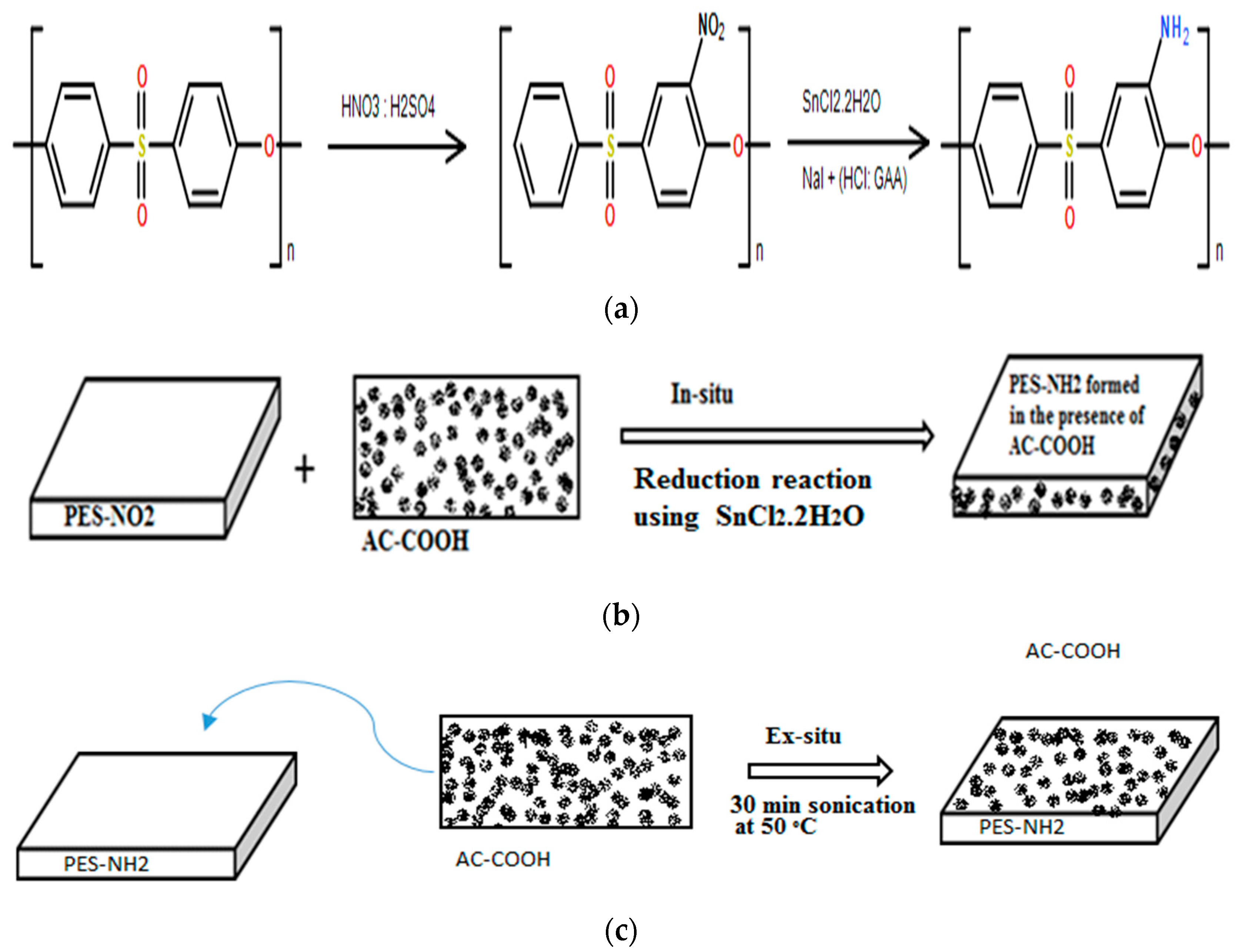
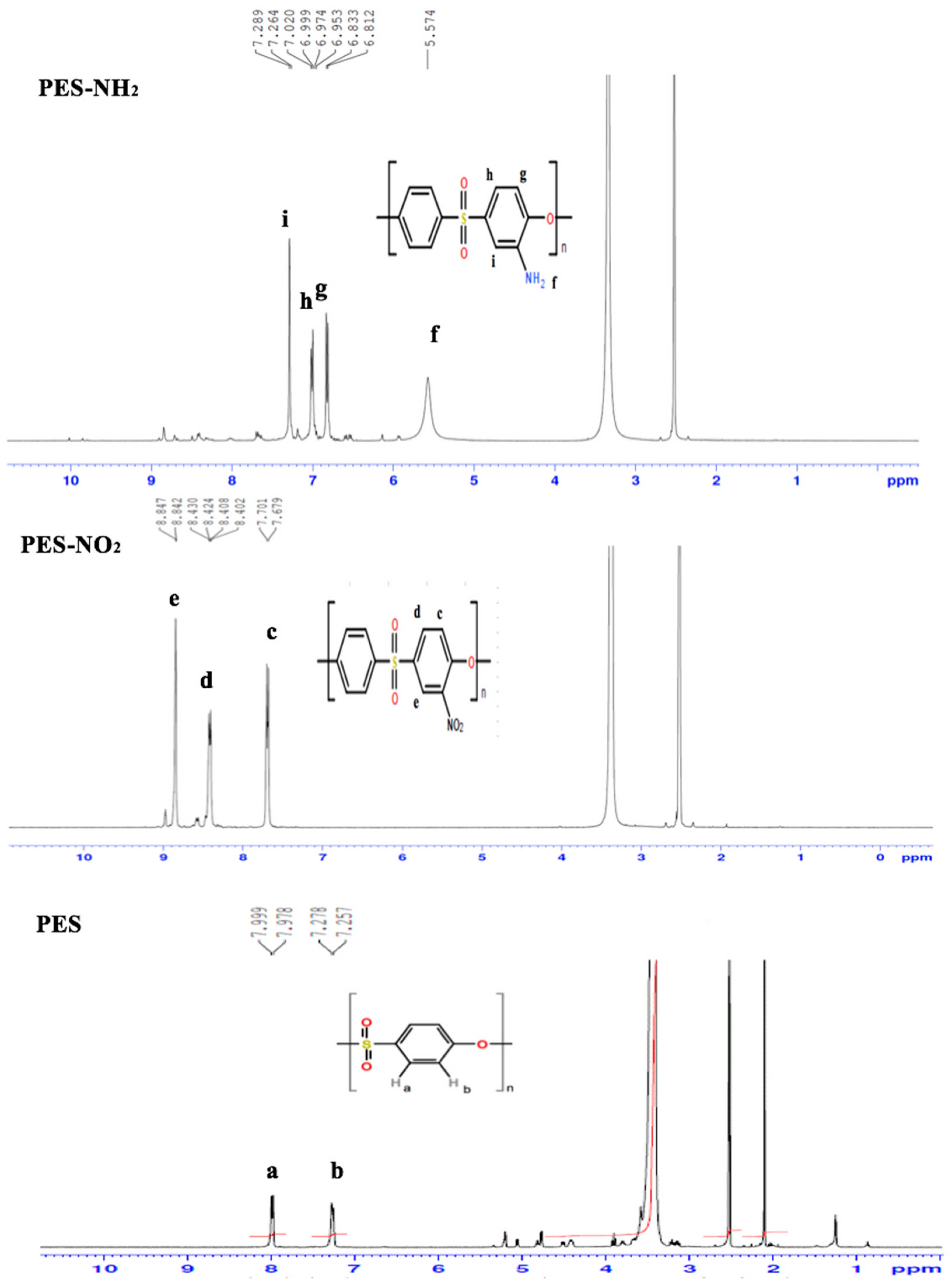
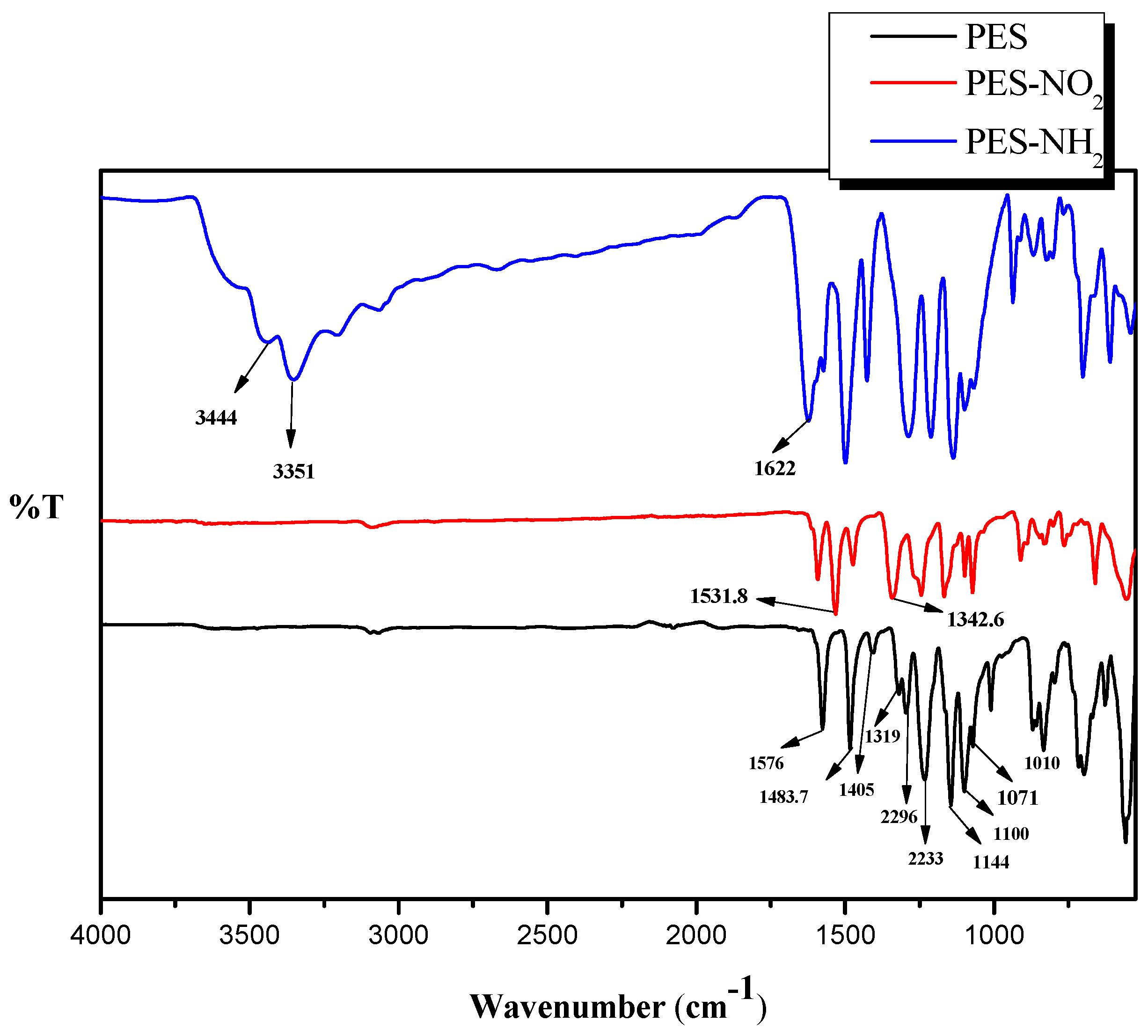
3.1. Characterization of PES–NH2 with AC–COOH
1H-NMR was used to confirm the chemical structure of PES, PES–NO
2, and PES–NH
2, respectively as shown in
Figure 1. According to
1H-NMR of the PES spectrum, two distinct doublet peaks were noticed at 7.9 ppm and 7.2 ppm accounting for two protons (a and b), respectively. After nitration, the
1H-NMR of PES–NO
2 spectrum showed three distinct peaks. The first peak was a double at 7.7 ppm (c) because of the ortho coupling for the proton that appeared at 8.8 ppm. The second distinct peak which appeared at 8.4 ppm (d) was a doublet of the two doublets of the protons that were ortho and meta coupled (at 8.8 ppm and 7.7 ppm, respectively), which suggested that the nitration of PES had occurred. The third peak, which appeared at 8.8 ppm (e), was a doublet because of the proton that showed meta coupling a shown in
Figure 1. Therefore, it can be concluded that the nitration occurred at the carbon that was on the ortho position to the ether oxygen. After the reduction of PES–NO
2, the
1H-NMR of PES–NH
2 spectrum showed four distinct peaks that were shifted to a lower filed because of the donating electron nature of the amine, unlike the nitro functions which have an electron withdrawing nature. The first peak of the four amine peaks was a single peak and it was noticed at 5.5 ppm rising from the amine protons. The second peak was noticed as a doublet at 6.8 ppm and this is because of the presence of the proton that showed ortho coupling. The third distinct peak was a doublet of doublets at 6.9 ppm because of the two protons that showed ortho and meta couplings. Lastly, the fourth peak was noticed as a doublet at 7.2 ppm and this is because of the presence of the proton that showed meta coupling. This
1H-NMR data were in agreement with the previous reported data [
14].
The chemical structures of PES, PES–NO
2, and PES–NH
2 were further confirmed using FT-IR spectroscopy as shown in
Figure 2. The chemical structure of the PES contains three important functions including benzene, ether, and sulfone. The presence of benzene rings should exhibit three peaks in the range of 1600 cm
−1 to 1400 cm
−1, and, in the PES spectrum, the three peaks were observed at 1576 cm
−1, 1483.7 cm
−1, and 1405 cm
−1. The presence of the ether function was confirmed due to the presence of its two starching peaks at 1319 cm
−1, as well as 1296 cm
−1. The two starching peaks indicating the presence of the sulfone group were also noticed at 1144 cm
−1 and 1100 cm
−1. Our data of the PES spectrum came in agreement with previously reported studies on PES FT-IR analysis [
12,
15]. The FT-IR spectrum of PES–NO
2 showed an asymmetric peak at 1531 cm
−1 and a symmetrical peak at 1342 cm
−1 which indicate the attachment of the –NO
2 functions on the PES. After the reduction of the –NO
2 groups to –NH
2 groups, the FT-IR of PES–NH
2 spectrum exhibited the formation of –NH
2 by showing two peaks at 3344 cm
−1, as well as 3451 cm
−1, and the deformation of –NH
2 by showing a peak at 1622 cm
−1. This confirms the attachment of the amine functions on PES. Both our FT-IR of the PES–NO
2 and PES–NH
2 spectra came in agreement with a previously reported study [
12]. The FT-IR spectra of PES–NH
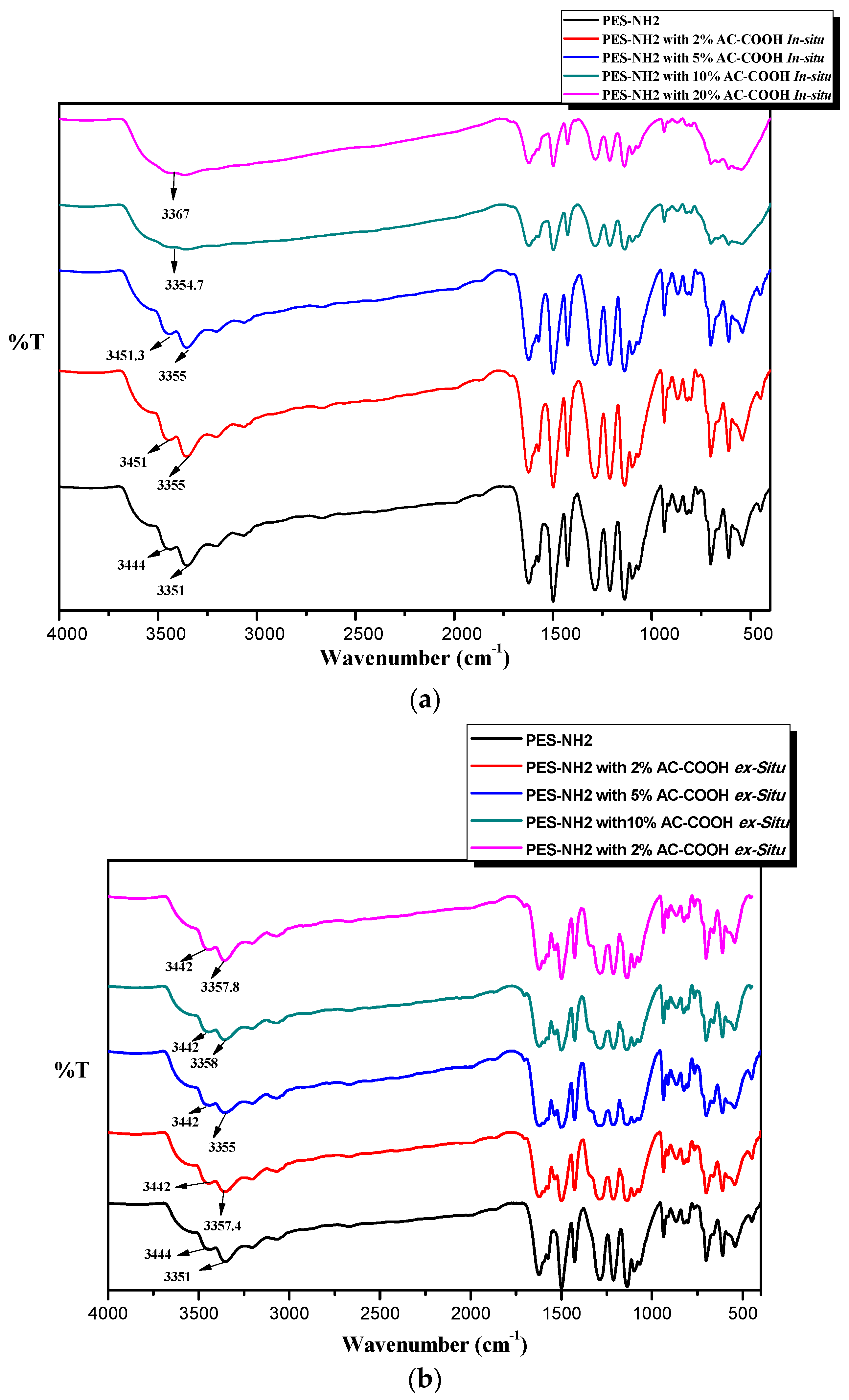
2 with different concentrations of AC–COOH using the in situ and ex situ techniques are shown in
Figure 3a,b, respectively. In
Figure 3a, it can be clearly seen that as the concentrations of AC–COOH increased, the shifting of the amine stretching peaks was noticed, which suggests that the interactions between AC–COOH and PES–NH
2 were formed. In addition, it was also noticed that the effects of increasing the concentrations of AC–COOH in PES–NH
2 resulted in overlapping peaks between the amine and –OH for the acid peaks until it became one peak at 3354 cm
−1 for using 10 wt % of AC–COOH and at 3367 cm
−1 for using 10 wt % of AC–COOH instead of two peaks for the amine functions. We suggest that the noncovalent interactions such as intermolecular forces, dipole–dipole, hydrogen bonding, and Van der Waals interactions between AC–COOH and PES–NH
2 took place during the synthesis of the polymer through the in situ technique. However, when the PES–NH
2 was coated with AC–COOH on its surface through the ex situ technique, the noncovalent interactions were weaker, which explained the FT-IR spectrum in
Figure 3b, in which less shifting was seen between the amine stretching peaks during the presence of AC–COOH on the surface of the PES–NH
2.
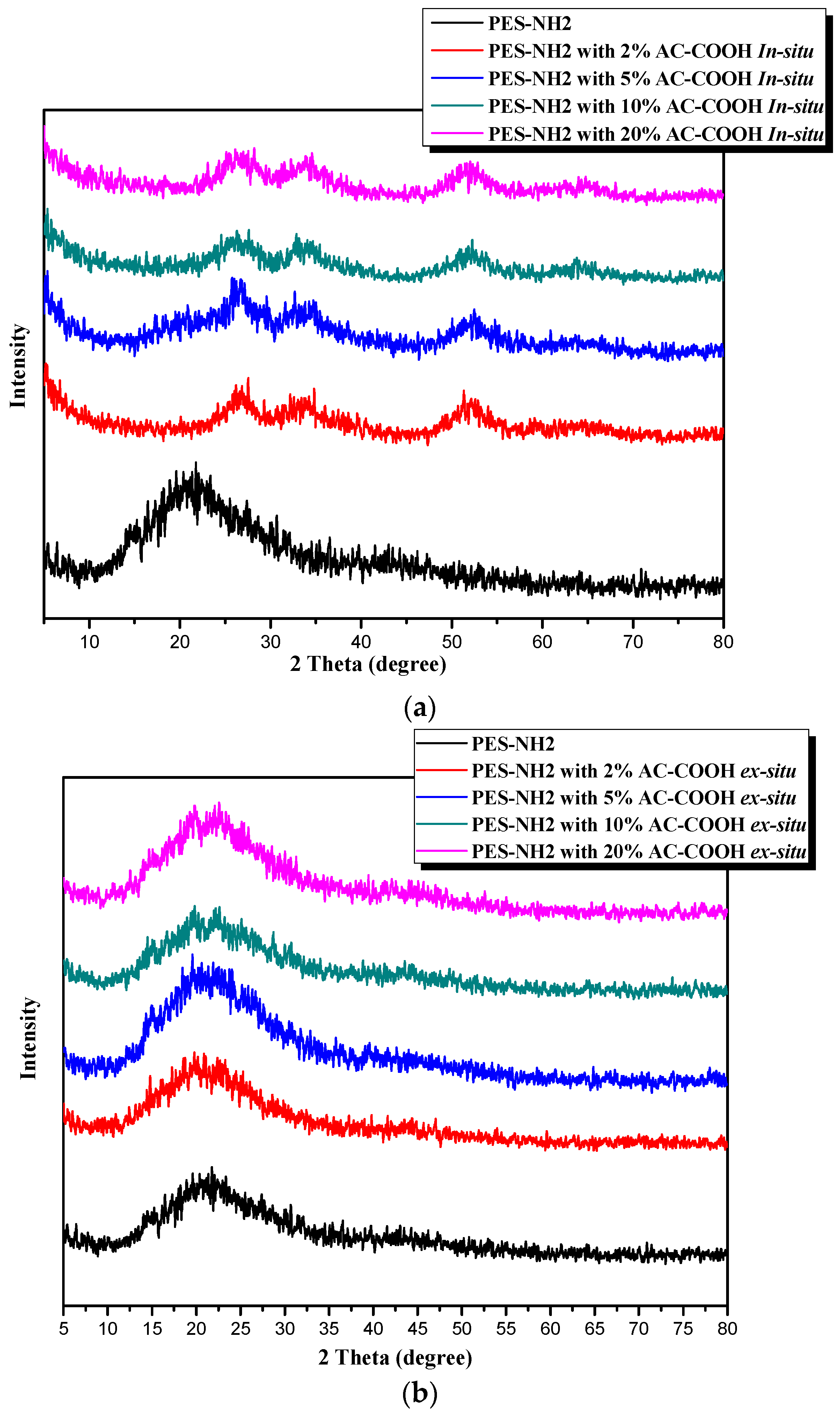
The XRD data confirmed the chemical compositions of the plan PES–NH
2 versus the PES–NH
2 in situ and the PES–NH
2 ex situ with AC–OOH, as shown in
Figure 4a,b, respectively. In
Figure 4a, the PES–NH
2 has an amorphous structure due to the presence of the benzene rings and the ether bonds, which show a broad peak at 2θ = 21° that came in agreement with previous data reported [
12,
15]. Two broad peaks appeared after blending the PES–NH
2 with AC–OOH at 2θ = 33° and 52°, which referred to the presence of AC–COOH and their interactions with PES–NH
2, observed from the XRD data. The XRD data of PES–NH
2 with AC–COOH in the ex situ technique did not show any change in the structure as shown in
Figure 4b, in which all the resultant polymers showed only one broad peak at 2θ = 21°, which is identical to the plan polymer. This suggests that PES–COOH was coated only on the surface of PES–NH
2, which appeared in XRD as one peak since no internal interaction was formed between the polymer and AC–COOH particles.
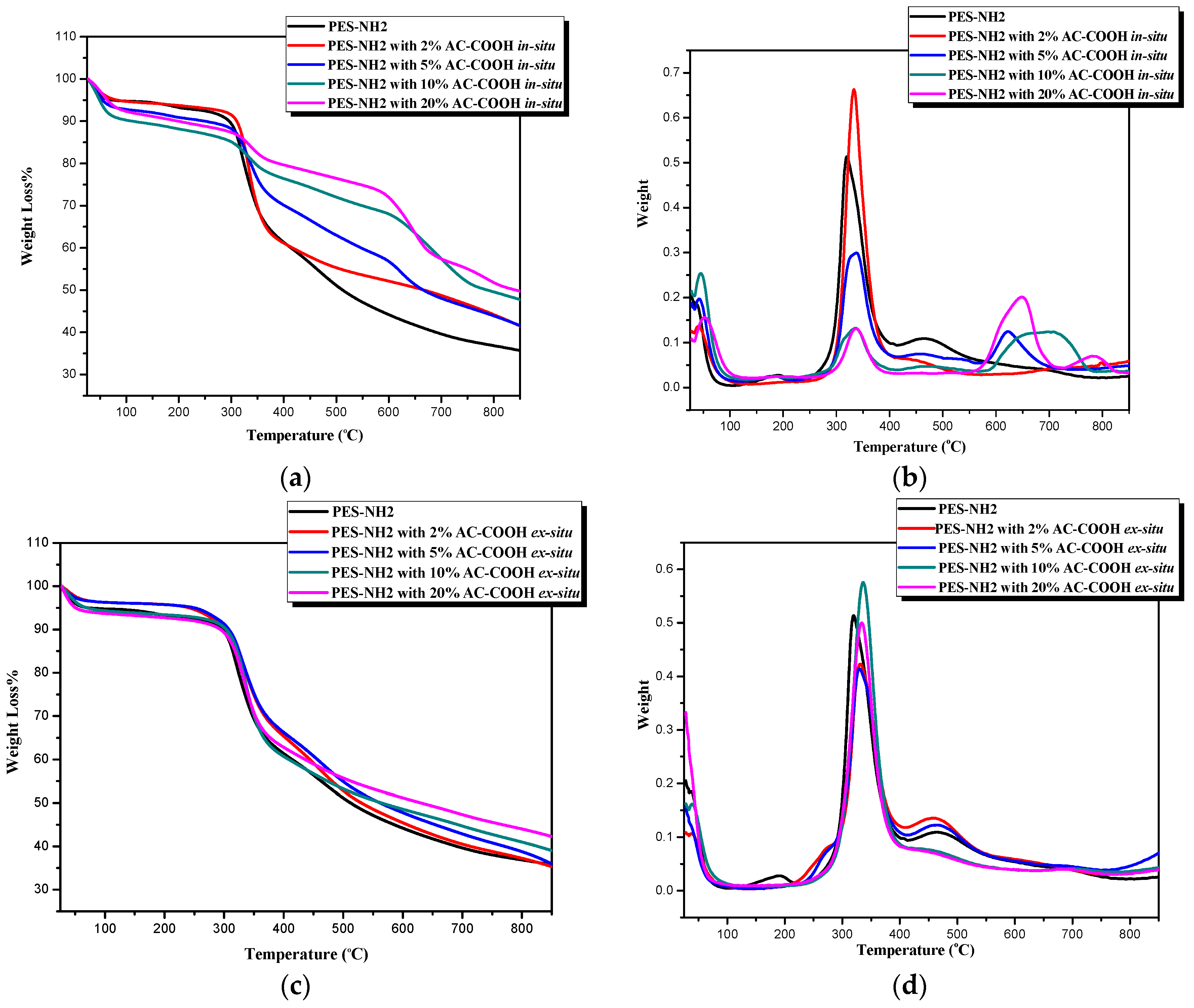
The thermogravimetric analysis (TGA) of the PES–NH
2 (control), as well as PES–NH
2 in situ and ex situ with AC–COOH, was performed with a heating rate at 10 °C/min and under nitrogen flow. The TGA curves of PES–NH
2 with different concentrations of AC–COOH underflow through the in situ technique appeared to be different from the plan PES–NH
2 curve as shown in
Figure 5a, whereas
Figure 5b shows the derivative thermogravimetric (DTG) analysis for the maximum weight loss. The differences of these curves are because there were variations in the chemical structures between the control (PES–NH
2) and PES–NH
2 with 2%, 5%, 10%, and 20% of AC–COOH using in the in situ method caused by the noncovalent strong interactions, which, in turn, also seem to decompose differently as they appeared in
Figure 5a. As the concentration of activated carbon increases, a better thermal stability was noticed as shown in
Table 1. The maximum weight loss in the control was at 320 °C whereas the PES–NH
2 with 20% AC–COOH was at the higher temperature of 340 °C. In addition, the control was decomposed (
T50) at a lower temperature, around 529 °C, while the decomposition of the PES–NH
2 with 20% AC–COOH was improved to be at 839 °C. The thermal stability of the PES–NH
2 in situ with 20% AC–COOH improved significantly in comparison to the control (
Table 1). The strong interactions formed during the synthesis between AC–OOH and PES–NH
2 have an impact in increasing the thermal stability of the polymer, which was in agreement with a previous study [
16], whereby an increase in the thermal stability of the activated carbon occurred when it was blended with cellulose acetate versus the thermal stability of activated carbon only.
On the other hand, the addition of AC–COOH to PES–NH
2 using the ex situ method, as shown in
Figure 5c,d, improved the thermal stability in a way less than in the in situ method, but with a higher thermal stability in comparison to with the control. This came in agreement with the XRD data, which showed a slight change in the chemical structures between the control and the PES–NH
2 with AC–COOH by the ex situ method.
Table 2 shows the enhancement of the thermal stability of the PES–NH
2 ex situ with AC–COOH in which the temperature of the maximum weight loss was 337 °C for the addition of 20% of AC–COOH to the PES–NH
2 whereas it was only 320 °C for the control. The decomposition of half the weight of the polymer (
T50) was only 534 °C for the control while it was 677 °C for the PES–NH
2 ex situ with 20% AC–COOH.
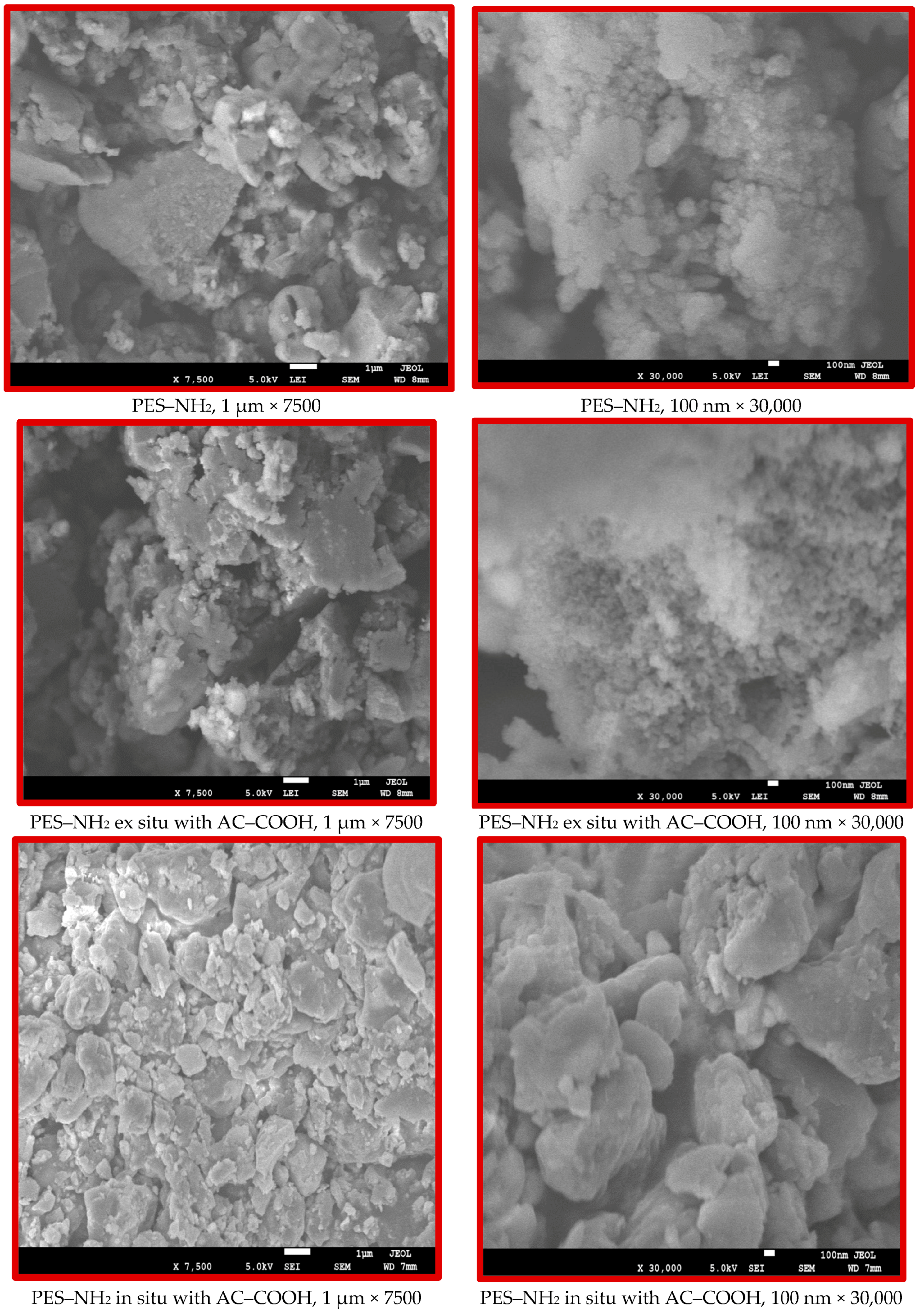
The morphology of PES–NH
2 versus the PES–NH
2 with 10% AC–COOH in situ and ex situ are shown in
Figure 6 on both the micro- and nanolevels. The SEM images show that PES–NH
2 (control) and the structural features of the PES–NH
2 with AC–COOH ex situ are similar, while the PES–NH
2 with AC–COOH in situ are different. The SEM images exhibited aggregations in the particles of PES–NH
2 with AC–COOH in situ, which clearly indicates that the in situ technique alters the morphological structure of the polymer unlike the ex situ method, which did not show any noticeable change on the morphology of PES–NH
2 in comparison with the control.
3.2. The Performance of PES–NH2 with AC–COOH in Dyes adsorption
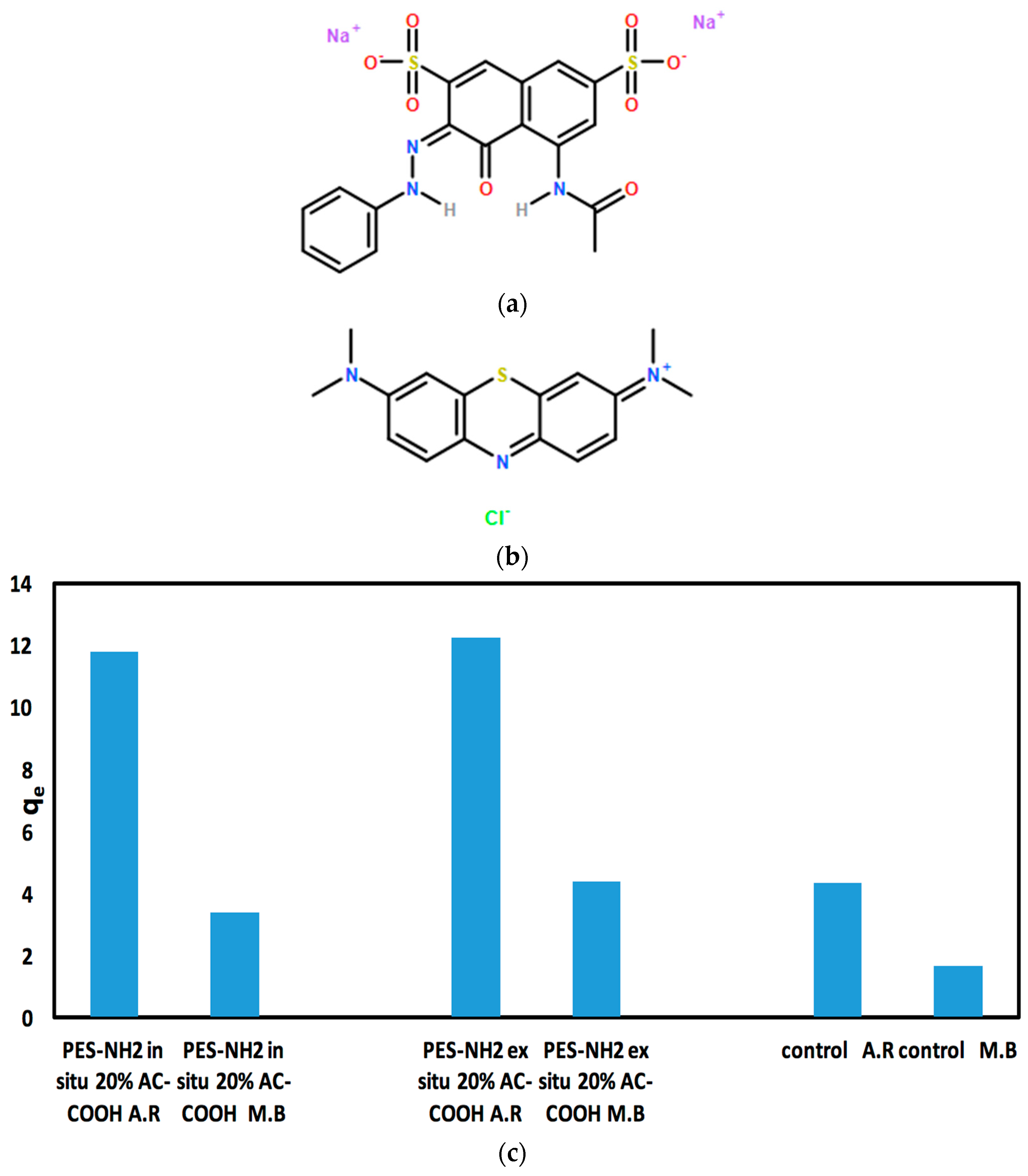
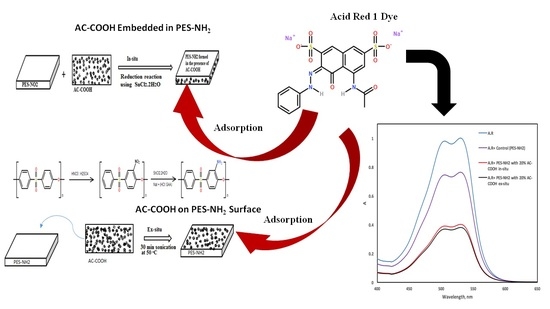
The performance PES–NH
2 in situ and ex situ with AC–COOH for the adsorption of dyes has been examined. In this work, acid red 1, an anionic dye, was used to test the capabilities of these polymers to adsorb it from aqueous solutions (
Figure 7a). As shown in
Table 3, as the concentration of AC increased in both techniques, a remarkable increase in the acid red dye adsorption was observed while the control polymer showed the lowest adsorption with only 21% in comparison with the PES–NH
2 in situ and ex situ technique with 20% AC–COOH, which showed 58% and 61% adsorption, respectively. Because PES–NH
2 with 20% AC–COOH gave the highest percentage of adsorption results in both techniques, these polymers were tested further with methylene blue (
Table 4) which is a cationic dye, to examine the performance of these polymers with cationic dyes. As shown in
Table 4, PES–NH
2 with 20% AC–COOH ex situ gave an 88% adsorption for methylene blue (5 ppm) in comparison with 60% for acid red (20 ppm). In addition, PES–NH
2 with 20% AC–COOH in situ showed 68% for methylene blue (5 ppm) and 60% for acid red (20 ppm) in comparison with the control PES–NH
2 that gave 1.6% only for methylene blue (5 ppm) and 21% for acid red (20 ppm).
Overall, both PES–NH
2 with AC–COOH in situ and ex situ showed excellent adsorption rates with both the acid red (anionic) and methylene blue (cationic) dyes in comparison with the control as shown in
Table 3 and 4, respectively. However, because acid red (20 ppm) showed a good adsorption capacity (
qe) up to 12 (
Figure 7c) in comparison with methylene blue (5 ppm) which gave
qe between 3 and 4 for PES–NH
2 with AC–COOH in situ and ex situ (
Figure 7c), the effect of different impact factors (including pH, temperature, time, and mass) was further examined on the adsorption of acid red dye by PES–NH
2 in situ and ex situ with AC–COOH.
3.3. Retention Outline of Acid Red from the Aqueous Solution onto Solid Phases PES–NH2 with In Situ and Ex Situ AC–COOH
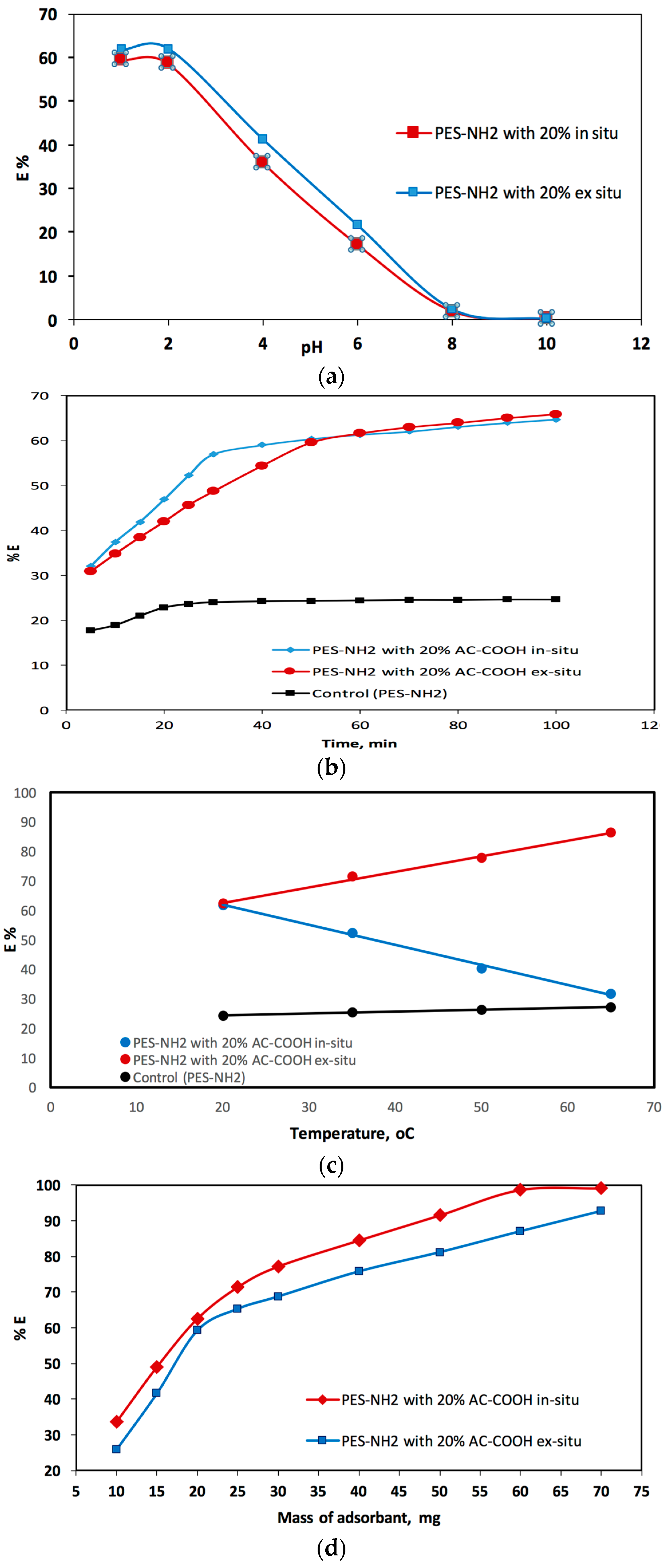
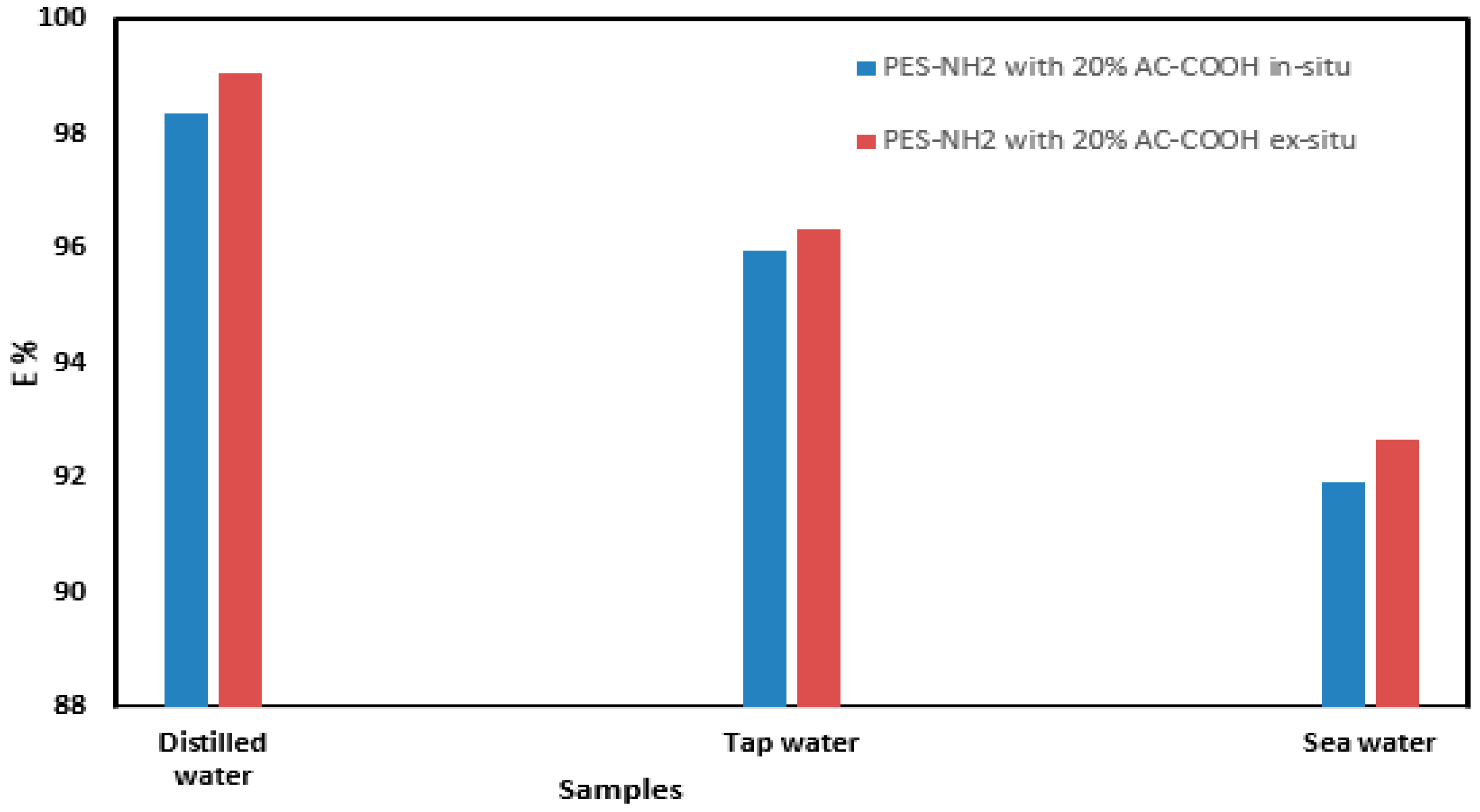
The adsorption of acid red dye in an aqueous medium with different pH values using PES–NH
2 in situ and ex situ with AC–COOH solid phase were investigated at r.t and after 1 h of shaking. At equilibrium, the acid red quantity in the aqueous medium was photometrically measured [
11]. It was observed when the % E was measured for the acid red adsorption onto the AC–COOH in situ and ex situ, there was a remarkable reduction in % E as the pH values increased as shown in
Figure 8a. Therefore, from this pH study, it was found that the pH values in the range of 1–2 were the best pH conditions to adsorb the acid red dye from the aqueous medium. The pH value of 1.5, which was obtained using HCl, was chosen for this current work.
The second impact factor was the contact time of the red acid analyte and PES–NH
2 with AC–COOH solid phase adsorbent.
Figure 8b shows the investigation study of the effect of the contact time for acid red dye adsorption by PES–NH
2 with AC–COOH in situ and ex situ. In this figure, there was an increase in the adsorption process when the contact time was increased. After 40 min of contact time, most of the acid red dye was adsorbed into the polymer solid phase. However, when the contact time was prolonged, an equilibrium of the percentage of adsorption (in 120 min) was observed. As shown in
Figure 8b, it can be clearly seen that there were two consecutive steps needed for the adsorption of acid red on PES–NH
2 with 20% of AC–COOH, in situ and ex situ. The first step was the fastest, in which the acid red transferred from the solution to the PES–NH
2 with a surface of 20% AC–COOH in situ and ex situ whereas the second one was the slowest in which the acid red dye was diffused in the PES–NH
2 with bundles of 20% AC–COOH in situ and ex situ.
In addition to the pH and contact time factors, the third impact factor that affected the adsorption mechanism was the temperature for the sample solutions. The impact of 20 °C, 35 °C, 50 °C, and 65 °C temperatures were chosen in this work in order to investigate the impact of these temperatures on the adsorption of acid red dye by the polymer solid phase. As shown in
Figure 8c, there was a remarkable increase in %adsorption for the acid red dye by PES–NH
2 with the 20% AC–COOH in situ and ex situ solid phase. However, PES–NH
2 with 20% AC–COOH in situ showed a reversed performance; meaning, as the solution temperature increased, the percentage of acid red adsorption decreased. The results for this adsorption process in
Figure 8c clearly showed the different thermodynamic behavior of PES–NH
2 with 20% AC–COOH in situ, which showed an endothermic nature in comparison with PES–NH
2 with 20% AC–COOH ex situ, which showed an exothermic nature similar to the control (PES–NH
2).
Lastly, the effect of the mass on %adsorption for the acid red adsorbed from the liquid medium was examined with an acid red concentration of 20 mg/L (
Figure 8d).
Figure 8d shows that the %adsorption for the acid red dye that was adsorbed from the liquid medium raised as the PES–NH
2 with 20% AC–COOH in situ and ex situ doses increased. This increase in the percentage is because of increasing number of activated carbon sites in the PES–NH
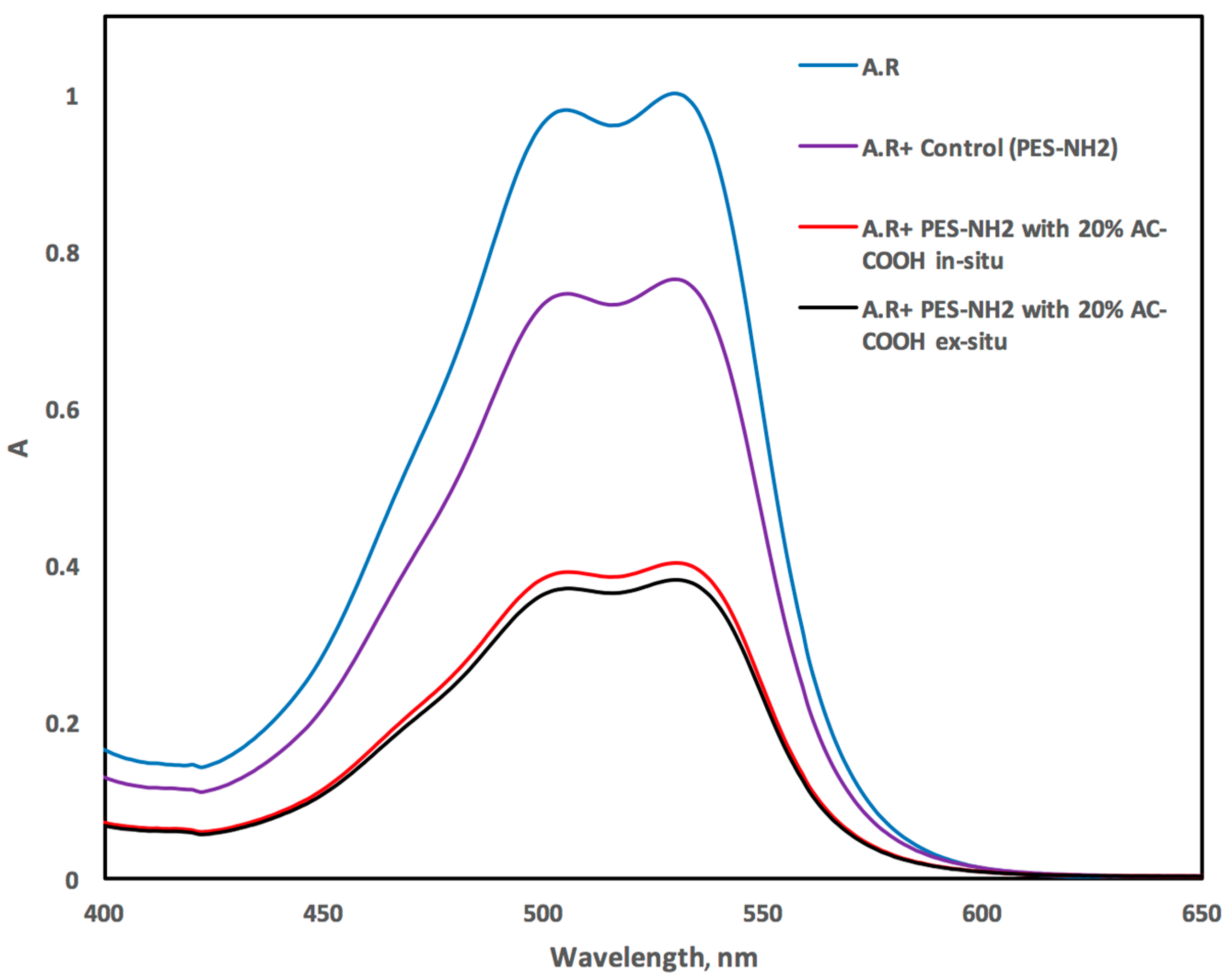
2 surface/matric for adsorbing the acid red dye from the aqueous medium. The VIS spectrum of the acid red recorded in the aqueous medium exhibited an absorption peak at 530 ± 2 nm as shown in
Figure 9. However, this peak decreased dramatically after shaking with PES–NH
2 with 20% AC–COOH (
Figure 9), and this behavior confirms the efficiency of PES–NH
2 in situ and ex situ with 20% AC–COOH for the acid red dye adsorption from the liquid medium.
3.4. The Adsorption Kinetic Performance for the Acid Red Dye by PES–NH2 with In Situ and Ex Situ 20% AC–COOH
The adsorption kinetics of acid red from aqueous solutions by PES–NH2 with the AC–COOH solid phase is necessary to deeply understand the adsorption reactions and the adsorption mechanisms. The intraparticle dispersal and film dispersal are two important factors that the retention of acid red adsorption on PES–NH2 with the AC–COOH solid phase relies on, and the faster the factor is, the likelier it will be the one that will govern the overall transport percentage.
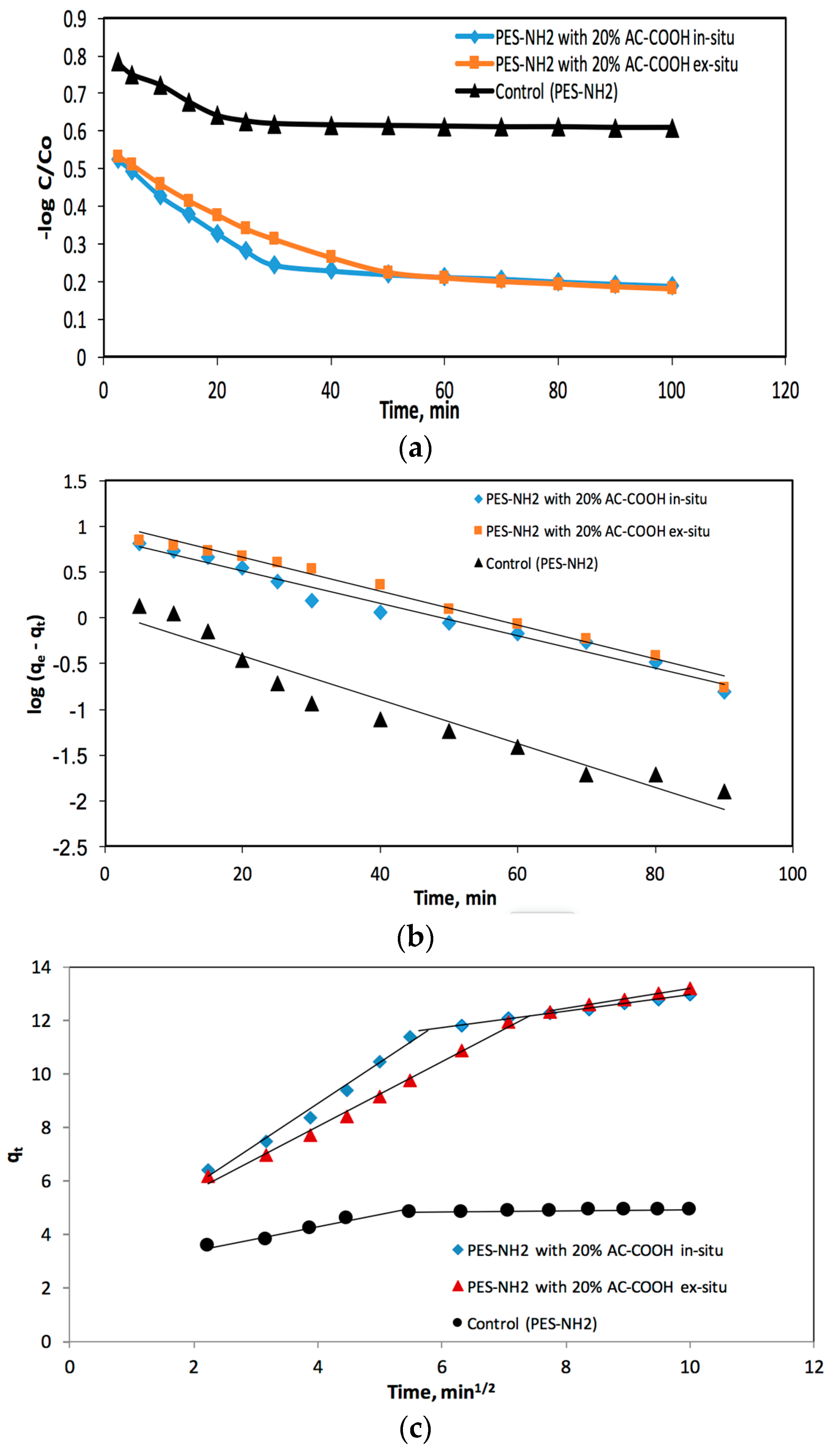
The acid red adsorbed process half-life time (
t1/2) by PES–NH
2 with the AC–COOH solid phase from the aquatic medium was determined by plotting log
C/
Co versus time as shown in
Figure 10a, in which
C and
Co are the red acid dye initial quantity before and after it was adsorbed, respectively. The value of
t1/2 was found to be 1.71 ± 0.07 min for PES–NH
2 in situ with 20% AC–COOH and 1.83 ± 0.06 for PES–NH
2 ex situ with 20% AC–COOH and 1.46 ± 0.04 for the control (PES–NH
2). This came in agreement with the values of
t1/2 reported recently [
17]. Consequently, the kinetics of acid red adsorption onto PES–NH
2 with AC–COOH adsorbent relies on both the intraparticle dispersal and film dispersal, in which the faster the factor is, the likelier it will be that the factor will govern the overall transport percentage.
The adsorption of acid red species on the PES–NH
2–AC–COOH solid phase was tested with a model developed by Weber and Morris [
18]:
qt is the acid red adsorption concentration in respect to time (
t), and
Rd refers to intra-particle transport rate values. The
qt versus time plot was presented in
Figure 10b. The values of
Rd were calculated from the two distinct slopes of the Weber–Morris plots (
Figure 10b). For PES–NH
2 in situ with 20% AC–COOH, the values were found to be about 1.536 and 0.307 mg/g while, for PES–NH
2 ex situ with 20% AC–COOH, the values were found to be about 1.207 and 0.421 mg/g, but for the control (PES–NH
2), the values were found to be about 0.424 and 0.025 mg/g, respectively.
The Lagergren equation is a well-known equation which defines the adsorption rate for aqueous phase systems. The variation of the acid red dye adsorption from liquid solutions onto PES–NH
2 with AC–COOH was also tested with a Lagergren equation [
19]:
The
qe stands for the acid red dye quantity that was adsorbed at equilibrium per sorbent mass, whereas
KLager stands for the first order total rate values for the retention mechanism, and t indicates the time. The log (
qe −
qt) versus the time plot in
Figure 10c was not a straight line and the calculated value of
KLager and
qe were found to be about 0.041 min
−1 and 7.43 mg/g for PES–NH
2 with 20% AC–COOH in situ, respectively, with a correlation coefficient of
R2 = 0.978, while the values for PES–NH
2 with 20% AC–COOH ex situ were found to equal 0.043 min
−1 and 10.68 mg/g, respectively, with a correlation coefficient of
R2 = 0.987. For the control (PES–NH
2), the values were about 0.055 min
−1 and 1.16 mg/g, respectively, with a correlation coefficient of
R2 = 0.937. All this data and the comparison between the calculated value of
qe with that measured experimentally (
qe, exp) confirmed that the first order kinetic model is not a suitable model to describe the adsorption of acid red species onto the used PES–NH
2 with AC–COOH sorbent.
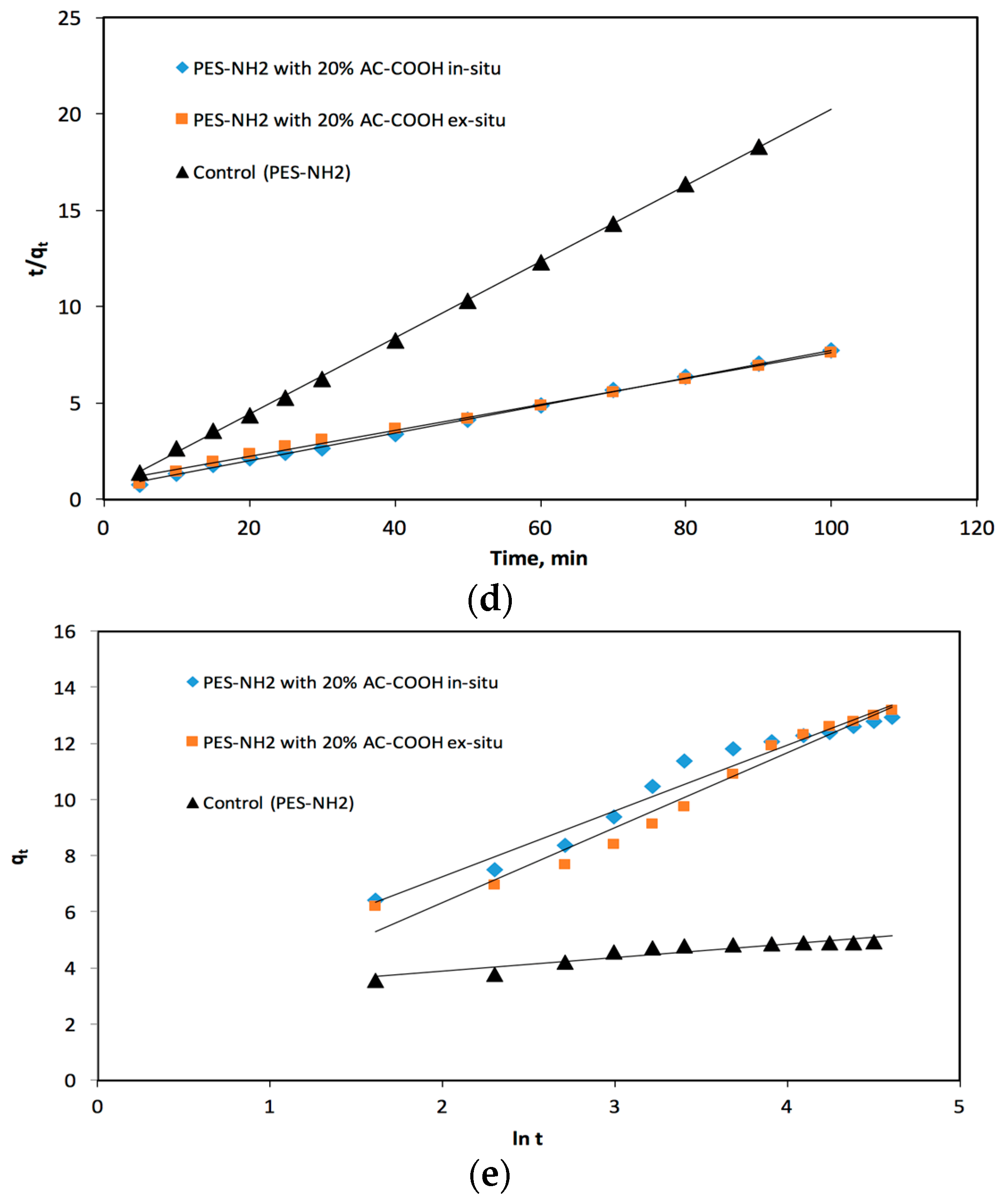
The pseudo-second-order model has been investigated as a Langmuir kinetics type [
19], taking into consideration two factors: the first is that the analyte concentration with respect to time is a constant and the second factor is that the binding sites rely on the analyte adsorbed quantity at equilibrium. The pseudo-second-order linear equation was stated as follows:
where
h is equal to
k2qe2 which refers to the initial adsorption level, whereas
qe and
qt are the quantity of adsorbed analyte per mass unit at any selected time at equilibrium. Under these conditions, the
versus the time (
t) plot was applied as shown in
Figure 10d, in which it was shown as linear. Then, from the slope and intercept, the second order constant (
k2) and equilibrium capacity (
qe) were obtained first for PES–NH
2 with 20% AC–COOH in situ and they were found to equal 8.84 × 10
−3 g·(mg·min)
−1 and 13.95 mg/g, respectively, with an excellent correlation (
R2 = 0.999), while for PES–NH
2 with 20% AC–COOH ex situ, they were found to be equal to 5.3 × 10
−3 g· (mg·min)
−1 and 14.79 mg/g, respectively, with an excellent correlation (
R2 = 0.995). For the control (PES–NH
2), the
k2 and
qe values were found to be equal to 8.8 × 10
−2 g·(mg·min)
−1 and 5.05 mg/g, respectively, with a superb correlation (
R2 =0.999). It is clearly seen from these reported data that all the experimental measured values were considered suitable values. The pseudo-second-order rate constant (
k2) values rely on different experimental factors including the initial PES–NH
2–AC–COOH concentration, the pH values, as well as the temperature [
19].
The adsorption capacity rate is usually determent using the Elovich equation [
19]. This equation is appropriate mostly for systems that show kinetics of chemisorptions and it can be also applied to a system in which the adsorbent surface area is heterogeneous. This equation can be calculated as follows:
The α value (g·mg
−1·min
−1) stands for the original adsorption rate value whereas the β value (mg·g
−1·min
−1) stands for the coefficient of desorption. The
qt against the ln
t plot was applied as shown
Figure 10e and the relationship between them was linear. The Elovich factors (the α as well as β values) were calculated from the intercepts and the slopes obtained from
Figure 10e. These values for the acid red dye were found to be about 1.12 g·mg
−1·min
−1 and 2.345 mg·g
−1·min
−1, respectively, adsorbed onto PES–NH
2 with 20% AC–COOH in situ, and they were found to be about 0.542 g·mg
−1·min
−1 and 2.671 mg·g
−1·min
−1, respectively, adsorbed onto PES–NH
2 with 20% AC–COOH ex situ. Meanwhile, the α, β values were found to equal 712.8 g·mg
−1·min
−1 and 0.493 mg·g
−1·min
−1, respectively adsorbed onto the control (PES–NH
2).
The above experimental data measured from different kinetic models (including the pseudo-first-order kinetic (Lagergren), the pseudo-second-order kinetic, as well as the Elovich kinetic) were applied and used in order to determine the kinetic behaviors of the acid red dye adsorption. From the comparisons between the experimental and calculated values of
qe as well as the values of the correlation coefficients that were all determined from these three models as summarized in
Table 5, it can be seen that the most suitable model for the kinetic behavior of the acid red dye that was adsorbed on PES–NH
2 with the AC–COOH solid phase was the pseudo-second-order kinetic model.
3.5. Thermodynamic Characteristics of Acid Red Retention onto the Solid Phase
The adsorption of acid red onto PES–NH
2 with the AC–COOH solid phase was investigated with various temperatures between 293 K and 338 K in order to find the acid red retention values of these polymers. The thermodynamic factors including Δ
H, Δ
S, and Δ
G were determined by utilizing the below equations [
20]:
The Δ
H stands for enthalpy, Δ
S refers to the entropy, and Δ
G defines the Gibbs free energy variations. T refers to the temperature in the Kelvin system, whereas the gas constant is abbreviated as
R (≈8.314 J·K
−1·mol
−1). The equilibrium constant (
Kc) relies on the adsorption mechanism for the fractional attainment which is abbreviated as Fe. The
Kc values were calculated in order to determine the acid red dye retention on the PES–NH
2 with the AC–COOH solid sorbent at equilibrium the below equation:
The ln
Kc versus 1000/T plot was applied for the retention of acid red dye onto the solid phase of PES–NH
2 with AC–COOH, in which the relationship was found to be linear as shown in
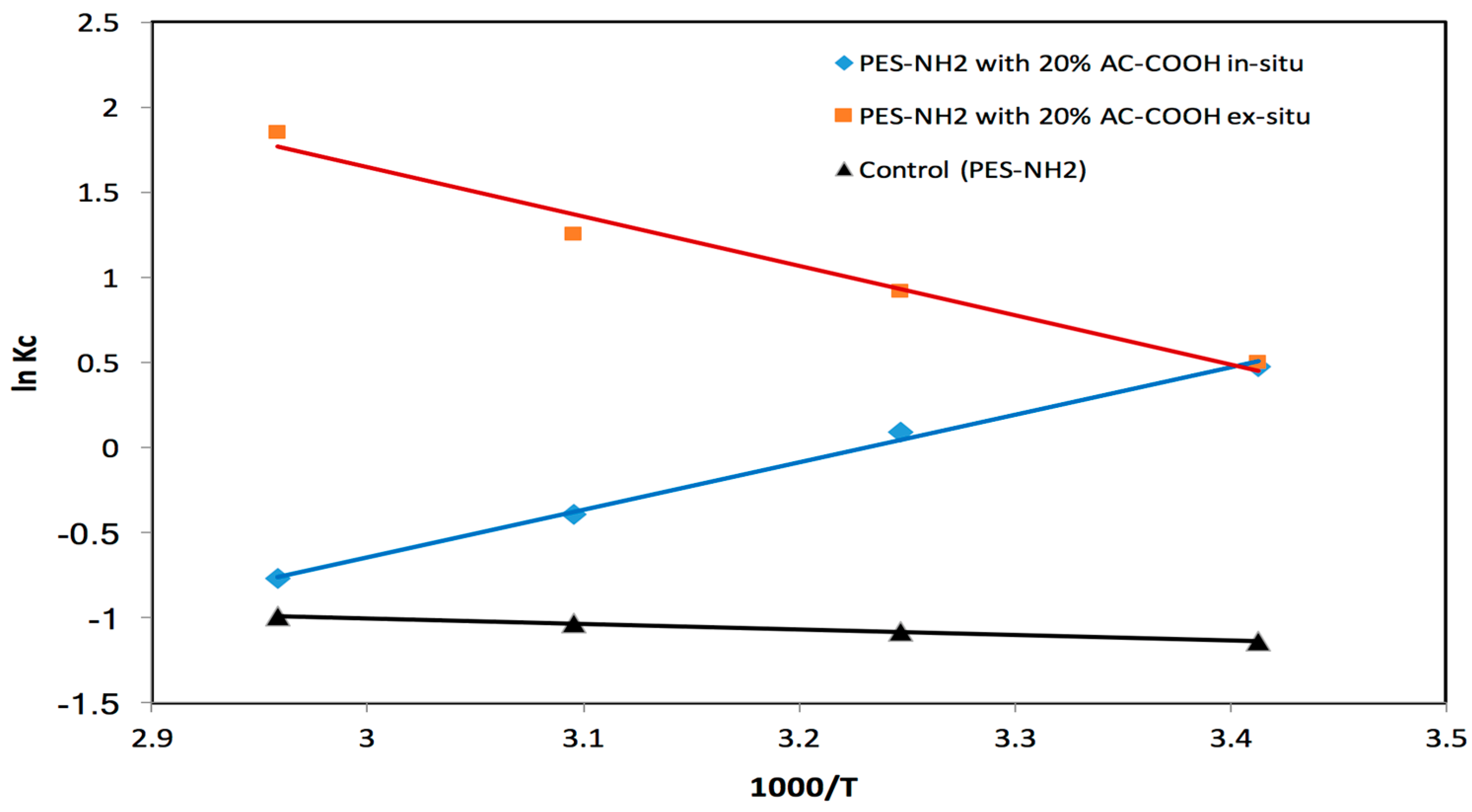
Figure 11 with various temperatures between 293 and 338 K. From
Figure 11, it can be seen that both the control (PES–NH
2) and the PES–NH
2 with 20% AC–COOH ex situ equilibrium constants increased when the temperature increased, which suggest that that the acid red dye retention on the solid phase sorbents is an endothermic mechanism. However, the PES–NH
2 with 20% AC–COOH using the in situ technique equilibrium constant decreased when the temperature increased which suggests that the acid red dye retention on the solid phase sorbents (PES–NH
2 in situ with 20% AC–COOH) is an exothermic mechanism. The Δ
H, Δ
S, and Δ
G values that were determined for acid red retention were determined from the ln
Kc versus 1000/T plot utilizing the slopes and intercepts as shown in
Figure 11. The Δ
H, Δ
S, and Δ
G values for control (PES–NH
2) were found to be around 2.78 ±0.1 kJ·mol
−1, 0.0025 ± 0.00003 J·mol
−1·K
−1, and 277 ± 0.02 kJ·mol
−1 (at 293 K), respectively, whereas for PES–NH
2 ex situ with 20% AC–COOH, they were found to be equal to 24.04 ± 0.5 kJ·mol
−1, 85.79 ± 0.8 J·mol
−1·K
−1, and −1.1 ± 0.06 kJ·mol
−1 (at 293 K), respectively. However, the values for PES–NH
2 with 20% AC–COOH in situ were found to be −23.16 ± 0.4 kJ·mol
−1, −74.85 ± 0.6 J·mol
−1·K
−1 and −1.23 ± 0.08 kJ·mol
−1 (at 293 K), correspondingly. The value of Δ
H for the control (PES–NH
2) and PES–NH
2 ex situ with 20% AC–COOH reveals that the uptake mechanism was endothermic, which exhibits the bond energy variations between the adsorbent solid phase and the analyte. However, the value of Δ
H for PES–NH
2 in situ with 20% AC–COOH reveals that the uptake mechanism was exothermic.
The ΔS positive values indicate there is an increase in the freedom degree at the solid-liquid system during the acid red binding, and this is because of the presence of water molecules that are released by hydration spheres occurred during the adsorption mechanism. On the other hand, the ΔS negative values indicate that the entropy (reorientation phase) is governed at the activation state and nonelectrostatics interactions between the analyte (acid red dye) and the adsorbed material (PES–NH2–AC–COOH). In addition, the ΔG negative values at a temperature of 293 K for both PES–NH2 with in situ 20% AC–COOH and PES–NH2 ex situ with 20% AC–COOH indicate that the interactions that form during the adsorption of acid red retention on PES–NH2 with AC–COOH solid phase were spontaneous. In contrast, the positive value of ΔG at 293 K for the control (PES–NH2) indicates that interactions that form during the adsorption of acid red retention on PES–NH2 solid phase were nonspontaneous.