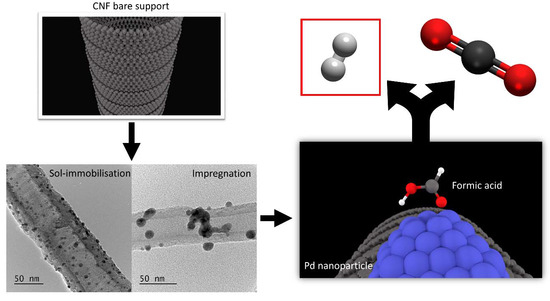
Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterisation of the Pd/CNF Series Catalysts
2.3. Catalytic Decomposition of Formic Acid and Analytical Methods
2.4. Product Analysis
2.5. Gas Analysis
3. Results
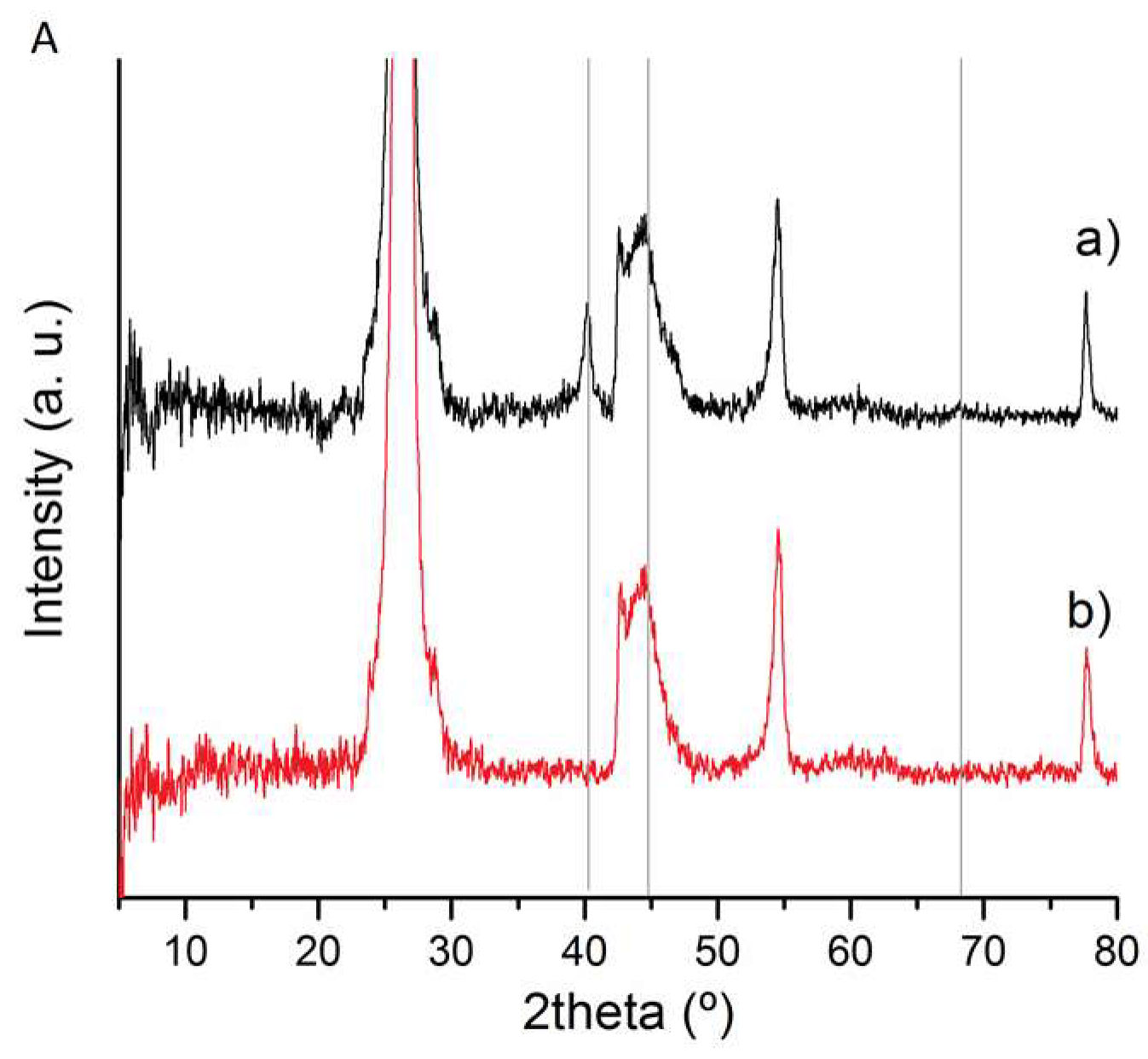
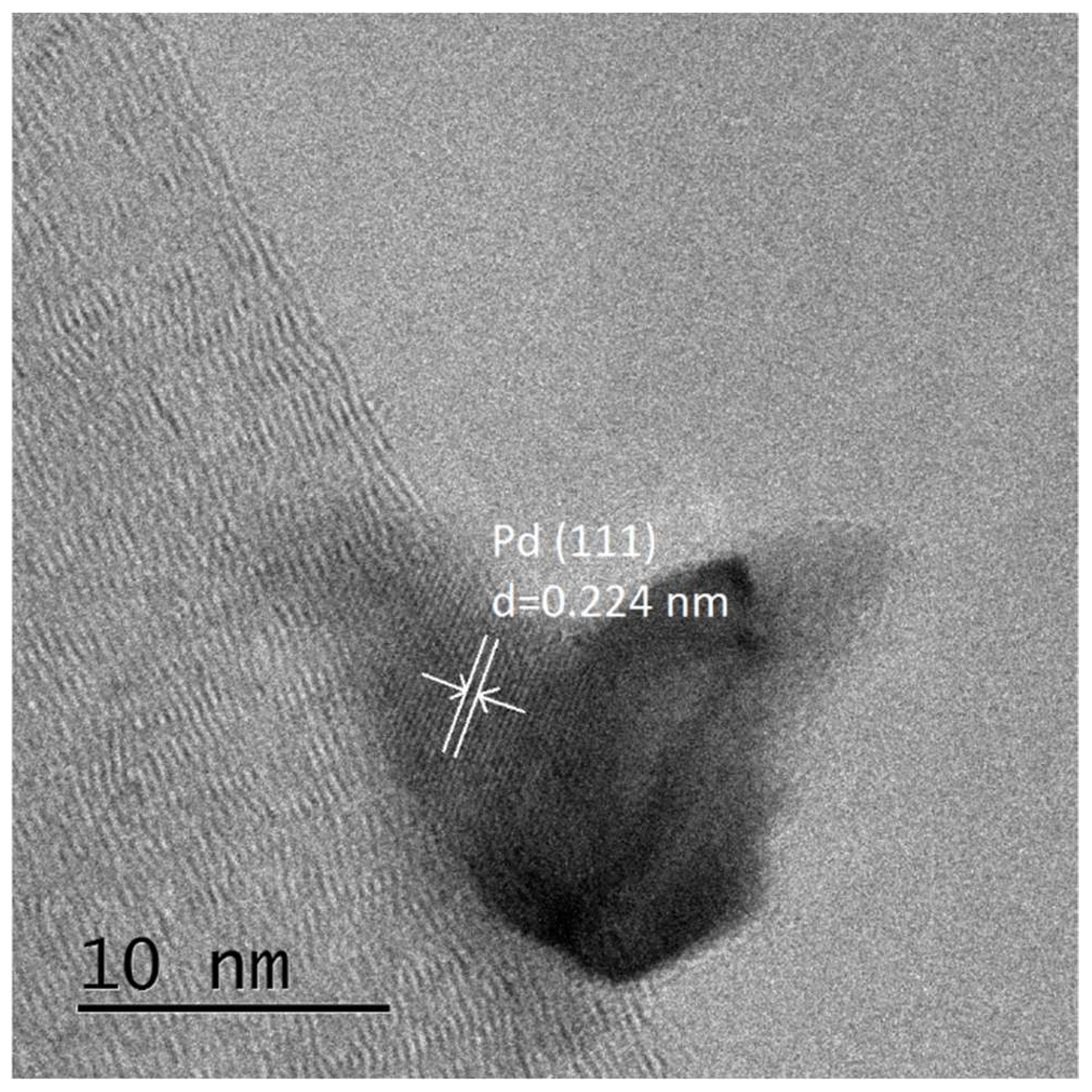
3.1. Catalysts Characterisation
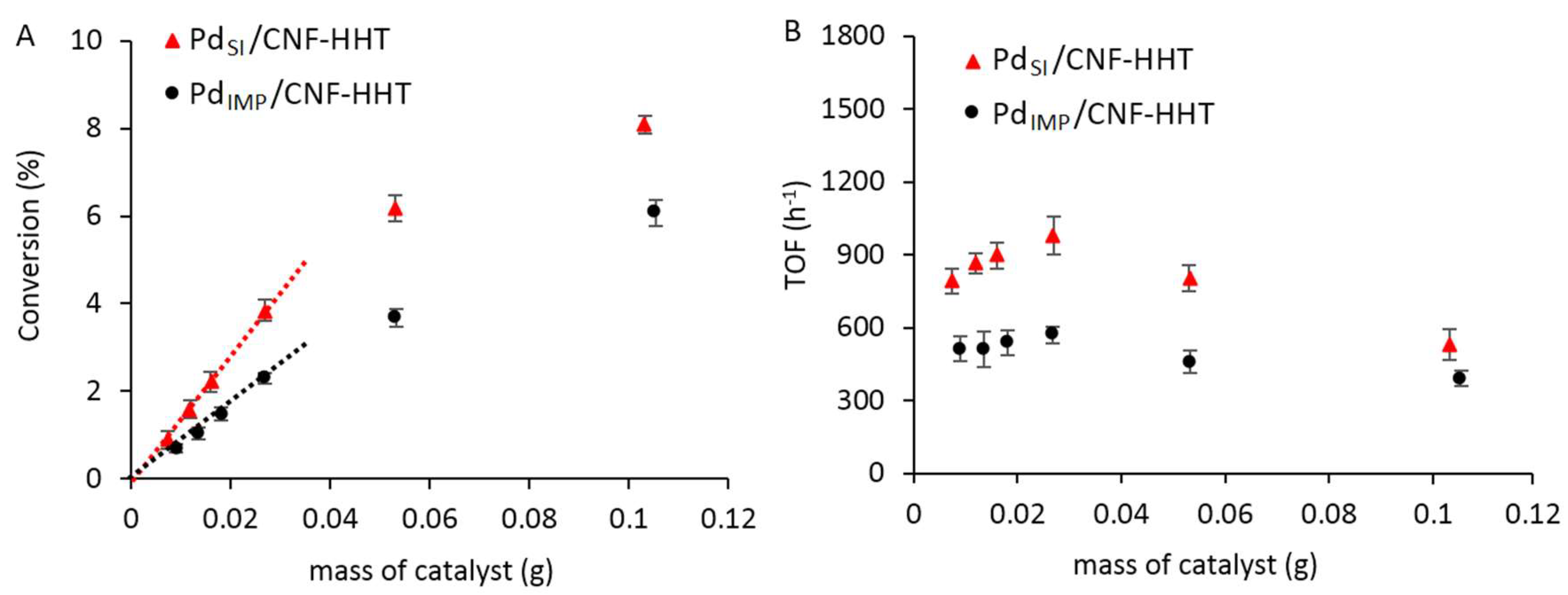
3.2. Catalytic Activity Comparison of the Pd/CNF Series Catalysts for the Formic Acid Dehydrogenation
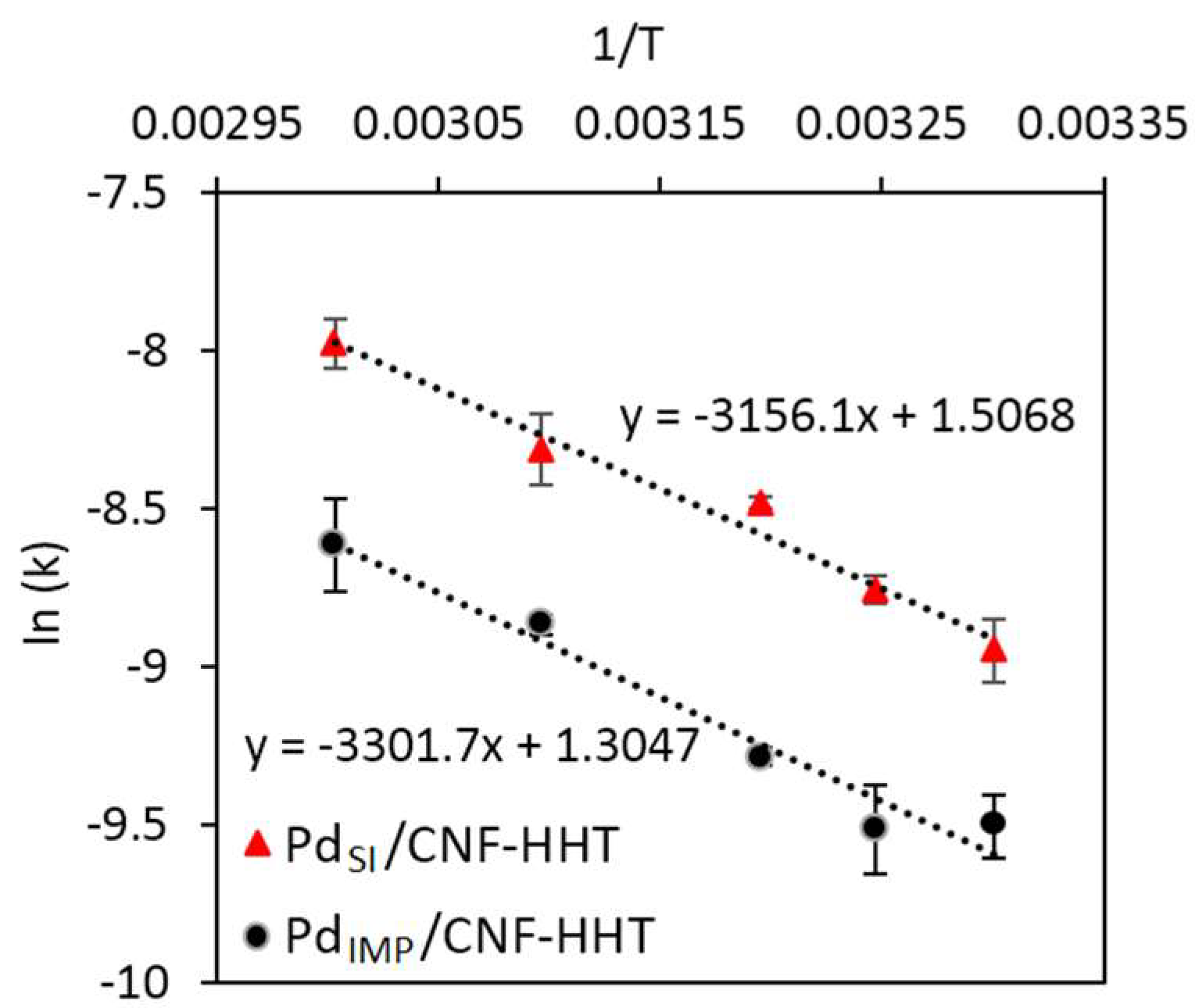
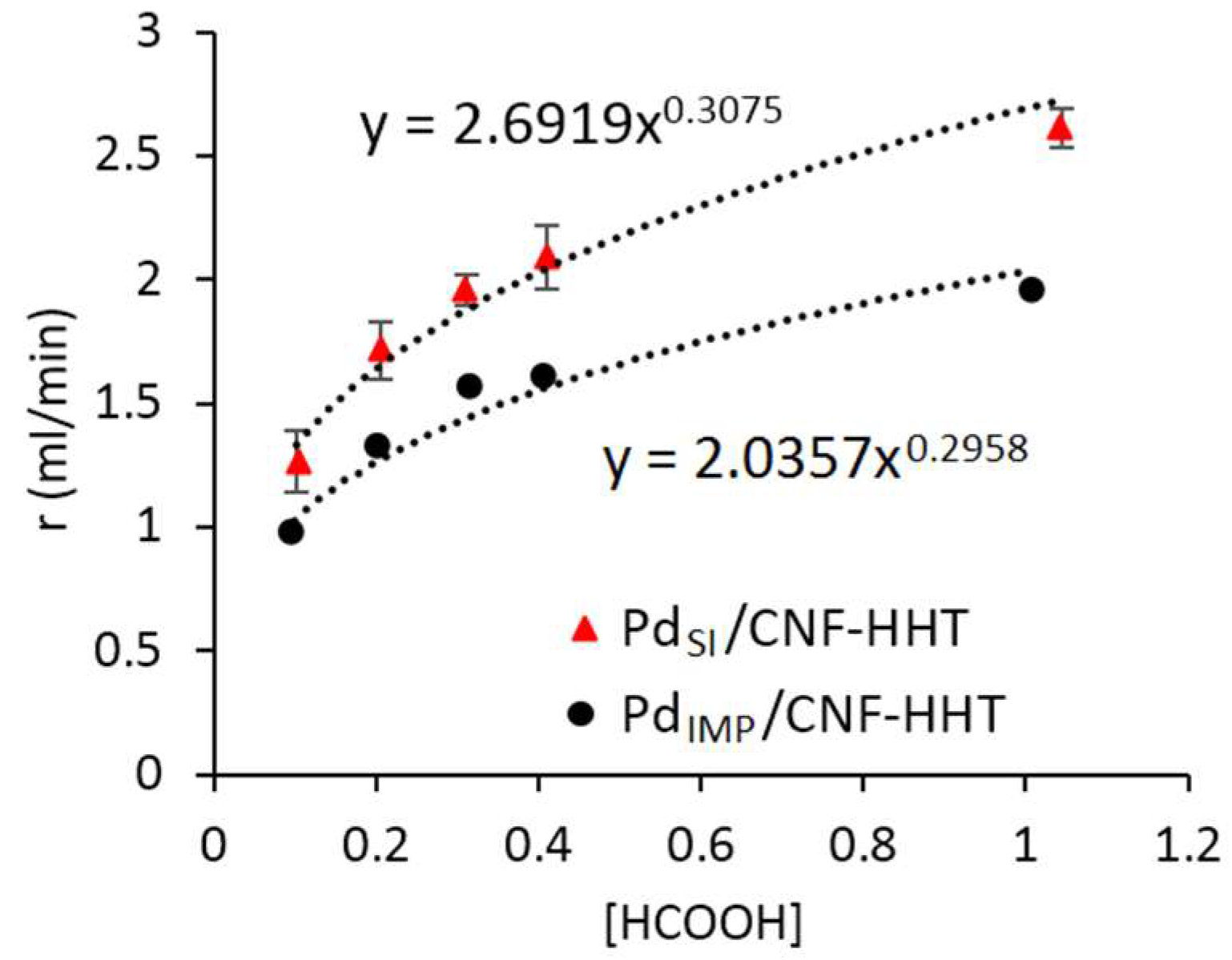
3.3. Kinetic Studies for Pd/CNF for Liquid Phase Formic Acid Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Veziroǧlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Mokaya, R. Enhanced hydrogen storage capacity of high surface area zeolite-like carbon materials. J. Am. Chem. Soc. 2007, 129, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Xia, B.; Wen, L.; Du, C.; Su, J.; Luo, W.; Cheng, G. Synergistic catalysis of AgPd@ZIF-8 on dehydrogenation of formic acid. Appl. Catal. B Environ. 2015, 165, 57–62. [Google Scholar] [CrossRef]
- Bae, D.; Park, H.; Kim, J.S.; Lee, J.B.; Kwon, O.Y.; Kim, K.Y.; Song, M.K.; No, K.T. Hydrogen adsorption in organic ion-exchanged zeolites. J. Phys. Chem. Solids 2008, 69, 1152–1154. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrogen storage in clathrate hydrates: Current state of the art and future directions. Appl. Energy 2014, 122, 112–132. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Hypercrosslinked polyanilines with nanoporous structure and high surface area: Potential adsorbents for hydrogen storage. J. Mater. Chem. 2007, 17, 4989–4997. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.-L.; Xu, Q. Highly active AuCo alloy nanoparticles encapsulated in the pores of metal-organic frameworks for hydrolytic dehydrogenation of ammonia borane. Catal. Sci. Technol. 2015, 5, 525–530. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; Hu, K.; Luo, W.; Cheng, G. Rh nanoparticles supported on graphene as efficient catalyst for hydrolytic dehydrogenation of amine boranes for chemical hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 1062–1070. [Google Scholar] [CrossRef]
- Manna, J.; Roy, B.; Sharma, P. Efficient hydrogen generation from sodium borohydride hydrolysis using silica sulfuric acid catalyst. J. Power Sources 2015, 275, 727–733. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Chemical hydrogen storage: “material” gravimetric capacity versus“system” gravimetric capacity. Energy Environ. Sci. 2011, 4, 3334–3341. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, R.E.; Trincado, M.; Vogt, M.; Tewes, F.; Santiso-Quinones, G.; Grützmacher, H. A homogeneous transition metal complex for clean hydrogen production from methanol–water mixtures. Nat. Chem. 2013, 5, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Cheng, R.; Chen, X.; Li, N.; Li, L.; Wang, X.; Zhang, T. A novel approach for CO-free H2 production via catalytic decomposition of hydrazine. Int. J. Hydrogen Energy 2005, 30, 1081–1089. [Google Scholar] [CrossRef]
- Metin, Ö.; Sun, X.; Sun, S. Monodisperse gold-palladium alloy nanoparticles and their composition-controlled catalysis in formic acid dehydrogenation under mild conditions. Nanoscale 2013, 5, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.; Iglesia, E. Formic acid dehydrogenation on au-based catalysts at near-ambient temperatures. Angew. Chem. Int. Ed. 2009, 48, 4800–4803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, Y.; Xing, W.; Liu, C.; Liao, J.; Lu, T. High-quality hydrogen from the catalyzed decomposition of formic acid by Pd-Au/C and Pd-Ag/C. Chem. Commun. 2008, 3540–3542. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hua, X.; Su, J.; Luo, W.; Chen, S.; Cheng, G. Highly efficient hydrogen generation from formic acid-sodium formate over monodisperse AgPd nanoparticles at room temperature. Appl. Catal. B Environ. 2015, 168–169, 423–428. [Google Scholar] [CrossRef]
- Lv, Q.; Feng, L.; Hu, C.; Liu, C.; Xing, W.; Wang, H.; Liu, Y.; Li, M.; Huang, H.; Xu, H.M.; et al. High-quality hydrogen generated from formic acid triggered by in situ prepared Pd/C catalyst for fuel cells. Catal. Sci. Technol. 2015, 5, 2581–2584. [Google Scholar] [CrossRef]
- Wang, X.; Qi, G.W.; Tan, C.H.; Li, Y.P.; Guo, J.; Pang, X.J.; Zhang, S.Y. Pd/C nanocatalyst with high turnover frequency for hydrogen generation from the formic acid-formate mixtures. Int. J. Hydrogen Energy 2014, 39, 837–843. [Google Scholar] [CrossRef]
- Hu, C.; Pulleri, J.K.; Ting, S.W.; Chan, K.Y. Activity of Pd/C for hydrogen generation in aqueous formic acid solution. Int. J. Hydrogen Energy 2014, 39, 381–390. [Google Scholar] [CrossRef]
- Navlani-García, M.; Martis, M.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Mori, K.; Yamashita, H. Investigation of Pd nanoparticles supported on zeolites for hydrogen production from formic acid dehydrogenation. Catal. Sci. Technol. 2015, 5, 364–371. [Google Scholar] [CrossRef]
- Mori, K.; Dojo, M.; Yamashita, H. Pd and Pd − Ag Nanoparticles within a Macroreticular Basic Resin: An Effi cient Catalyst for Hydrogen Production from Formic Acid Decomposition. ACS Catal. 2013, 3, 1114–1119. [Google Scholar] [CrossRef]
- Sponholz, P.; Mellmann, D.; Junge, H.; Beller, M. Towards a practical setup for hydrogen production from formic acid. ChemSusChem 2013, 6, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Cao, N.; Yang, L.; Su, J.; Luo, W.; Cheng, G. AgPd nanoparticles supported on MIL-101 as high performance catalysts for catalytic dehydrogenation of formic acid. J. Mater. Chem. A 2014, 2, 11060–11064. [Google Scholar] [CrossRef]
- Gao, S.-T.; Liu, W.; Feng, C.; Shang, N.-Z.; Wang, C. A Ag–Pd alloy supported on an amine-functionalized UiO-66 as an efficient synergetic catalyst for the dehydrogenation of formic acid at room temperature. Catal. Sci. Technol. 2016, 6, 869–874. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Dimitratos, N.; Su, D.; Trevisan, V.; Prati, L. Pd on carbon nanotubes for liquid phase alcohol oxidation. Catal. Today 2010, 150, 8–15. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Spontoni, P.; Arrigo, R.; Su, D.; Prati, L. Nitrogen functionalized carbon nanostructures supported Pd and Au-Pd NPs as catalyst for alcohols oxidation. Catal. Today 2010, 157, 89–93. [Google Scholar] [CrossRef]
- Chinthaginjala, J.K.; Villa, A.; Su, D.S.; Mojet, B.L.; Lefferts, L. Nitrite reduction over Pd supported CNFs: Metal particle size effect on selectivity. Catal. Today 2012, 183. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.L.; Chen, D.; Zhu, J.; Zhao, T.J.; Zhou, X.G. CNFs-supported Pt catalyst for hydrogen evolution from decalin. Catal. Commun. 2009, 10, 815–818. [Google Scholar] [CrossRef]
- Lazaro, M.P.; Garcia-Bordeje, E.; Sebastian, D.; Lazaro, M.J.; Moliner, R. In situ hydrogen generation from cycloalkanes using a Pt/CNF catalyst. Catal. Today 2008, 138, 203–209. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J. Mol. Catal. A Chem. 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Karousis, N.; Tsotsou, G.-E.; Evangelista, F.; Rudolf, P.; Ragoussis, N.; Tagmatarchis, N. Carbon Nanotubes Decorated with Palladium Nanoparticles: Synthesis, Characterization, and Catalytic Activity. J. Phys. Chem. C 2008, 112, 13463–13469. [Google Scholar] [CrossRef]
- Baek, J.B.; Lyons, C.B.; Tan, L.S. Grafting of vapor-grown carbon nanofibers via in-situ polycondensation of 3-Phenoxybenzoic acid in poly(phosphoric acid). Macromolecules 2004, 37, 8278–8285. [Google Scholar] [CrossRef]
- Patton, R.D.; Pittman, C.U.; Wang, L.; Hill, J.R. Vapor grown carbon fiber composites with epoxy and poly(phenylene sulfide) matrices. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1081–1091. [Google Scholar] [CrossRef]
- Finegan, I.C.; Tibbetts, G.G.; Glasgow, D.G.; Ting, J.-M.; Lake, M.L. Surface treatments for improving the mechanical properties of carbon nanofiber/thermoplastic. J. Mater. Sci. 2003, 38, 3485–3490. [Google Scholar] [CrossRef]
- Zacharska, M.; Bulusheva, L.G.; Lisitsyn, A.S.; Beloshapkin, S.; Guo, Y.; Chuvilin, A.L.; Shlyakhova, E.V.; Podyacheva, O.Y.; Leahy, J.J.; Okotrub, A.V.; et al. Factors Influencing the Performance of Pd/C Catalysts in the Green Production of Hydrogen from Formic Acid. ChemSusChem 2017, 10, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.-P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon N. Y. 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Pyrograf Products Inc. Available online: http://pyrografproducts.com/nanofiber.html (accessed on 16 January 2018).
- Huang, C.J.; Pan, F.M.; Tzeng, T.C.; Li, C.; Sheu, J.T. Growth and Field Emission of Reactive Sputtered Pd-PdO Core-Shell Nanoflakes on Platinum. J. Electrochem. Soc. 2009, 156, J28–J31. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Yan, J.-M.; Wang, H.-L.; Ping, Y.; Jiang, Q. Pd/C Synthesized with Citric Acid: An Efficient Catalyst for Hydrogen Generation from Formic Acid/Sodium Formate. Sci. Rep. 2012, 2, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Militello, M.C.; Simko, S.J. Elemental Palladium by XPS. Surf. Sci. Spectra 1994, 3, 387–394. [Google Scholar] [CrossRef]
- Militello, M.C. Palladium Oxide (PdO) by XPS. Surf. Sci. Spectra 1994, 3, 395. [Google Scholar] [CrossRef]
- Pattamakomsan, K.; Suriye, K.; Dokjampa, S.; Mongkolsiri, N. Effect of mixed Al2O3 structure between h-and a-Al2O3 on the properties of Pd/Al2O3 in the selective hydrogenation of 1,3-butadiene. Catal. Commun. 2010, 11, 311–316. [Google Scholar] [CrossRef]
- Lara, L.R.S.; Zottis, A.D.; Elias, W.C.; Faggion, D.; de Campos, C.E.M.; Acuña, J.J.S.; Domingos, J.B. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Chan-Thaw, C.E.; Campisi, S.; Veith, G.M.; Prati, L. The confinement effect on the activity of Au NPs in polyol oxidation. Catal. Sci. Technol. 2016, 6, 598–601. [Google Scholar] [CrossRef]
- Sánchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen generation from additive-free formic acid decomposition under mild conditions by Pd/C: Experimental and DFT studies. Top. Catal. 2018, 61, 254–266. [Google Scholar] [CrossRef]
- Li, S.J.; Zhou, X.; Tian, W.Q. Theoretical mechanism investigations on the HCOOH catalyzed by Pd cluster. J. Phys. Chem. A 2012, 116, 11745–11752. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Q.; Bai, R.; Li, X.; Guo, G.; Yu, J. In Situ Confinement of Ultrasmall Pd Clusters within Nanosized Silicalite-1 Zeolite for Highly Efficient Catalysis of Hydrogen Generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Z.Y.; Wang, X.C.; Zeng, J.; Li, Y.M. DFT study of hydrogen production from formic acid decomposition on Pd-Au alloy nanoclusters. Appl. Surf. Sci. 2017, 426, 194–205. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Bulusheva, L.G.; Beloshapkin, S.; O’Connor, T.; Okotrub, A.V.; Ryan, K.M. Pd clusters supported on amorphous, low-porosity carbon spheres for hydrogen production from formic acid. ACS Appl. Mater. Interfaces 2015, 7, 8719–8726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Metin, O.; Su, D.; Sun, S. Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew. Chem. Int. Ed. 2013, 52, 3681–3684. [Google Scholar] [CrossRef] [PubMed]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Zahmakiran, M.; Kivrak, H.; Gulcan, M.; Kaya, M. Pd-MnOx nanoparticles dispersed on amine-grafted silica: Highly efficient nanocatalyst for hydrogen production from additive-free dehydrogenation of formic acid under mild conditions. Appl. Catal. B Environ. 2015, 164, 324–333. [Google Scholar] [CrossRef]
- Ho, S.F.; Mendoza-Garcia, A.; Guo, S.; He, K.; Su, D.; Liu, S.; Metin, O.; Sun, S. A facile route to monodisperse MPd (M = Co or Cu) alloy nanoparticles and their catalysis for electrooxidation of formic acid. Nano Lett. 2012, 12, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Tsumori, N.; Xu, Q. Sodium hydroxide-assisted growth of uniform Pd nanoparticles on nanoporous carbon MSC-30 for efficient and complete dehydrogenation of formic acid under ambient conditions. Chem. Sci. 2014, 5, 195–199. [Google Scholar] [CrossRef]




| Catalyst | Pd3d5/2 | Pd Content (at. %) | O Content (at. %) | ||
|---|---|---|---|---|---|
| Pd0 | Pd2+ | ||||
| PdIMP/CNF | BE | 334.9 | 336.5 | 0.25 | 0.94 |
| % | 25.9 | 74.1 | |||
| PdSI/CNF | BE | 335.0 | 336.4 | 0.77 | 2.72 |
| % | 61.5 | 38.5 | |||
| PdSI/AC | BE | 335.6 | 337.2 | 3.68 | 24.51 |
| % | 74.2 | 25.8 | |||
| Catalyst | Statistical Median (nm) | Standard Deviation |
|---|---|---|
| PdIMP/CNF | 5.4 | 0.9 |
| PdSI/CNF | 3.9 | 1.2 |
| PdSI/AC | 3.7 | 0.9 |
| Catalyst | Pd Loading (wt. %) | |
|---|---|---|
| EDX | AAS | |
| PdIMP/CNF | 1.03 | 0.99 |
| PdSI/CNF | 0.91 | 0.95 |
| PdSI/AC | 1.15 | 1.10 |
| Catalyst | T (°C) | Reagent | TOF (h−1) | Activation Energy (KJ/mol) | Ref. | |
|---|---|---|---|---|---|---|
| Initial | 2 h | |||||
| PdIMP/CNF | 30 | Formic acid (0.5 M) | 563.2 | 27.5 | This work | |
| PdSI/CNF | 30 | 979.1 | 26.2 | |||
| PdSI/AC | 30 | 240.5 | ||||
| Pd/C | 21 | Formic acid (1.33 M) | 18 | 15 a | 53.7 | [21] |
| 30 | 48 | 28 a | ||||
| Pd/C (citric acid) | 25 | Formic acid | 64 b | [41] | ||
| Pd/C | 30 | Formic acid:Sodium formate 1:9 | 228.3 | [20] | ||
| Au41Pd59/C | 50 | Formic acid (1 M) | 230 | 28 ± 2 | [13] | |
| Ag@Pd (1:1) | 35 | Formic acid | 156 c | 30 | [15] | |
| 50 | 252 c | |||||
| Ag/Pd alloy (1:1) | 20 | 144 c | ||||
| Ag42Pd58 | 50 | Formic acid (1 M) | 382 | 22 ± 1 | [52] | |
| Pd-MnOx/SiO2-NH2 | 20 | Formic acid (0.265 M) | 140 | 61.9 | [53] | |
| 50 | 1300 | |||||
| Ag0.1Pd0.9/rGO | 25 | Formic acid | 105 | [54] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, F.; Motta, D.; Bocelli, L.; Albonetti, S.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition. C 2018, 4, 26. https://doi.org/10.3390/c4020026
Sanchez F, Motta D, Bocelli L, Albonetti S, Roldan A, Hammond C, Villa A, Dimitratos N. Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition. C. 2018; 4(2):26. https://doi.org/10.3390/c4020026
Chicago/Turabian StyleSanchez, Felipe, Davide Motta, Ludovica Bocelli, Stefania Albonetti, Alberto Roldan, Ceri Hammond, Alberto Villa, and Nikolaos Dimitratos. 2018. "Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition" C 4, no. 2: 26. https://doi.org/10.3390/c4020026
APA StyleSanchez, F., Motta, D., Bocelli, L., Albonetti, S., Roldan, A., Hammond, C., Villa, A., & Dimitratos, N. (2018). Investigation of the Catalytic Performance of Pd/CNFs for Hydrogen Evolution from Additive-Free Formic Acid Decomposition. C, 4(2), 26. https://doi.org/10.3390/c4020026