An Innovative Solution Method for the Evaluation of CO2 Disposal in the Seafloor Environment
Abstract
1. Introduction
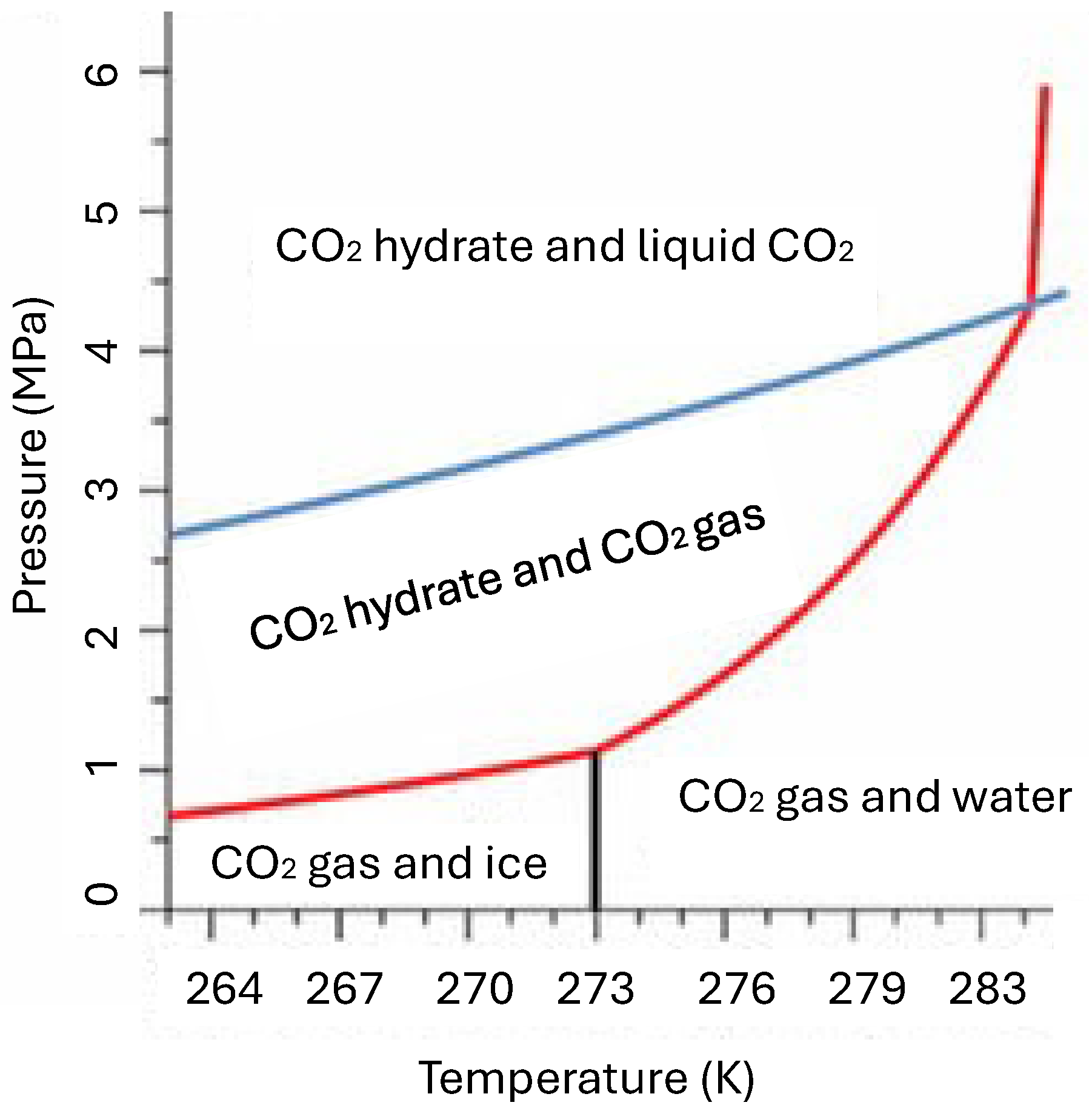
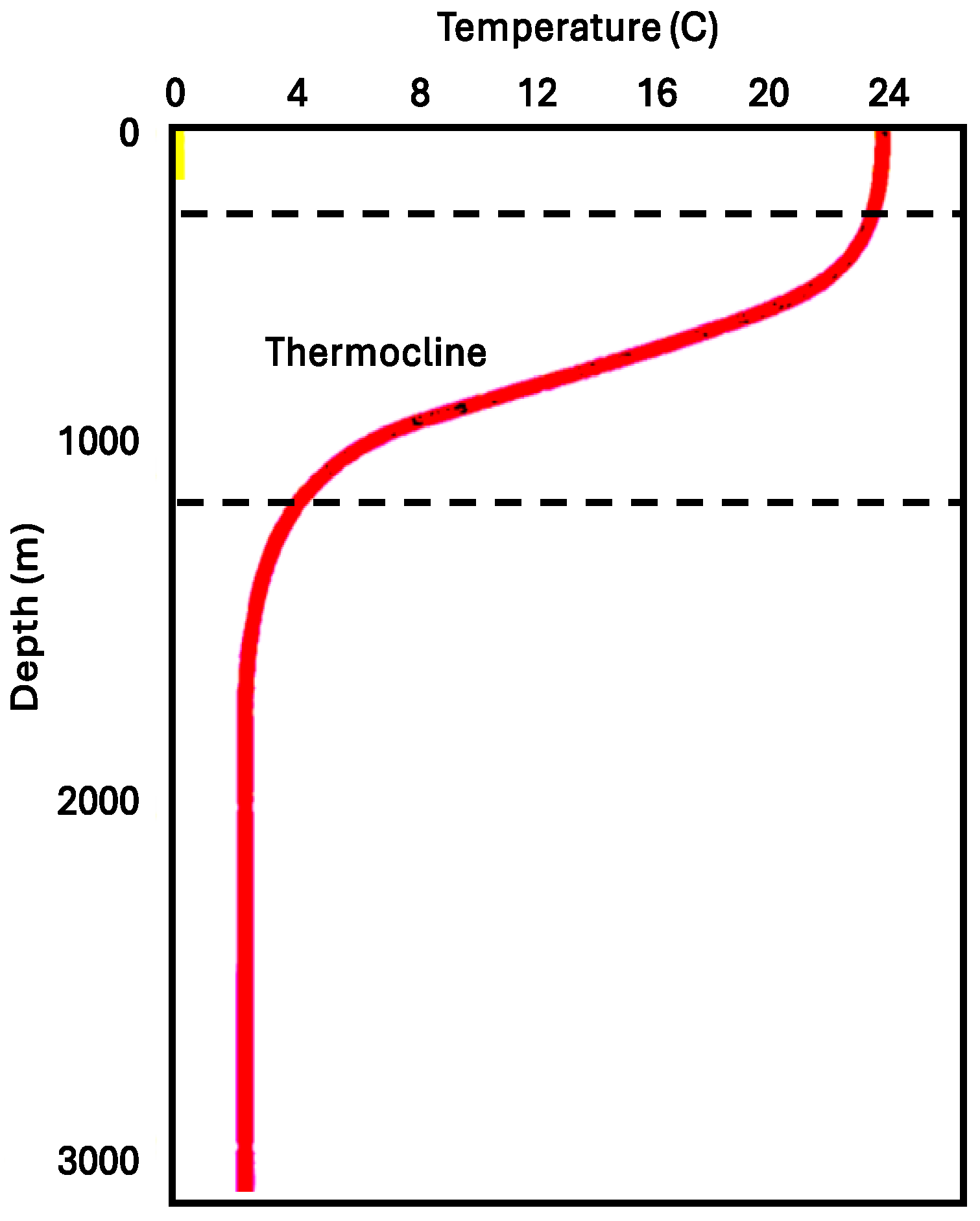
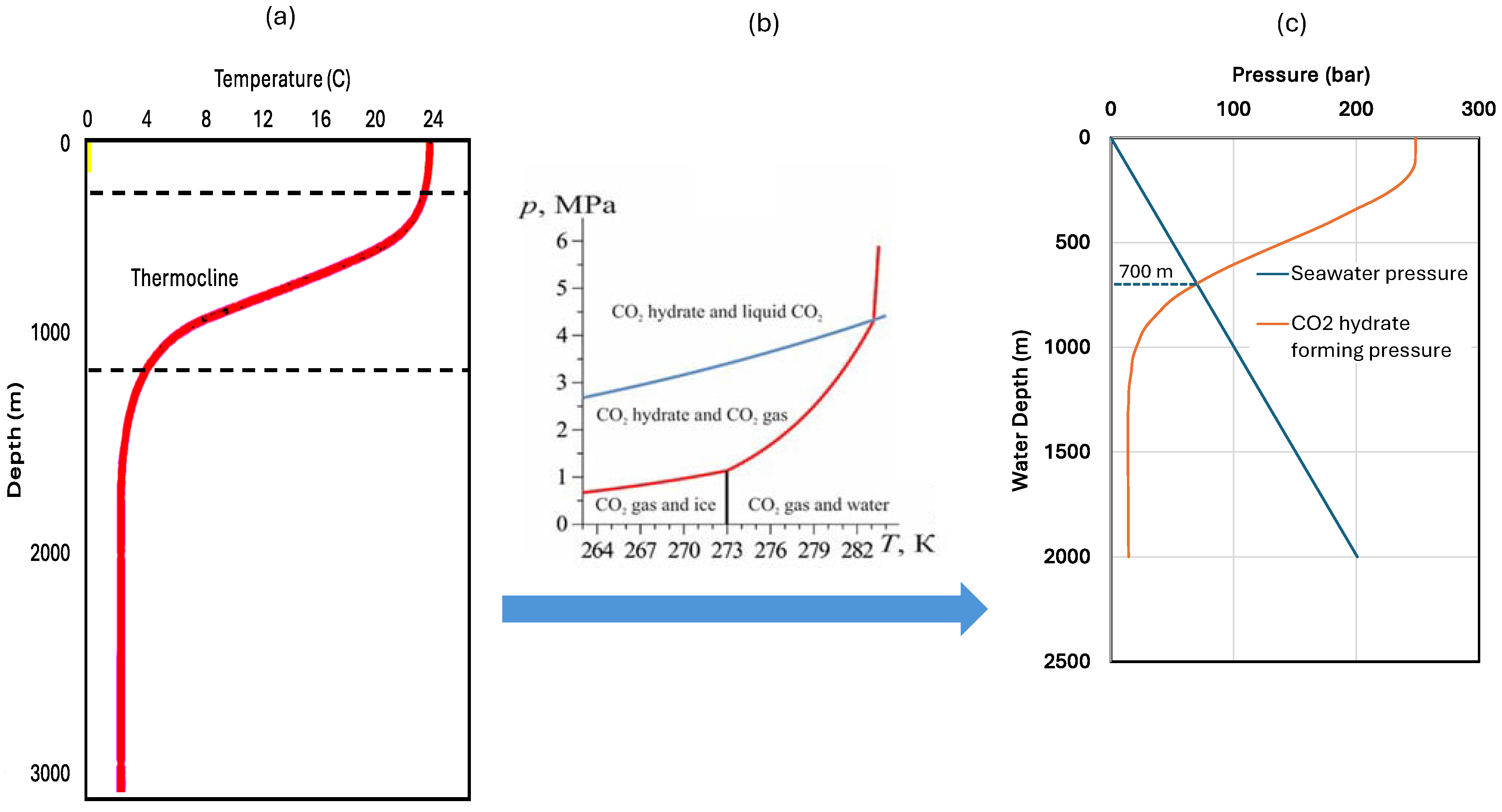
2. Identification of Suitable Seawater Depths
3. Analytical Method
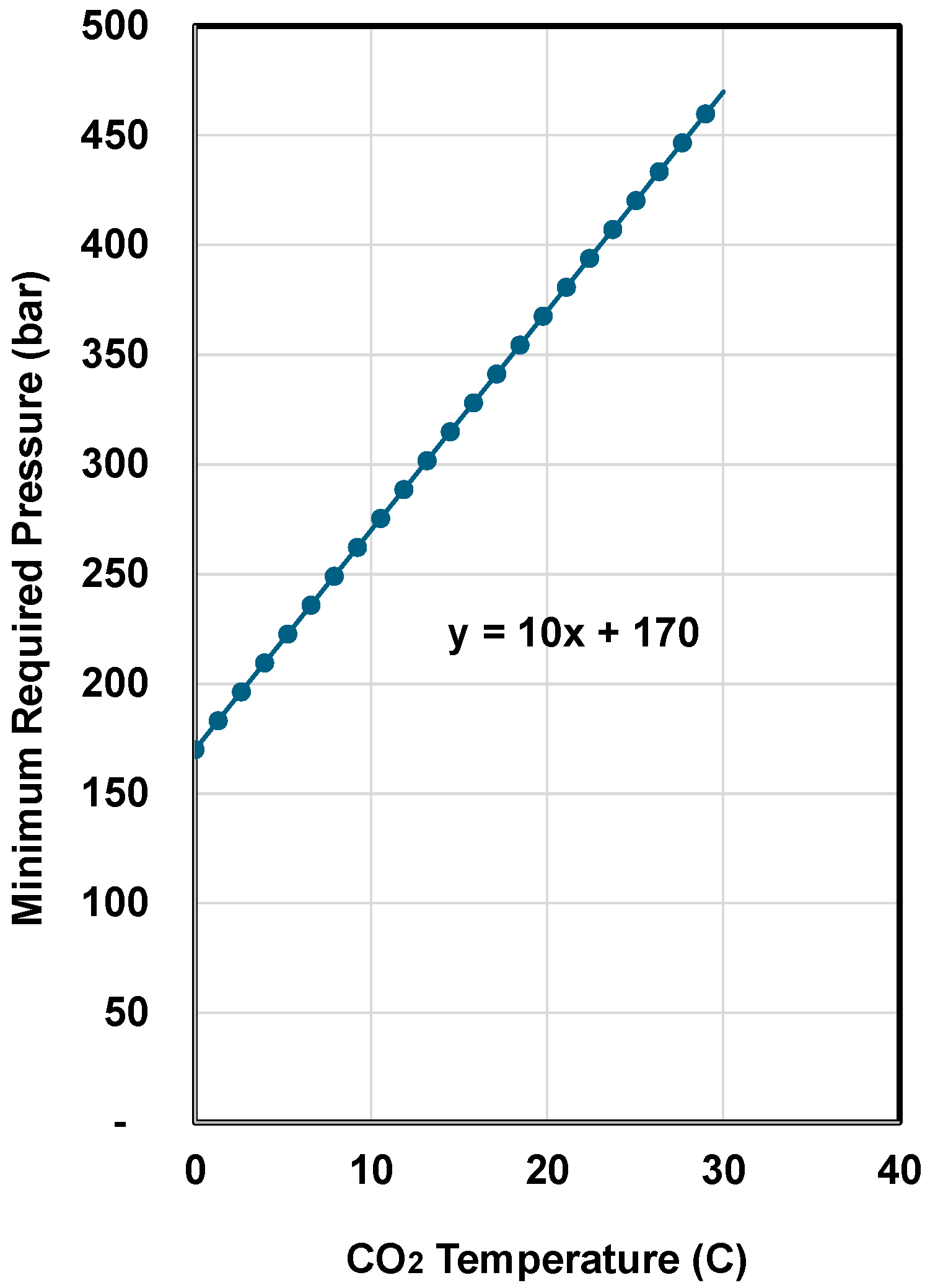
4. System Requirement
- 1
- Select a seafloor site that is deeper than the critical water depth determined in the procedure shown in Figure 5. Designthe seawater depth for CO2 placement.
- 2
- Calculate the hydrostatic pressure of seawater pdn at the water depth of CO2 placement. This pressure is considered to be the downstream pressure of the jetting nozzles.
- 3
- Calculate the required upstream pressure pup of the jetting nozzles using
5. Discussion
- Conducting experimental investigations of the proposed jet-cooling process to validate the concept. This is being designed and prepared using a high-pressure–low-temperature windowed cell to observe the settling of CO2 hydrates and CO2 liquid due to gravitational segregation.
- Performing a quantitative hydrate formation kinetics analysis to determine the minimum required residence time of the jet plume for full solidification under the seawater conditions of the proposed process. This information will be used for designing the geometry (diameter and length) of the buffer chamber to ensure 100% conversion of CO2 to hydrates before the CO2 plume exits the buffer chamber.
- Carrying out research work to analyze how CO2 liquid and hydrates might be disturbed by bottom sea currents, interact with sediments and nearby man-made structures, warming scenarios of geological events (e.g., earthquake and volcano rupture), extreme weather (e.g., hurricanes and tsunamis), and human activities such as the aviation of artificial objects.
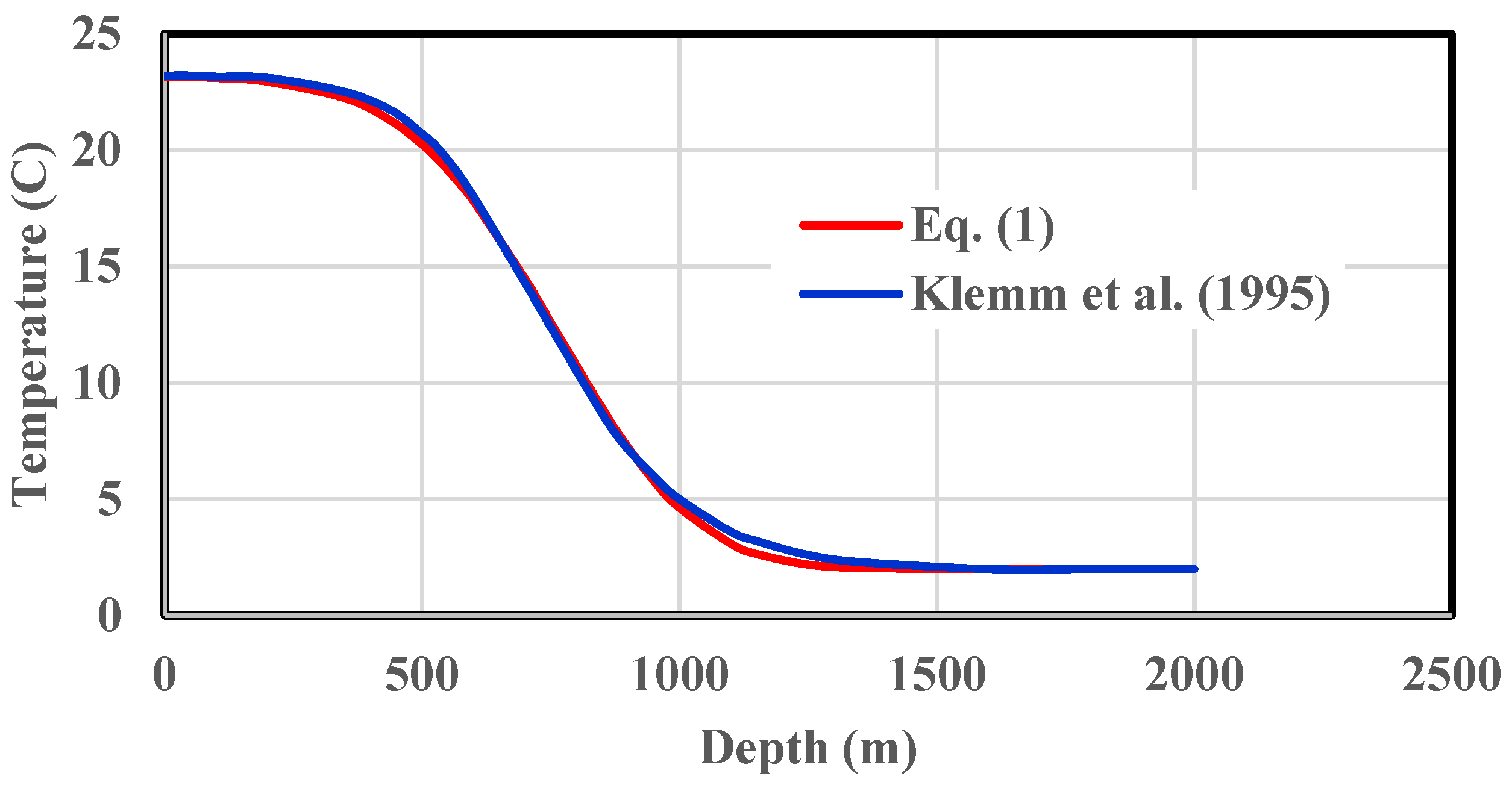
- It is understood that Equation (1) was established based on the data in Figure 2. Under other seawater conditions, either the actual temperature data from local survey or Equation (1) validated using the actual data should be used to determine the critical water depth for CO2 hydrate formation.
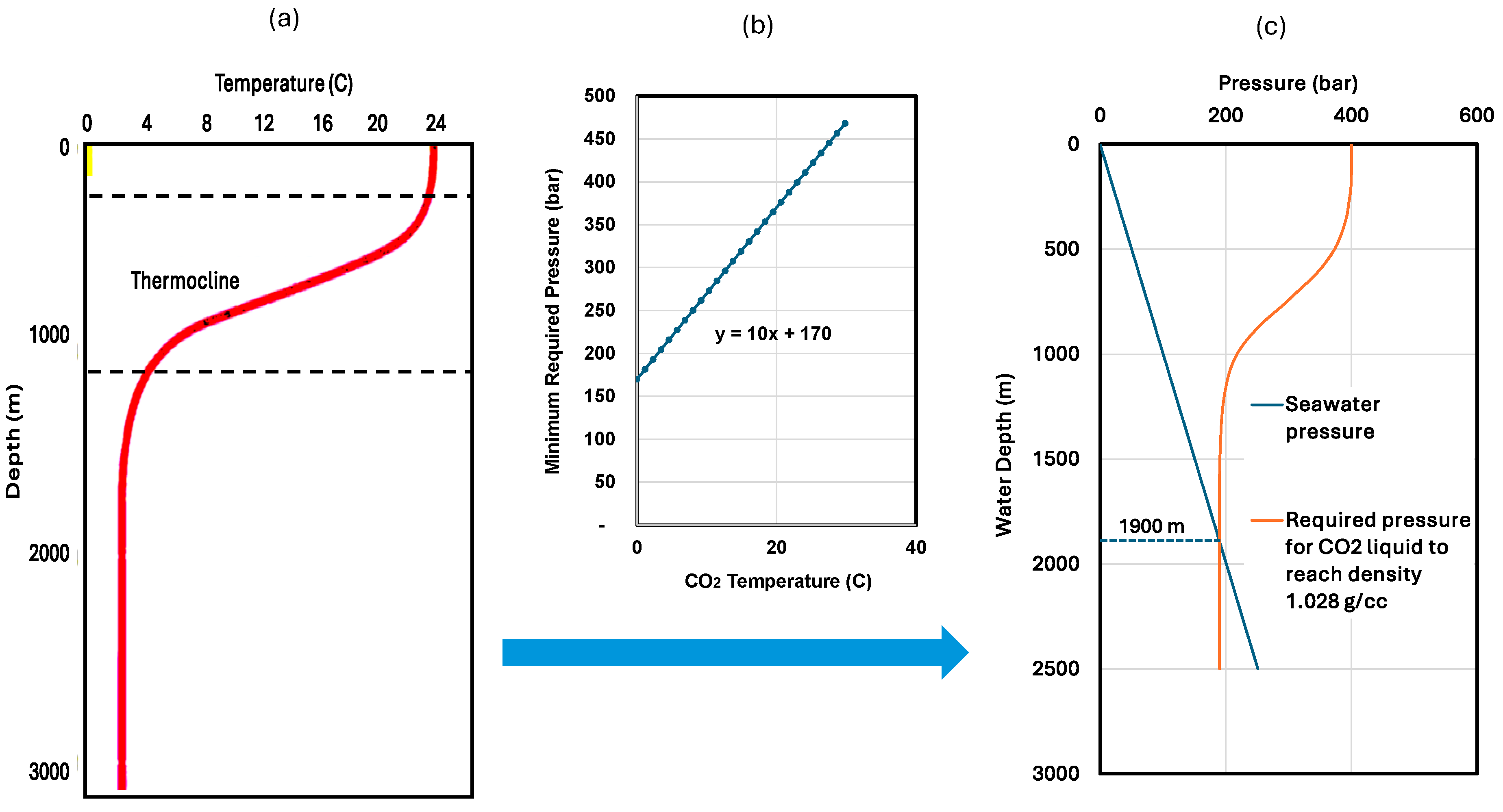
- Application of the proposed technique is limited to disposal water depths greater than the critical disposal water depths, typically 700 m for storage in hydrate form and 1900 m for storage in liquid form. Local studies need to be conducted in real applications. The major drawback of the technique is that it requires significant investment in building the CO2-disposing ship for CO2 disposal and CO2 carrier for CO2 delivery.
6. Conclusions
- CO2 can be stored on the seafloor in its liquid-to-hydrate form if the seawater is deep enough. In a typical seawater environment, if the seafloor depth is greater than 6200 ft (1900 m), CO2 can be injected directly onto the seafloor in liquid form. The liquid CO2 will then form hydrates and stay on the seafloor.
- CO2 can be stored on the seafloor in its hydrate form if the seafloor is deep enough. In a typical seawater environment, if the seafloor depth is greater than 700 m, CO2 can be injected through nozzles to reduce its temperature and generate hydrates. The generated hydrates will then be deposited on the sea floor.
- The nozzle for reducing the CO2 temperature to generate hydrate is the key component of the proposed technology suite. The nozzles should be sized based on the required upstream pressure and CO2 flow rate. Sonic (critical) flow condition is preferred to maximize the cooling efficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, B.; Wang, B.; Li, X.; Aishan, M.; Ju, Y. CO2 storage in depleted oil and gas reservoirs: A review. Adv. Geo-Energy Res. 2023, 9, 76–93. [Google Scholar] [CrossRef]
- Duguid, A.; Guo, B.; Nygaard, R. Well integrity assessment of monitoring wells at an active CO2-EOR flood. Energy Proc. 2017, 114, 5118–5138. [Google Scholar] [CrossRef]
- Guo, B. Statistical Analysis of CO2 Exposed Wells to Predict Long Term Leakage Through the Development of an Integrated Neural-Genetic Algorithm; Report to U.S. DOE project DE FE0009284; University of Louisiana at Lafayette: Lafayette, LA, USA, 2017. [Google Scholar]
- Li, Q.; Li, Q.; Wang, F.; Xu, N.; Wang, Y.; Bai, B. Settling behavior and mechanism analysis of kaolinite as a fracture proppant of hydrocarbon reservoirs in CO2 fracturing fluid. Colloids Surf. A Physicochem. Eng. Asp. 2025, 724, 137463. [Google Scholar] [CrossRef]
- Aoyagi, R. Study on role of simulation of possible leakage from geological CO2 storage in sub-seabed for environmental safety. Energy Procedia 2011, 4, 3881–3888. [Google Scholar] [CrossRef]
- University of Oslo. Storage of CO2 in Safe Geological Formations in the Subsurface Can Be an Important Strategy to Reduce CO2 Gasses in the Atmosphere. 19 January 2024. Available online: https://www.mn.uio.no/geo/english/research/groups/co2-storage/index.html (accessed on 1 May 2025).
- Ogawa, T.; Nakanishi, S.; Shidahara, T.; Okumura, T.; Hayashi, E. Saline-aquifer CO2 sequestration in Japan-methodology of storage capacity assessment. Int. J. Greenh. Gas Control 2011, 5, 318–326. [Google Scholar] [CrossRef]
- Pilisi, N.; Ghorbani, D.; Vasantharajan, S. CO2 Sequestration in Deepwater Sediments Offshore Japan. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012. [Google Scholar] [CrossRef]
- JOGMEC. Overview of Request for Proposal on “Business Feasibility Study on Japanese Advanced CCS Project” in FY2023. (30 March 2023). Available online: https://www.jogmec.go.jp/english/news/release/news_10_00036.html (accessed on 15 December 2024).
- Anchondo, C. Offshore CO2 Storage Project Nabs $26M DOE Grant. E$E News, 11/19/2024. 2024. Available online: https://www.eenews.net/articles/offshore-co2-storage-project-nabs-26m-doe-grant/ (accessed on 20 April 2025).
- Genetti, D. 2024: A Breakout Year for Our Carbon Capture and Storage Business, 9 January 2025. Available online: https://corporate.exxonmobil.com/news/viewpoints/a-breakout-year-for-our-carbon-capture-and-storage-business (accessed on 25 June 2025).
- Aramis, 1024. A Large-Scale CO2 Transport and Storage Service. Available online: https://www.aramis-ccs.com/ (accessed on 15 March 2025).
- EniSpA. UK Announces the Launch of the Bacton Thames Net Zero Cooperation Agreement. 21 November 2022. Available online: https://www.eni.com/en-IT/media/press-release/2022/11/eni-uk-announces-launch-bacton-thames-net-zero-cooperation-agreement.html (accessed on 12 April 2025).
- Ineos. Project Greensand. 2025. Available online: https://www.ineos.com/news/shared-news/ineos-led-greensand-to-become-the-first-full-scale-co2-storage-facility-in-eu-to-help-mitigate-climate-change/ (accessed on 12 April 2025).
- L10 CCS. Safe CO2 Storage in the North Sea at Low Cost. Available online: https://l10ccs-storage.com (accessed on 12 April 2025).
- CIEL (Center for International Environmental Law). Deep Trouble: The Risks of Offshore Carbon Capture and Storage. CIEL Report (November 2023), Reissued July 2024. Available online: https://www.ciel.org/reports/deep-trouble-the-risks-of-offshore-carbon-capture-and-storage-november-2023/ (accessed on 12 April 2025).
- IEEFA; Roberston, B.; Mousavian, M. The Carbon Capture Crux: Lessons Learned, 1 September 2022. Available online: https://www.ieefa.org/resources/carbon-capture-crux-lessons-learned (accessed on 12 April 2025).
- IEEFA; Mattei, S.; Schlissel, D. The Ill-Fated Petra Nova CCS Project: NRG Energy Throws in the Towel, 5 October 2023. Available online: https://ieefa.org/resources/ill-fated-petra-nova-ccs-project-nrg-energy-throwstowel (accessed on 12 April 2025).
- NETL: Kemper County Energy Facility: Demonstration of a Coal-Based Transport Gasifier, September 2017. Available online: https://www.netl.doe.gov/sites/default/files/netl-file/NT42391.pdf (accessed on 12 April 2025).
- Rastelli, E.; Corinaldesi, C.; Dell’Anno, A.; Amaro, T.; Queirós, A.M.; Widdicombe, S.; Danovaro, R. Impact of CO2 leakage from sub-seabed carbon dioxide capture and storage (CCS) reservoirs on benthic virus–prokaryote interactions and functions. Front. Microbiol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Vinca, A.; Emmerling, J.; Tavoni, M. Bearing the Cost of Stored Carbon Leakage. Front. Energy Res. 2018, 6, 40. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Skovborg, P.; Rasmussen, P. A mass transport limited model for the growth of methane and ethane gas hydrates. Chem. Eng. Sci. 1994, 49, 1131–1143. [Google Scholar] [CrossRef]
- Mohebbi, V.; Naderifar, A.; Behbahani, R. A mass transfer study of methane and ethane during hydrate formation. Pet. Sci. Technol. 2014, 32, 1418–1425. [Google Scholar] [CrossRef]
- Kashchiev, D.; Firoozabadi, A. Driving force for crystallization of gas hydrates. J. Cryst. Growth 2002, 241, 220–230. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Natarajan, V.; Bishnoi, P.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075–2087. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Lee, H.; Moudrakovski, I.L.; Ripmeester, J.A.; Seo, Y. Kinetics of methane hydrate replacement with carbon dioxide and nitrogen gas mixture using in situ NMR spectroscopy. Environ. Sci. Technol. 2015, 49, 1964–1971. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Xu, J.; Teng, Y.; Ling, Z.; Zhang, Y.; Jiang, L.; Song, Y. A review of the gas hydrate phase transition with a microfluidic approach. Energy Rev. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Rahman, M.J.; Fawad, M.; Jahren, J.; Mondol, N.H. Influence of depositional and diagenetic processes on caprock properties of CO2 storage sites in the northern North Sea, offshore Norway. Geosciences 2022, 12, 181. [Google Scholar] [CrossRef]
- Brewer, P.G.; Peltzer, E.T.; Walz, P.M.; Coward, E.K.; Stern, L.A.; Kirby, S.H.; Pinkston, J. Deep-Sea Field Test of the CH4 Hydrate to CO2 Hydrate Spontaneous Conversion Hypothesis. Energy Fuels 2014, 28, 7061–7069. [Google Scholar] [CrossRef]
- Niu, M.; Yao, Y.; Yin, Z.; Liu, K.; Bian, P.; Zi, M.; Chen, D. Synergistic CH4 hydrate recovery and CO2 storage by coupling depressurization with CO2/N2 injection: A pilot-scale investigation. Chem. Eng. J. 2023, 475, 146216. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Q. Construction of self-reliance technology system for development of green ocean energy. Res. Front. 2022, 16, 1–8. [Google Scholar]
- University of Texas at Austin. Gulf Coast Ready to Develop Carbon Storage Hub. Phys.Org. 6 July 2021. Available online: https://phys.org/news/2021-07-gulf-coast-ready-carbon-storage.html (accessed on 12 April 2025).
- Guo, B.; Zhang, P. Theoretical assessment of CO2 injection into low-temperature water zones for non-leaking storage in hydrate form. Adv. Geo-Energy Res. 2023, 10, 1–6. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, P. Injectivity Assessment of Radial-Lateral Wells for CO2 Storage in Marine Gas Hydrate Reservoirs. Energies 2023, 16, 7987. [Google Scholar] [CrossRef]
- Guo, B.; Islam, M.; Mahmood, M. Carbon dioxide hydrate formation in porous media under dynamic conditions. Energy Sci. Eng. 2024, 12, 5266–5271. [Google Scholar] [CrossRef]
- Mahmood, M.; Guo, B. A Feasibility Study of Using Geothermal Energy to Enhance Natural Gas Production from Offshore Gas Hydrate Reservoirs by CO2 Swapping. Energy Eng. 2023, 120, 2707. [Google Scholar] [CrossRef]
- Khasanov, M.K.; Musakaev, N.G.; Stolpovsky, M.V.; Kildibaeva, S.R. Mathematical model of decomposition of methane hydrate during the injection of liquid carbon dioxide into a reservoir saturated with methane and its hydrate. Mathematics 2020, 8, 1482. [Google Scholar] [CrossRef]
- Klemm, E.B.; Pottenger, F.M.; Speitel, T.W.; Reed, S.A.; Coopersmith, F.A. The Fluid Earth; Curriculum Research & Development Group: Honolulu, HI, USA, 1990. [Google Scholar]
- Klemm, E.B.; Reed, S.A.; Pottenger, F.M.; Porter, C.; Speitel, T.W. The Living Ocean; Curriculum Research & Development Group: Honolulu, HI, USA, 1995. [Google Scholar]
- Guo, B.; Mahmood, M.N. Early assessment of carbon dioxide storage on seafloor using jet-cooling technology. J. Environ. Prot. 2025, 16, 695–705. [Google Scholar] [CrossRef]
- Calsep. Characterization of CO2 Rich Fluids. Available online: https://calsep.com/23-characterization-of-co2-rich-fluids/ (accessed on 12 April 2025).
- Kamath, V.A. Study of Heat Transfer Characteristics During Dissociation of Gas Hydrates in Porous Media. Ph.D. Dissertation, University of Pittsburgh, Ann Arbor, MI, USA, 1984. [Google Scholar]
- Guo, B.; Ghalambor, A. Natural Gas Engineering Handbook, 2nd ed.; Gulf Publishing Company: Houston, TX, USA, 2012; pp. 81–85. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Islam, M.T.; Amponsah, V.N.B. An Innovative Solution Method for the Evaluation of CO2 Disposal in the Seafloor Environment. C 2025, 11, 81. https://doi.org/10.3390/c11040081
Guo B, Islam MT, Amponsah VNB. An Innovative Solution Method for the Evaluation of CO2 Disposal in the Seafloor Environment. C. 2025; 11(4):81. https://doi.org/10.3390/c11040081
Chicago/Turabian StyleGuo, Boyun, Muhammad Towhidul Islam, and Vincent Nana Boah Amponsah. 2025. "An Innovative Solution Method for the Evaluation of CO2 Disposal in the Seafloor Environment" C 11, no. 4: 81. https://doi.org/10.3390/c11040081
APA StyleGuo, B., Islam, M. T., & Amponsah, V. N. B. (2025). An Innovative Solution Method for the Evaluation of CO2 Disposal in the Seafloor Environment. C, 11(4), 81. https://doi.org/10.3390/c11040081






