The Role of Carbon on Copper–Carbon Composites for the Electrooxidation of Alcohols in an Alkaline Medium
Abstract
1. Introduction
2. Results and Discussion
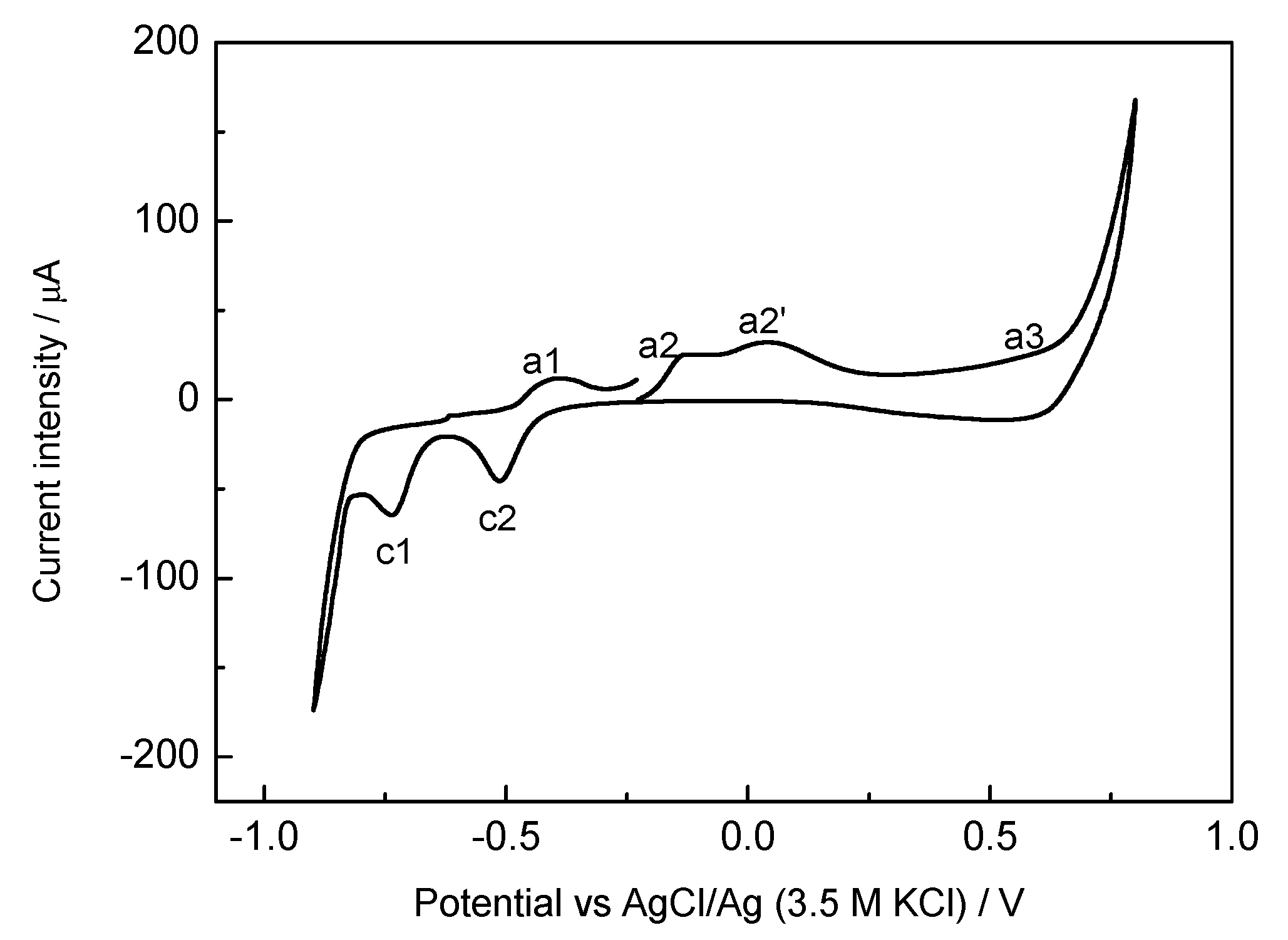
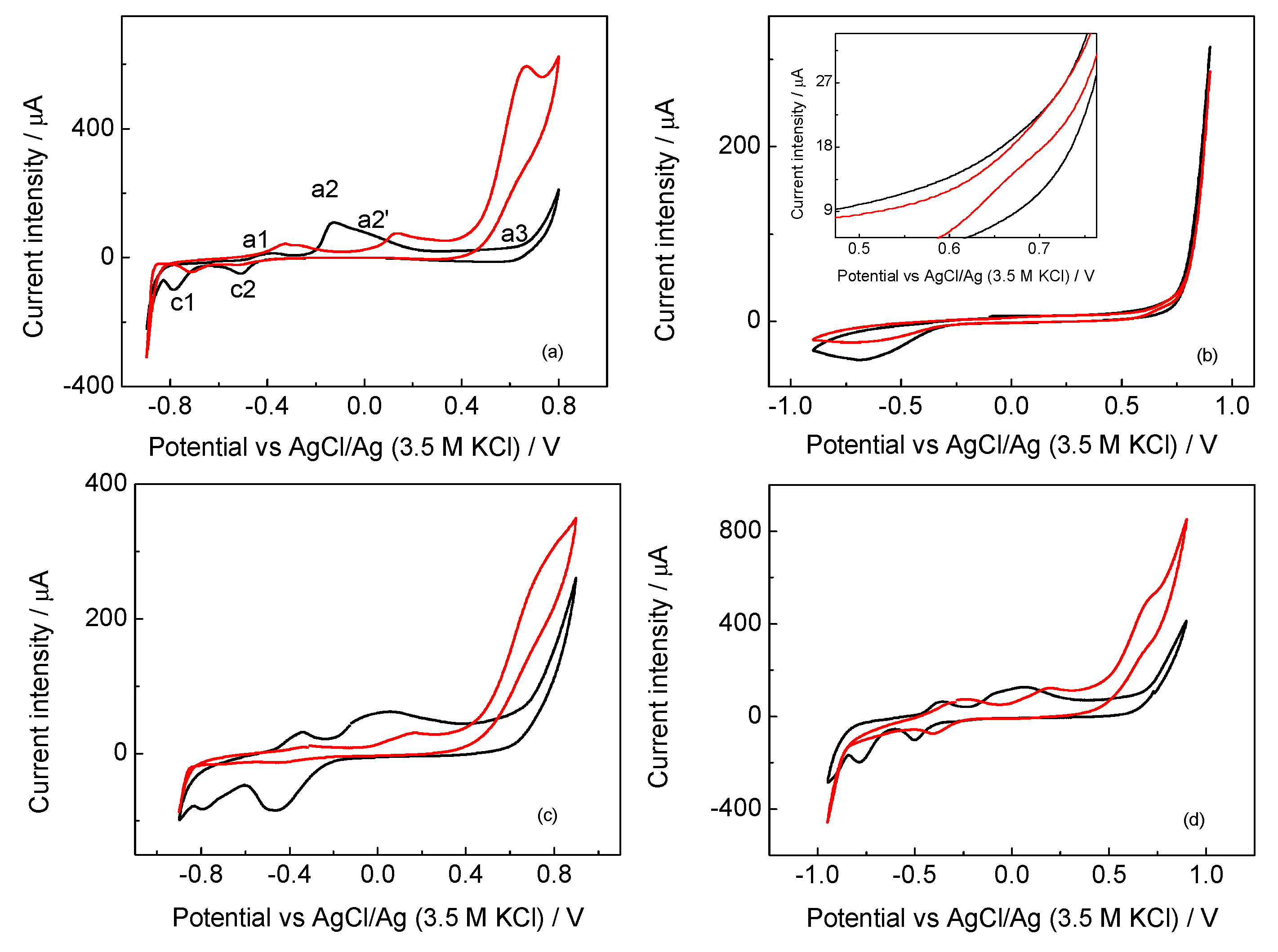
2.1. Electrochemical Characterization of the Prepared Materials
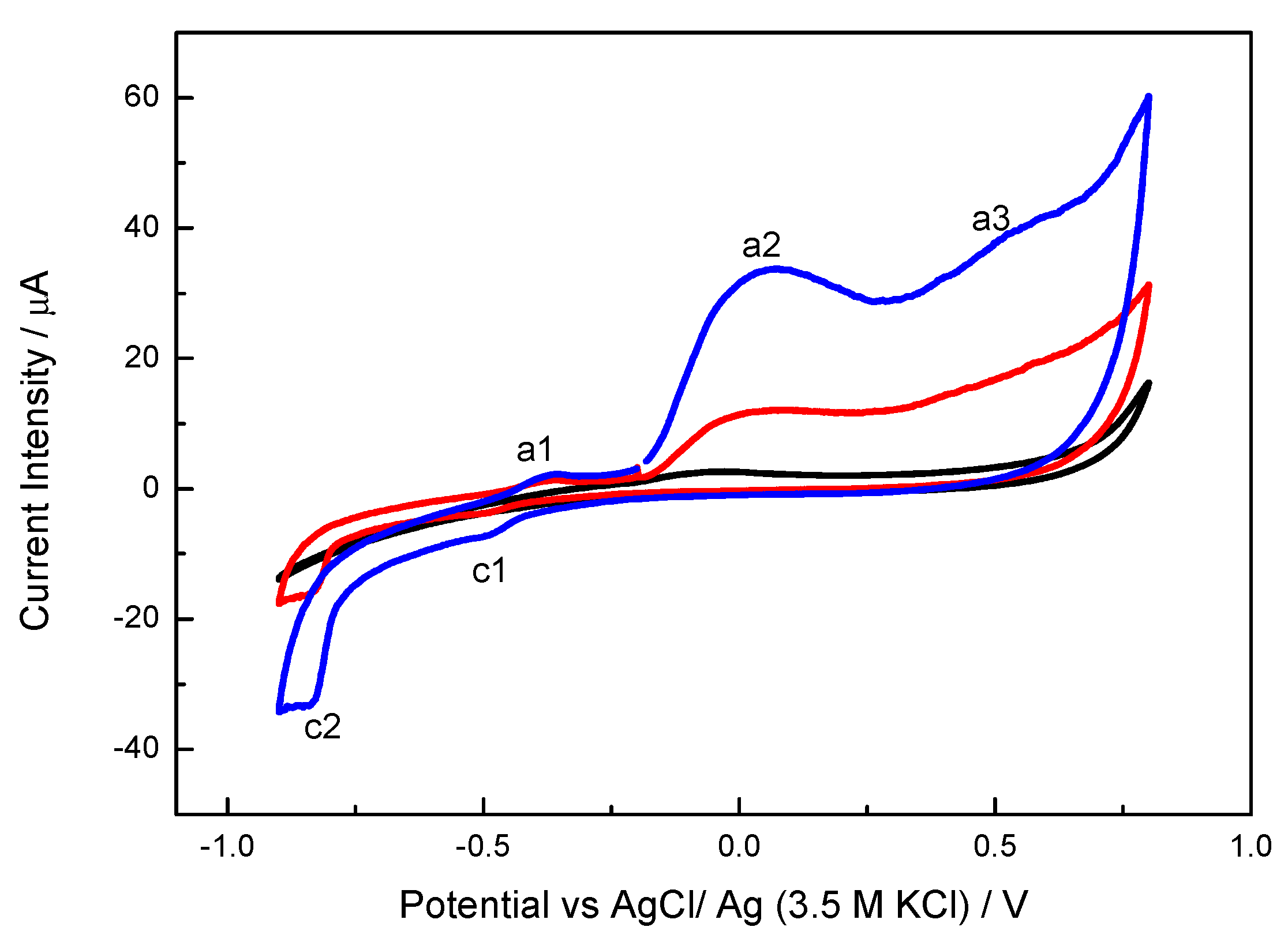
2.2. Electrocatalytic Oxidation of Propargylic Alcohol
2.3. Effect of the Ionomer
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Carbons
3.3. Preparation of the Anodes
3.4. Characterization Techniques
3.5. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Zamani, P.; Yu, A.P.; Chen, Z.W. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z.W. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Chen, Z.W.; Higgins, D.; Yu, A.P.; Zhang, L.; Zhang, J.J. A review on non-precious metal electrocatalysts for pem fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Ozoemena, K.I. Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: Catalyst design, principles and applications. RSC Adv. 2016, 6, 89523–89550. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Electrochemical study of bulk and monolayer copper in alkaline solution. J. Electrochem. Soc. 2016, 163, I1252–I1259. [Google Scholar] [CrossRef]
- Miller, B. Split-ring disk study of anodic processes at a copper electrode in alkaline solution. J. Electrochem. Soc. 1969, 116, 1675–1680. [Google Scholar] [CrossRef]
- Dechialvo, M.R.G.; Marchiano, S.L.; Arvia, A.J. The mechanism of oxidation of copper in alkaline-solutions. J. Appl. Electrochem. 1984, 14, 165–175. [Google Scholar] [CrossRef]
- Ambrose, J.; Barradas, R.G.; Shoesmit, D.W. Rotating copper disk electrode studies of mechanism of dissolution-passivation step on copper in alkaline solutions. J. Electroanal. Chem. 1973, 47, 65–80. [Google Scholar] [CrossRef]
- Ambrose, J.; Shoesmit, D.W. Investigations of copper in aqueous alkaline solutions by cyclic voltammetry. J. Electroanal. Chem. 1973, 47, 47–64. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J. A simple route of modifying copper electrodes for the determination of methanol and ethylene glycol. Electroanalysis 2012, 24, 1639–1645. [Google Scholar] [CrossRef]
- Luo, M.Z.; Baldwin, R.P. Characterization of carbohydrate oxidation at copper electrodes. J. Electroanal. Chem. 1995, 387, 87–94. [Google Scholar] [CrossRef]
- Gao, X.H.; Du, C.; Zhang, C.M.; Chen, W. Copper nanoclusters on carbon supports for the electrochemical oxidation and detection of hydrazine. ChemElectroChem 2016, 3, 1266–1272. [Google Scholar] [CrossRef]
- Priya, S.; Berchmans, S. Cuo microspheres modified glassy carbon electrodes as sensor materials and fuel cell catalysts. J. Electrochem. Soc. 2012, 159, F73–F80. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karim-Nezhad, G.; Mahjani, M.G.; Jafarian, M.; Shadjou, N.; Khalilzadeh, B.; Saghatforoush, L.A. A study of the electrocatalytic oxidation of cyclohexanol on copper electrode. Catal. Commun. 2008, 10, 295–299. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Saghatforoush, L.; Khalilzadeh, B.; Kazeman, I. Electro-oxidation of cyclohexanol on a copper electrode modified by copper-dimethylglyoxime complex formed by electrochemical synthesis. Bull. Korean Chem. Soc. 2009, 30, 2943–2948. [Google Scholar] [CrossRef]
- Heli, H.; Jafarian, M.; Mahjani, M.G.; Gobal, F. Electro-oxidation of methanol on copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Martin, C.; Huser, H.; Servat, K.; Kokoh, K.B. Electrosynthesis of lactic acid on copper and lead cathodes in aqueous media. Electrochim. Acta 2005, 51, 111–117. [Google Scholar] [CrossRef]
- Li, T.T.; Cao, S.; Yang, C.; Chen, Y.; Lv, X.J.; Fu, W.F. Electrochemical water oxidation by in situ-generated copper oxide film from cu(teoa)(H2O)(2) SO4 complex. Inorg. Chem. 2015, 54, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, H.F.; Sun, Z.J.; Han, A.; Du, P.W. Earth-abundant copper-based bifunctional electrocatalyst for both catalytic hydrogen production and water oxidation. ACS Catal. 2015, 5, 1530–1538. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.S.; Sun, Z.J.; Du, P.W. Robust and highly active copper-based electrocatalyst for hydrogen production at low overpotential in neutral water. Chem. Commun. 2015, 51, 12954–12957. [Google Scholar] [CrossRef] [PubMed]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of co2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M.T.M.; Mul, G.; Baltrusaitis, J. Electrochemical CO2 reduction on Cu2o-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 2014, 16, 12194–12201. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.I.; Malaibari, Z.O.; Atieh, M.; Abussaud, B. Electrochemical reduction of CO2 to methanol over mwcnts impregnated with Cu2o. Chem. Eng. Sci. 2016, 152, 468–477. [Google Scholar] [CrossRef]
- Jia, F.L.; Yu, X.X.; Zhang, L.Z. Enhanced selectivity for the electrochemical reduction of co2 to alcohols in aqueous solution with nanostructured cu-au alloy as catalyst. J. Power Sources 2014, 252, 85–89. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Selective formation of C2 compounds from electrochemical reduction of co2 at a series of copper single crystal electrodes. J. Phys. Chem. B 2002, 106, 15–17. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local ph. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Reske, R.; Mistry, H.; Behafarid, F.; Roldan Cuenya, B.; Strasser, P. Particle size effects in the catalytic electroreduction of co2 on cu nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Q.; Huber, C.O. Electrocatalysis and amperometric detection using an electrode made of copper-oxide and carbon paste. Anal. Chem. 1991, 63, 1714–1719. [Google Scholar] [CrossRef]
- Jafarian, M.; Avei, M.R.; Danaee, I.; Gobal, F.; Mahjani, M.G. Electrochemical oxidation of saccharose on copper (hydr)oxide-modified electrode in alkaline media. Chin. J. Catal. 2010, 31, 1351–1357. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Soggia, F.; Zanicchi, G.; Scavino, G. Influence of rare earth metals addition on the corrosion behaviour of copper in alkaline environment. Corros. Sci. 2012, 58, 139–144. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Malik, R.; Alvarez, N.; White, L.; Shanov, V.N.; Schulz, M.; Collins, B.; Sankar, J.; Yun, Y. Aligned carbon nanotube/copper sheets: A new electrocatalyst for co2 reduction to hydrocarbons. RSC Adv. 2014, 4, 16362–16367. [Google Scholar] [CrossRef]
- Liu, C.B.; Zhang, H.; Tang, Y.H.; Luo, S.L. Controllable growth of graphene/cu composite and its nanoarchitecture-dependent electrocatalytic activity to hydrazine oxidation. J. Mater. Chem. A 2014, 2, 4580–4587. [Google Scholar] [CrossRef]
- Heli, H.; Jabbari, A.; Zarghan, M.; Moosavi-Movahedi, A.A. Copper nanoparticles–carbon microparticles nanocomposite for electrooxidation and sensitive detection of sotalol. Sens. Actuators B Chem. 2009, 140, 245–251. [Google Scholar] [CrossRef]
- Ania, C.O.; Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. New copper/go based material as an efficient oxygen reduction catalyst in an alkaline medium: The role of unique cu/rgo architecture. Appl. Catal. B Environ. 2015, 163, 424–435. [Google Scholar] [CrossRef]
- Garcia-Cruz, L.; Saez, A.; Ania, C.O.; Solla-Gullon, J.; Thiemann, T.; Iniesta, J.; Montiel, V. Electrocatalytic activity of ni-doped nanoporous carbons in the electrooxidation of propargyl alcohol. Carbon 2014, 73, 291–302. [Google Scholar] [CrossRef]
- Yan, X.Y.; Tong, X.L.; Zhang, Y.F.; Han, X.D.; Wang, Y.Y.; Jin, G.Q.; Qin, Y.; Guo, X.Y. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 2012, 48, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- El Din, A.M.S.; El Wahab, F.M.A. The behaviour of the copper electrode in alkaline solutions upon alternate anodic and cathodic polarization. Electrochim. Acta 1964, 9, 113–121. [Google Scholar] [CrossRef]
- Lorimer, J.P.; Mason, T.J.; Plattes, M.; Walton, D.J. Passivation phenomena during sonovoltammetric studies on copper in strongly alkaline solutions. J. Electroanal. Chem. 2004, 568, 379–390. [Google Scholar] [CrossRef]
- Marsh, H.; RodriguezReinoso, F. Activated Carbon; Elsevier Science Bv: Amsterdam, The Netherlands, 2006; pp. 1–536. [Google Scholar]
- Garcia-Cruz, L.; Iniesta, J.; Thiemann, T.; Montiel, V. Surprising electrooxidation of propargyl alcohol to (z)-3-(2-propynoxy)-2-propenoic acid at a niooh electrode in alkaline medium. Electrochem. Commun. 2012, 22, 200–202. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Alkaline direct alcohol fuel cells using an anion exchange membrane. J. Power Sources 2005, 150, 27–31. [Google Scholar] [CrossRef]
- Garcia-Cruz, L.; Casado-Coterillo, C.; Irabien, A.; Montiel, V.; Iniesta, J. Performance assessment of a polymer electrolyte membrane electrochemical reactor under alkaline conditions—A case study with the electrooxidation of alcohols. Electrochim. Acta 2016, 206, 165–175. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material Cu-3(tma)(2)(H2O)(3) (n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal-organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]

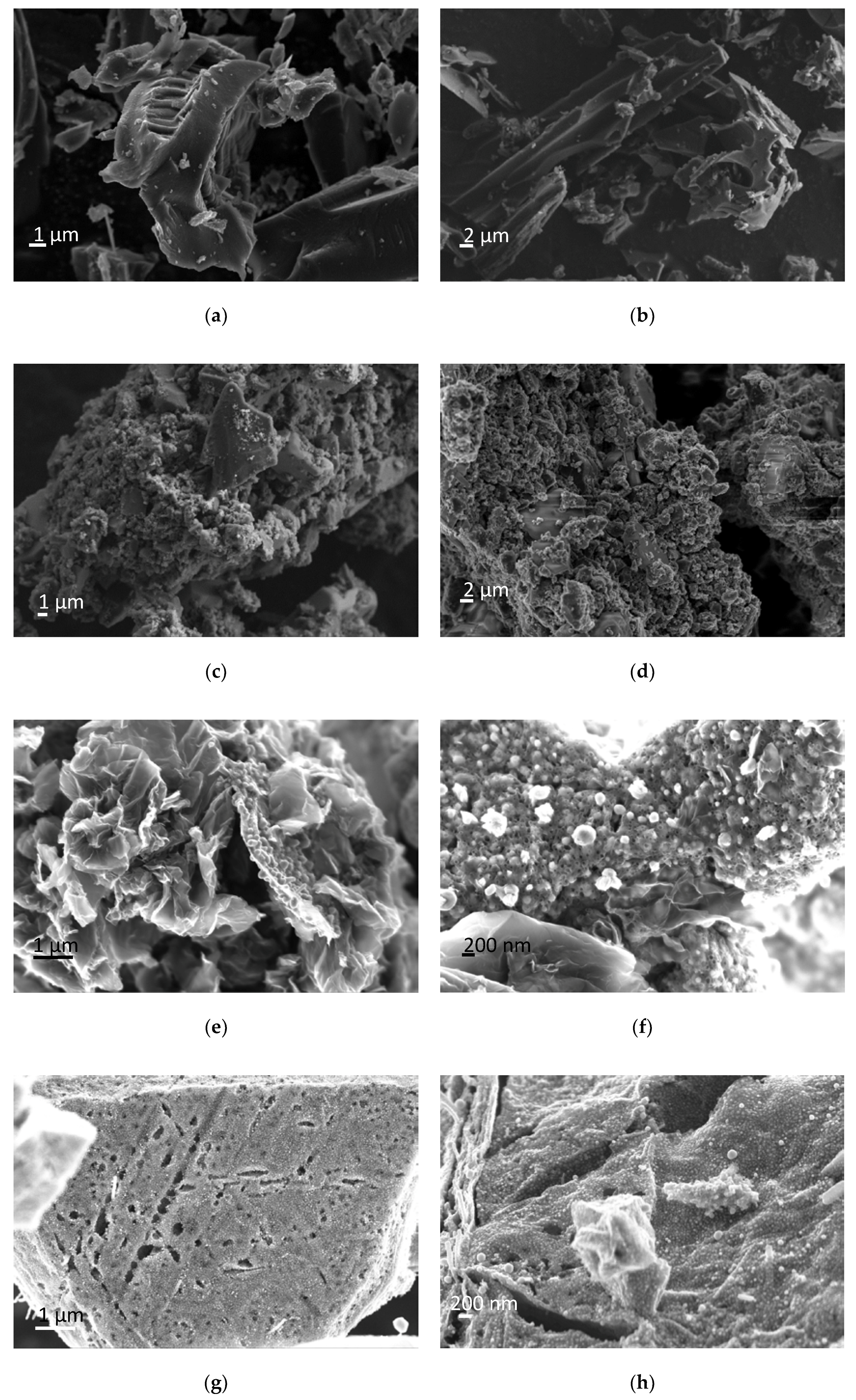

| Sample | SBET (m2/g) | VPORES A (cm3/g) | VMIC B (cm3/g) | pH |
|---|---|---|---|---|
| S | 970 | 0.45 | 0.41 | 8.3 |
| SCu | 26 | 0.07 | 0.01 | 8.2 |
| CMCu | 117 | 0.14 | 0.06 | 8.2 |
| CMCuGr | 53 | 0.10 | 0.03 | 7.7 |
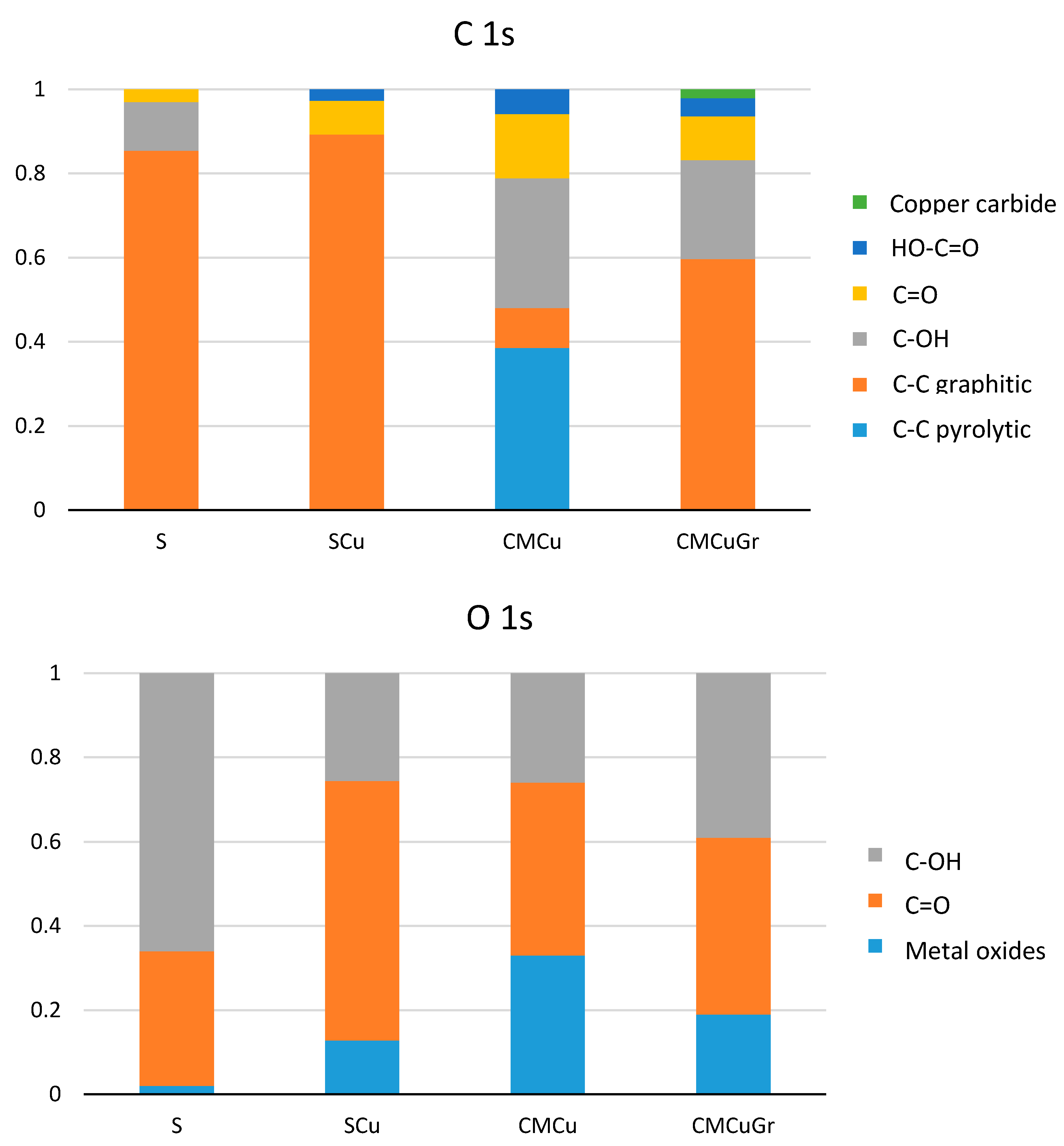
| Sample | C 1s | O 1s | Cu 2p |
|---|---|---|---|
| S | 87.0 | 13.4 | n.d. |
| SCu | 69.4 | 12.6 | 18.5 |
| CMCu | 90.86 | 7.40 | 1.75 |
| CMCuGr | 94.65 | 4.58 | 0.77 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Cruz, L.; Ania, C.O.; Carvalho, A.P.; Bandosz, T.J.; Montiel, V.; Iniesta, J. The Role of Carbon on Copper–Carbon Composites for the Electrooxidation of Alcohols in an Alkaline Medium. C 2017, 3, 36. https://doi.org/10.3390/c3040036
García-Cruz L, Ania CO, Carvalho AP, Bandosz TJ, Montiel V, Iniesta J. The Role of Carbon on Copper–Carbon Composites for the Electrooxidation of Alcohols in an Alkaline Medium. C. 2017; 3(4):36. https://doi.org/10.3390/c3040036
Chicago/Turabian StyleGarcía-Cruz, Leticia, Conchi O. Ania, Ana Paula Carvalho, Teresa J. Bandosz, Vicente Montiel, and Jesús Iniesta. 2017. "The Role of Carbon on Copper–Carbon Composites for the Electrooxidation of Alcohols in an Alkaline Medium" C 3, no. 4: 36. https://doi.org/10.3390/c3040036
APA StyleGarcía-Cruz, L., Ania, C. O., Carvalho, A. P., Bandosz, T. J., Montiel, V., & Iniesta, J. (2017). The Role of Carbon on Copper–Carbon Composites for the Electrooxidation of Alcohols in an Alkaline Medium. C, 3(4), 36. https://doi.org/10.3390/c3040036








