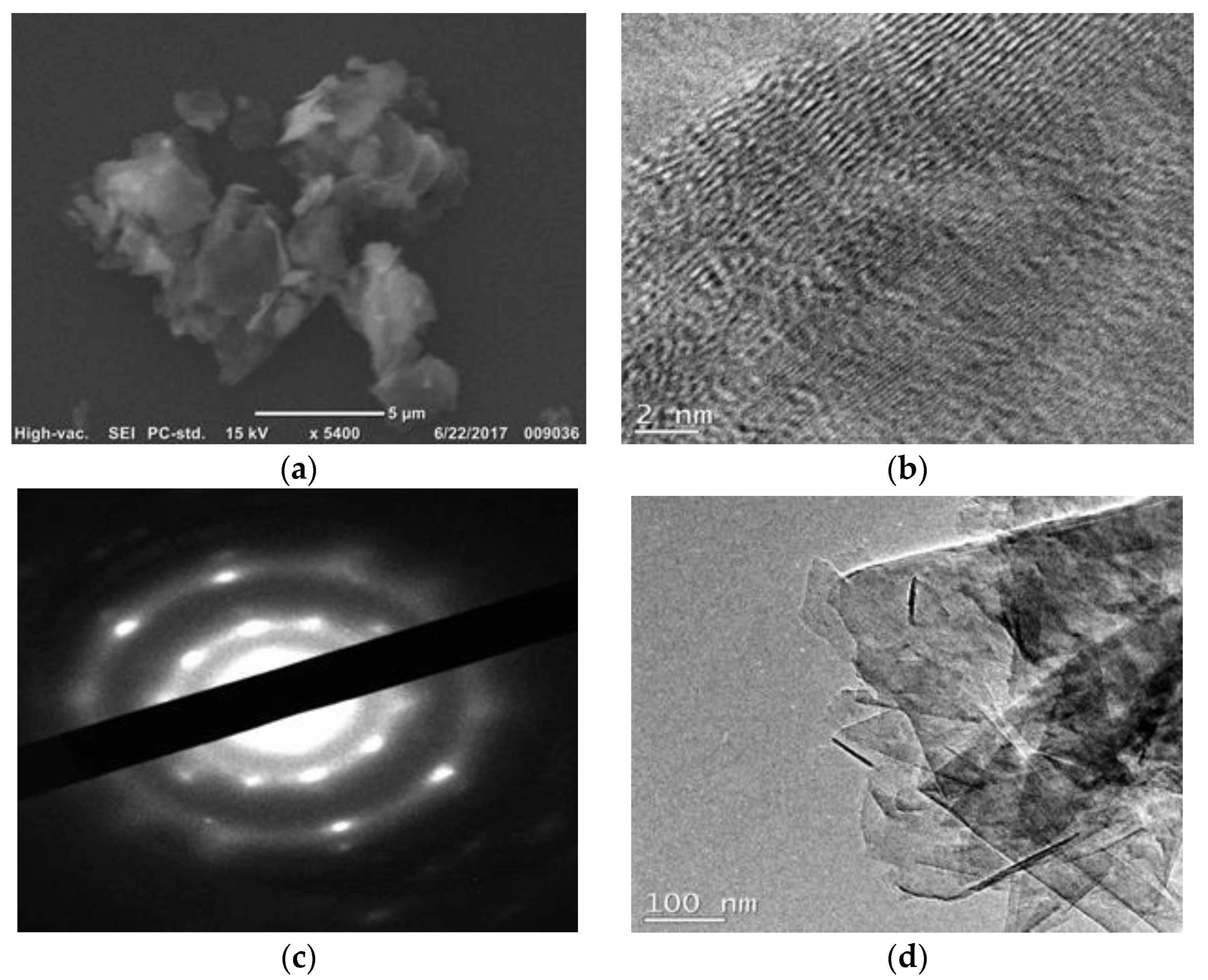
3.5. Scannning and Transmission Electron Microscopy Imaging
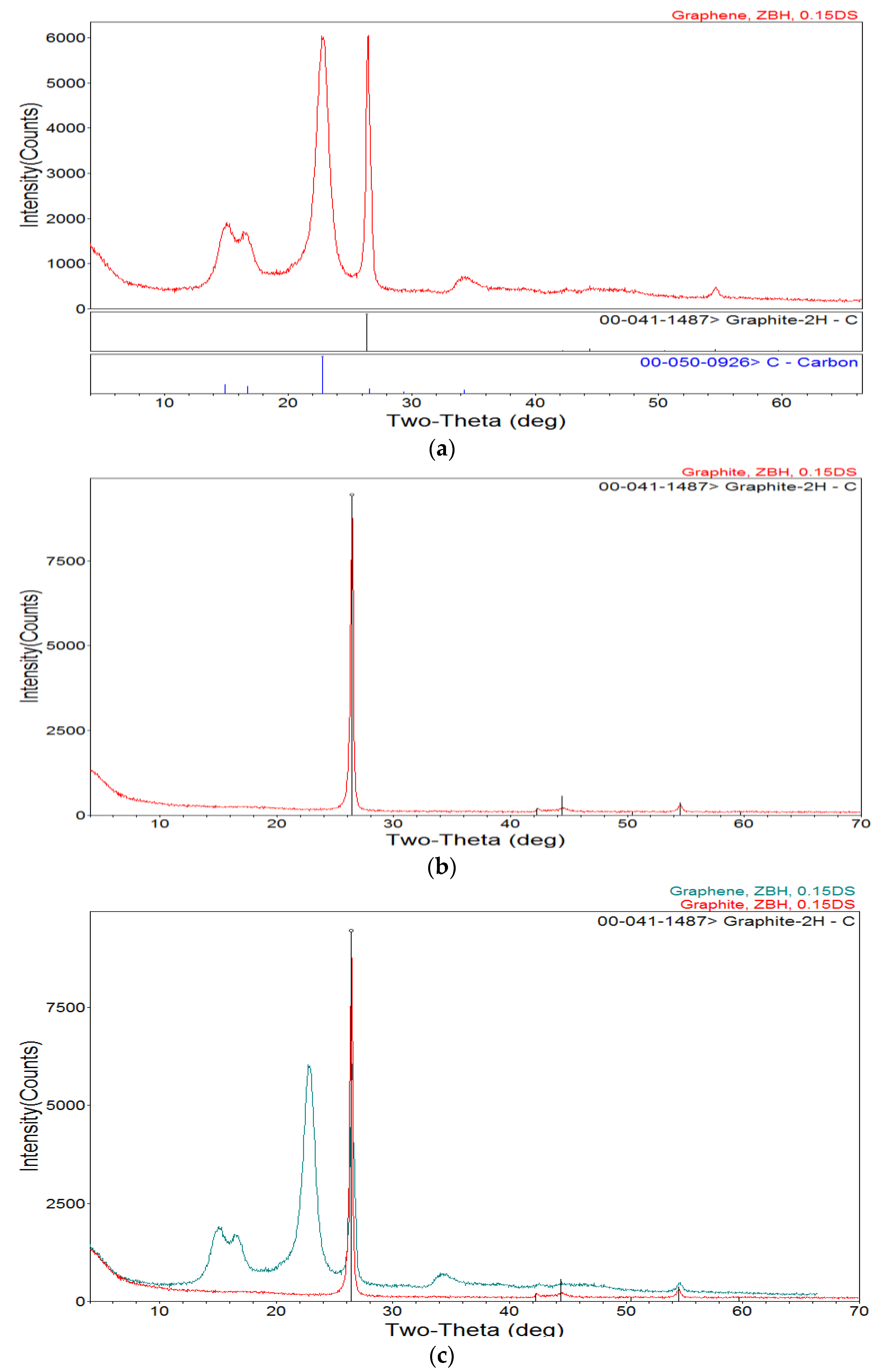
3.6. X-ray Diffraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.A.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Yan, T.; Wang, H.; Shi, L.; Zhang, J.; Zhang, D. Graphene-like carbon nanosheets prepared by a Fe-catalyzed glucose-blowing method for capacitive deionization. J. Mater. Chem. A 2015, 3, 5934–5941. [Google Scholar] [CrossRef]
- Tan, R.K.; Reeves, S.P.; Hashemi, N.; Thomas, D.G.; Kavak, E.; Montazami, R.; Hashemi, N.N. Graphene as a flexible electrode: Review of fabrication approaches. J. Mater. Chem. A 2017, 5, 17777–17803. [Google Scholar] [CrossRef]
- Rao, C.E.; Sood, A.E.; Subrahmanyam, K.E.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Nuvoli, D.; Valentini, L.; Alzari, V.; Scognamillo, S.; Bon, S.B.; Piccinini, M.; Illescas, J.; Mariani, A. High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid. J. Mater. Chem. 2011, 21, 3428–3431. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Low, C.T.; Walsh, F.C.; Chakrabarti, M.H.; Hashim, M.A.; Hussain, M.A. Electrochemical approaches to the production of graphene flakes and their potential applications. Carbon 2013, 54, 1–21. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.X.; Wang, J.Z.; Lim, A.L.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Manga, K.K.; Bao, Q.; Loh, K.P. High-yield synthesis of few-layer graphene flakes through electrochemical expansion of graphite in propylene carbonate electrolyte. J. Am. Chem. Soc. 2011, 133, 8888–8891. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Müllen, K. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Lu, A.Y.; Xu, Y.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Mullen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.G.; Papakonstantinou, P.; Sharma, S.; Lubarsky, G.; Li, M.; McNeill, D.W.; Quinn, A.J.; Zhou, W.; Blackley, R. Controllable selective exfoliation of high-quality graphene nanosheets and nanodots by ionic liquid assisted grinding. Chem. Commun. 2012, 48, 1877–1879. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.; Zhen, S.G.; Poh, C.K.; Chaurasia, A.; Luo, J.; Wu, X.; Yeow, E.K.; Sahoo, N.G.; Lin, J.; Shen, Z. A green approach to the synthesis of high-quality graphene oxide flakes via electrochemical exfoliation of pencil core. RSC Adv. 2013, 3, 11745–11750. [Google Scholar] [CrossRef]
- Zheng, Y.; Ni, G.X.; Bae, S.; Cong, C.X.; Kahya, O.; Toh, C.T.; Kim, H.R.; Im, D.; Yu, T.; Ahn, J.H.; Hong, B.H. Wafer-scale graphene/ferroelectric hybrid devices for low-voltage electronics. EPL (Europhys. Lett.) 2011, 93, 17002. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Yan, T.; Wen, X.; Shi, L.; Zhang, J. Graphene prepared via a novel pyridine–thermal strategy for capacitive deionization. J. Mater. Chem. 2012, 22, 23745–23748. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.; Na, W.; Hong, S.M.; Koo, C.M. Fabrication of high quality graphene nanosheets via a spontaneous electrochemical reaction process. Carbon 2015, 91, 527–534. [Google Scholar] [CrossRef]
- Lithium Element Facts. Available online: https://www.chemicool.com/elements/lithium.html (accessed on 4 July 2018).
- Seyfried, W.E., Jr.; Janecky, D.R.; Mottl, M.J. Alteration of the oceanic crust: Implications for geochemical cycles of lithium and boron. Geochim. Cosmochim. Acta 1984, 48, 557–569. [Google Scholar] [CrossRef]
- Ellis, B.L.; Makahnouk, W.R.; Makimura, Y.; Toghill, K.; Nazar, L.F. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries. Nat. Mater. 2007, 6, 749. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; Duesberg, G.S. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Xu, F.; Lee, J.H.; Jung, C.; Hong, S.M.; Zhang, Q.M.; Koo, C.M. A fast and efficient pre-doping approach to high energy density lithium-ion hybrid capacitors. J. Mater. Chem. A 2014, 2, 10029–10033. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.; Xu, F.; Jung, C.; Hong, S.M.; Koo, C.M. In situ synchrotron wide-angle X-ray scattering study on rapid lithiation of graphite anode via direct contact method for Li-ion capacitors. J. Power Sources 2015, 283, 68–73. [Google Scholar] [CrossRef]
- Huang, H.; Xia, Y.; Tao, X.; Du, J.; Fang, J.; Gan, Y.; Zhang, W. Highly efficient electrolytic exfoliation of graphite into graphene sheets based on Li ions intercalation-expansion-microexplosion mechanism. J. Mater. Chem. 2012, 22, 10452–10456. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Yoon, G.; Kim, H.; Park, K.Y.; Park, M.S.; Yoon, W.S.; Kang, K. Sodium intercalation chemistry in graphite. Energy Environ. Sci. 2015, 8, 2963–2969. [Google Scholar] [CrossRef]
- Charlier, J.C.; Gonze, X.; Michenaud, J.P. Graphite interplanar bonding: Electronic delocalization and van der Waals interaction. EPL (Europhys. Lett.) 1994, 28, 403. [Google Scholar] [CrossRef]
- Briggs, J. Science in Focus Chemistry for Secondary 5 Normal (Academic): Theory Workbook; Pearson Education South Asia: Singapore, 2005. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Vidano, R.P.; Fischbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Krishnan, R.S.; Shankar, R.K. Raman effect: History of the discovery. J. Raman Spectrosc. 1981, 10, 1–8. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Pimenta, M.A. Measuring the absolute Raman cross section of nanographites as a function of laser energy and crystallite size. Phys. Rev. B 2007, 76, 064304. [Google Scholar] [CrossRef]
- Pardanaud, C.; Martin, C.; Roubin, P. Multiwavelength Raman spectroscopy analysis of a large sampling of disordered carbons extracted from the Tore Supra tokamak. Vib. Spectrosc. 2014, 70, 187–192. [Google Scholar] [CrossRef]
- Li, Z.; Han, J.; Fan, L.; Guo, R. In-situ controllable growth of α-Ni(OH)2 with different morphologies on reduced graphene oxide sheets and capacitive performance for supercapacitors. Colloid Polym. Sci. 2016, 294, 681–689. [Google Scholar] [CrossRef]
- Carbon Non-Metals. Available online: https://xpssimplified.com/elements/carbon.php (accessed on 4 July 2018).
- Shevitski, B. Structural Properties of Graphene and Carbon Nanotubes. Procedia IUTAM 2010, 28, 1–8. [Google Scholar]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing graphene sheets from restacking for high-capacitance performance. J. Phys. Chem. C 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene, graphene oxide and other graphenoids. Electrochem. Commun. 2013, 36, 14–18. [Google Scholar] [CrossRef]
- Tanuma, S.I.; Palnichenko, A. Synthesis of low density carbon crystal “carbolite” by quenching of carbon gas. J. Mater. Res. 1995, 10, 1120–1125. [Google Scholar] [CrossRef]
- Bai, Z.; Lin, H.; Johnson, J.; Gui, S.C.; Imakita, K.; Montazami, R.; Fujii, M.; Hashemi, N. The single-band red upconversion luminescence from morphology and size controllable Er3+/Yb3+ doped MnF2 nanostructures. J. Mater. Chem. C 2014, 2, 1736–1741. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, D.-G.; Kavak, E.; Hashemi, N.; Montazami, R.; Hashemi, N.N. Synthesis of Graphene Nanosheets through Spontaneous Sodiation Process. C 2018, 4, 42. https://doi.org/10.3390/c4030042
Thomas D-G, Kavak E, Hashemi N, Montazami R, Hashemi NN. Synthesis of Graphene Nanosheets through Spontaneous Sodiation Process. C. 2018; 4(3):42. https://doi.org/10.3390/c4030042
Chicago/Turabian StyleThomas, Deepak-George, Emrah Kavak, Niloofar Hashemi, Reza Montazami, and Nicole N. Hashemi. 2018. "Synthesis of Graphene Nanosheets through Spontaneous Sodiation Process" C 4, no. 3: 42. https://doi.org/10.3390/c4030042
APA StyleThomas, D.-G., Kavak, E., Hashemi, N., Montazami, R., & Hashemi, N. N. (2018). Synthesis of Graphene Nanosheets through Spontaneous Sodiation Process. C, 4(3), 42. https://doi.org/10.3390/c4030042