Formation and Melting of Hydrate with Binary CO2/C2H6 Mixtures in Silica Sand: Comparison Between Dissociation Data and Phase Equilibrium of Pure CO2 and C2H6 Hydrates
Abstract
1. Introduction
2. Materials and Methods
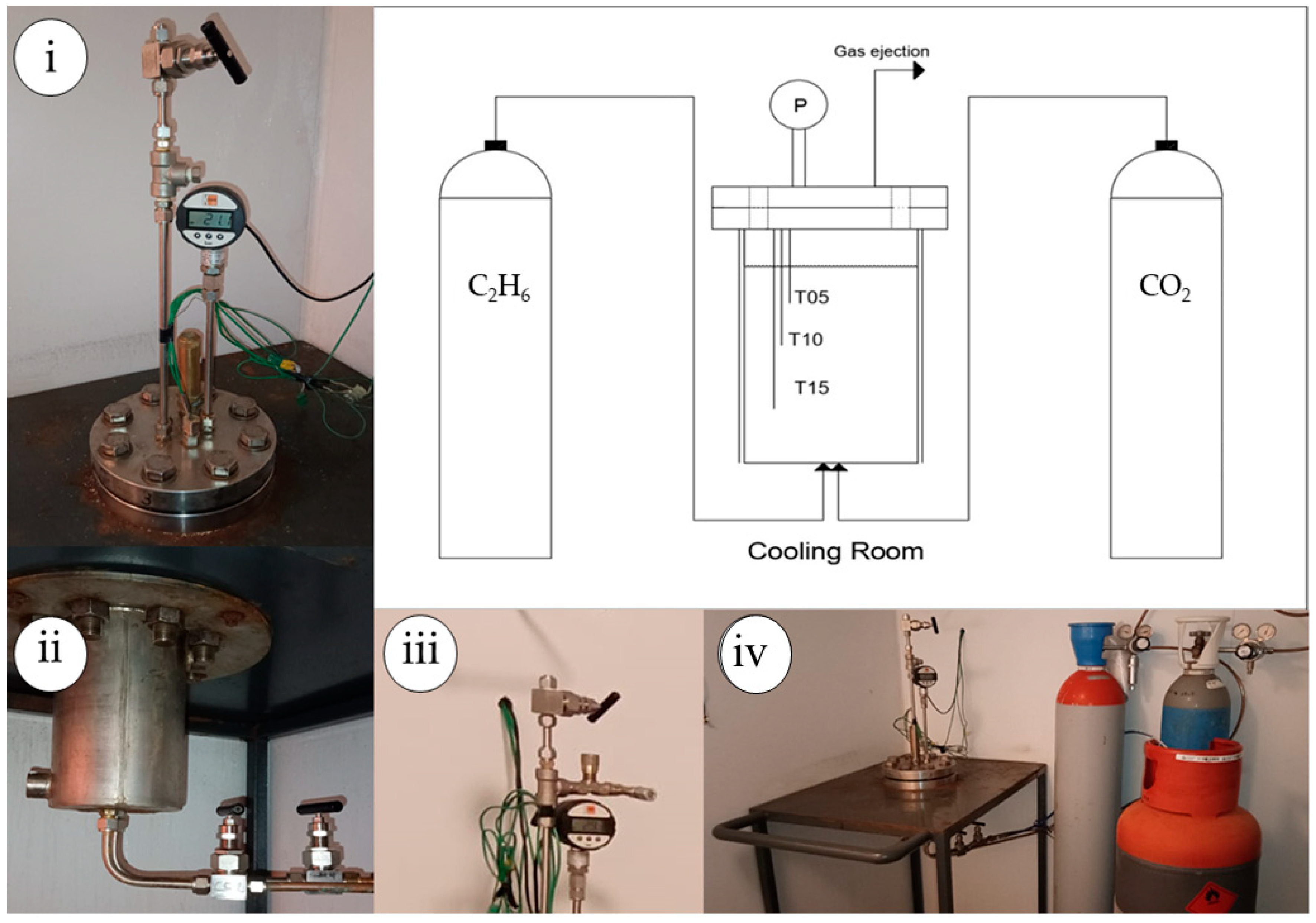
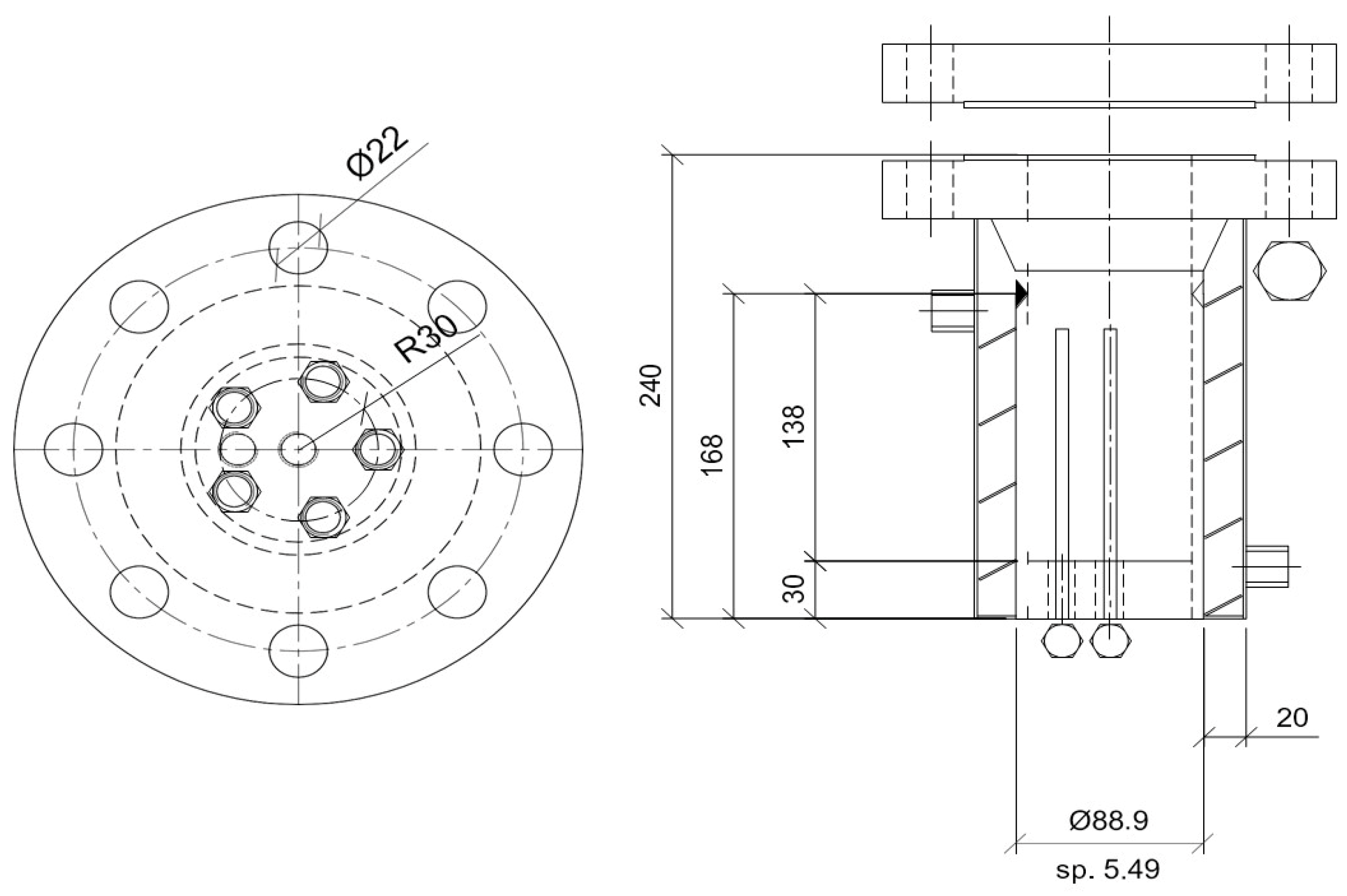
2.1. Experimental Apparatus and Materials
2.2. Experimental Procedure
- (1)
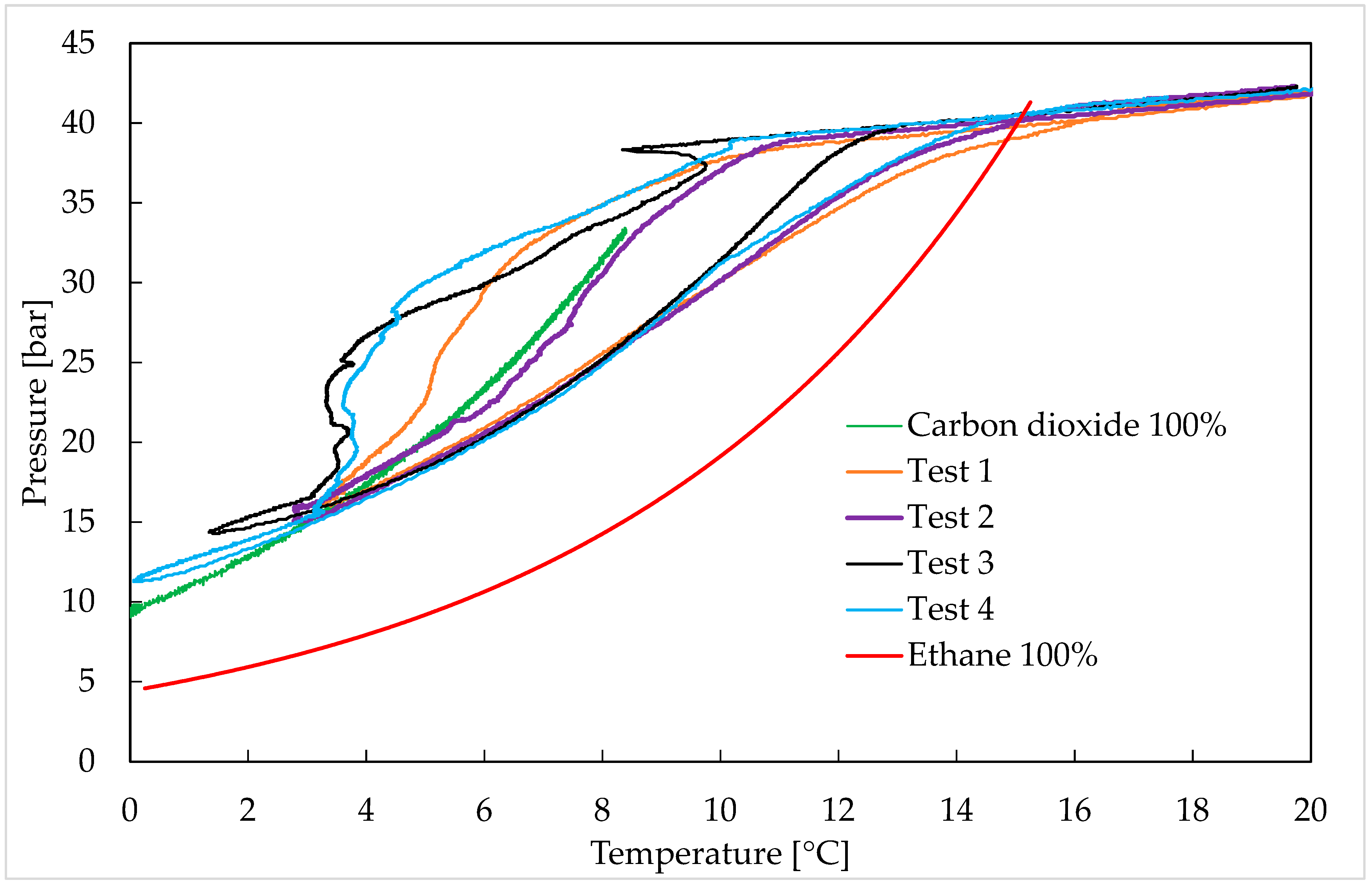
- CO2/C2H6 (75/25) vol%: Tests 1–4;
- (2)
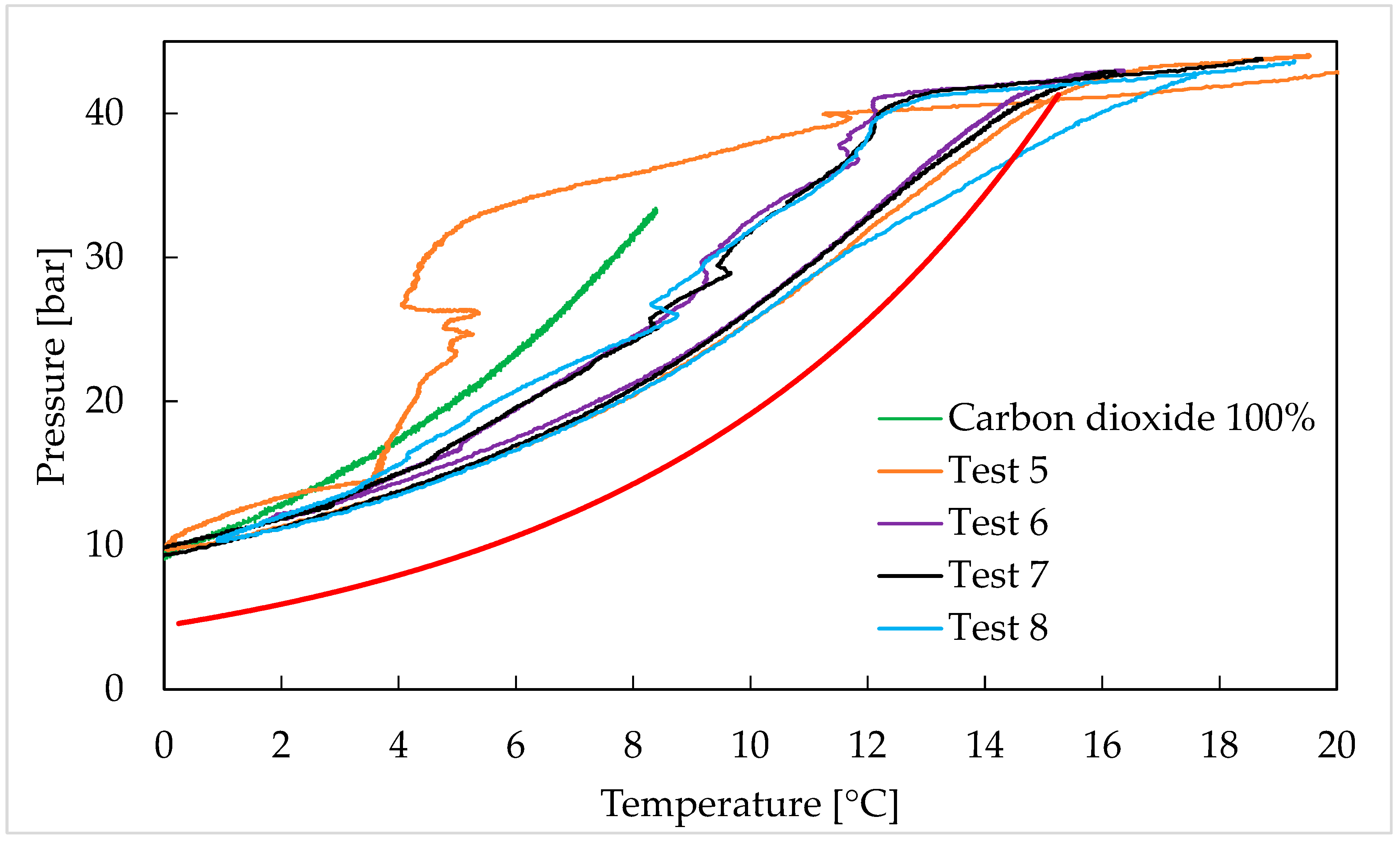
- CO2/C2H6 (50/50) vol%: Tests 5–8;
- (3)
- CO2/C2H6 (25/75) vol%: Tests 9–12.
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, C.A.; Sloan, E.D. Natural gas hydrates: Recent advances and challenges in energy and environmental applications. AIChE J. 2007, 53, 1636–1643. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Thermodynamic and kinetic characterization of methane hydrate nucleation, growth and dissociation processes, according to the Labile Cluster Theory. Chem. Eng. J. 2021, 425, 130706. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Goel, N. In Situ methane hydrate dissociation with carbon dioxide sequestration: Current knowledge and issues. J. Petrol. Sci. Eng. 2009, 51, 169–184. [Google Scholar] [CrossRef]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangway, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combination. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Xuan, K.; Yi, W.; Li, X.S.; Zhang, Y.; Chen, Z.Y. Influence of heat conduction and heat convection on hydrate dissociation by depressurization in a pilot-scale hydrate simulator. Appl. Energy 2019, 251, 113405. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Go, W.; Yun, S.; Lee, D.; Seo, Y. Experimental and computational investigation of hydrophilic monomeric substances as novel CO2 hydrate inhibitors and potential synergists. Energy 2022, 244, 123136. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, C.; Wang, H.; Yang, L.; Zhang, L.; Zhao, J.; Song, Y. Atomistic insight into the performance of thermodynamic inhibitors in the nucleation of methane hydrate. Chem. Eng. J. 2022, 431, 133479. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Natural gas hydrates: Comparison between two different applications of thermal stimulation for performing CO2 replacement. Energy 2019, 172, 423–434. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 2008. [Google Scholar]
- Jeffrey, J.A. Hydrate inclusion compounds. J. Incl. Phenom. 1984, 1, 211–222. [Google Scholar] [CrossRef]
- Pauling, L.; Marsh, R.E. The structure for inert gas hydrates. Proc. Natl. Acad. Sci. USA 1952, 38, 1425–1426. [Google Scholar] [CrossRef]
- Claussen, W.F. A second water structure for inert gas hydrates. J. Chem. Phys. 1951, 19, 1425–1426. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Ailin, J. Progress and prospects of natural gas development technologies in China. Nat. Gas Ind. B 2018, 5, 547–557. [Google Scholar] [CrossRef]
- Demirbas, A. Natural gas. In Methane Gas Hydrate; Chapter 2; Springer: London, UK, 2010; pp. 57–76. [Google Scholar]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, X.; Zhang, F.; Zhang, Z.; Hooman, K.; Zhang, H.; Wang, X. A biomimetic red blood cell inspired encapsulation design for advanced hydrate-based carbon capture. Energy 2023, 271, 126985. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Castellani, B.; Rossi, A.; Minicucci, M.; Zannotti, M.; Li, Y.; Rossi, F. May sediments affect the inhibiting properties of NaCl on CH4 and CO2 hydrates formation? An experimental report. J. Mol. Liq. 2022, 359, 119300. [Google Scholar] [CrossRef]
- Dhamu, V.; Qureshi, M.F.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Evaluating liquid CO2 hydrate formation kinetics, morphology, and stability in oceanic sediments on a lab scale using top injection. Chem. Eng. J. 2023, 478, 147200. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kawamura, T.; Yamamoto, Y.; Yoon, J.H. Phase equilibrium studies of tetrahydrofuran (THF) + CH4, THF + CO2, CH4 + CO2, and THF + CO2 + CH4 hydrates. J. Chem. Eng. Data 2012, 57, 3543–3548. [Google Scholar] [CrossRef]
- Yi, L.; Liang, D.; Zhou, X.; Li, D. Molecular dynamics simulations for the growth of CH4-CO2 mixed hydrate. J. Energy Chem. 2014, 23, 747–754. [Google Scholar] [CrossRef]
- Dornas, P.; Alavi, S.; Woo, T.K. Free energies of carbon dioxide sequestration and methane recovery in clathrate hydrates. J. Chem. Phys. 2007, 127, 124510. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Ding, Y.L.; Xu, C.G.; Yu, Y.S.; Li, X.S. Methane recovery from natural gas hydrate with simulated IGCC syngas. Energy 2017, 120, 192–198. [Google Scholar] [CrossRef]
- .Gambelli, A.M.; Presciutti, A.; Rossi, F. Kinetic considerations and formation rate for carbon dioxide hydrate, formed in presence of a natural silica-based porous medium: How initial thermodynamic conditions may modify the process kinetic. Thermochim. Acta 2021, 705, 179039. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Kong, Y.; Liu, C.; Liu, Y. Preliminary study on measurement technology for hydrate phase equilibrium. Fluid Phase Equilibr. 2015, 403, 60–69. [Google Scholar]
- Gambelli, A.M.; Rossi, F.; Gigliotti, G. Methane replacement by using CO2/C3H8 mixtures for carbon storage and enhanced methane recovery in gas hydrates. Gas Sci. Eng. 2023, 115, 205028. [Google Scholar] [CrossRef]
- Ricaurte, M.; Torré, J.P.; Diaz, J.; Dicharry, C. In Situ injection of THF to trigger gas hydrate crystallization: Application to the evaluation of a kinetic hydrate promoter. Chem. Eng. Res. Des. 2014, 92, 1674–1680. [Google Scholar] [CrossRef]
- Torré, J.P.; Ricaurte, M.; Dicharry, C.; Broseta, D. CO2 enclathration in the presence of water-soluble hydrate promoters: Hydrate phase equilibria and kinetic studies in quiescent conditions. Chem. Eng. Sci. 2012, 82, 1–13. [Google Scholar] [CrossRef]
- Babu, P.; Yang, T.; Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrate phase equilibrium of ternary gas mixture containing carbon dioxide, hydrogen and propane. J. Chem. Thermodyn. 2013, 61, 58–63. [Google Scholar] [CrossRef]
- Lang, F.; Servio, P. Solubility measurements for the CH4 + C2H6 + H2O system under hydrate-liquid-vapour equilibrium. J. Nat. Gas Sci. Eng. 2015, 26, 130–134. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, Y.; Sun, C.; Chen, G. Structural transitions range of methane + ethane gas hydrates during the decomposition process below the ice point. Energy Proc. 2019, 158, 5201–5206. [Google Scholar] [CrossRef]
- Bhawangirkar, D.R.; Adhikari, J.; Sangway, J.S. Thermodynamic modelling of phase equilibria of clathrate hydrates formed from CH4, CO2, C2H6 and C3H8, with different equations of state. J. Chem. Thermodyn. 2018, 117, 180–192. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Bowler, K.E.; Stadterman, L.L.; Dec, S.F.; Koh, C.A.; Sloan, E.D. Dissociation studies of CH4-C2H6 and CH4-CO2 binary gas hydrates. Fluid Phase Equilibr. 2007, 261, 407–413. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivation and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J.; Zhou, Y. Large scale reactor details and results for the formation and decomposition of methane hydrates via thermal stimulation dissociation. J. Pet. Sci. Eng. 2012, 94–95, 19–27. [Google Scholar] [CrossRef]
- Yu, S.H.; Zhou, S.D.; Li, X.S.; Wang, S.L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibr. 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Cao, X.; Yang, K.; Xia, W.; Tang, G.; Bian, J. Dissociation experiment and dissociation rate model of CO2 hydrate. Nat. Gas Ind. B 2021, 8, 607–614. [Google Scholar] [CrossRef]
- Jarrahian, A.; Nakhaee, A. Hydrate-liquid-vapor equilibrium condition for N2 + CO2 + H2O system: Measurement and modelling. Fuel 2019, 237, 769–774. [Google Scholar] [CrossRef]
- Kyung, D.; Lee, K.; Kim, H.; Lee, W. Effect of marine environmental factors on the phase equilibrium of CO2 hydrate. Int. J. Greenh. Gas Control 2014, 20, 285–292. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Hendriks, E.M.; Edmond, B.; Moorwood, R.A.S.; Szcepanski, R. Hydrate structure stability in simple and mixed hydrates. Fluid Phase Equilibr. 1996, 117, 193–200. [Google Scholar] [CrossRef]
- Subramanian, S.; Kini, R.A.; Dec, S.F.; Sloan, E.D. Evidence of structure II hydrate formation from methane + ethane mixtures. Chem. Eng. Sci. 2000, 55, 1981–1999. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhang, G.B.; Carroll, J.J.; Li, S.L.; Jiang, S.H.; Guo, W. Experimental investigation into gas recovery from CH4-C2H6 C3H8 hydrates by CO2 replacement. Appl. Energy 2018, 229, 625–636. [Google Scholar] [CrossRef]
- Sundramoorthy, J.D.; Hammonds, P.; Lal, B.; Philips, G. Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Eng. 2016, 148, 870–877. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F.; Gigliotti, G. Hydrates production with binary CO2/C3H8 gaseous mixtures (90/10, 85/15, 80/20 vol%) in batch and unstirred conditions: The role of propane on the process thermodynamics. Chem. Eng. Sci. 2024, 298, 120441. [Google Scholar] [CrossRef]
- Giovannetti, R.; Gambelli, A.M.; Rossi, A.; Castellani, B.; Minicucci, M.; Zannotti, M.; Nicolini, A.; Rossi, F. Thermodynamic assessment and microscale Raman spectroscopy of binary CO2/CH4 hydrates produced during replacement applications in natural reservoirs. J. Mol. Liq. 2022, 368, 120739. [Google Scholar] [CrossRef]
- Gambelli, A.M. Methane replacement into hydrate reservoirs with carbon dioxide: Main limiting factors and influence of the gaseous phase composition, over hydrates, on the process. Chem. Eng. J. 2023, 478, 147247. [Google Scholar] [CrossRef]
- Waite, W.F.; Gilbert, L.Y.; Winters, W.J.; Mason, D.H. Thermal property measurements in tetrahydrofuran (THF) hydrate between −25 and +4 °C and their application to methane hydrate. In Proceedings of the 5th International Conference on Gas Hydrates, Trondheim, Norway, 12–16 June 2005. [Google Scholar]
- Stoll, R.D.; Bryan, G.M. Physical properties of sediments containing gas hydrates. J. Geophys. Res. 1979, 84, 1629–1634. [Google Scholar] [CrossRef]
- Max, M.D. Natural Gas Hydrates in Oceanic and Permafrost Environments; Kluwer Academic Publisher: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lu, H.; Matsumoto, R. Preliminary experimental results of the stable P-T conditions of methane hydrate in a nannofossil-rich claystone column. Geochem. J. 2002, 36, 21–30. [Google Scholar] [CrossRef]
- Di Profio, P.; Arca, S.; Savelli, G. Novel nanostructured media for gas storage and transport: Clathrate hydrates of methane and hydrogen. J. Fuel Cell Sci. Technol. 2007, 4, 49–55. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Di Schino, A.; Rossi, f. Experimental characterization of CH4 and CO2 hydrates formation in presence of porous quartz and Cu gas-atomized particles: Thermodynamic analyses and evidences about the feasibility of CH4/CO2 reverse replacement. Chem. Eng. Res. Des. 2022, 186, 511–524. [Google Scholar] [CrossRef]
- Hachikubo, A.; Takeya, S.; Chuvilin, E.; Istomin, V. Preservation phenomena of methane hydrate in pore spaces. PCCP 2011, 13, 17449–17452. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Proc. Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Robustillo, M.D.; de Menezes, D.E.S.; de Alcântara Pessôa Filho, P. Phase equilibrium of double-guest clathrates of methane and CO2, ethane, or propane as measured by high-pressure microcalorimetry. J. Mol. Liq. 2023, 387, 122609. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Review on the characteristics and advantages related to the use of flue-gas as CO2/N2 mixture for gas hydrate production. Fluid Phase Equilibr. 2021, 541, 113077. [Google Scholar] [CrossRef]
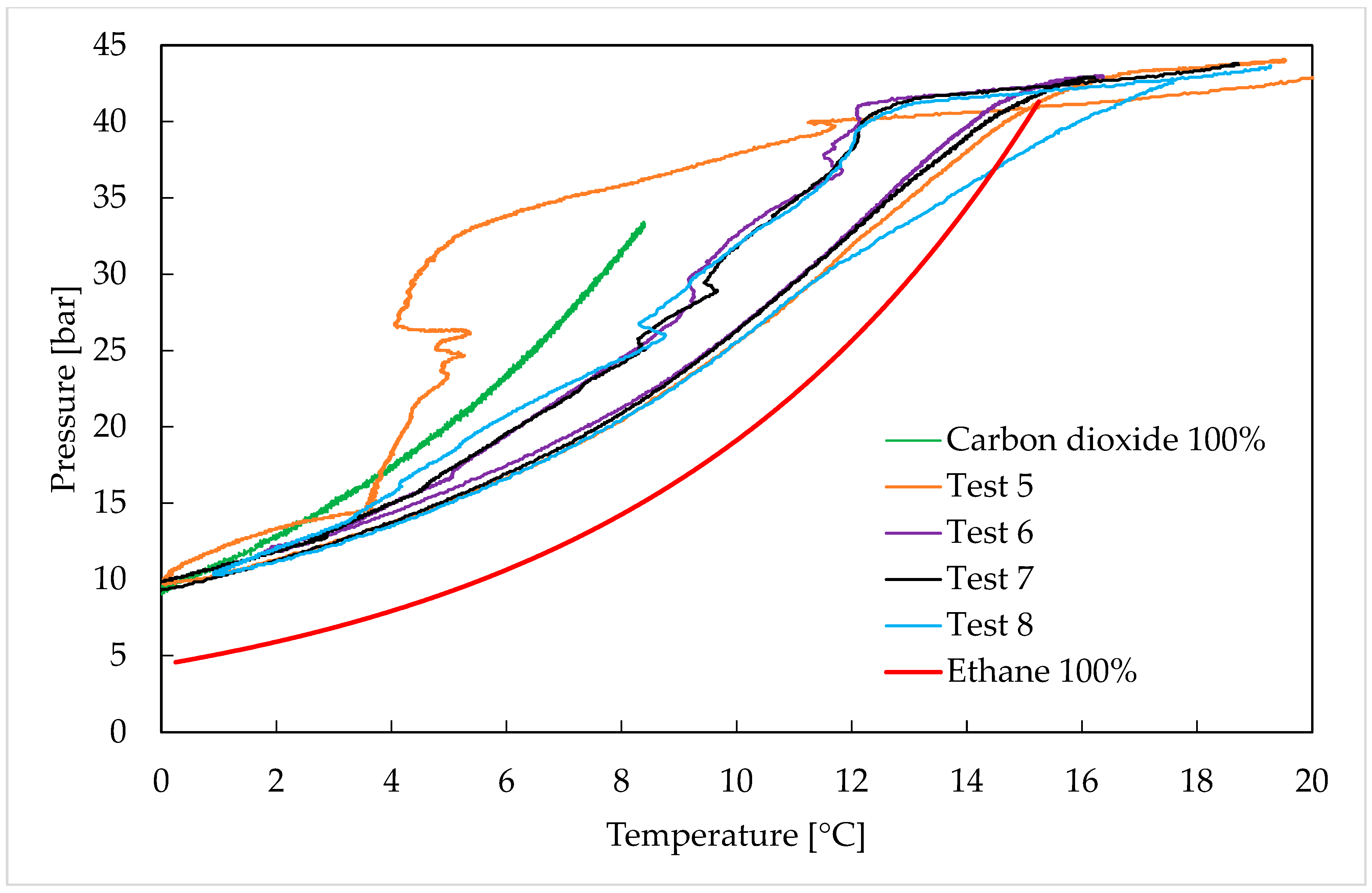
| T [°C] | P [bar] | T [°C] | P [bar] | T [°C] | P [bar] | T [°C] | P [bar] |
|---|---|---|---|---|---|---|---|
| 0.1 | 11.26 | 3.4 | 15.46 | 6.7 | 21.6 | 9.9 | 29.85 |
| 0.2 | 11.33 | 3.5 | 15.57 | 6.8 | 21.85 | 10 | 30.13 |
| 0.3 | 11.35 | 3.6 | 15.74 | 6.9 | 22.01 | 10.1 | 30.38 |
| 0.4 | 11.42 | 3.7 | 15.88 | 7 | 22.33 | 10.2 | 30.64 |
| 0.5 | 11.43 | 3.8 | 16.16 | 7.1 | 22.52 | 10.3 | 30.86 |
| 0.6 | 11.56 | 3.9 | 16.27 | 7.2 | 22.82 | 10.4 | 30.97 |
| 0.7 | 11.67 | 4 | 16.49 | 7.3 | 23.01 | 10.5 | 31.16 |
| 0.8 | 11.75 | 4.1 | 16.58 | 7.4 | 23.17 | 10.6 | 31.46 |
| 0.9 | 11.83 | 4.2 | 16.84 | 7.5 | 23.51 | 10.7 | 31.74 |
| 1 | 11.94 | 4.3 | 16.99 | 7.6 | 23.79 | 10.8 | 31.99 |
| 1.1 | 12.07 | 4.4 | 17.08 | 7.7 | 24 | 10.9 | 32.2 |
| 1.2 | 12.24 | 4.5 | 17.35 | 7.8 | 24.26 | 11 | 32.5 |
| 1.3 | 12.32 | 4.6 | 17.35 | 7.9 | 24.6 | 11.1 | 32.69 |
| 1.4 | 12.43 | 4.7 | 17.68 | 8 | 24.79 | 11.2 | 32.86 |
| 1.5 | 12.65 | 4.8 | 17.76 | 8.1 | 25.17 | 11.3 | 33.1 |
| 1.6 | 12.73 | 4.9 | 18.09 | 8.2 | 25.41 | 11.4 | 33.34 |
| 1.7 | 12.92 | 5 | 18.18 | 8.3 | 25.69 | 11.5 | 33.58 |
| 1.8 | 13.01 | 5.1 | 18.37 | 8.4 | 25.98 | 11.6 | 33.75 |
| 1.9 | 13.26 | 5.2 | 18.45 | 8.5 | 26.36 | 11.7 | 34.07 |
| 2 | 13.34 | 5.3 | 18.68 | 8.6 | 26.67 | 11.8 | 34.26 |
| 2.1 | 13.42 | 5.4 | 18.95 | 8.7 | 26.94 | 11.9 | 34.42 |
| 2.2 | 13.53 | 5.5 | 19.08 | 8.8 | 27.27 | 12 | 34.66 |
| 2.3 | 13.75 | 5.6 | 19.27 | 8.9 | 27.63 | 12.1 | 34.82 |
| 2.4 | 13.95 | 5.7 | 19.46 | 9 | 27.85 | 12.2 | 35.07 |
| 2.5 | 14.03 | 5.8 | 19.68 | 9.1 | 28.18 | 12.3 | 35.32 |
| 2.6 | 14.17 | 5.9 | 19.95 | 9.2 | 28.45 | 12.4 | 35.51 |
| 2.7 | 14.34 | 6 | 20.03 | 9.3 | 28.53 | 12.5 | 35.74 |
| 2.8 | 14.45 | 6.1 | 20.28 | 9.4 | 28.87 | 12.6 | 35.93 |
| 2.9 | 14.62 | 6.2 | 20.44 | 9.5 | 28.95 | 12.7 | 36.16 |
| 3 | 14.77 | 6.3 | 20.76 | 9.6 | 29.27 | 12.8 | 36.3 |
| 3.1 | 14.96 | 6.4 | 21.01 | 9.7 | 29.45 | 12.9 | 36.53 |
| 3.2 | 15.16 | 6.5 | 21.17 | 9.8 | 29.63 | 13 | 36.75 |
| 3.3 | 15.3 | 6.6 | 21.44 |
| T [°C] | P [bar] | T [°C] | P [bar] | T [°C] | P [bar] | T [°C] | P [bar] | T [°C] | P [bar] |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 9.74 | 2.7 | 12.06 | 5.3 | 15.56 | 7.9 | 20.27 | 10.6 | 27.27 |
| 0.2 | 9.79 | 2.8 | 12.23 | 5.4 | 15.65 | 8 | 20.43 | 10.7 | 27.43 |
| 0.3 | 9.87 | 2.9 | 12.31 | 5.5 | 15.87 | 8.1 | 20.75 | 10.8 | 27.75 |
| 0.4 | 9.91 | 3 | 12.56 | 5.6 | 15.98 | 8.2 | 20.92 | 10.9 | 28.17 |
| 0.5 | 9.94 | 3.1 | 12.64 | 5.7 | 16.15 | 8.3 | 21.16 | 11 | 28.45 |
| 0.6 | 10.06 | 3.2 | 12.73 | 5.8 | 16.4 | 8.4 | 21.43 | 11.1 | 28.86 |
| 0.7 | 10.06 | 3.3 | 12.84 | 5.9 | 16.57 | 8.5 | 21.73 | 11.2 | 29.03 |
| 0.8 | 10.12 | 3.4 | 13 | 6 | 16.65 | 8.6 | 21.92 | 11.3 | 29.45 |
| 0.9 | 10.21 | 3.5 | 13.14 | 6.1 | 16.9 | 8.7 | 22.25 | 11.4 | 29.77 |
| 1 | 10.29 | 3.6 | 13.25 | 6.2 | 16.99 | 8.8 | 22.41 | 11.5 | 30.05 |
| 1.1 | 10.43 | 3.7 | 13.41 | 6.3 | 17.18 | 8.9 | 22.59 | 11.6 | 30.46 |
| 1.2 | 10.54 | 3.8 | 13.53 | 6.4 | 17.34 | 9 | 22.89 | 11.7 | 30.88 |
| 1.3 | 10.62 | 3.9 | 13.66 | 6.5 | 17.48 | 9.2 | 23.5 | 11.8 | 31.16 |
| 1.4 | 10.71 | 4 | 13.78 | 6.6 | 17.76 | 9.3 | 23.69 | 11.9 | 31.46 |
| 1.5 | 10.82 | 4.1 | 13.94 | 6.7 | 17.87 | 9.4 | 23.91 | 12 | 31.88 |
| 1.6 | 10.96 | 4.2 | 14.03 | 6.8 | 18.09 | 9.5 | 24.1 | 12.1 | 32.27 |
| 1.7 | 11.04 | 4.3 | 14.16 | 6.9 | 18.28 | 9.6 | 24.51 | 12.2 | 32.5 |
| 1.8 | 11.13 | 4.4 | 14.25 | 7 | 18.37 | 9.7 | 24.67 | 12.3 | 32.8 |
| 1.9 | 11.24 | 4.5 | 14.36 | 7.1 | 18.67 | 9.8 | 25 | 12.4 | 33.18 |
| 2 | 11.32 | 4.6 | 14.53 | 7.2 | 18.86 | 9.9 | 25.27 | 12.5 | 33.34 |
| 2.1 | 11.4 | 4.7 | 14.62 | 7.3 | 18.94 | 10 | 25.53 | 12.6 | 33.72 |
| 2.2 | 11.54 | 4.8 | 14.87 | 7.4 | 19.26 | 10.1 | 25.85 | 12.7 | 34.07 |
| 2.3 | 11.74 | 4.9 | 14.96 | 7.5 | 19.34 | 10.2 | 26.08 | 12.8 | 34.34 |
| 2.4 | 11.74 | 5 | 15.15 | 7.6 | 19.67 | 10.3 | 26.36 | 12.9 | 34.66 |
| 2.5 | 11.82 | 5.1 | 15.29 | 7.7 | 19.86 | 10.4 | 26.66 | 13 | 35.07 |
| 2.6 | 11.92 | 5.2 | 15.39 | 7.8 | 20.03 | 10.5 | 26.85 |
| T [°C] | P [bar] | T [°C] | P [bar] |
|---|---|---|---|
| 7 | 17.36 | 10 | 21.33 |
| 7.1 | 17.38 | 10.1 | 21.52 |
| 7.2 | 17.42 | 10.2 | 21.74 |
| 7.3 | 17.47 | 10.3 | 22.12 |
| 7.4 | 17.48 | 10.4 | 22.42 |
| 7.5 | 17.5 | 10.5 | 22.52 |
| 7.6 | 17.54 | 10.6 | 22.9 |
| 7.7 | 17.57 | 10.7 | 23.09 |
| 7.8 | 17.61 | 10.8 | 23.51 |
| 7.9 | 17.64 | 10.9 | 23.7 |
| 8 | 17.66 | 11 | 24 |
| 8.1 | 17.69 | 11.1 | 24.2 |
| 8.2 | 17.77 | 11.2 | 24.6 |
| 8.3 | 17.88 | 11.3 | 24.79 |
| 8.4 | 18.10 | 11.4 | 25.17 |
| 8.5 | 18.30 | 11.5 | 25.5 |
| 8.6 | 18.38 | 11.6 | 25.77 |
| 8.7 | 18.60 | 11.7 | 26.09 |
| 8.8 | 18.79 | 11.8 | 26.36 |
| 8.9 | 18.88 | 11.9 | 26.67 |
| 9 | 18.96 | 12 | 27.08 |
| 9.1 | 19.08 | 12.1 | 27.35 |
| 9.2 | 19.27 | 12.2 | 27.68 |
| 9.3 | 19.46 | 12.3 | 27.95 |
| 9.4 | 19.72 | 12.4 | 28.37 |
| 9.5 | 19.96 | 12.5 | 28.67 |
| 9.6 | 20.28 | 12.6 | 29.04 |
| 9.7 | 20.44 | 12.7 | 29.37 |
| 9.8 | 20.77 | 12.8 | 29.63 |
| 9.9 | 21.04 | 12.9 | 30.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rossi, F.; Gigliotti, G. Formation and Melting of Hydrate with Binary CO2/C2H6 Mixtures in Silica Sand: Comparison Between Dissociation Data and Phase Equilibrium of Pure CO2 and C2H6 Hydrates. C 2025, 11, 63. https://doi.org/10.3390/c11030063
Gambelli AM, Rossi F, Gigliotti G. Formation and Melting of Hydrate with Binary CO2/C2H6 Mixtures in Silica Sand: Comparison Between Dissociation Data and Phase Equilibrium of Pure CO2 and C2H6 Hydrates. C. 2025; 11(3):63. https://doi.org/10.3390/c11030063
Chicago/Turabian StyleGambelli, Alberto Maria, Federico Rossi, and Giovanni Gigliotti. 2025. "Formation and Melting of Hydrate with Binary CO2/C2H6 Mixtures in Silica Sand: Comparison Between Dissociation Data and Phase Equilibrium of Pure CO2 and C2H6 Hydrates" C 11, no. 3: 63. https://doi.org/10.3390/c11030063
APA StyleGambelli, A. M., Rossi, F., & Gigliotti, G. (2025). Formation and Melting of Hydrate with Binary CO2/C2H6 Mixtures in Silica Sand: Comparison Between Dissociation Data and Phase Equilibrium of Pure CO2 and C2H6 Hydrates. C, 11(3), 63. https://doi.org/10.3390/c11030063








