Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
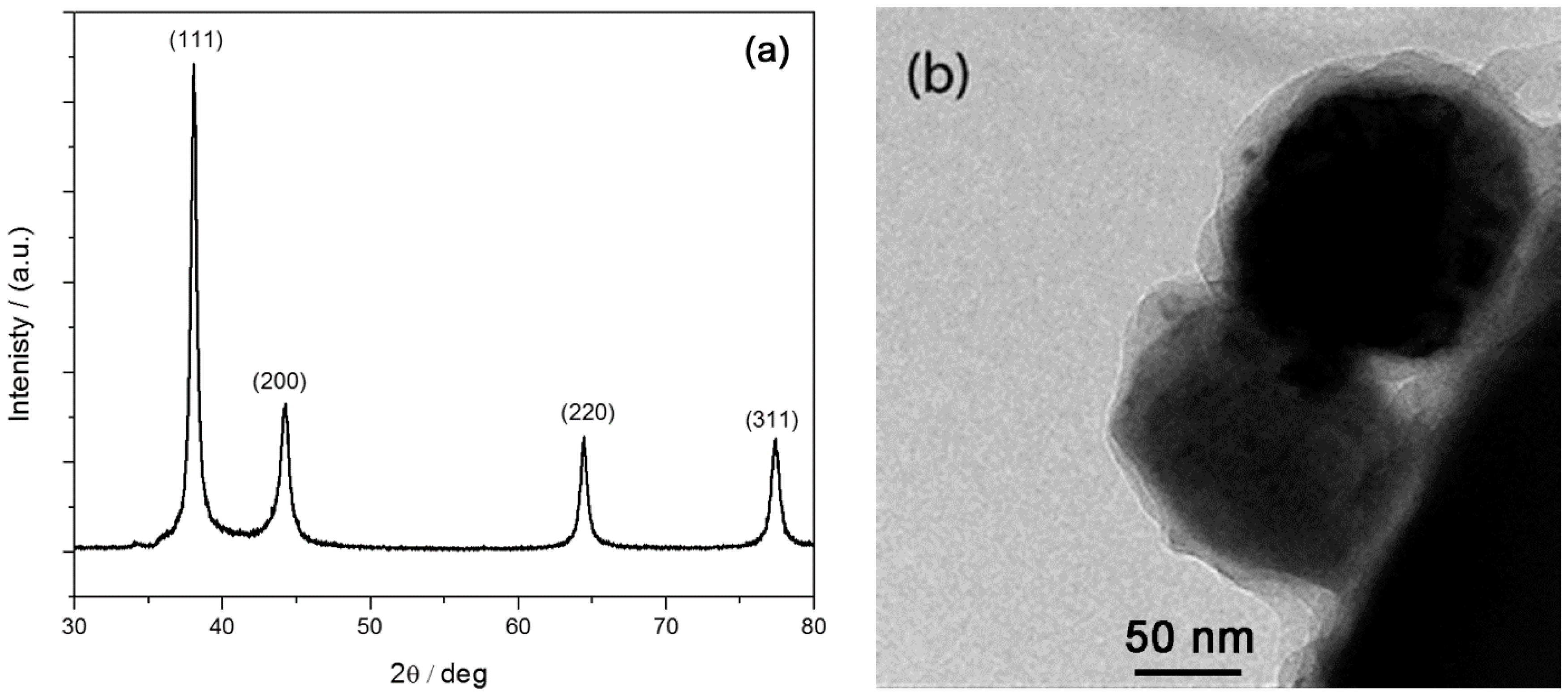
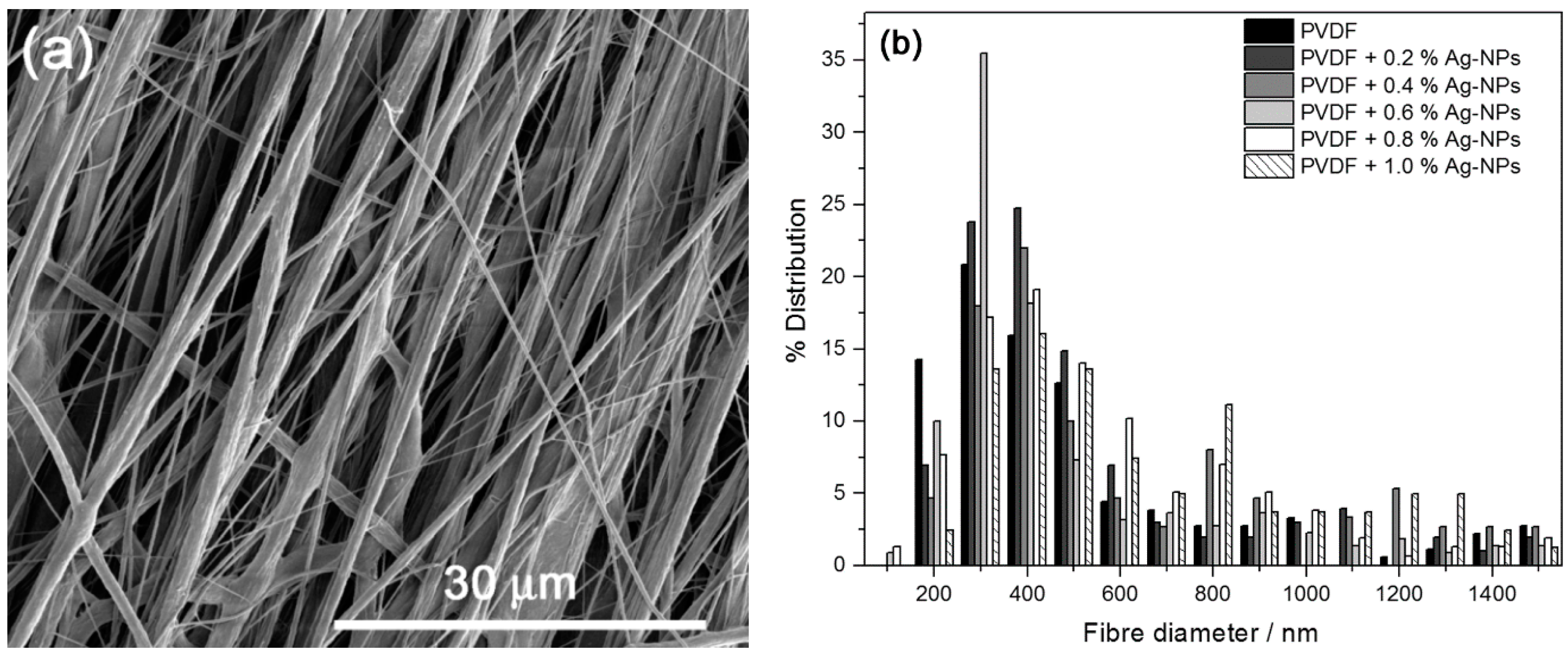
2.1. Morphology
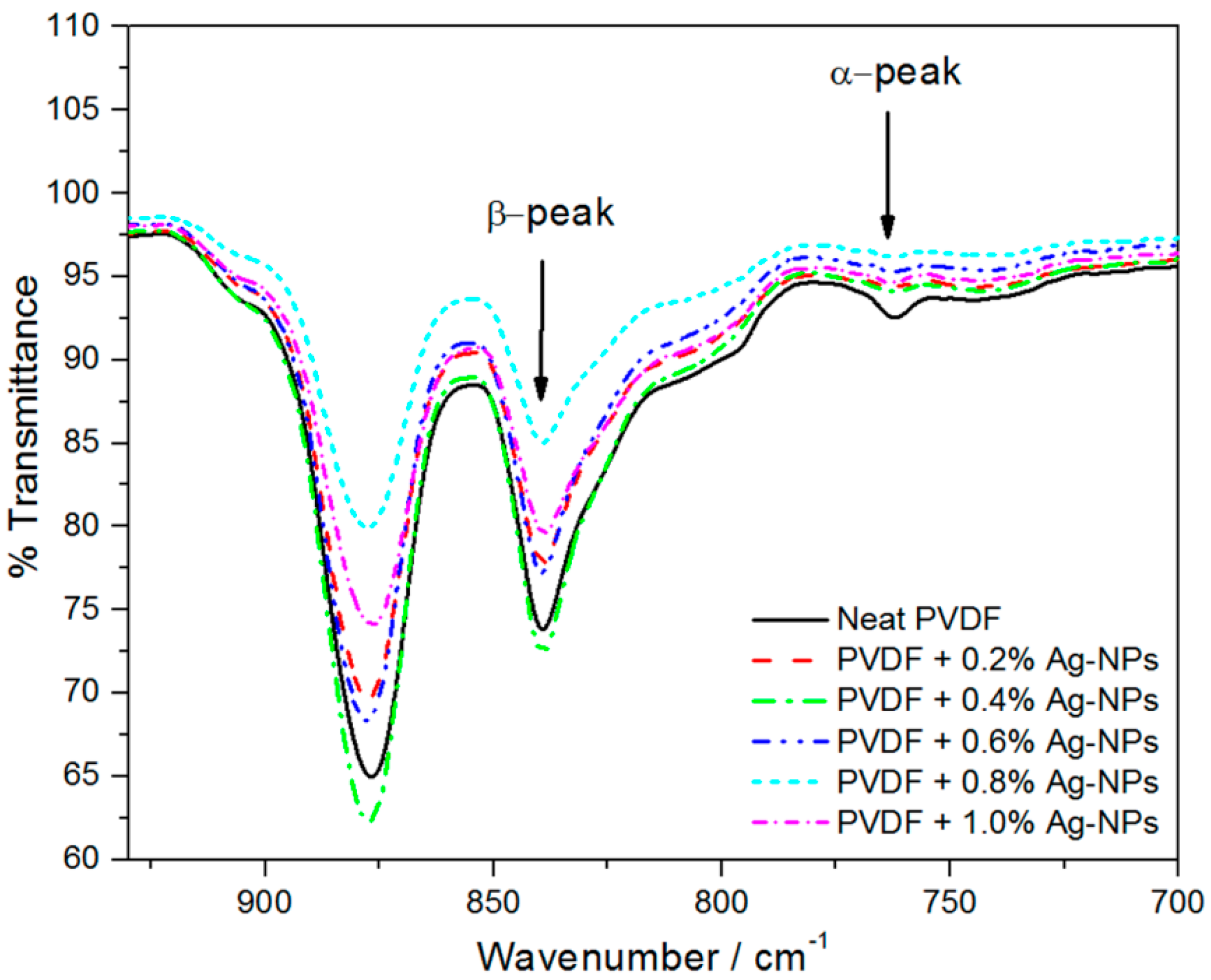
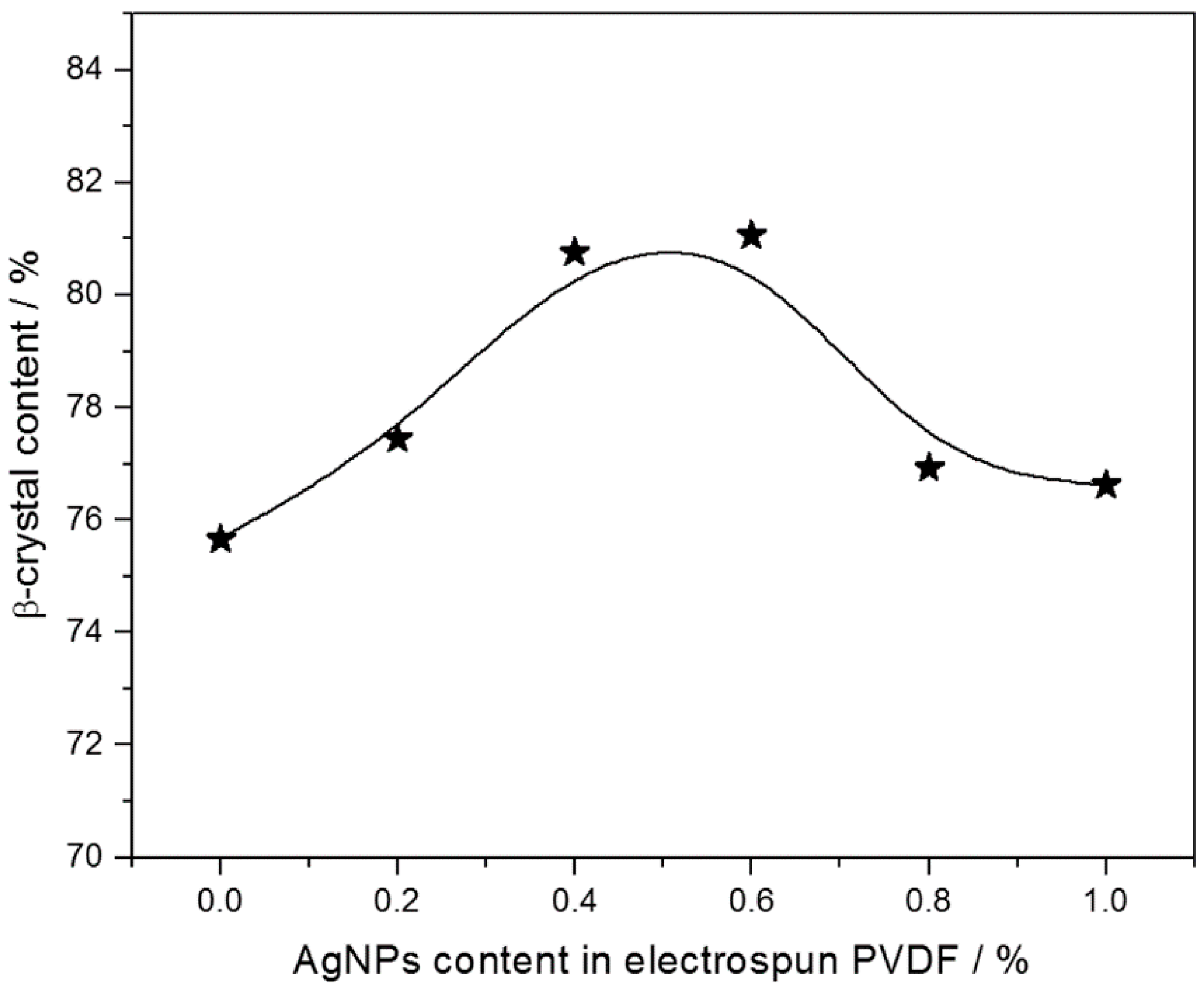
2.2. β-Phase Content in the PVDF/AgNPs Composites
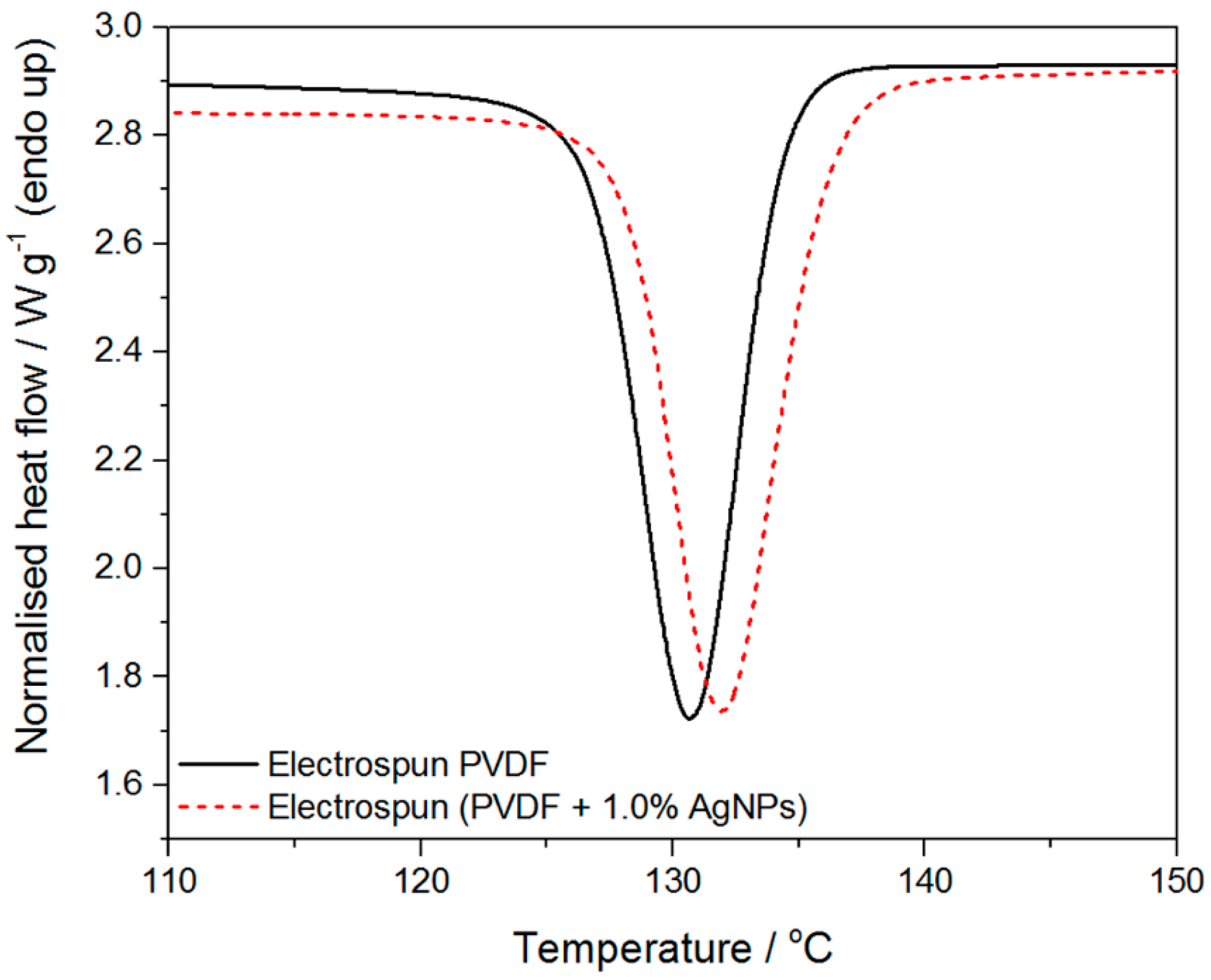
2.3. Crystallinity
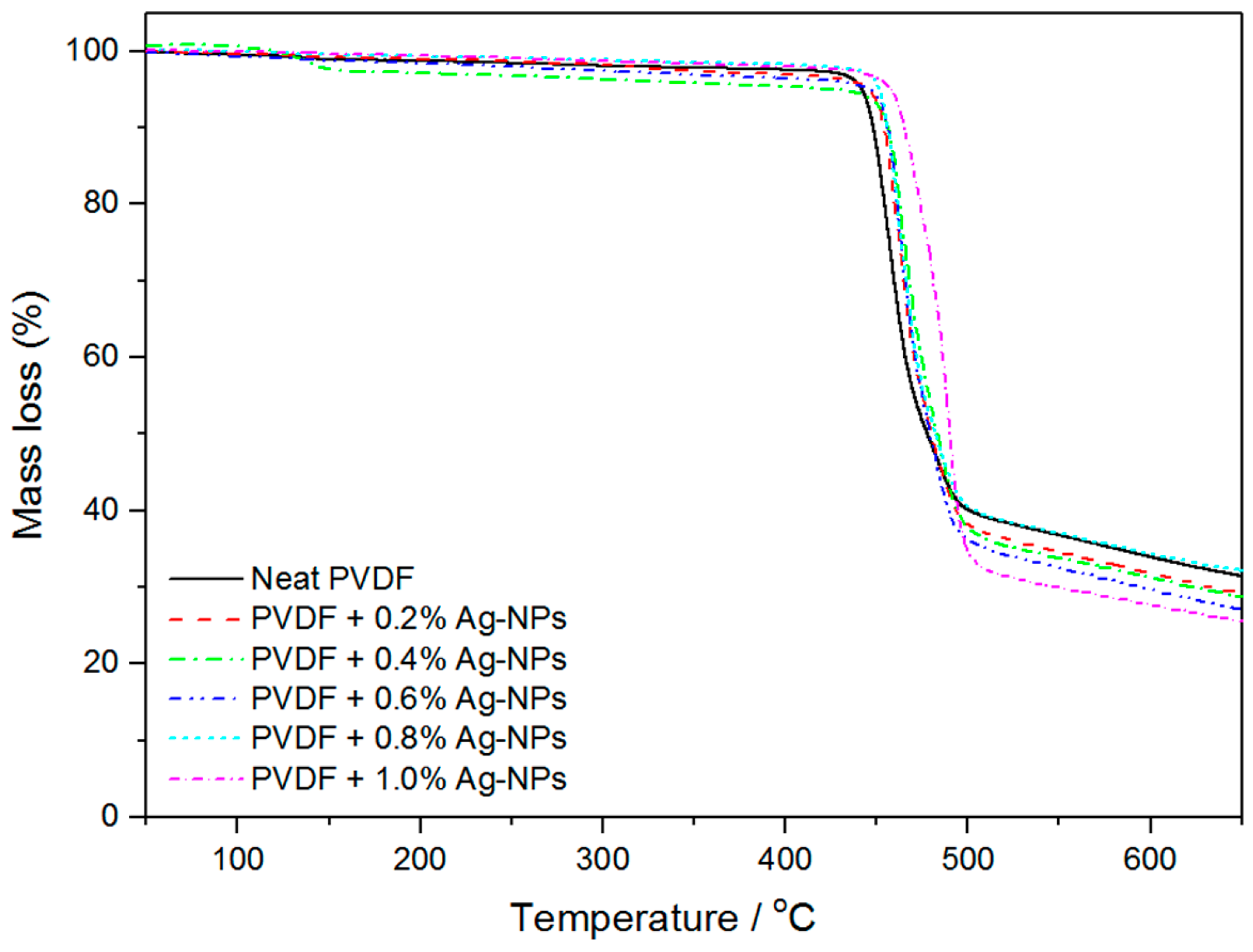
2.4. Thermal Degradation
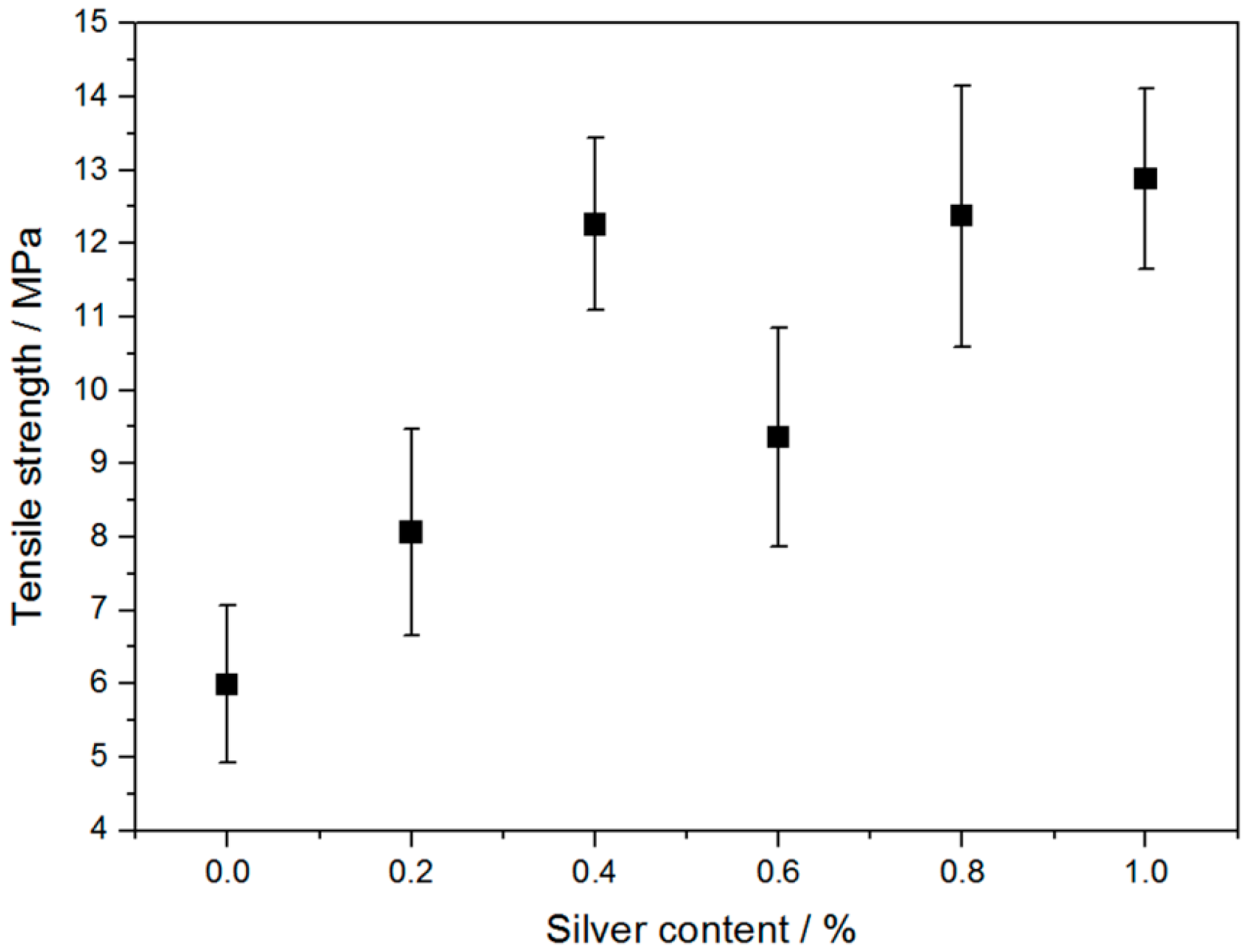
2.5. Tensile Properties
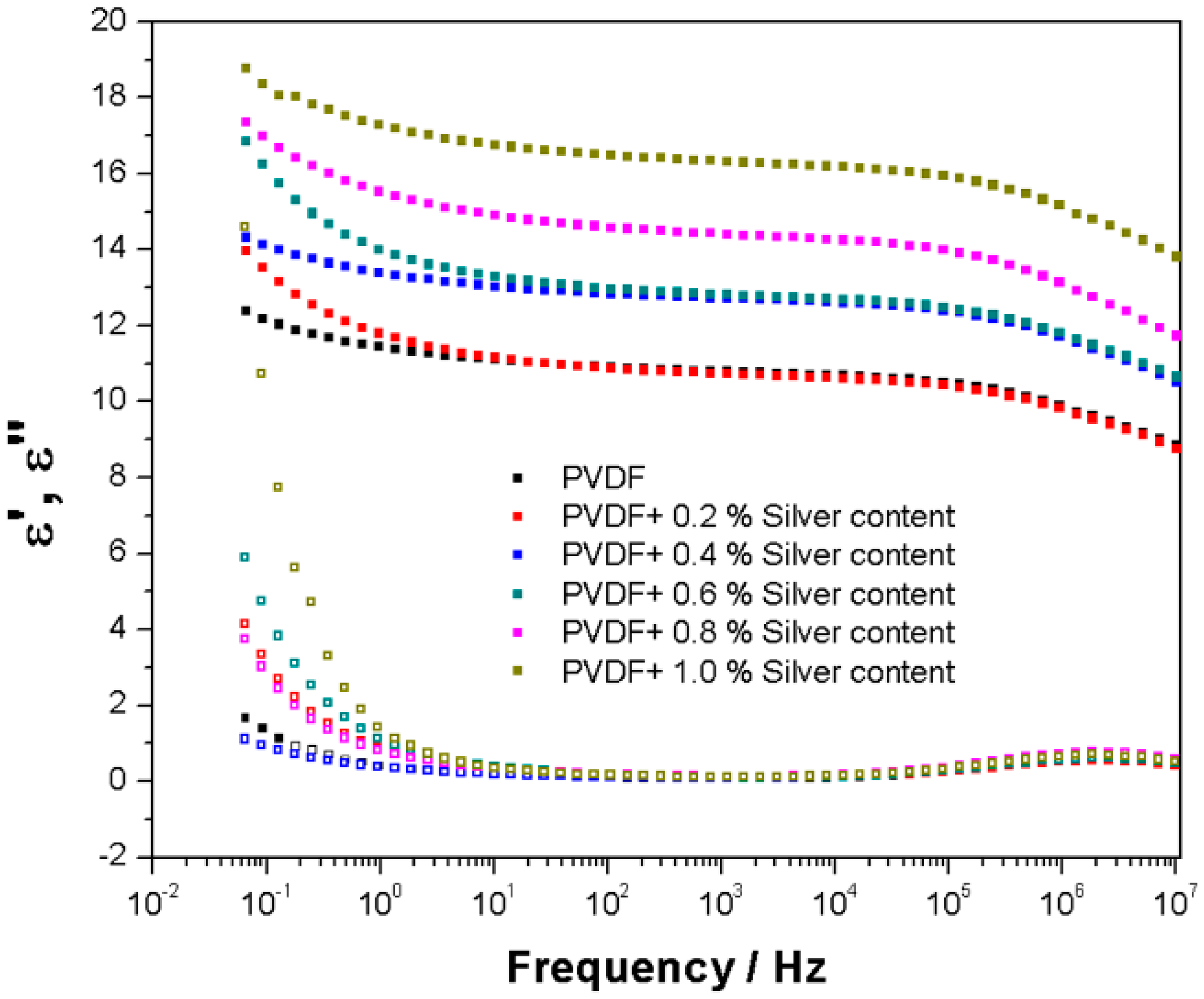
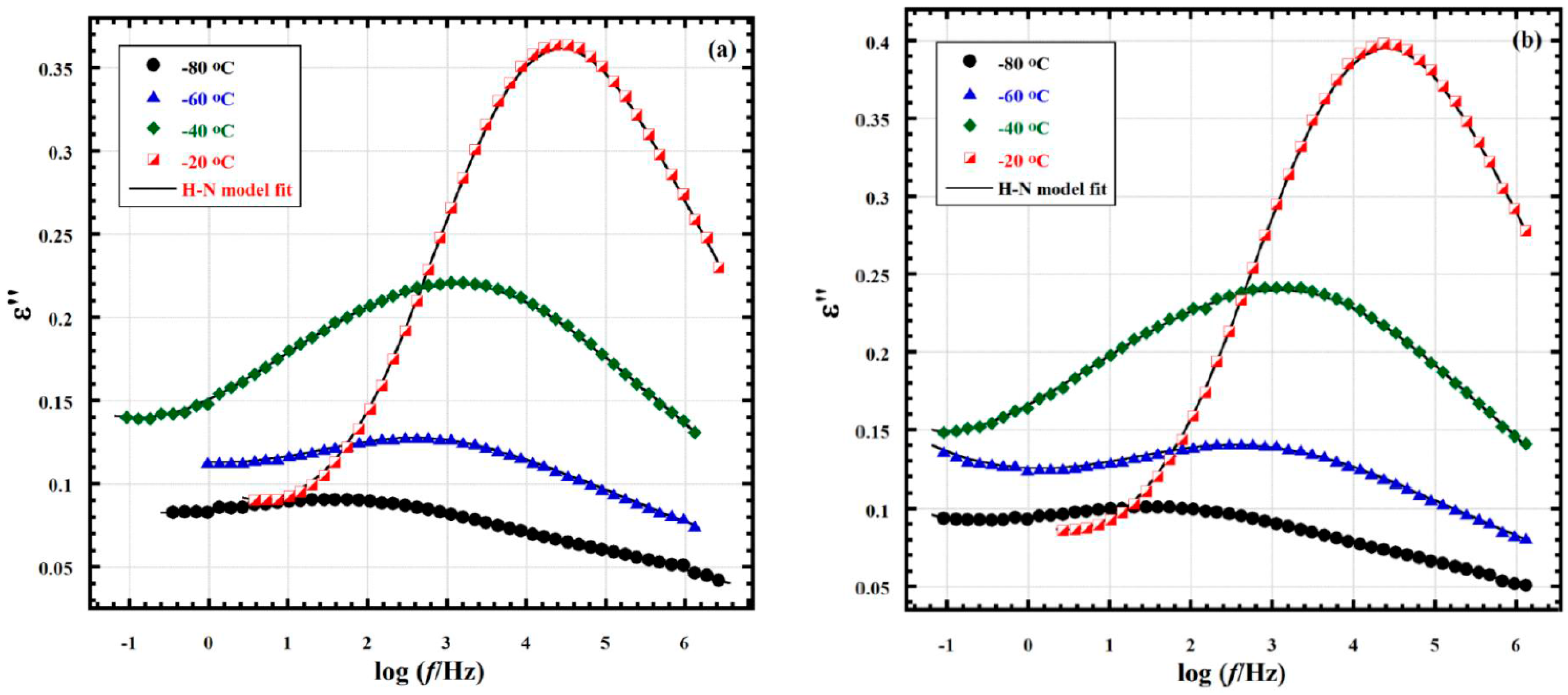
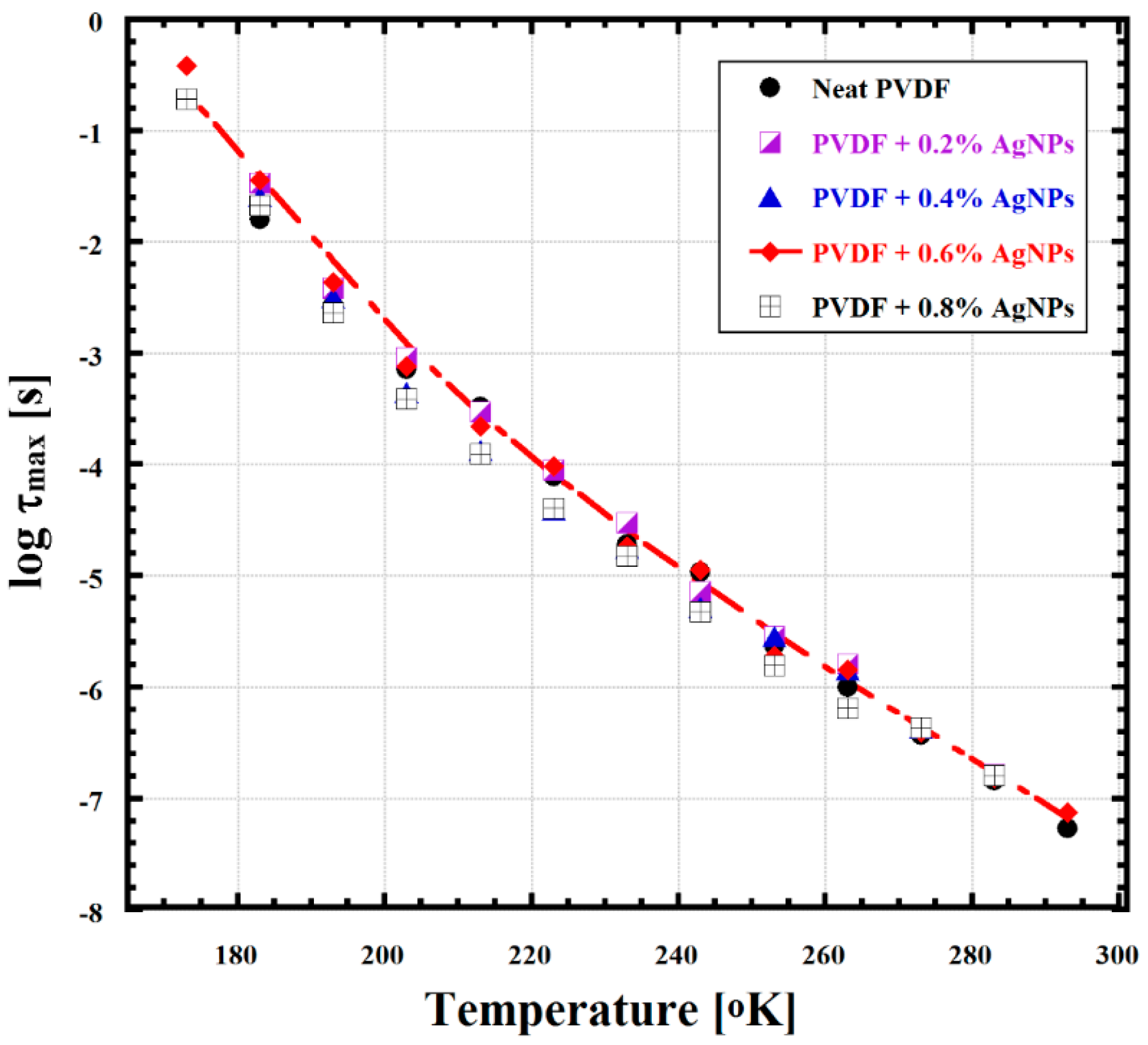
2.6. Broadband Dielectric Analysis
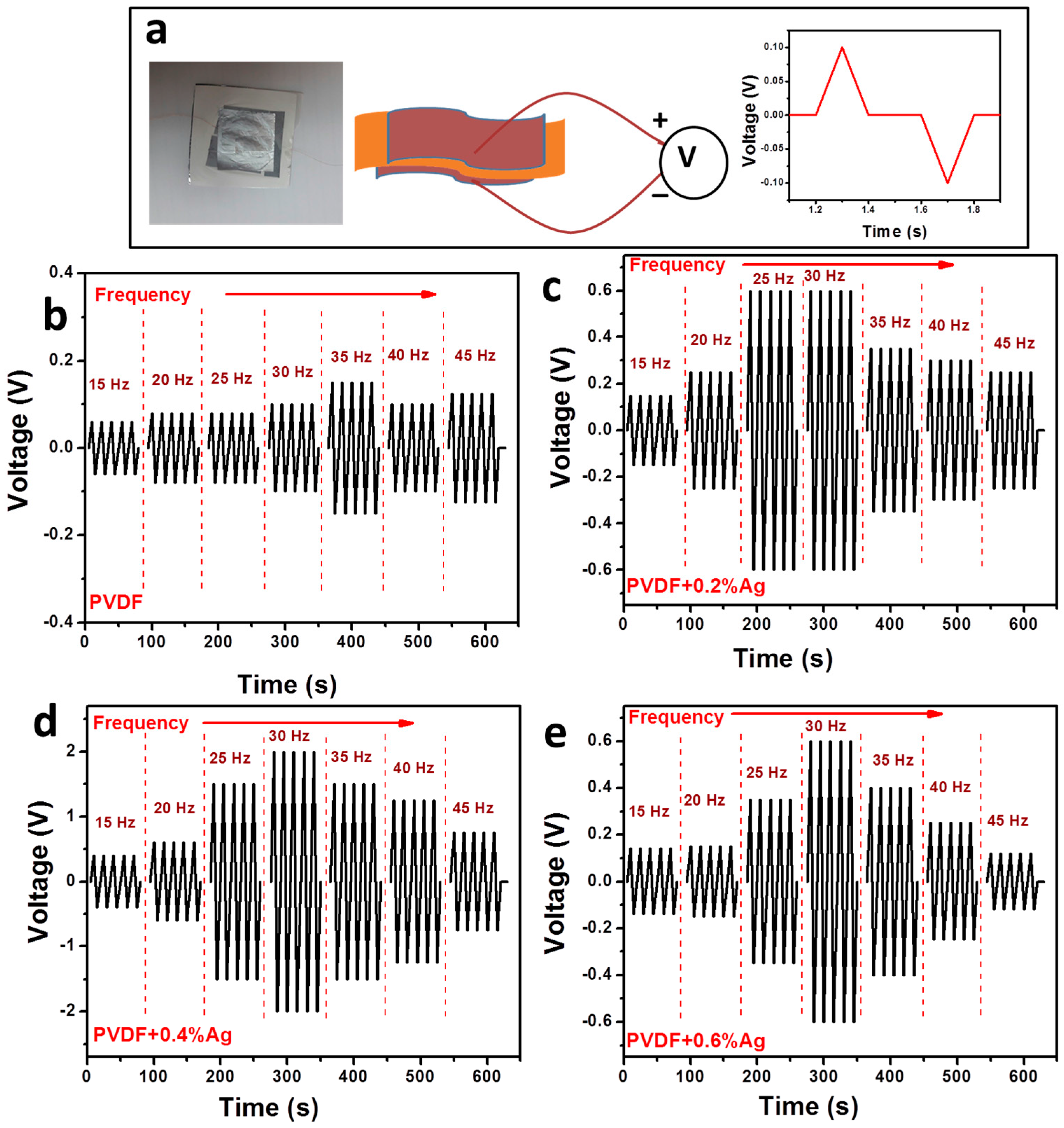
2.7. Piezoelectric Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of Nanofibre Mats
3.3. Characterization and Analysis Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Rajesh, P.S.M.; Bodkh, S.; Kamle, S.; Verma, V. Enhancing β-Phase in PVDF through Physicochemical Modification of Cellulose. Electron. Mater. Lett. 2014, 10, 315–319. [Google Scholar] [CrossRef]
- Gonçalves, P.M.M.R.; Caparrós, C.; Martins, P.; Benelmekki, A.M.; Botelho, G.; Lanceros-Mendez, S.; Lasheras, D.A.; Gutiérrez, J.; Barandiarán, J.M. Nucleation of the electroactive β-phase, dielectric and magnetic response of poly(vinylidene fluoride) composites with Fe2O3 nanoparticles. J. Non-Cryst. Solids 2013, 361, 93–99. [Google Scholar] [CrossRef]
- Yee, W.A.; Kotaki, M.; Liu, Y.; Lu, X. Morphology, polymorphism behavior and molecular orientation of electrospun poly(vinylidene fluoride) fibers. Polymer 2007, 48, 512–521. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Kakimoto, K.-I.; Fukata, K.; Ogawab, H. Fabrication of fibrous BaTiO3-reinforced PVDF composite sheet for transducer application. Sens. Actuators A 2013, 200, 21–25. [Google Scholar] [CrossRef]
- Low, Y.K.A.; Tan, L.Y.; Tan, L.P.; Boey, F.Y.C.; Ng, K.W. Increasing solvent polarity and addition of salts promote β-phase poly(vinylidene fluoride) formation. J. Appl. Polym. Sci. 2013, 128, 2902–2910. [Google Scholar] [CrossRef]
- Chen, D.; Sharma, T.; Zhang, J.X.J. Mesoporous surface control of PVDF thin films for enhanced piezoelectric energy generation. Sens. Actuators A 2014, 216, 196–201. [Google Scholar] [CrossRef]
- Layek, R.K.; Das, A.K.; Park, M.J.; Kim, N.H.; Lee, J.H. Enhancement of physical, mechanical, and gas barrier properties in noncovalently functionalized graphene oxide/poly(vinylidene fluoride) composites. Carbon 2015, 81, 329–338. [Google Scholar] [CrossRef]
- An, N.; Liu, S.; Fang, C.; Yu, R.; Zhou, X.; Cheng, Y. Preparation and properties of β-phase graphene oxide/PVDF composite films. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Bodkhe, S.; Rajesh, P.S.M.; Kamle, S.; Verma, V. β-phase enhancement in polyvinylidene fluoride through filler addition: Comparing cellulose with carbon nanotubes and clay. J. Polym. Res. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Shi, N.; Duan, J.; Su, J.; Huang, F.; Xue, W.; Zheng, C.; Qian, Y.; Chen, S.; Xie, L.; Huang, W. Crystal polymorphism and enhanced dielectric performance of composite nanofibers of poly(vinylidene fluoride) with silver nanoparticles. J. Appl. Polym. Sci. 2013, 128, 1004–1010. [Google Scholar] [CrossRef]
- Chiolerio, A.; Lombardi, M.; Guerriero, A.; Canavese, G.; Stassi, S.; Gazia, R.; Cauda, V.; Manfredi, D.; Chiodoni, A.; Verna, A.; et al. Effect of the fabrication method on the functional properties of BaTiO3:PVDF nanocomposites. J. Mater. Sci. 2013, 48, 6943–6951. [Google Scholar] [CrossRef]
- Matabola, K.P.; Moutloali, R.M. The influence of electrospinning parameters on the morphology and diameter of poly(vinyledene fluoride) nanofibers—Effect of sodium chloride. J. Mater. Sci. 2013, 48, 5475–5482. [Google Scholar] [CrossRef]
- Lafrance, C.-P.; Prud’homme, R.E.; Guidoin, R. Identification and quantification of the crystalline structures of poly(vinylidene fluoride) sutures by wide-angle X-ray scattering and differential scanning calorimetry. J. Biomed. Mater. Res. 1998, 39, 184–189. [Google Scholar]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103–225112. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, J.; Xing, Z.; Shen, J.; Kang, I.K.; Byun, H. Electrospinning of antibacterial poly(vinylidene fluoride) nanofibers containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 668–672. [Google Scholar] [CrossRef]
- Li, B.; Xu, C.; Zheng, J.; Xu, C. Sensitivity of Pressure Sensors Enhanced by Doping Silver Nanowires. Sensors 2014, 14, 9889–9899. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.-M.; You, S.-S.; Zha, J.-W.; Song, H.-T.; Li, S.-T. Effect of shell-layer thickness on dielectric properties in Ag@TiO2 core@shell nanoparticles filled ferroelectric poly(vinylidene fluoride) composites. Phys. Status Solidi 2010, 207, 739–742. [Google Scholar] [CrossRef]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Compton, J.; Kranbuehl, D.; Martin, G.; Espuche, E.; David, L. In situ formation of a uniform distribution of silver nanoparticles in PVDF: Kinetics of formation and resulting properties. Macromol. Symp. 2007, 247, 182–189. [Google Scholar] [CrossRef]
- Chae, D.W.; Hwang, S.S.; Hong, S.M.; Hong, S.P.; Cho, B.G.; Kim, B.C. Influence of high contents of silver nanoparticles on the physical properties of poly(vinylidene fluoride). Mol. Cryst. Liq. Cryst. 2007, 464, 233–241. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline polymorphism in poly(vinylidenefluoride) membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.; Luyt, A.S.; Mrlik, M.; Hassan, M.K. Investigation of the physico-mechanical properties of electrospun PVDF/cellulose (nano)fibers. J. Appl. Polym. Sci. 2016, 133, 43594. [Google Scholar] [CrossRef]
- Greco, T.; Wang, F.; Wegener, M. Multifunctional silver poly(vinylidene fluoride) nanocomposites: Nanoparticle synthesis, film processing, and structural characterization. Ferroelectrics 2010, 405, 85–91. [Google Scholar] [CrossRef]
- Botelho, G.; Lanceros-Mendez, S.; Gonçalves, A.M.; Sencadas, V.; Rocha, J.G. Relationship between processing conditions, defects and thermal degradation of poly(vinylidene fluoride) in the β-phase. J. Non-Cryst. Solids 2008, 354, 72–78. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.A.A.S.; Mrlík, M.; Luyt, A.S. Electrospun PVDF graphene oxide composite fiber mats with tunable physical properties. J. Polym. Res. 2016, 23, 232. [Google Scholar] [CrossRef]
- Li, X.; Niitsoo, O.; Couzis, A. Electrostatically assisted fabrication of silver-dielectric core/shell nanoparticles thin film capacitor with uniform metal nanoparticle distribution and controlled spacing. J. Colloid Interface Sci. 2016, 465, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tawansi, A.; Oraby, A.H.; Badr, S.I.; Elashmawi, I.S. Physical properties and β-phase increment of AgNO3-filled poly(vinylidene fluoride) films. Polym. Int. 2004, 53, 370–377. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, Y.; Bi, J.; Wang, S.; Li, M.; Zhang, Z. Tunable BT@SiO2 core@shell filler reinforced polymer composite with high breakdown strength and release energy density. Compos. Part A Appl. Sci. Manuf. 2016, 85, 172–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Deng, Y.; Li, M.; Bai, J. Enhanced dielectric properties of ferroelectric polymer composites induced by metal-semiconductor Zn-ZnO core-shell structure. ACS Appl. Mater. Interfaces 2012, 4, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Mahendia, S.; Tomar, A.K.; Kumar, S. Electrical conductivity and dielectric spectroscopic studies of PVA-Ag nanocomposite films. J. Alloy Compd. 2010, 508, 406–411. [Google Scholar] [CrossRef]
- Yu, S.; Qin, F.; Wang, G. Improving the dielectric properties of poly(vinylidene fluoride) composites by using poly(vinyl pyrrolidone)-encapsulated polyaniline nanorods. J. Mater. Chem. C 2016, 4, 1504–1510. [Google Scholar] [CrossRef]
- Zhou, W.; Dong, L.; Sui, X.; Wang, Z.; Zuo, J.; Cai, H.; Chen, Q. High dielectric permittivity and low loss in PVDF filled by core-shell Zn@ZnO particles. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, Z.; Zhu, H. Dielectric properties of Ag@C/PVDF composites. J. Appl. Polym. Sci. 2013, 129, 3411–3416. [Google Scholar] [CrossRef]
- Paleo, A.J.; Martínez-Boubeta, C.; Balcells, L.; Costa, C.M.; Sencadas, V.; Lanceros-Mendez, S. Thermal, dielectrical and mechanical response of α and β-poly(vinilydene fluoride)/Co-MgO nanocomposites. Nanoscale Res. Lett. 2011, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, K.; Mandal, B.P.; Tyagi, A.K. Enhancement of dielectric permittivity and ferroelectricity of a modified cobalt nanoparticle and polyvinylidene fluoride based composite. RSC Adv. 2015, 5, 8591–8597. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Hassan, M.K.; Mauritz, K.A.; Bunkley, S.L.; Buchanan, R.K.; Buchanan, J.P. Dielectric properties of C60 and Sc3N@C80 fullerenol containing polyurethane nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40577. [Google Scholar] [CrossRef]
- Manna, S.; Batabyal, S.K.; Nandi, A.K. Preparation and characterization of silver-poly(vinylidene fluoride) nanocomposites: Formation of piezoelectric polymorph of poly(vinylidene fluoride). J. Phys. Chem. B 2006, 110, 12318–12326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Liu, X.; Zhang, C.; Li, X.; Zhang, W.; Wang, C. Ultra-low dielectric performance of polymer electrospun nanofiber mats. Appl. Phys. A Mater. Sci. Process. 2010, 100, 207–212. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Sencadas, V.; Lanceros-Méndez, S.; Sabater, R.; Serra, I.; Balado, A.A.; Gómez, J.L. Ribelles Relaxation dynamics of poly(vinylidene fluoride) studied by dynamical mechanical measurements and dielectric spectroscopy. Eur. Phys. J. E 2012, 35, 41. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, R., Jr.; Ueno, E.M. Effect of crystalline phase, orientation and temperature on the dielectric properties of poly (vinylidene fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Hahn, B.; Wendorff, J.; Yoon, D.Y. Dielectric relaxation of the crystal-amorphous interphase in poly(vinylidene fluoride) and its blends with poly(methyl methacrylate). Macromolecules 1985, 18, 718–721. [Google Scholar] [CrossRef]
- Ando, Y.; Hanada, T.; Saitoh, K. Quantitative confirmation of the crystal-amorphous interphase in semicrystalline poly(vinylidene fluoride) and poly(vinylidene fluoride)/poly(ethyl methacrylate) blends, Journal of Polymer Science. Part B Polym. Phys. 1994, 32, 179–185. [Google Scholar] [CrossRef]
- Vogel, H. The law of the relation between the viscosity of liquids and the temperature. Physik. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Sharma, M.; Srinivas, V.; Madras, G.; Bose, S. Outstanding dielectric constant and piezoelectric coefficient in electrospun nanofiber mats of PVDF containing silver decorated multiwall carbon nanotubes: Assessing through piezoresponse force microscopy. RSC Adv. 2016, 6, 6251–6258. [Google Scholar] [CrossRef]
- Sinha, T.K.; Ghosh, S.K.; Maiti, R.; Jana, S.; Adhikari, B.; Mandal, D.; Ray, S.K. Graphene-silver-induced self-polarized PVDF-based flexible plasmonic nanogenerator toward the realization for new class of self powered optical sensor. ACS Appl. Mater. Interfaces 2016, 8, 14986–14993. [Google Scholar] [CrossRef] [PubMed]
| Sample | Viscosity/cP | Conductivity/μS cm−1 |
|---|---|---|
| Solvent (60:40 DMF:acetone) | 0.45 | 2.1 |
| Solvent + PVDF | 361.1 | 12.4 |
| Solvent + PVDF + 0.2% AgNPs | 340.8 | 15.0 |
| Solvent + PVDF + 0.4% AgNPs | 350.9 | 15.0 |
| Solvent + PVDF + 0.6% AgNPs | 343.3 | 14.2 |
| Solvent + PVDF + 0.8% AgNPs | 338.2 | 15.8 |
| Solvent + PVDF + 1.0% AgNPs | 338.2 | 16.3 |
| Sample | Ntotal | Mean Diameter/nm | Standard Deviation/nm |
|---|---|---|---|
| PVDF | 166 | 506 | 393 |
| PVDF + 0.2% AgNPs | 97 | 470 | 302 |
| PVDF + 0.4% AgNPs | 138 | 576 | 374 |
| PVDF + 0.6% AgNPs | 208 | 432 | 312 |
| PVDF + 0.8% AgNPs | 153 | 508 | 302 |
| PVDF + 1.0% AgNPs | 78 | 648 | 372 |
| Phase | α-Phase | β-Phase | γ-Phase | References |
|---|---|---|---|---|
| FTIR (corresponding wavenumbers/cm−1) | 408, 532, 614, 766, 795, 855, 976 | 440, 485, 510, 840, 1279 | 431, 512, 776, 812, 833, 840, 1234 | [1,9,10,25] |
| Sample | ∆Hm/J∙g−1 | Tm/°C | ∆Hc/J∙g−1 | Tc/°C |
|---|---|---|---|---|
| Electrospun PVDF | 42.8 | 160.1 | −35.2 | 130.7 |
| Electrospun PVDF + 1% AgNPs | 48.4 | 159.0 | −37.3 | 131.9 |
| Parameter | Value |
|---|---|
| Silver content | 0.2%, 0.4%, 0.6%, 0.8%, 1.0% |
| Flow rate | 2 mL∙h−1 |
| Voltage | 8.0 kV (adjusted *) |
| Distance | 10 cm |
| Collector/speed | Rotating disk/1700–1900 rpm |
| Spinneret (needle) | G22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, A.A.; Al-Maadeed, M.A.; Luyt, A.S.; Ponnamma, D.; Hassan, M.K. Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles. C 2017, 3, 30. https://doi.org/10.3390/c3040030
Issa AA, Al-Maadeed MA, Luyt AS, Ponnamma D, Hassan MK. Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles. C. 2017; 3(4):30. https://doi.org/10.3390/c3040030
Chicago/Turabian StyleIssa, Ahmed A., Mariam A. Al-Maadeed, Adriaan S. Luyt, Deepalekshmi Ponnamma, and Mohammad K. Hassan. 2017. "Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles" C 3, no. 4: 30. https://doi.org/10.3390/c3040030
APA StyleIssa, A. A., Al-Maadeed, M. A., Luyt, A. S., Ponnamma, D., & Hassan, M. K. (2017). Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles. C, 3(4), 30. https://doi.org/10.3390/c3040030







