Characterisation of a Biodegradable Electrode Substrate Based on Psyllium Husk–Carbon Nanoparticle Composites
Abstract
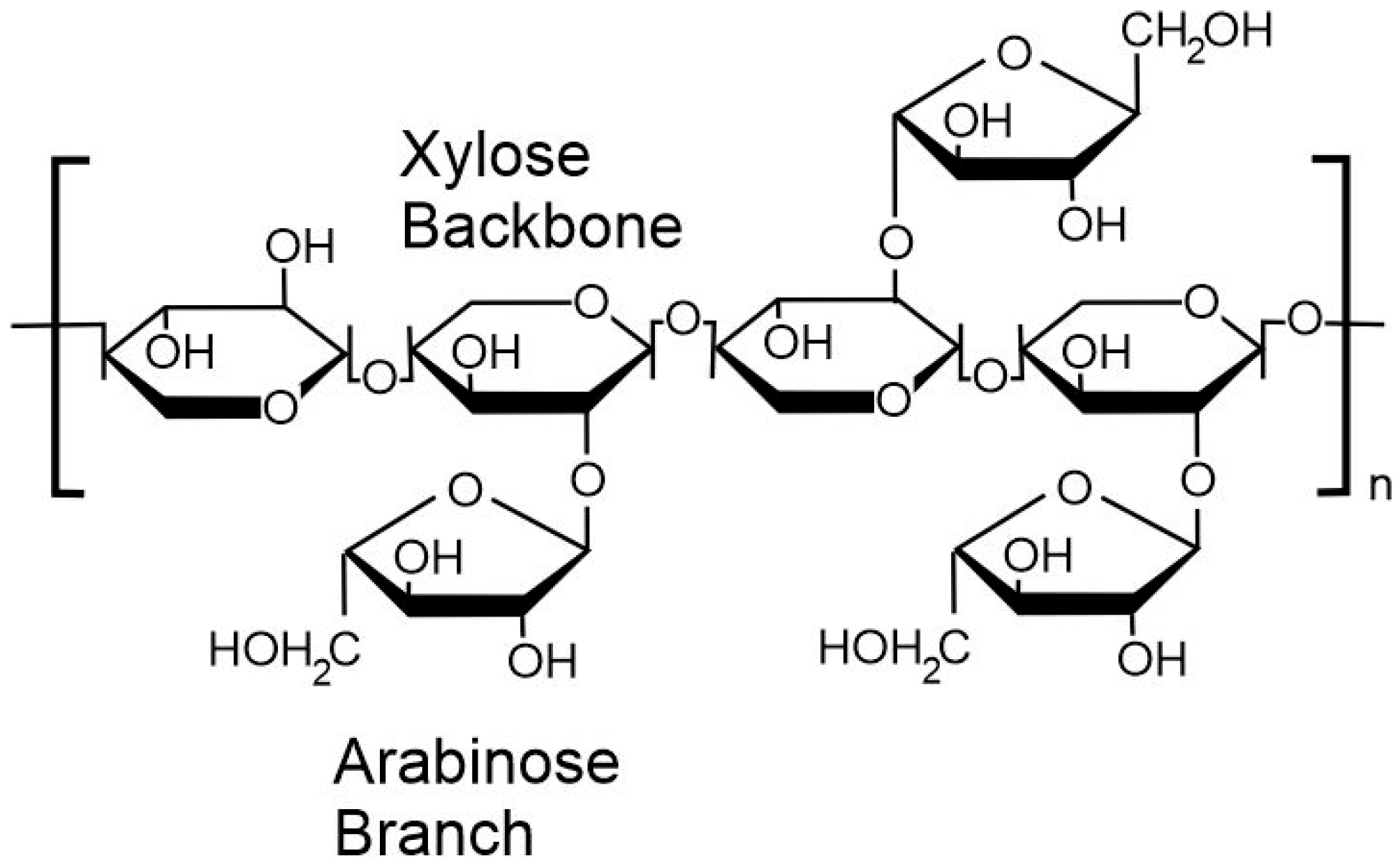
1. Introduction
2. Materials and Methods
2.1. Preparation of Carbon Composite Films
2.2. Preparation of Psyllium Husk–Carbon Film Electrodes
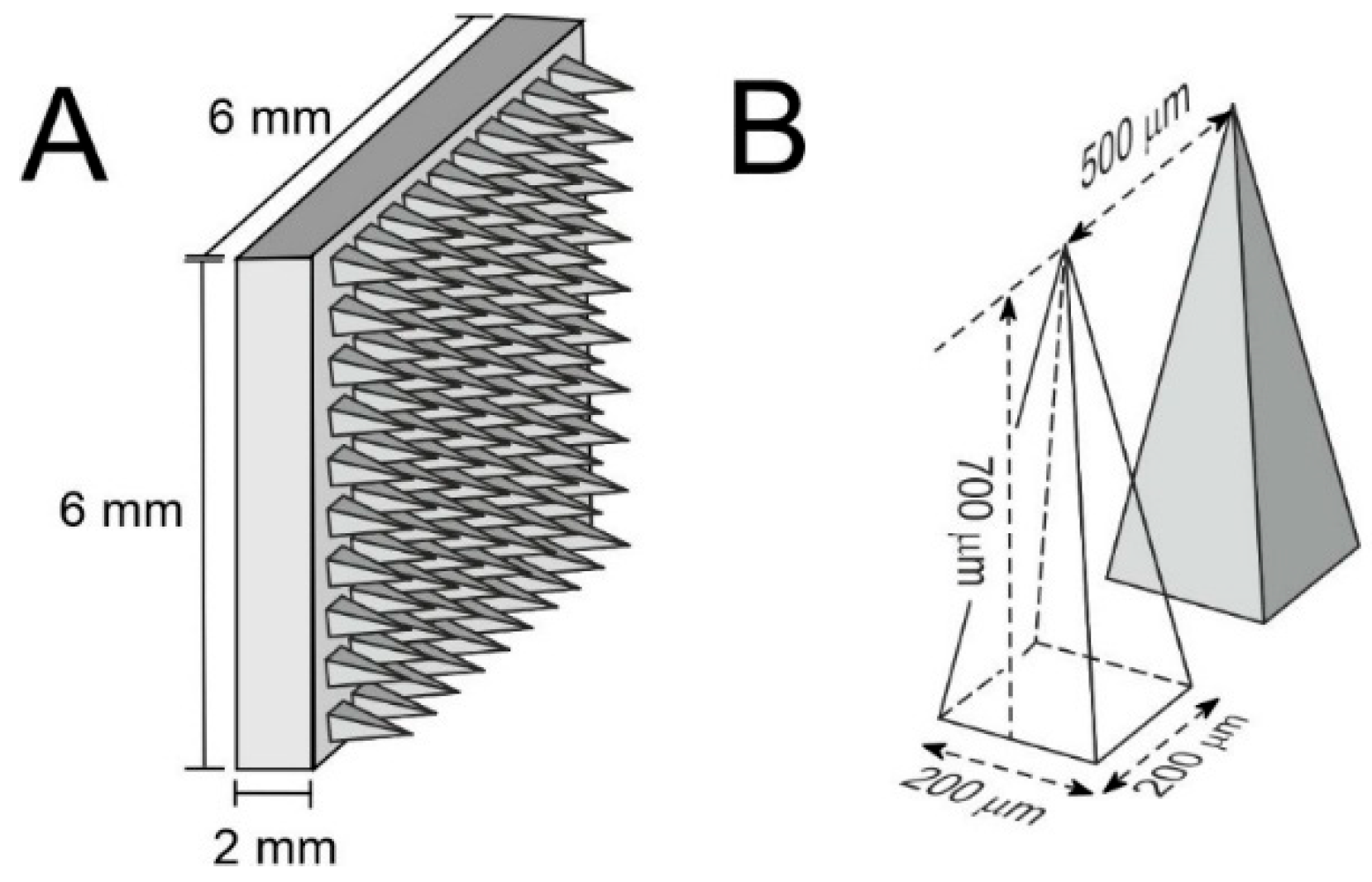
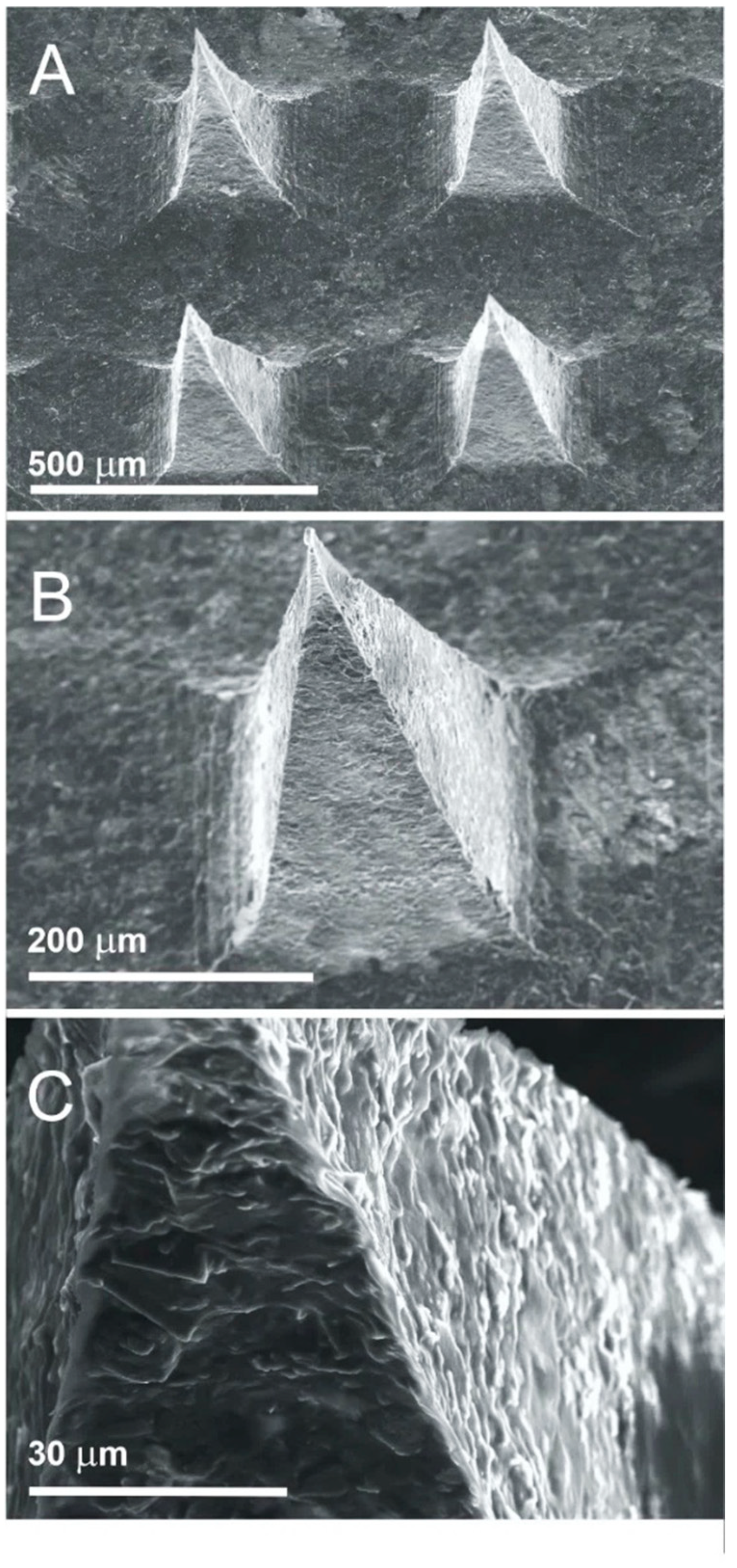
2.3. Preparation of Psyllium Husk–Carbon Microneedles
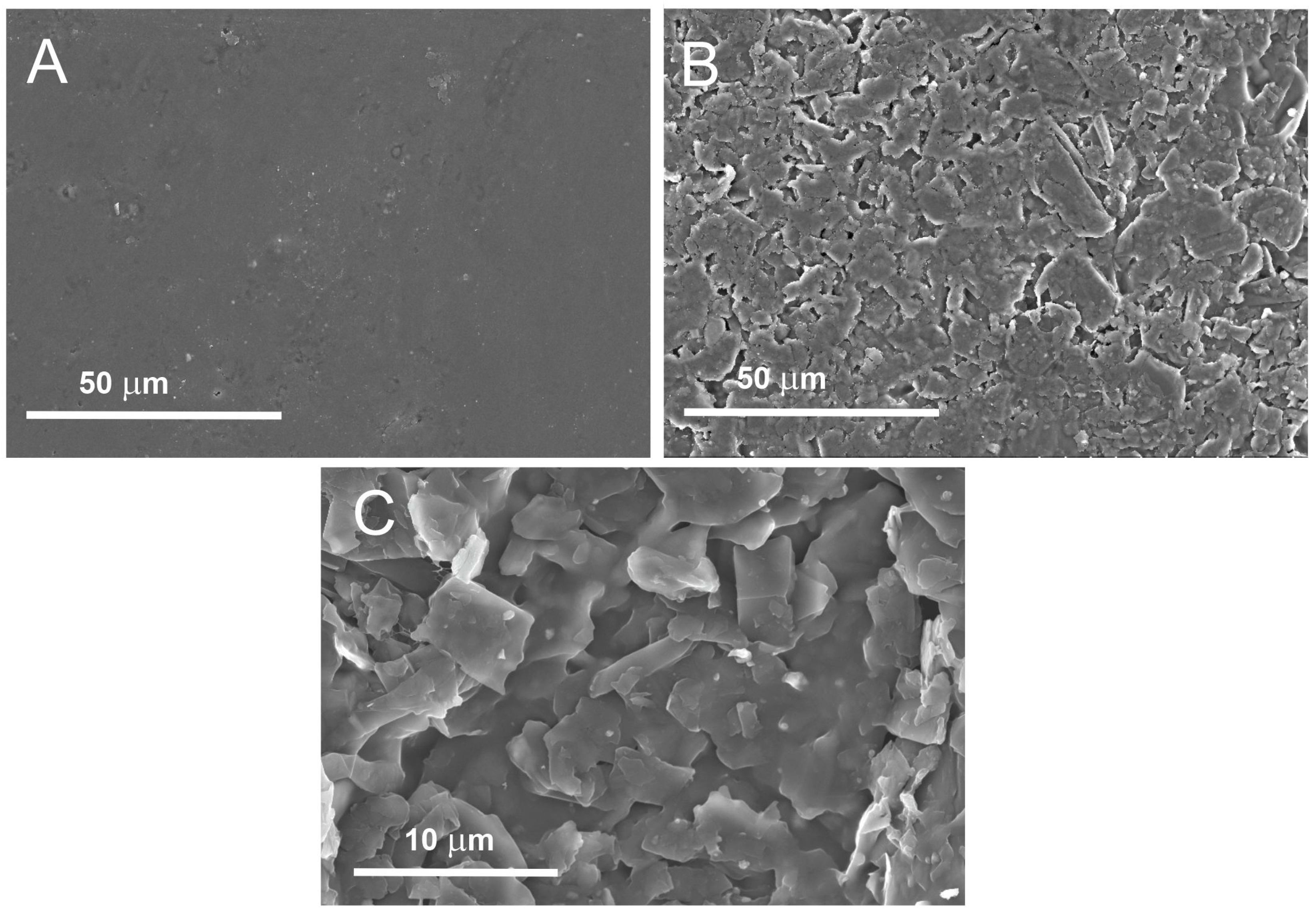
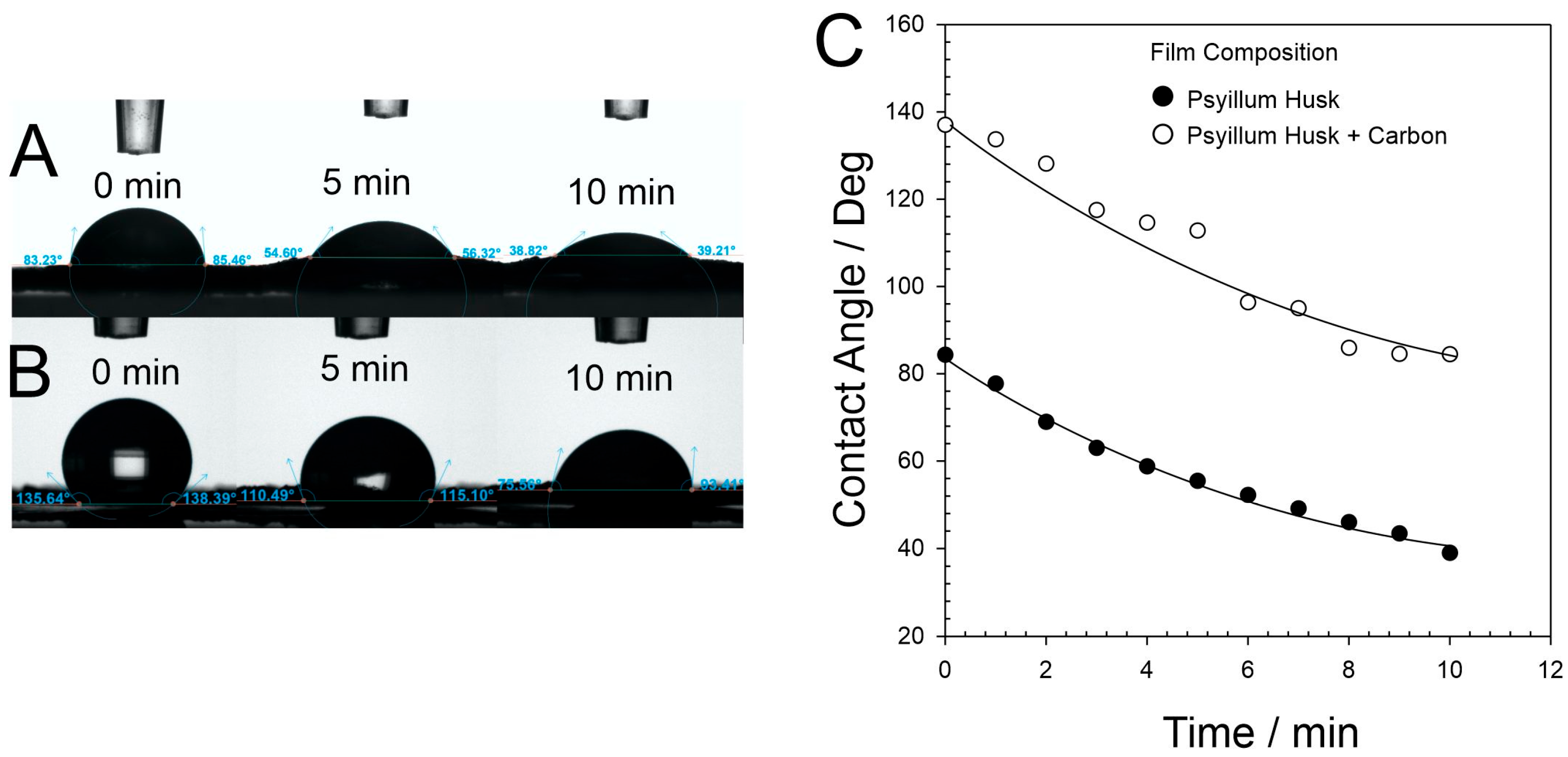
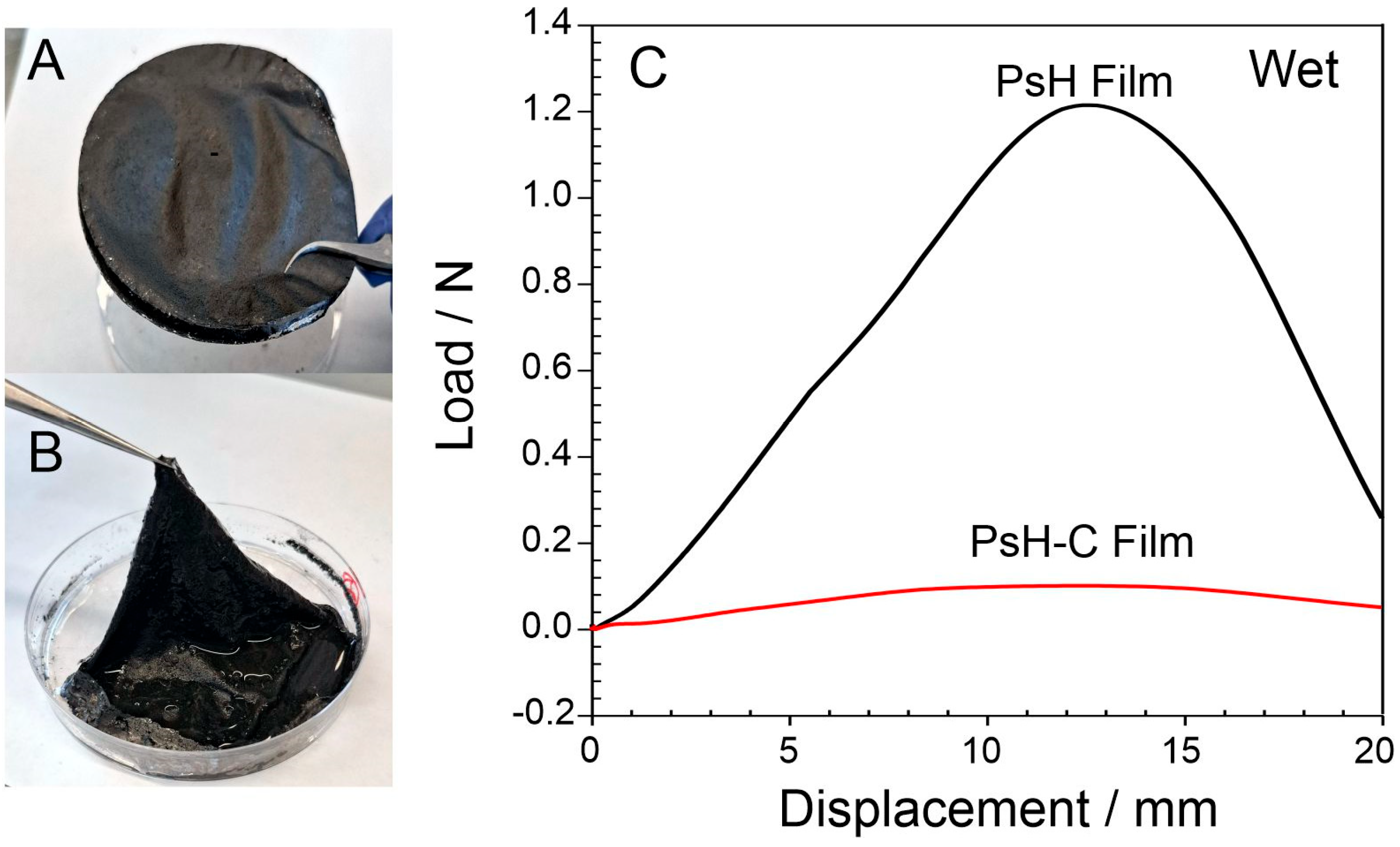
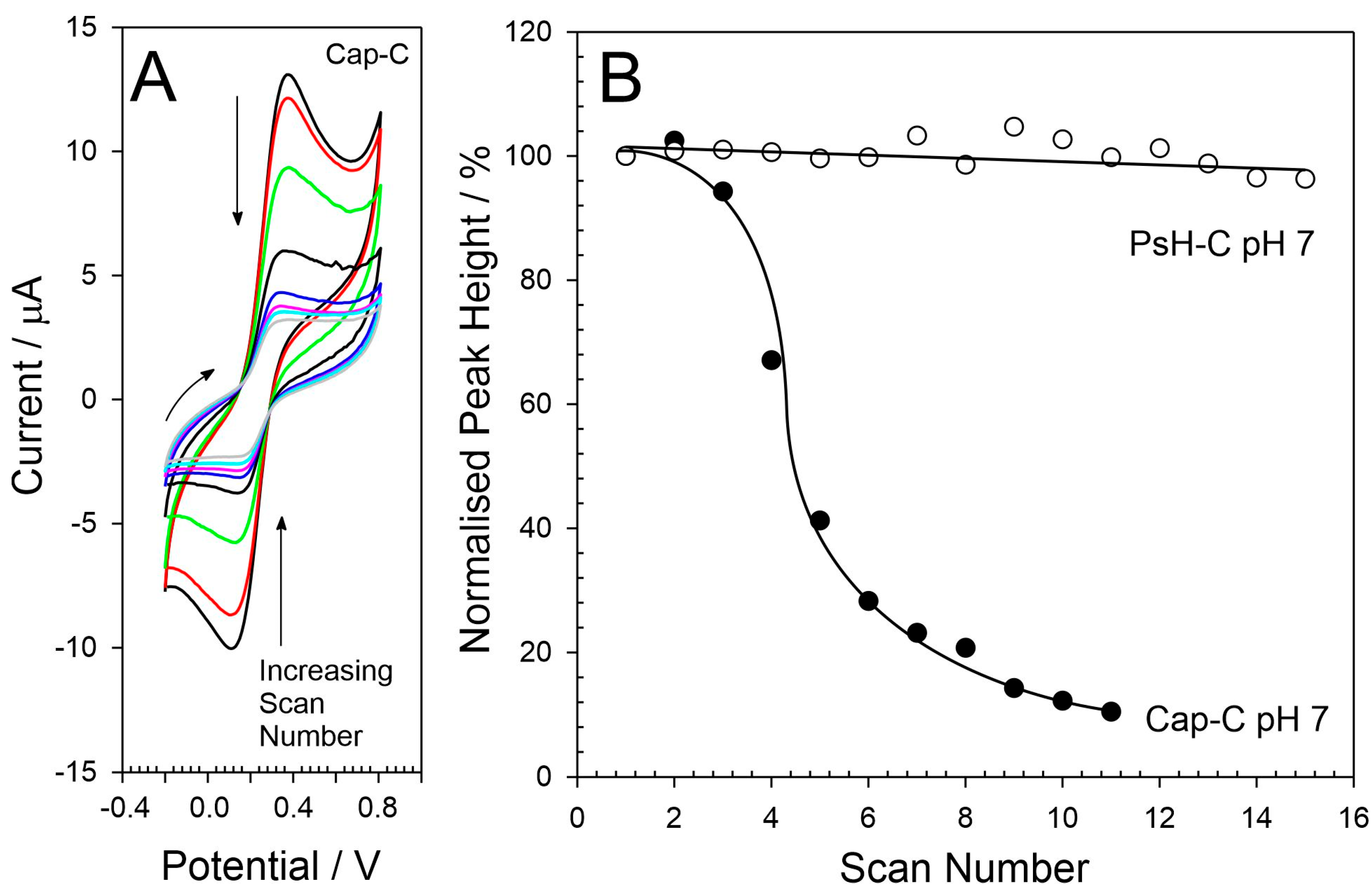
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Singh, A.; Saini, R.; Kulriya, P.; Kumar, R. Advancements in graphene-based nanostructured conducting polymer hybrid composite electrodes for high-performance supercapacitors. J. Power Sources 2025, 630, 236176. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, M.S.; Adak, B.; Rahman, M.M.; Moni, K.K.; Nur, A.S.M.; Hong, H.P.; Younes, H.; Mukhopadhyay, S. Recent advancements in carbon-based composite materials as electrodes for high-performance supercapacitors. J. Energy Sources 2025, 107, 114838. [Google Scholar] [CrossRef]
- Imran, M.; Afzal, A.M.; Iqbal, M.W.; Fouda, A.M.; Hegazy, H.H.; Mumtaz, S. Unlocking the potential of biodegradable and environment-friendly electrode materials for applications in energy storage devices. Ceram. Int. 2024, 50, 47529–47548. [Google Scholar] [CrossRef]
- Suganthi, S.; Ahmad, K.; Oh, T.H. Progress in Graphitic Carbon Nitride-Based Composite Materials for Electrochemical Sensing and Energy Storage Applications. ChemistrySelect 2025, 10, e202404670. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Wu, A.B.; Zhang, Y.T.; Duan, H.Y.; Zhu, P.C.; Mao, Y.C. Recent progress of nanomaterials-based composite hydrogel sensors for human-machine interactions. Discov. Nano. 2025, 20, 60. [Google Scholar] [CrossRef]
- Devine, A.; Hegarty, E.; Hegarty, C.; Davis, J. Conductive Composite Microneedle Sensors Based on Cellulose Acetate Phthalate: Investigating Performance and Biodegradability. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Karami-Mosammam, M.; Danninger, D.; Schiller, D.; Kaltenbrunner, M. Stretchable and Biodegradable Batteries with High Energy and Power Density. Adv. Mater. 2022, 34, 2204457. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.P.; Trzcinski, K.; Zarach, Z.; Li, J.; Roda, D.; Szkoda, M. Poly(hydroxybutyrate-co-hydroxyvalerate) as a biodegradable binder in a negative electrode material for lithium-ion batteries. Appl. Surf. Sci. 2022, 606, 154933. [Google Scholar] [CrossRef]
- Chen, T.; Lau, K.; Singh, A.; Zhang, Y.X.; Salari, M.; Naguib, H.E.; Morshead, C.M. Biodegradable stimulating electrodes for resident neural stem cell activation in vivo. Biomaterials 2025, 315, 122957. [Google Scholar] [CrossRef] [PubMed]
- Fryn, P.; Jewłoszewicz, B.; Bogdanowicz, K.A.; Przybył, W.; Gonciarz, A.; Pich, R.; Marzec, M.; Iwan, A. Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes. Materials 2020, 13, 2480. [Google Scholar] [CrossRef]
- Martin, C.; Dejardin, T.; Hart, A.; Riehle, M.O.; Cumming, D.R.S. Directed Nerve Regeneration Enabled by Wirelessly Powered Electrodes Printed on a Biodegradable Polymer. Adv. Healthc. Mater. 2014, 3, 1001–1006. [Google Scholar] [CrossRef]
- Meng, L.; Fu, Q.; Hao, S.; Xu, F.; Yang, J. Self-adhesive, biodegradable silk-based dry electrodes for epidermal electrophysiological monitoring. Chem. Eng. J. 2022, 427, 131999. [Google Scholar] [CrossRef]
- Molinnus, D.; Janus, K.A.; Fang, A.C.; Drinic, A.; Achtsnicht, S.; Köpf, M.; Keusgen, M.; Schöning, M.J. Thick-Film Carbon Electrode Deposited onto a Biodegradable Fibroin Substrate for Biosensing. Phys. Status Solidi A 2022, 219, 2200100. [Google Scholar] [CrossRef]
- Cui, S.W. Polysaccharide Gums from Agricultural Products; Taylor and Francis: Abingdon, UK, 2019; ISBN 9780367397999. [Google Scholar]
- Hussain, M.A.; Muhammad, G.; Jantan, I.; Bukhari, S.N.A. Psyllium Arabinoxylan: A Versatile Biomaterial for Potential Medicinal and Pharmaceutical Applications. Polym. Rev. 2016, 56, 1–30. [Google Scholar] [CrossRef]
- Akbar, S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-16806-3. [Google Scholar]
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Zhi, Q. Physicochemical, mechanical and structural properties of composite edible films based on whey protein isolate/psyllium seed gum. Int. J. Biol. Macromol. 2020, 153, 892–901. [Google Scholar] [CrossRef]
- Suresh, H.; Mikhael, M.; Ho, V.; Zhou, J. A HPLCESI-Q-ToF-MS Method for the Analysis of Monomer Constituents in PHGG, Gum Arabic And Psyllium Husk Prebiotic Dietary Fibre Supplements. Int. J. Food Prop. 2022, 25, 1650–1667. [Google Scholar] [CrossRef]
- Poddar, S.; Agarwal, P.S.; Sahi, A.K.; Varshney, N.; Vajanthri, K.Y.; Mahto, S.K. Fabrication and characterization of electrospun psyllium husk-based nanofibers for tissue regeneration. J. Appl. Polym. Sci. 2021, 138, e50569. [Google Scholar] [CrossRef]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Maity, P.P.; Ghosh, S.; Dhara, S.; Das, N. Design of psyllium-g-poly(acrylic acid-co-sodium acrylate)/cloisite 10A semi-IPN nanocomposite hydrogel and its mechanical, rheological and controlled drug release behaviour. Int. J. Biol. Macromol. 2018, 111, 983–998. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Goff, H.D.; Smith, A. Microstructure and rheological properties of psyllium polysaccharide gel. Food Hydrocoll. 2009, 23, 1542–1547. [Google Scholar] [CrossRef]
- Belorio, M.; Gomez, M.J. Psyllium: A useful functional ingredient in food systems. Crit. Rev. Food Nutr. 2022, 62, 527–538. [Google Scholar] [CrossRef]
- Fernandes, C.; Acharya, P.C.; Bhatt, S. Preparation of Lauroyl Grafted Alginate-Psyllium Husk Gel Composite Film with Enhanced Physicochemical, Mechanical and Antimicrobial Properties. Sci. Rep. 2018, 8, 17213. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Chand, N.; Agrawal, A. Carboxymethylpsyllium (CMPsy)/poly(acrylamide)(poly(AAm)) hydrogels for oral delivery of anti-diabetic drug Gliclazide. J. Macromol. Sci. Part A 2017, 54, 221–227. [Google Scholar] [CrossRef]
- Singh, B.; Kanwar, J.S.; Kumari, P. Modification of Dietary Fiber Psyllium with Poly(vinyl pyrrolidone) through Network Formation for Use in Slow Drug Delivery Application. Polym. Sci. Ser. B 2018, 60, 331–348. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sharma, P.P.; Mazumder, B.; Bhatnagar, A.; Subramaniyan, V.; Fuloria, S.; Kumar Fuloria, N. Mucoadhesive microspheres of glutaraldehyde crosslinked mucilage of Isabgol husk for sustained release of gliclazide. J. Biomater. Sci. Polym. Ed. 2021, 32, 1420–1449. [Google Scholar] [CrossRef]
- Van Craeyveld, V.; Delcour, J.A.; Courtin, C.M. Extractability and chemical and enzymic degradation of psyllium (Plantago ovata Forsk) seed husk arabinoxylans. Food Chem. 2009, 112, 812–819. [Google Scholar] [CrossRef]
- Beer-Lech, K.; Skic, A.; Skic, K.; Stropek, Z.; Arczewska, M. Effect of Psyllium Husk Addition on the Structural and Physical Properties of Biodegradable Thermoplastic Starch Film. Materials 2022, 15, 4459. [Google Scholar] [CrossRef]
- Casimero, C.; Hegarty, C.; McGlynn, R.J.; Davis, J. Ultrasonic exfoliation of carbon fiber: Electroanalytical perspectives. J. Appl. Electrochem. 2020, 50, 383–394. [Google Scholar] [CrossRef]
- Lindner, E.; Guzinski, M.; Pendley, B.; Chaum, E. Plasticized PVC Membrane Modified Electrodes: Voltammetry of Highly Hydrophobic Compounds. Membranes 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, D.; Chen, X.; Zhu, Z.; Li, W. Three-phase interface-assisted advanced electrochemistry-related applications. Cell Rep. Phys. Sci. 2021, 2, 100602. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, J.; Zhao, W.; Wu, H.B. Antiflooding Gas Diffusion Electrodes Enabled by Liquid–Solid–Liquid Interfaces for Durable CO2 Electrolysis. ACS Appl. Energy Mater. 2024, 7, 9394–9401. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, J.; Rajabpour, S.; Joshi, K.; Kowalik, M.; Croom, B.; Schwab, Y.; Zhang, L.; Bumgardner, C.; Brown, K.R.; et al. Graphene Reinforced Carbon Fibers. Sci. Adv. 2020, 6, eaaz4191. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Martinez, J.; Carnagarin, R.; Dass, C.R. In-Vitro Evaluation of Enteric Coated Insulin Tablets Containing Absorption Enhancer and Enzyme Inhibitor. J. Pharm. Pharmacol. 2017, 69, 285–294. [Google Scholar] [CrossRef]
- Hanafi, A.; Nograles, N.; Abdullah, S.; Shamsudin, M.N.; Rosli, R. Cellulose Acetate Phthalate Microencapsulation and Delivery of Plasmid DNA to the Intestines. J. Pharm. Sci. 2013, 102, 617–626. [Google Scholar] [CrossRef]
- Anderson, A.; Hegarty, C.; Casimero, C.; Davis, J. Electrochemically Controlled Dissolution of Nanocarbon–Cellulose Acetate Phthalate Microneedle Arrays. ACS Appl. Mater. Interfaces 2019, 11, 35540–35547. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, C.; McConville, A.; McGlynn, R.J.; Mariotti, D.; Davis, J. Design of composite microneedle sensor systems for the measurement of transdermal pH. Mater. Chem. Phys. 2019, 227, 340–346. [Google Scholar] [CrossRef]
- Abbas, M.F.; Karim, D.K.; Kareem, H.R.; Kamil, M.M.; Al-Musawi, M.H.; Asker, M.H.; Ghanamid, M.; Shahriari-Khalajid, M.; Sattare, M.; Mirhajf, M.; et al. Fucoidan and its derivatives: From extraction to cutting-edge biomedical applications. Carbohydr. Polym. 2025, 357, 123468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Yue, J. Dissolving microneedles: A transdermal drug delivery system for the treatment of rheumatoid arthritis. Int. J. Pharm. 2025, 671, 125206. [Google Scholar] [CrossRef]
- Senobari, F.; Abolmaali, S.S.; Farahavr, G.; Tamaddon, A.M. Targeting inflammation with hyaluronic acid-based micro- and nanotechnology: A disease-oriented review. Int. J. Biol. Macromol. 2024, 280, 135923. [Google Scholar] [CrossRef] [PubMed]
- Waleed, M.; Saeed, F.; Afzaal, M.; Niaz, B.; Raza, M.A.; Hussain, M.; Tufail, T.; Rasheed, A.; Ateeq, H.; Al Jbawi, E. Structural and nutritional properties of psyllium husk arabinoxylans with special reference to their antioxidant potential. Int. J. Food Prop. 2022, 25, 2505–2513. [Google Scholar] [CrossRef]
- Bleija, M.; Platnieks, O.; Macutkevic, J.; Starkova, O.; Gaidukovs, S. Comparison of Carbon-Nanoparticle-Filled Poly(Butylene Succinate-co-Adipate) Nanocomposites for Electromagnetic Applications. Nanomaterials 2022, 12, 3671. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Golokhvast, K. Colloidal Behavior and Biodegradation of Engineered Carbon-Based Nanomaterials in Aquatic Environment. Nanomaterials 2022, 12, 4149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, Z.; Liu, Z.; Huang, J.; Xiao, R.; Shao, B.; Liu, Y.; Liu, Y.; Tang, W.; Zeng, G.; et al. Effects of carbon nanotubes on biodegradation of pollutants: Positive or negative. Ecotoxicol. Environ. Saf. 2020, 189, 109914. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Taguchi, F.; Hori, K. Contribution of the Fenton reaction to the degradation of carbon nanotubes by enzymes. Front. Environ. Sci. 2023, 11, 1184257. [Google Scholar] [CrossRef]
- Takahashi, S.; Hori, K. Long-term continuous degradation of carbon nanotubes by a bacteria-driven Fenton reaction. Front. Microbiol. 2023, 14, 1298323. [Google Scholar] [CrossRef]
- Mokhtari-Farsani, A.; Hasany, M.; Lynch, I.; Mehrali, M. Biodegradation of Carbon-Based Nanomaterials: The Importance of “Biomolecular Corona” Consideration. Adv. Funct. Mater. 2022, 32, 2105649. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCann, C.; Gilpin, V.; McMath, R.; Gill, C.I.R.; McCreadie, K.; Uhomoibhi, J.; Papakonstantinou, P.; Davis, J. Characterisation of a Biodegradable Electrode Substrate Based on Psyllium Husk–Carbon Nanoparticle Composites. C 2025, 11, 64. https://doi.org/10.3390/c11030064
McCann C, Gilpin V, McMath R, Gill CIR, McCreadie K, Uhomoibhi J, Papakonstantinou P, Davis J. Characterisation of a Biodegradable Electrode Substrate Based on Psyllium Husk–Carbon Nanoparticle Composites. C. 2025; 11(3):64. https://doi.org/10.3390/c11030064
Chicago/Turabian StyleMcCann, Cliodhna, Victoria Gilpin, Regan McMath, Chris I. R. Gill, Karl McCreadie, James Uhomoibhi, Pagona Papakonstantinou, and James Davis. 2025. "Characterisation of a Biodegradable Electrode Substrate Based on Psyllium Husk–Carbon Nanoparticle Composites" C 11, no. 3: 64. https://doi.org/10.3390/c11030064
APA StyleMcCann, C., Gilpin, V., McMath, R., Gill, C. I. R., McCreadie, K., Uhomoibhi, J., Papakonstantinou, P., & Davis, J. (2025). Characterisation of a Biodegradable Electrode Substrate Based on Psyllium Husk–Carbon Nanoparticle Composites. C, 11(3), 64. https://doi.org/10.3390/c11030064







