A Constructed 2D-Cu2O/Carbon Nitride Heterojunction for Efficient CO2 Photoreduction to CH4
Abstract
1. Introduction
2. Experimental Procedures
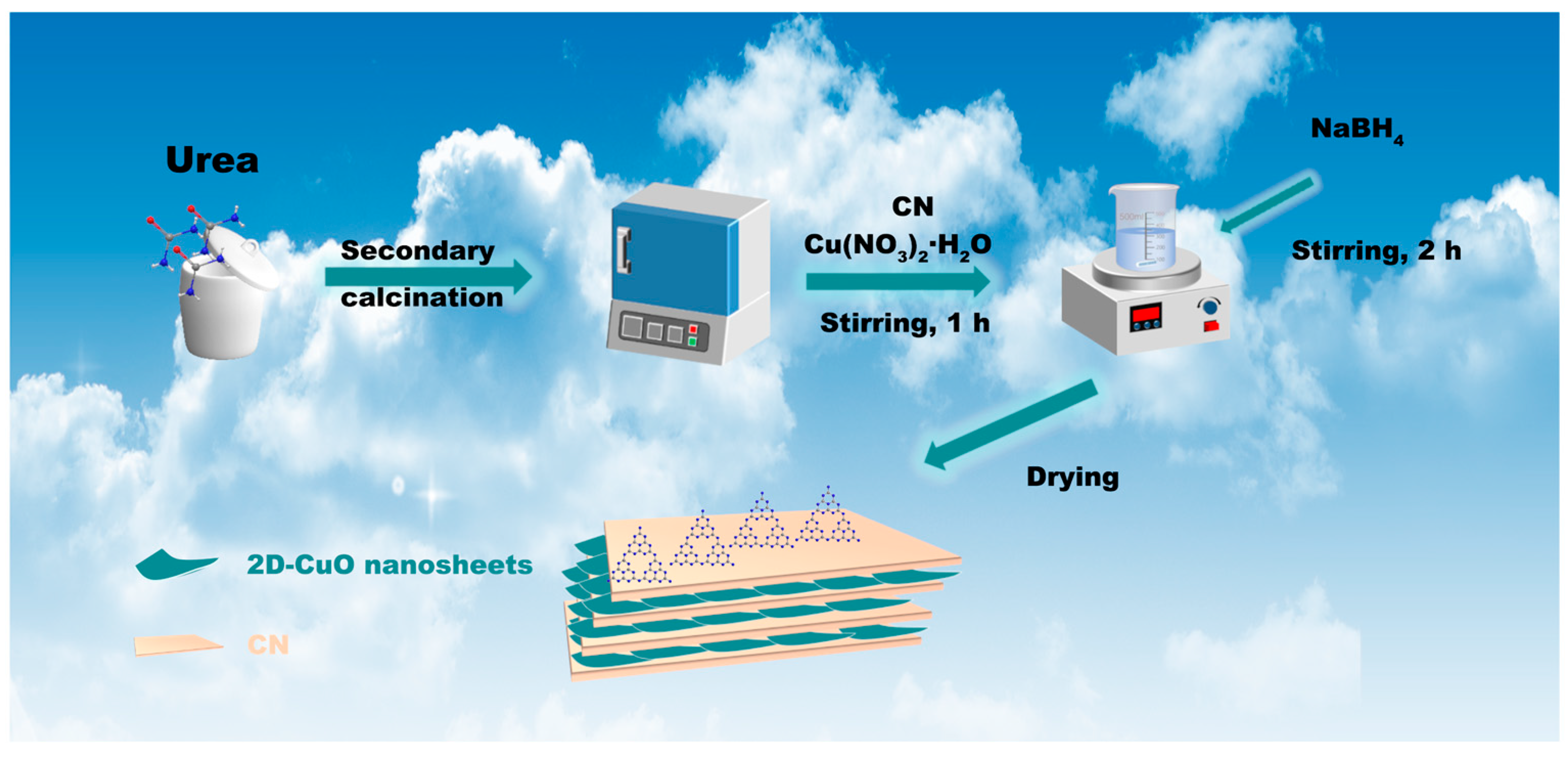
2.1. Synthesis of CN and 2D-Cu2O/CN
2.2. Photocatalytic CO2 Reduction
2.3. Photoelectrochemical Measurements
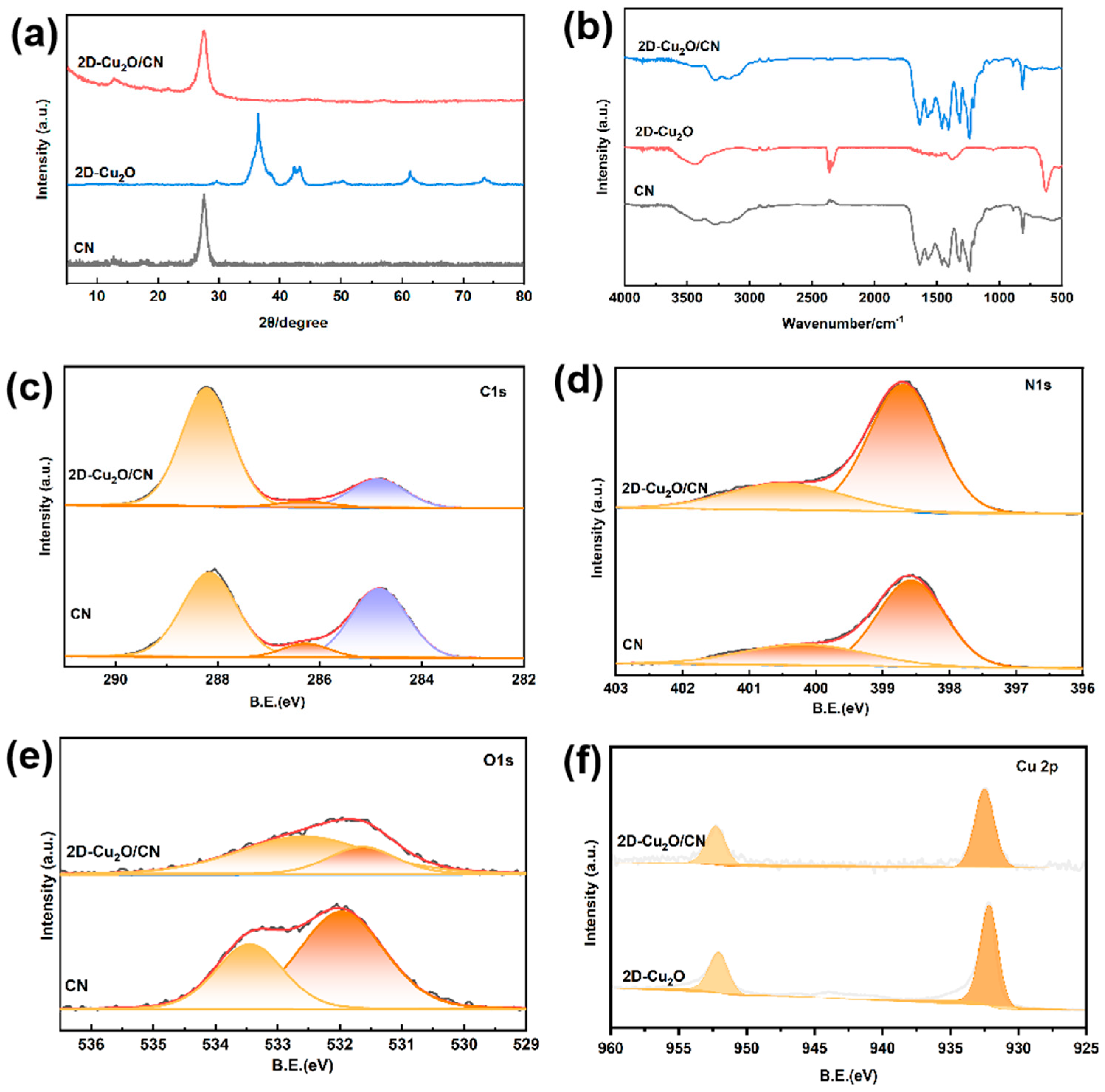
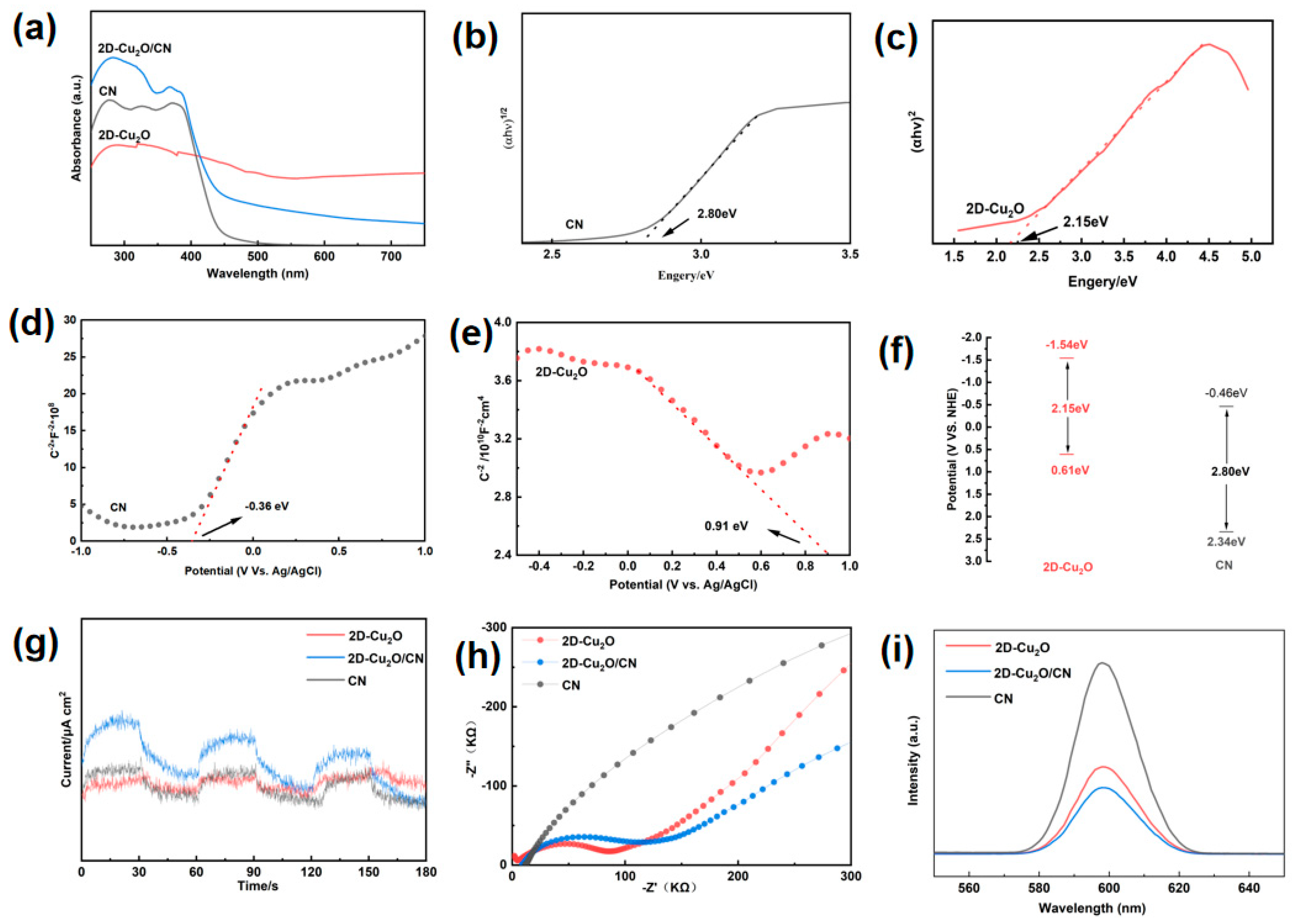
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Grim, R.G.; Ferrell, J.R., III; Huang, Z.; Tao, L. The feasibility of direct CO2 conversion technologies on impacting mid-century climate goals. Joule 2023, 7, 1684–1699. [Google Scholar] [CrossRef]
- Yuan, Z.M.; Zhu, X.L.; Gao, X.Q.; An, C.H.; Wang, Z.; Zuo, C. Enhancing photocatalytic CO2 reduction with TiO2-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnology 2024, 20, 100368. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Liu, F.; Lin, Y.; Chen, C.; Zou, D. Efficiency of CO2 photoreduction to hydrocarbons with K2Fe2O4/rGO heterojunction as a photocatalyst. J. CO2 Util. 2024, 85, 102858. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Cai, M.J.; Wu, Z.Y.; Li, Z. Greenhouse-inspired supra-photothermal CO2 catalysis. Nat. Energy 2021, 6, 807–814. [Google Scholar] [CrossRef]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, L.; Yuan, N.; Zhang, G. Adsorption and activation, active site and reaction pathway of photocatalytic CO2 reduction: A review. Chem. Eng. J. 2024, 481, 148754. [Google Scholar] [CrossRef]
- Wang, L.Q.; Si, W.P.; Tong, Y.Y.; Hou, F. Graphitic carbon nitride (g-C3N4)-based nanosized heteroarrays: Promising materials for photoelectrochemical water splitting. Carbon Energy 2020, 2, 223–250. [Google Scholar] [CrossRef]
- Vuong, H.T.; Nguyen, D.V.; Phuong, P.; Phan, P.D.; Bao, M.; Ho, N.; Nguyen, H.A. Nitrogen-rich graphitic carbon nitride (g-C3N5): Emerging low-bandgap materials for photocatalysis. Carbon Neutralization 2023, 2, 425–457. [Google Scholar] [CrossRef]
- Tang, J.; Guo, C.Y.; Wang, T.T.; Cheng, X.L.; Huo, L.H.; Zhang, X.F.; Huang, C.B. A review of g-C3N4-based photocatalytic materials for photocatalytic CO2 reduction. Carbon Neutralization 2024, 3, 557–583. [Google Scholar] [CrossRef]
- Cao, S.; Liu, H.; Jia, Z. Controllable adsorption groups on amine-functionalized carbon nitride for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2023, 455, 140746. [Google Scholar] [CrossRef]
- Michalska, M.; Pavlovsky, J.; Martynkova, G.S.; Kratosova, G.; Hornok, V.; Nagy, P.B.; Novak, V.; Szabo, T. Comparative study of photocatalysis with bulk and nanosheet graphitic carbon nitrides enhanced with silver. Sci. Rep. 2024, 14, 11512. [Google Scholar] [CrossRef]
- Ren, F.Y.; Sun, Z.; Ma, T.; Zhang, H.; Wei, M.; Chen, S. Recent research progress in photocatalytic reduction of CO2 using g-C3N4-based heterostructures. Fuel Chem. Technol. 2025, 53, 40–52. [Google Scholar] [CrossRef]
- Khan, I.; Sun, Y.S.; Khan, F.; Jing, Z.G.; Kareem, A.; Naseem, M.; Ali, Z. Synthesis, mechanism and environmental applications of g-C3N4 composites: A synergistic approach to adsorption and photocatalysis. Sep. Puri Cation Technol. 2025, 359, 130472. [Google Scholar] [CrossRef]
- Sadanandan, A.; Yang, J.; Devtade, V.; Singh, G.; Dharmarajan, N.; Fawaz, M.; Lee, J.; Tavakkoli, E.; Jeon, C.; Kumar, P. Carbon nitride based nanoarchitectonics for nature-inspired photocatalytic CO2 reduction. Prog. Mater. Sci. 2024, 142, 101242. [Google Scholar] [CrossRef]
- Mo, Z.; Zhu, X.; Jiang, Z.; Song, Y.; Liu, D.; Li, H.; Yang, X.; She, Y.; Lei, Y.; Yuan, S.; et al. Porous nitrogen-rich g-C3N4 nanotubes for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. Energy 2019, 256, 117854. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Qi, M.Y.; Tang, Z.R. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B Environ. Energy 2020, 268, 118380. [Google Scholar] [CrossRef]
- Fu, W.L.; Liu, Z.; Wang, T.Y.; Liang, J.S. Promoting C2+ Production from Electrochemical CO2 Reduction on Shape-Controlled Cuprous Oxide Nanocrystals with High-Index Facets. ACS Sustain. Chem. Eng. 2020, 8, 15223–15229. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2017, 300, 160–172. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Hu, J. Polymeric carbon nitride loaded with atomic Cu sites for improved CO2 photocatalytic conversion performance. J. Power Sources 2023, 577, 233188. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Mane, G.; Rane, V.; Tripathi, A.; Tyagi, A. Selective CO2 Photoreduction with Cu-Doped TiO2 Photocatalyst: Delineating the Crucial Role of Cu-Oxidation State and Oxygen Vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Lee, D.; Satyanarayana, M.; Bhosale, R.; Jo, W.; Tonda, S. Cu–Ni core–shell bimetallic cocatalyst decorated polymeric carbon nitride for highly efficient and selective methane production from photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 599, 153973. [Google Scholar] [CrossRef]
- Li, N.; Li, H.; Xu, C.; Zhou, Z.; Rao, T.; Ji, R.; Lin, S.; Du, J.; Xu, S.; Lyu, S.; et al. Synergistic enhanced activation of peroxymonosulfate by heterojunction Co3O4-CuO@CN for removal of oxytetracycline: Performance, mechanism, and stability. Environ. Res. 2023, 234, 116517. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.W.; Yu, J.G.; Jiang, C.J.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Kong, M.D.; Li, P.; Lu, J.; Zhou, J.W.; Wu, J.K.; Xu, J.X.; Lin, H.F.; Wang, H.; Li, Y.Y.; Wang, L. Synergistic spatial charge separation of intimate 2D/2D/2D dual heterojunctions for efficient visible-light photocatalysis. J. Alloys Compd. 2025, 1038, 182769. [Google Scholar] [CrossRef]
- Cao, S.W.; Shen, B.J.; Tong, T.; Fu, J.W.; Yu, J.G. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Guan, Z.J.; Pan, J.W.; Li, Q.Y.; Li, G.Q.; Yang, J.J. Boosting Visible-Light Photocatalytic Hydrogen Evolution with an Efficient CuInS2/ZnIn2S4 2D/2D Heterojunction. ACS Sustain. Chem. Eng. 2019, 7, 7736–7742. [Google Scholar] [CrossRef]
- Bao, Y.C.; Pan, J.K.; Wu, H.; Zhang, Z.S.; Li, Y.J.; Wang, Z.L.; Hui, T.T.; Yang, B.; Li, J.N.; Hu, H.T.; et al. Designed 2D/2D/2D Bi2WO6/Ti3C2/SnNb2O6 Z-scheme heterojunction via interfacial chemical bond with enhanced photocatalytic activity. J. Alloys Compd. 2023, 948, 169762. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, J.; Jin, Z. Reduced graphene oxide assisted g-C3N4/rGO/NiAl-LDHs type II heterostructure with high performance photocatalytic CO2 reduction. Chem. Eng. J. 2022, 450, 138108. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Sellers, M.C.K.; Seebauer, E.G. Measurement method for carrier concentration in TiO2 via the Mott–Schottky approach. Thin Solid Films 2011, 519, 2103–2110. [Google Scholar] [CrossRef]
- Wang, J.M.; Kim, E.; Kumar, D.P.; Rangappa, A.P.; Kim, Y.; Zhang, Y.X.; Kim, T.K. Highly Durable and Fully Dispersed Cobalt Diatomic Site Catalysts for CO2 Photoreduction to CH4. Angew. Chem. Int. Ed. 2022, 61, e202113044. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, Y.Y.; Zhang, W.S.; Zhang, X.J.; Han, D.X. Nanoengineering Construction of Cu2O Nanowire Arrays Encapsulated with g-C3N4 as 3D Spatial Reticulation All-Solid-State Direct Z-Scheme Photocatalysts for Photocatalytic Reduction of Carbon Dioxide. ACS Catal. 2020, 10, 6367–6376. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Yin, H.B. Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction. Catalysts 2025, 15, 599. [Google Scholar] [CrossRef]
- Zhu, C.; Zhong, K.; Zhu, B.; Li, S.; Li, H.; Yang, J.; Xu, H. Engineering the S-scheme heterojunction modulating the charge density of the central carbon atom of half-metallic carbon nitride for boosting CO2 photoreduction. Appl. Catal. B Environ. Energy 2025, 371, 125200. [Google Scholar] [CrossRef]
- Li, X.; Guan, J.; Jiang, H.; Song, X.; Huo, P.; Wang, H. rGO modified R-CeO2/g-C3N4 multi-interface contact S-scheme photocatalyst for efficient CO2 photoreduction. Appl. Surf. Sci. 2021, 563, 150042. [Google Scholar] [CrossRef]
- Sabir, M.; Qiu, G.G.; Yang, S.Y.; Tahir, M. Femtosecond transient absorption spectroscopy investigation on Cu-MOF/g-C3N4 S-scheme heterojunction for photocatalytic CO2 reduction. J. Environ. Chem. Eng. 2025, 13, 119415. [Google Scholar] [CrossRef]
- Tao, F.F.; Dong, Y.L.; Yang, L. A NiO/g-C3N4 p-n Heterojunctions Wrapped by rGO for the Enhanced CO2 Photocatalytic Reduction. ACS Sustain. Chem. Eng. 2024, 12, 6709–6718. [Google Scholar] [CrossRef]
- Manikandan, M.; Anandharam, E.; Manikandan, E. Engineering NiO/g-C3N4 and NiO/rGO composites for dual applications in electrochemical water splitting and energy storage. Sci. Rep. 2025, 15, 36708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, J.; Xu, Q.; Duan, L.; Guo, H. Understanding inclusive quantum dots hollow CN@CIZS heterojunction for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 604, 154601. [Google Scholar] [CrossRef]
- Dong, Y.H.; Pan, A.Z. Fabricating a type II heterojunction by growing lead-free perovskite Cs2AgBiBr6 in situ on graphite-like g-C3N4 nanosheets for enhanced photocatalytic CO2 reduction. Nanoscale 2023, 15, 15619–15625. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Liu, J.; Zhang, X.; Gao, J.; Liu, X. A Constructed 2D-Cu2O/Carbon Nitride Heterojunction for Efficient CO2 Photoreduction to CH4. C 2026, 12, 6. https://doi.org/10.3390/c12010006
Liu J, Zhang X, Gao J, Liu X. A Constructed 2D-Cu2O/Carbon Nitride Heterojunction for Efficient CO2 Photoreduction to CH4. C. 2026; 12(1):6. https://doi.org/10.3390/c12010006
Chicago/Turabian StyleLiu, Jialiang, Xiaoxuan Zhang, Jiaxuan Gao, and Xuanhe Liu. 2026. "A Constructed 2D-Cu2O/Carbon Nitride Heterojunction for Efficient CO2 Photoreduction to CH4" C 12, no. 1: 6. https://doi.org/10.3390/c12010006
APA StyleLiu, J., Zhang, X., Gao, J., & Liu, X. (2026). A Constructed 2D-Cu2O/Carbon Nitride Heterojunction for Efficient CO2 Photoreduction to CH4. C, 12(1), 6. https://doi.org/10.3390/c12010006




