Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing
Abstract
:1. Introduction
2. Results and Discussion
2.1. GUITAR Coated Graphite Felt Electrode
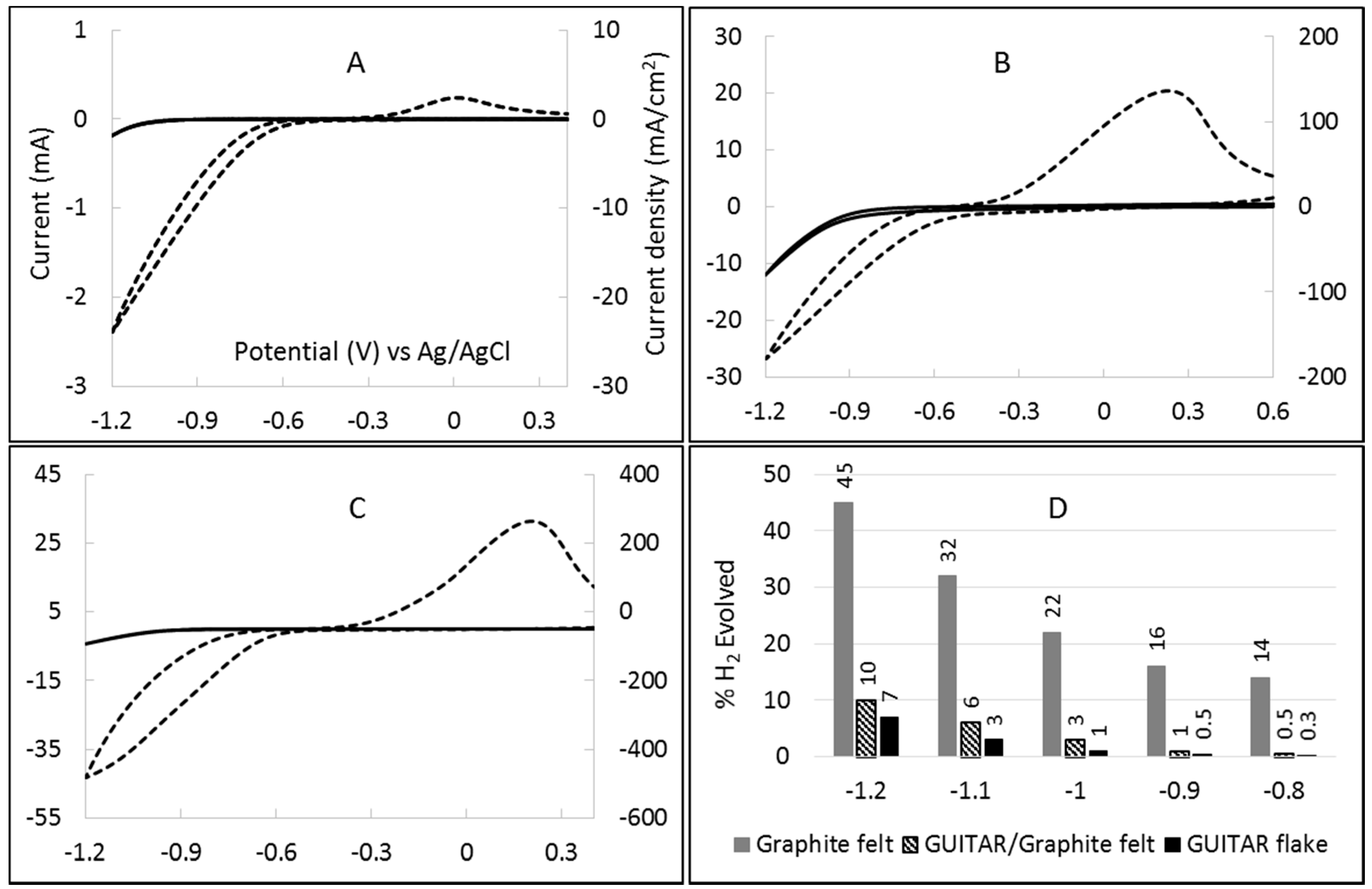
2.2. Estimation of Hydrogen Overpotential by Cyclic Voltammetry in 1 M H2SO4
2.3. Estimation of V3+/2+ HET Rates by Cyclic Voltammetry (CV)
2.4. Percentage Hydrogen Evolution
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GUITAR | Graphene from the University of Idaho Thermolyzed Asphalt Reaction |
| HET | Heterogeneous electron transfer |
| DOS | Density of electronic states |
| RFB | Redox flow batteries |
| VRFB | Vanadium redox flow battery |
| SHE | Standard hydrogen electrode |
| SCE | Saturated calomel electrode |
| EP | Edge plane |
| BP | Basal plane |
References
- Gyan, I.O.; Cheng, I.F. Electrochemical study of biologically relevant molecules at electrodes constructed from GUITAR, a new carbon allotrope. Microchem. J. 2015, 122, 39–44. [Google Scholar] [CrossRef]
- Cheng, I.F.; Xie, Y.; Gyan, I.O.; Nicholasa, N.W. Highest measured anodic stability in aqueous solutions: Graphenic electrodes from the thermolyzed asphalt reaction. RSC Adv. 2013, 3, 2379–2384. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furuta, M.; Kuriyama, K.; Kuwabara, R.; Katsuki, Y.; Kondo, T.; Fujishima, A.; Honda, K. Electrochemical properties of N-doped hydrogenated amorphous carbon films fabricated by plasma-enhanced chemical vapor deposition methods. Electrochim. Acta 2011, 56, 1172–1181. [Google Scholar] [CrossRef]
- Martin, H.B.; Argoitia, A.; Landau, U.; Anderson, A.B.; Angus, J.C. Hydrogen and Oxygen Evolution on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 1996, 143, L133–L136. [Google Scholar] [CrossRef]
- Ndlovu, T.; Sampath, O.A.A.S.; Krause, R.W.; Mamba, B.B. Reactivities of Modified and Unmodified Exfoliated Graphite Electrodes in Selected Redox Systems. Int. J. Electrochem. Sci. 2012, 7, 9441–9453. [Google Scholar]
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A Stable Vanadium Redox-Flow Battery with High Energy Density for Large-Scale Energy Storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Graphite-graphite oxide composite electrode for vanadium redox flow battery. Electrochim. Acta 2011, 56, 5290–5294. [Google Scholar] [CrossRef]
- Joerissen, L.; Garche, J.; Fabjan, C.; Tomazic, G. Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems. J. Power Sources 2004, 127, 98–104. [Google Scholar] [CrossRef]
- Fetyan, A.; Derr, I.; Kayarkatte, M.K.; Langner, J.; Bernsmeier, D.; Kraehnert, R.; Roth, C. Electrospun Carbon Nanofibers as Alternative Electrode Materials for Vanadium Redox Flow Batteries. ChemElectroChem 2015. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q.H. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.L.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Agar, E.; Dennison, C.R.; Knehr, K.W.; Kumbur, E.C. Identification of performance limiting electrode using asymmetric cell configuration in vanadium redox flow batteries. J. Power Sources 2013, 225, 89–94. [Google Scholar] [CrossRef]
- Aaron, D.; Sun, C.; Bright, M.; Papandrew, A.B.; Mench, M.M.; Zawodzinski, T.A. In Situ Kinetics Studies in All-Vanadium Redox Flow Batteries. ECS Electrochem. Lett. 2013, 2, A1–A3. [Google Scholar] [CrossRef]
- Agar, E.; Dennison, C.R.; Knehr, K.W.; Kumbur, E.C. Asymmetric performance testing of carbon felt electrodes to identify the limiting electrode in vanadium redox flow batteries. ECS Trans. 2013, 53, 69–73. [Google Scholar] [CrossRef]
- Chen, F.; L, J.; Chen, H.; Yan, C. Study on Hydrogen Evolution Reaction at a Graphite Electrode in the All-Vanadium Redox Flow Battery. Int. J. Electrochem. Sci. 2012, 7, 3750–3764. [Google Scholar]
- Sun, C.; Delnick, F.M.; Baggetto, L.; Veith, G.M.; Zawodzinski, T.A. Hydrogen evolution at the negative electrode of the all-vanadium redox flow batteries. J. Power Sources 2014, 248, 560–564. [Google Scholar] [CrossRef]
- Surez, D.J.; Gonzlez, Z.; Blanco, C.; Granda, M.; Menndez, R.; Santamara, R. Graphite Felt Modified with Bismuth Nanoparticles as Negative Electrode in a Vanadium Redox Flow Battery. ChemSusChem 2014, 7, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Wu, X.W.; Tomoo, Y.; Suguru, O.; Zhang, Q.X.; Lv, F.C.; Liu, C.M.; Shirasaki, K.; Satoh, I.; Shikama, T.; Lu, D. Acceleration of the redox kinetics of VO2+/VO2+ and V3+/V2+ couples on carbon paper. J. Appl. Electrochem. 2011, 41, 1183–1190. [Google Scholar] [CrossRef]
- Gyan, I.O.; Wojcik, P.M.; Aston, D.E.; McIlroy, D.N.; Cheng, I.F. A Study of the Electrochemical Properties of a New Graphitic Material: GUITAR. ChemElectroChem 2015, 2, 700–706. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Huang, K.-L.; Li, X.-G.; Liu, S.-Q.; Tan, N.; Chen, L.-Q. Research progress of vanadium redox flow battery for energy storage in China. Renew. Energy 2008, 33, 186–192. [Google Scholar] [CrossRef]
- Zhong, S.; Kazacos, P.M.; Skyllas-Kazacos, M. Comparison of the physical, chemical and electrochemical properties of rayon and polyacrylonitrile-based graphite felt electrodes. J. Power Sources 1993, 45, 29–41. [Google Scholar] [CrossRef]
- Kaneko, H.; Nozaki, K.; Wada, Y.; Aoki, T.; Negishi, A.; Kamimoto, M. Vanadium redox reactions and carbon electrodes for vanadium redox flow battery. Electrochim. Acta 1991, 36, 1191–1196. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazakos, M. Chemical modification and electrochemical behaviour of graphite fibre in acidic vanadium solution. Electrochim. Acta 1991, 36, 513–517. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H. Development of nitrogen-doped carbons using the hydrothermal method as electrode materials for vanadium redox flow batteries. J. Appl. Electrochem. 2013, 43, 553–557. [Google Scholar] [CrossRef]
- Han, P.; Yue, Y.; Liu, Z.; Xu, W.; Zhang, L.; Xu, H.; Dong, S.; Cui, G. Graphene oxide nanosheets/multi-walled carbon nanotubes hybrid as an excellent electrocatalytic material towards VO2+/VO2+ redox couples for vanadium redox flow batteries. Energy Environ. Sci. 2011, 4, 4710–4717. [Google Scholar] [CrossRef]
- Cheng, I.F.; Xie, Y.; Gonzales, R.A.; Gonzales, P.R.; Sundararajan, J.P.; Fouetio Kengne, B.A.; Aston, D.E.; McIlroy, D.N.; Foutch, J.D.; Griffiths, P.R. Synthesis of graphene paper from pyrolyzed asphalt. Carbon 2011, 49, 2852–2861. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. Vanadium Flow Battery for Energy Storage: Prospects and Challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, S.; He, Z.; Shi, L. Influence of antimony ions in negative electrolyte on the electrochemical performance of vanadium redox flow batteries. Electrochim. Acta 2015, 151, 297–305. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Lu, S.; Wu, C.; Liu, Y.; Tan, Q.; Liang, D.; Xiang, Y. Titanium nitride as an electrocatalyst for V(II)/V(III) redox couples in all-vanadium redox flow batteries. Electrochim. Acta 2015, 182, 834–840. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Smith, R.E.G.; Davies, T.J.; Baynes, N.B.; Nichols, R.J. The electrochemical characterisation of graphite felts. J. Electroanal. Chem. 2015, 747, 29–38. [Google Scholar] [CrossRef]
- Flox, C.; Rubio-Garcia, J.; Nafria, R.; Zamani, R.; Skoumal, M.; Andreu, T.; Arbiol, J.; Cabot, A.; Morante, J.R. Active nano-CuPt3 electrocatalyst supported on graphene for enhancing reactions at the cathode in all-vanadium redox flow batteries. Carbon 2012, 50, 2347–2374. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000; p. 455. [Google Scholar]
- Rice, R.J.; McCreery, R.L. Quantitative Relationship between Electron Transfer Rate and Surface Microstructure of Laser-Modified Graphite Electrodes. Anal. Chem. 1989, 61, 1637–1641. [Google Scholar] [CrossRef]
- Pour, N.; Kwabi, D.G.; Carney, T.; Darling, R.M.; Perry, M.L.; Shao-Horn, Y. Influence of Edge- and Basal-Plane Sites on the Vanadium Redox Kinetics for Flow Batteries. J. Phys. Chem. C 2015, 119, 5311–5318. [Google Scholar] [CrossRef]
- Smith, R.E.G.; Davies, T.J.; de B. Baynes, N.; Nichols, R.J. The electrochemical characterisation of graphite felts. J. Electroanal. Chem. 2015, 747, 29–38. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, T.; Watanabe, N.; Yano, T.; Shiokawa, Y. Electron-Transfer Kinetics of Np3+/Np4+, NpO2+/NpO22+, V2+/V3+, and VO2+/VO2+ at Carbon Electrodes. J. Electrochem. Soc. 2005, 152, A830–A836. [Google Scholar] [CrossRef]
- McDermott, C.A.; Kneten, K.R.; McCreery, R.L. Electron Transfer Kinetics of Aquated Fe+3/+2, Eu+3/+2, and V+3/+2 at Carbon Electrodes: Inner Sphere Catalysis by Surface Oxides. J. Electrochem. Soc. 1993, 140, 2593–2599. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Zhang, Y.M.; Yue, L.; Li, W.S.; Li, G.L.; Shu, D.; Chen, H.Y. Graphite-carbon nanotube composite electrodes for all vanadium redox flow battery. J. Power Sources 2008, 184, 637–640. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.-J.; Kim, J.-H.; Park, M.-S. The effects of surface modification on carbon felt electrodes for use in vanadium redox flow batteries. Mater. Chem. Phys. 2011, 131, 547–553. [Google Scholar] [CrossRef]
- Sum, E.; Skyllas-kazacos, M. A study of the V(II)/V(III) redox couple for redox flow cell applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.; Sun, F.; Zhao, L.; Xing, L. Highly hydroxylated carbon fibers as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, T.; Li, X.; Liu, Z. Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J. Power Sources 2012, 218, 455–461. [Google Scholar] [CrossRef]
- Xie, Y.; McAllister, S.D.; Hyde, S.A.; Sundararajan, J.P.; FouetioKengne, B.A.; McIlroy, D.N.; Cheng, I.F. Sulfur as an important co-factor in the formation of multilayer graphene in the thermolyzed asphalt reaction. J. Mater. Chem. 2012, 22, 5723–5729. [Google Scholar] [CrossRef]
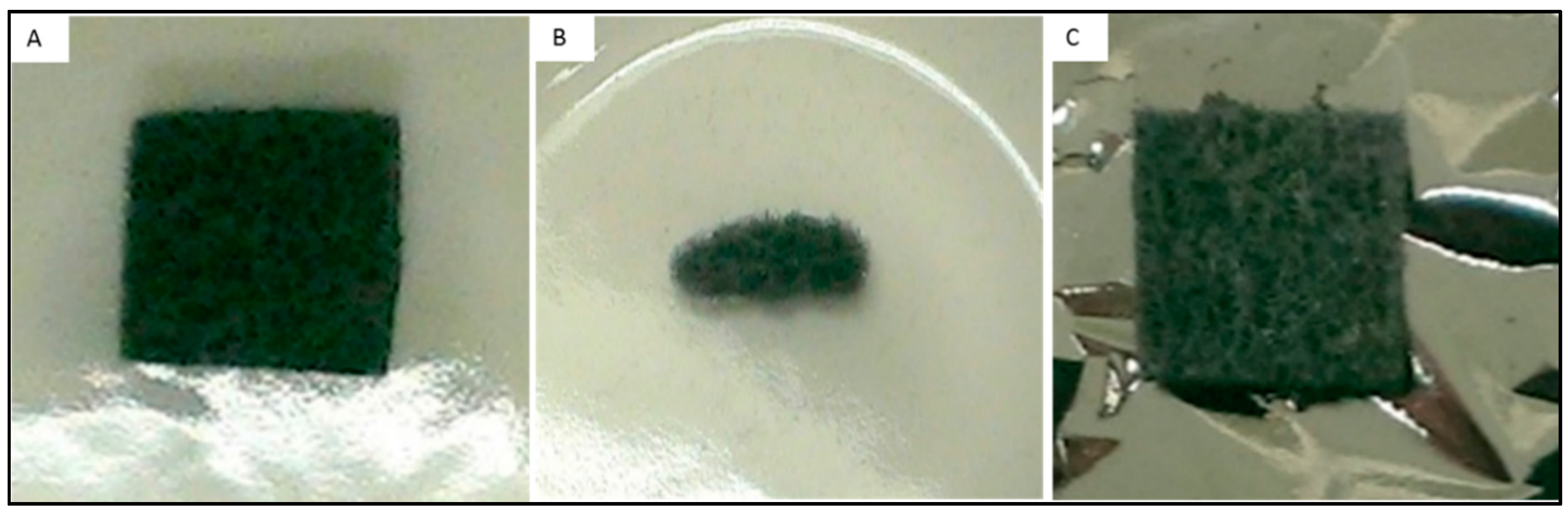

| Material | Cathodic Limit (V) vs. SHE | Reference |
|---|---|---|
| GUITAR | −0.90 ± 0.07 (n = 5) | This work |
| GUITAR/KFD graphite felt | −0.75 ± 0.05 (n = 5) | This work |
| Pyrolytic Graphite | −0.52 ± 0.06 | [22] |
| KFD Graphite felt | −0.40 ± 0.05 (n = 5) | This work |
| Graphite * | −0.4 to −0.5 | [3,4,5] |
| Glassy carbon | −0.3 to −0.5 | [3,4,5] |
| Material | Geometric Surface Area (cm2) | True Surface Area (cm2) | HET Rate Constant (k⁰) for V3+/2+ (cm/s) | Reference |
|---|---|---|---|---|
| GUITAR/KFD graphite felt | 0.10 | 6.1 | 8.6 × 10−6 | This work |
| GUITAR flake | 0.10 | 0.10 | 4.8 × 10−6 | This work |
| KFD graphite felt | 0.16 | 10.3 | 8.2 × 10−7 | This work |
| Non-porous flat electrodes | ||||
| Edge plane pyrolytic graphite | 3.5 × 10−5–5.5 × 10−4 | [21,41] | ||
| Glassy carbon | 0.07 | 1.7 × 10−5–5.4 × 10−5 | [21,41] | |
| Basal plane highly ordered pyrolytic graphite | 0.02 | <3.0 × 10−6 | [42] | |
| High surface area electrodes | ||||
| Graphite reinforcement carbon | 0.08 | 4.8 × 10−3–9.7 × 10−3 | [26] | |
| Plastic formed carbon | 5.3 × 10−4 | [41] | ||
| Carbon felt | 3.0 | 1.5 × 10−7 | [14] | |
| Carbon paper | 0.13 | 128 | 1.07 × 10−3–3.28 × 10−3 | [21] |
| Material | % H2 | Potential (V) | Conditions | How Calculated? and Ref. |
|---|---|---|---|---|
| GUITAR flake | 1 | −1.0 vs. Ag/AgCl | 0.05 M V3+ 1 M H2SO4 | Figure 3 This work |
| 0.3 | −0.8 vs. Ag/AgCl | |||
| GUITAR coated KFD graphite felt | 3 | −1.0 vs. Ag/AgCl | ||
| 0.5 | −0.8 vs. Ag/AgCl | |||
| KFD graphite felt | 22 | −1.0 vs. Ag/AgCl | ||
| 14 | −0.8 vs. Ag/AgCl | |||
| Graphite | 78 | −1.0 vs. SCE | 0.1 M V3+ 2 M H2SO4 | Stated Figure 2b in ref. [17] |
| 22 | −0.8 vs. SCE | |||
| Graphite | 20 | −0.65 vs. SCE | 1 M V3+ 5 M H2SO4 | Calculated Figure 1a,b in ref. [43] |
| Graphite felt | 5–8 | −0.65 vs. Ag/AgCl | 0.05 M V3+ 1 M H2SO4 | Calculated Figures 2b, 4 in ref. [19] |
| Carbon felt | 20 | 2 M V3+ 2.5 M H2SO4 | Stated Figure 6b in ref. [44] | |
| Glassy carbon | 80 | −1.0 vs. SCE | 0.08 M V3+ 1.8 M H2SO4 | Calculated Figure 2 in ref. [45] |
| Porous carbon paper | 10–22 | 1 M V3+ H2SO4:HNO3=3:1 | Stated Table 5 in ref. [46] | |
| Carbon nanotubes | 15 | −0.65 vs. SCE | 1 M V3+ 5 M H2SO4 | Calculated Figure 1a,b in ref. [44] |
| WO3/ASC/CP * | 5–9 | 0.05 M V3+ 3 M H2SO4 | Stated Table 3 in ref. [47] | |
| Titanium nitride/Carbon paper | 12 | −0.7 vs. SCE | 0.1 M V3+, 1 M H2SO4 | Calculated Figure 2 in ref. [33] |
| Graphite plate | 10 | −0.75 vs. SCE | 1.6 M V3+ 3 M H2SO4 | Calculated Figures 3,6 in ref. [32] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, H.; Gyan, I.O.; Foutch, J.D.; Zhu, H.; Cheng, I.F. Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing. C 2016, 2, 13. https://doi.org/10.3390/c2020013
Kabir H, Gyan IO, Foutch JD, Zhu H, Cheng IF. Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing. C. 2016; 2(2):13. https://doi.org/10.3390/c2020013
Chicago/Turabian StyleKabir, Humayun, Isaiah O. Gyan, Jeremy D. Foutch, Haoyu Zhu, and I. Francis Cheng. 2016. "Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing" C 2, no. 2: 13. https://doi.org/10.3390/c2020013
APA StyleKabir, H., Gyan, I. O., Foutch, J. D., Zhu, H., & Cheng, I. F. (2016). Application of GUITAR on the Negative Electrode of the Vanadium Redox Flow Battery: Improved V3+/2+ Heterogeneous Electron Transfer with Reduced Hydrogen Gassing. C, 2(2), 13. https://doi.org/10.3390/c2020013








