Graphene-like Carbon Materials from King Grass Biomass via Catalytic Pyrolysis Using K3[Fe(CN)6] as a Dual Catalyst and Activator
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for Carbonaceous Material Synthesis
2.2. Biomass Feedstock Collection
2.3. Cellulose Extraction
2.4. Synthesis of Carbonaceous Materials
2.5. Experimental Conditions for the Physicochemical Characterization of the Carbonaceous Materials
3. Results and Discussion
3.1. Characterization of King Grass Biomass and Extracted Cellulose
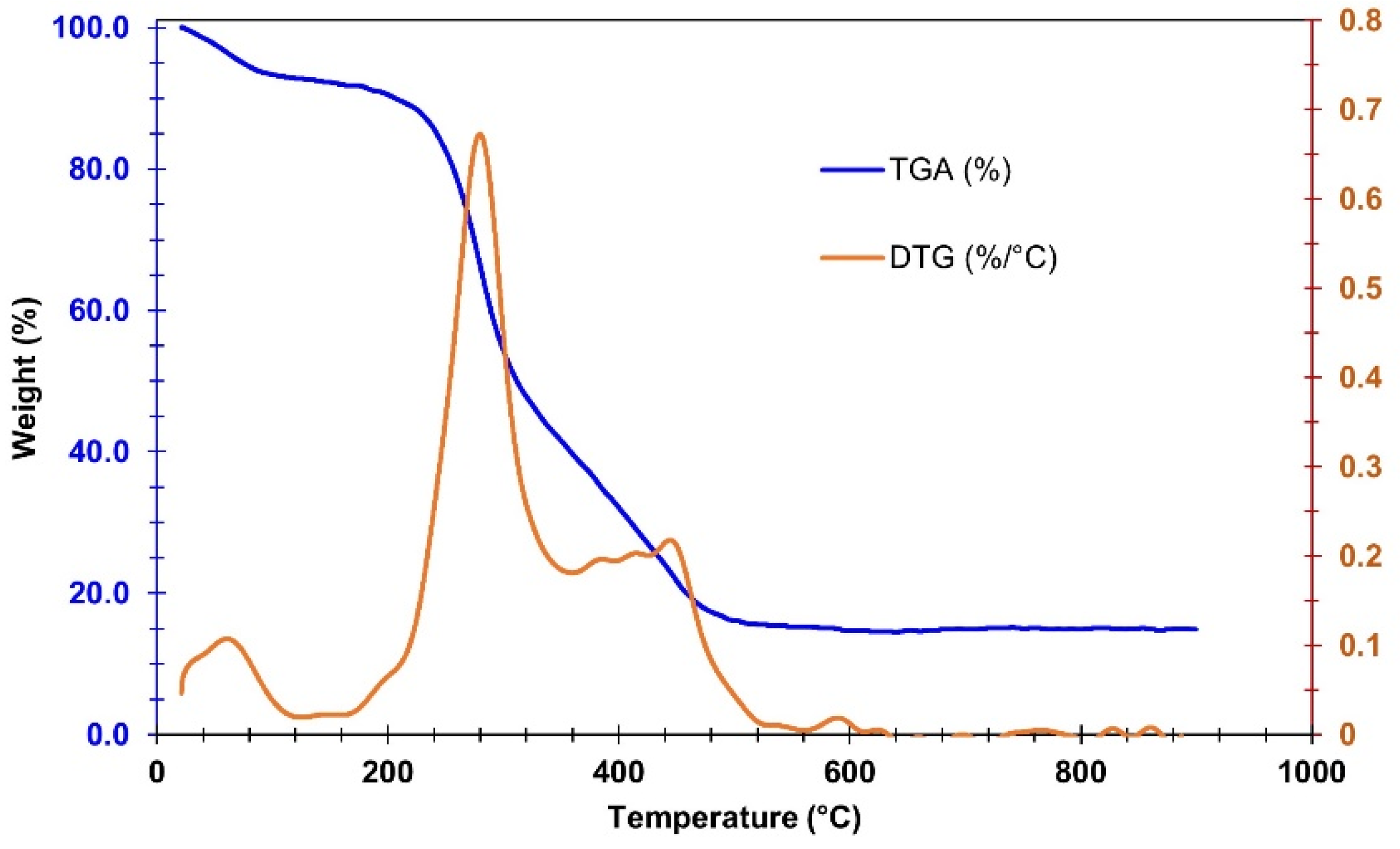
3.1.1. TGA/DTA
3.1.2. Proximate and Elemental Analysis
3.1.3. Extractives, Structural Carbohydrates and Lignin Composition
3.1.4. Yields of the Production of Carbonaceous Materials
3.2. Physicochemical Characterization of the Carbonaceous Materials
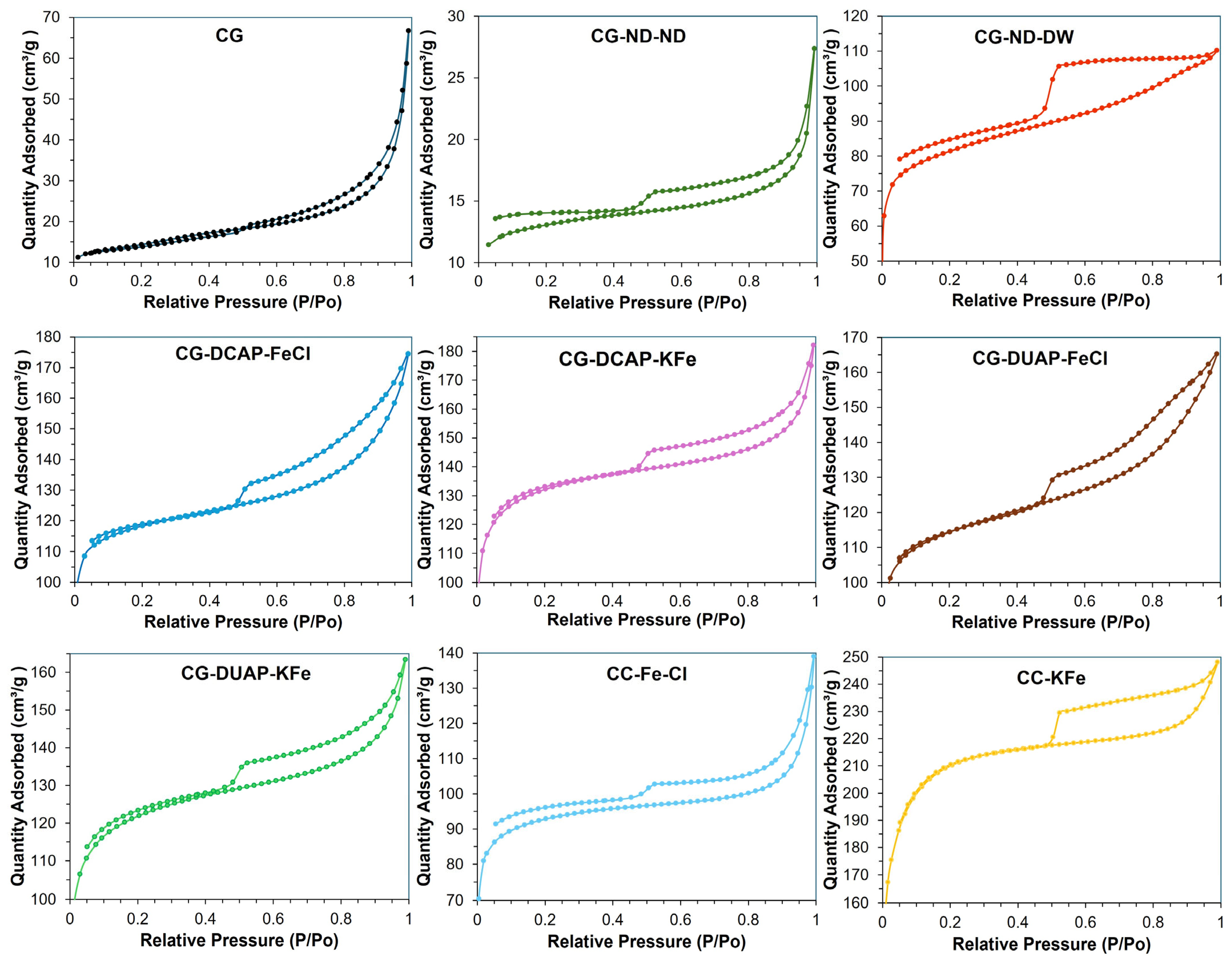
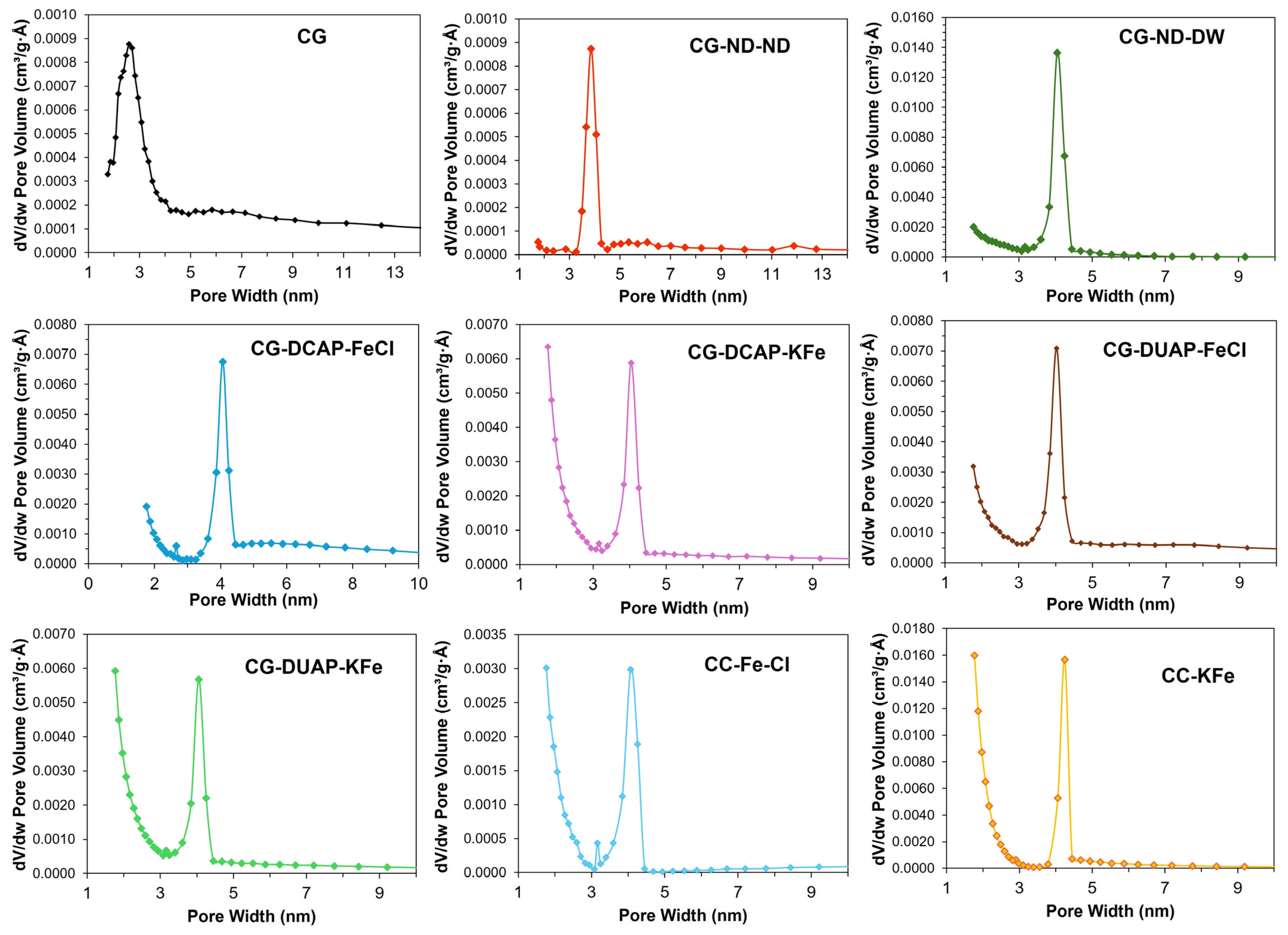
3.2.1. Textural Properties
3.2.2. Fe and K Content
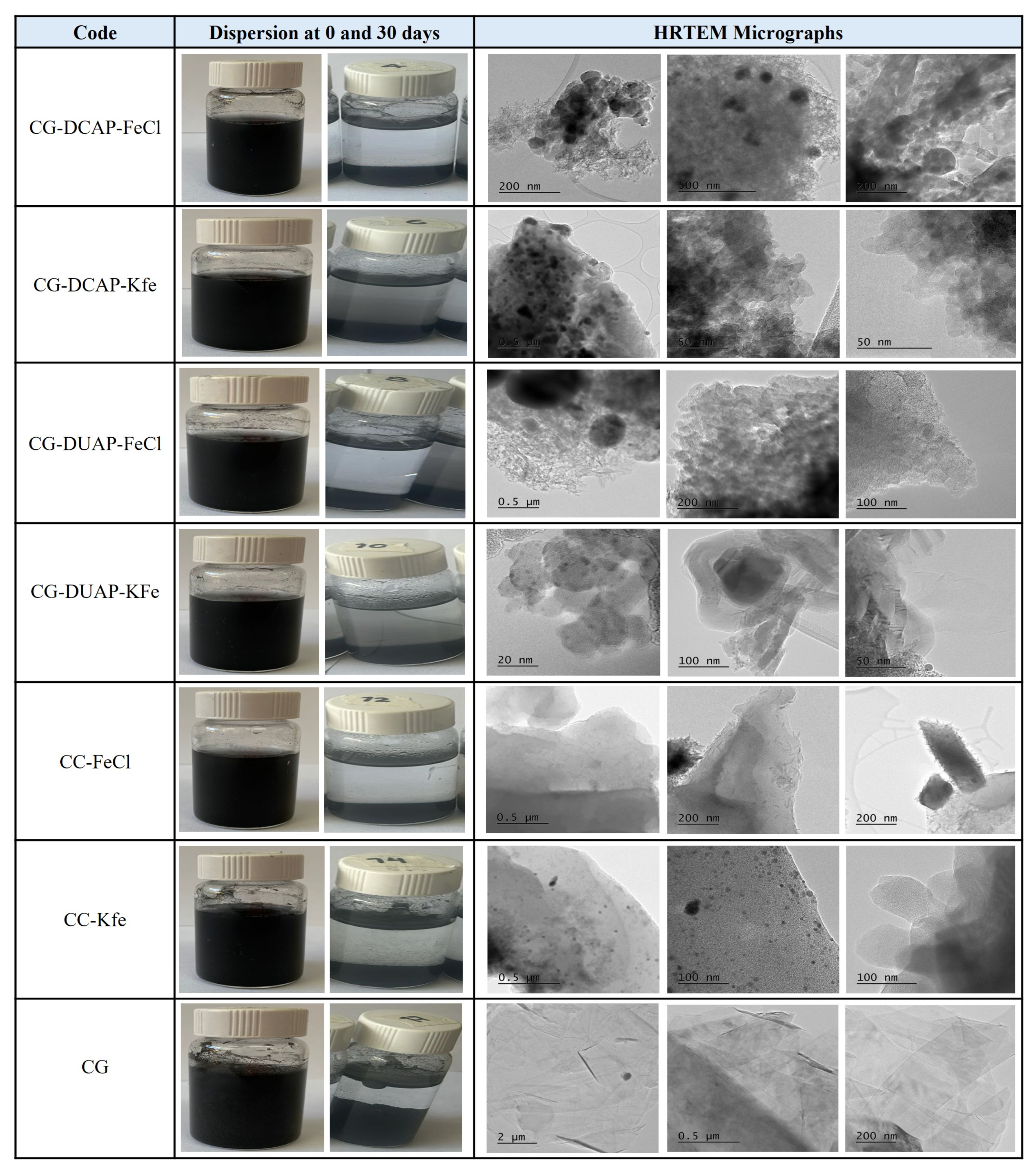
3.2.3. Dispersion pH and Zeta Potential
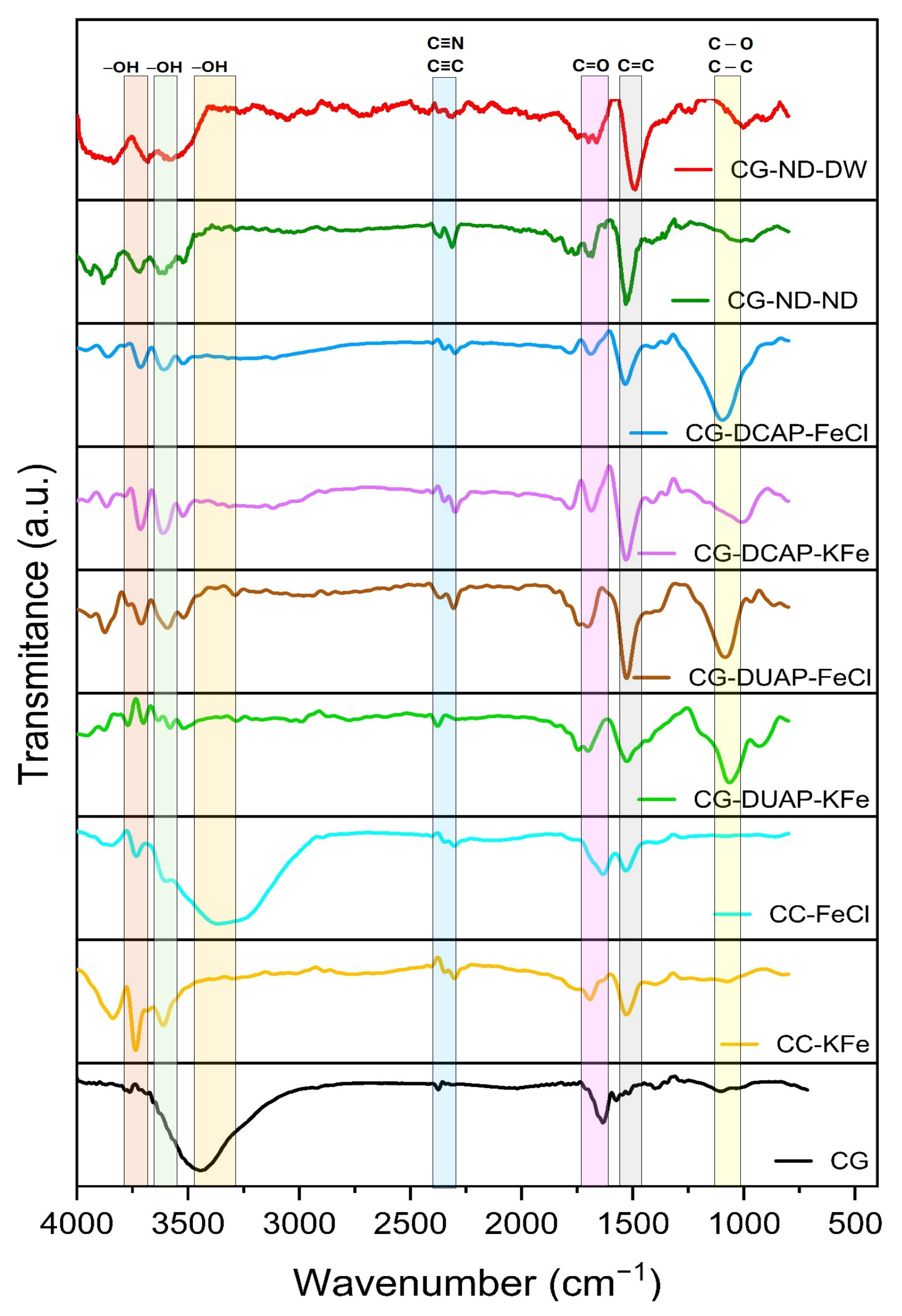
3.2.4. FT-IR Analysis
3.2.5. H2-TPR Analysis
3.2.6. XRD Analysis
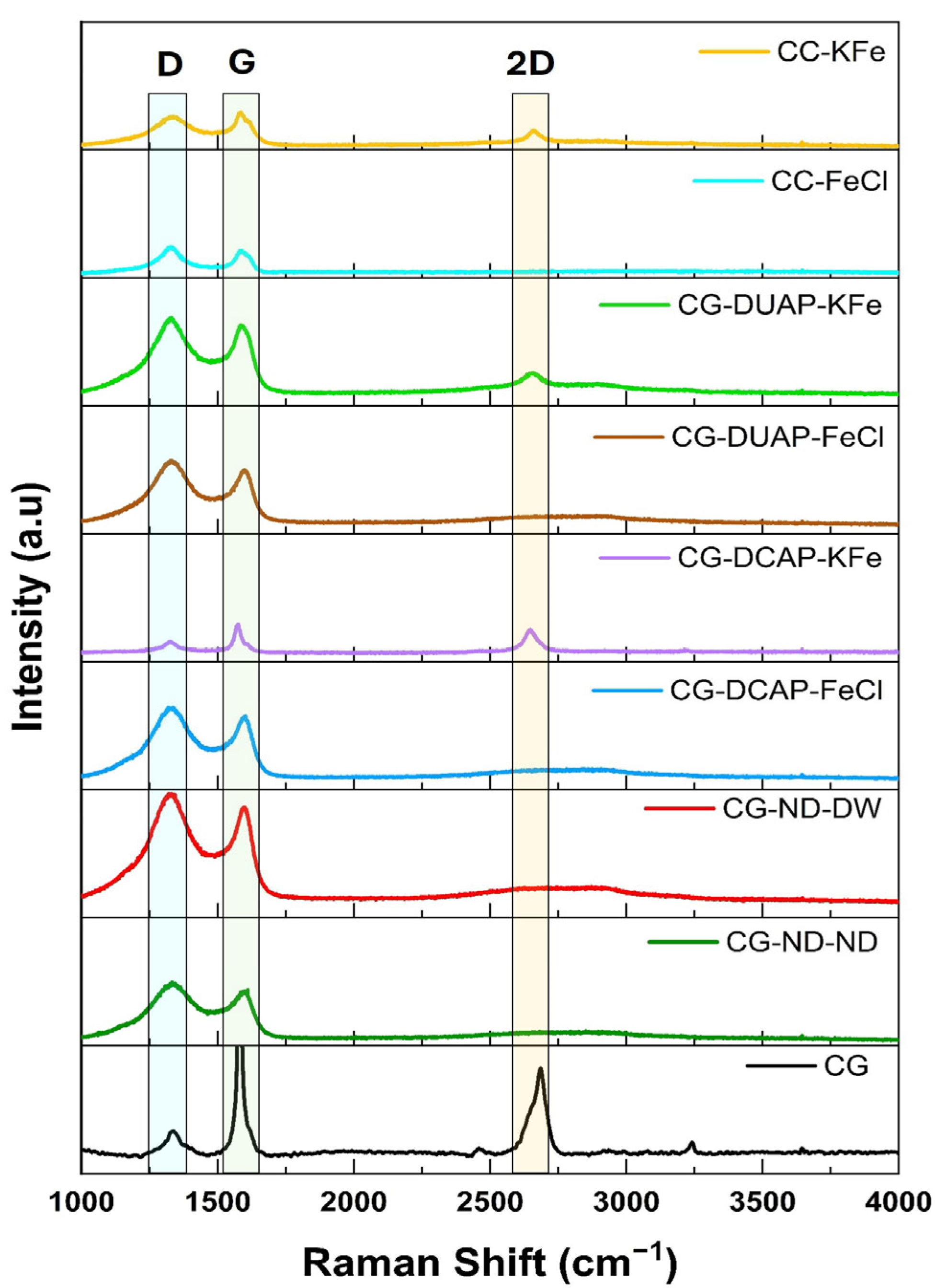
3.2.7. Raman Analysis
3.2.8. HRTEM Analysis
3.3. Mechanistic Discussion of the Dual Precursor Effect on Structure and Porosity
3.4. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safian, M.T.; Haron, U.S.; Mohamad Ibrahim, M.N. A Review on Bio-Based Graphene Derived from Biomass Wastes. BioResources 2020, 15, 9756–9785. [Google Scholar] [CrossRef]
- Bahmei, F.; Bahramifar, N.; Ghasemi, S.; Younesi, H.; Weil, M. Comparison of Environmental Impacts in the Production of Graphene from Biomass Waste and the Hummers’ Method. J. Clean. Prod. 2025, 497, 145145. [Google Scholar] [CrossRef]
- Cheng, K.; Wallaert, S.; Ardebili, H.; Karim, A. Advanced Triboelectric Nanogenerators Based on Low-Dimension Carbon Materials: A Review. Carbon 2022, 194, 81–103. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-Waste Derived Graphene Quantum Dots and Their Applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-Based Catalysts for Fischer–Tropsch Synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef]
- Atakoohi, S.E.; Spennati, E.; Riani, P.; Carpanese, M.P.; Garbarino, G. Graphene-Based Materials for Wastes, Biomass and CO2 Valorization in Catalysis: A Technological Perspective via Molten Salt Synthesis. Catal. Today 2024, 441, 114848. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Lugovoy, Y.V.; Kosivtsov, Y.Y.; Matveeva, V.G.; Sulman, M.G. Plant-Biomass-Derived Carbon Materials as Catalyst Support, A Brief Review. Catal. Rev. 2023, 13, 655. [Google Scholar] [CrossRef]
- He, H.; Zhang, R.; Zhang, P.; Wang, P.; Chen, N.; Qian, B.; Zhang, L.; Yu, J.; Dai, B. Functional Carbon from Nature: Biomass-Derived Carbon Materials and the Recent Progress of Their Applications. Adv. Sci. 2023, 10, 2205557. [Google Scholar] [CrossRef] [PubMed]
- Ruwona, W.; Danha, G.; Muzenda, E. A Review on Material and Energy Recovery from Waste Tyres. Procedia Manuf. 2019, 35, 216–222. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhang, X. The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules 2024, 29, 1825. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, N.; Thangavel, S.; Venugopal, G. A Short Review on Preparation of Graphene from Waste and Bioprecursors. Appl. Mater. Today 2017, 7, 246–254. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, T.S. Graphene Sheets Fabricated from Disposable Paper Cups as a Catalyst Support Material for Fuel Cells. J. Mater. Chem. A 2013, 1, 183–187. [Google Scholar] [CrossRef]
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valorization 2022, 13, 1385–1429. [Google Scholar] [CrossRef]
- Ramos, L.G.V. Materiales de Carbón Micro-mesoporosos Obtenidos del Brosimum Alicastrum Para Aplicaciones en Generación de Energía. Master’s Thesis, Universidad Nacional de Ingeniería, Yucatán, México, 2018. Volume 1. [Google Scholar]
- Burkholder, M.B.; Rahman, F.B.A.; Chandler, E.H.; Regalbuto, J.R.; Gupton, B.F.; Tengco, J.M.M. Metal Supported Graphene Catalysis: A Review on the Benefits of Nanoparticular Supported Specialty Sp2 Carbon Catalysts on Enhancing the Activities of Multiple Chemical Transformations. Carbon Trends 2022, 9, 100196. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, M.; He, C.; Liu, C.; Li, Z.; Liu, Y. Preparation of Graphene by Catalytic Pyrolysis of Lignin and Its Electrochemical Properties. Mater. Lett. 2020, 274, 128047. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Andou, Y. A Route towards Graphene from Lignocellulosic Biomass: Technicality, Challenges, and Their Prospective Applications. J. Clean. Prod. 2022, 380, 135090. [Google Scholar] [CrossRef]
- Betancur, S.B.; Villaseñor, E.A.; Arias, A.N.A. Preliminary Economic Feasibility Study for Graphene Synthesis from King Grass at Laboratory Scale. J. Eng. Res. 2023, 3, 2–14. [Google Scholar] [CrossRef]
- Botero-Londono, J.M.; Celis-Celis, E.M.; Botero-Londono, M.A. Nutritional Quality, Nutrient Uptake and Biomass Production of Pennisetum Purpureum Cv. King Grass. Sci. Rep. 2021, 11, 13799. [Google Scholar] [CrossRef] [PubMed]
- Cardona, E.; Rios, J.; Peña, J.; Peñuela, M.; Rios, L. King Grass: A Very Promising Material for the Production of Second Generation Ethanol in Tropical Countries. Biomass Bioenergy 2016, 95, 206–213. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory (NREL), U.S. Department of Energy: Golden, CO, USA, 2011; pp. 1–14. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory (NREL), U.S. Department of Energy: Golden, CO, USA, 2005; pp. 1–9. [Google Scholar]
- ASTM D1104-56; Method of Test for Holocellulose in Wood. ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 1997.
- Ardila, A.N.; González, E.A.-V.E.E.V.; Guerrero, H.E.G.; Hernández-Maldonado, J.A.; Villa, E.G.-P.; Villa, C.C. Enhanced Cellulose Extraction from Banana Pseudostem Waste: A Comparative Analysis Using Chemical Methods Assisted by Conventional and Focused Ultrasound. Polymers 2024, 16, 2785. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.Á.; Díaz, C.P.G.; Folgueras, M.B. Caracterización Química de Biomasa y Su Relación Con El Poder Calorífico; Universidad de Oviedo: Oviedo, Spain, 2012; pp. 1–12. [Google Scholar]
- Shetty, A.; Molahalli, V.; Sharma, A.; Hegde, G. Biomass-Derived Carbon Materials in Heterogeneous Catalysis: A Step towards Sustainable Future. Catalysts 2023, 13, 20. [Google Scholar] [CrossRef]
- Batista, R.R.; Gomes, M.M. Effects of Chemical Composition and Pyrolysis Process Variables on Biochar Yields: Correlation and Principal Component Analysis. Floresta Ambient. 2021, 28, e20210007. [Google Scholar] [CrossRef]
- Yang, D.P.; Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications. ACS Sustain. Chem. Eng. 2019, 7, 4564–4585. [Google Scholar] [CrossRef]
- Li, J.; Yin, D.; Qin, Y. Carbon Materials: Structures, Properties, Synthesis and Applications. Manuf. Rev. 2023, 10, 13. [Google Scholar] [CrossRef]
- Zhao, N.; Feng, Y.; Xiong, M.; Fu, P. Renewable Biomass Bougainvillea Petals-Derived Porous Carbon through KOH Activation and B/N Co-Doping: Synergistic Contribution and Charge Storage Mechanism. Appl. Surf. Sci. 2024, 672, 160852. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Issahaku, I.; Tetteh, A.Y. Recent Advances in Synthesis, Characterization, and Environmental Applications of Activated Carbons and Other Carbon Derivatives. Carbon Trends 2024, 14, 100328. [Google Scholar] [CrossRef]
- Chagas, J.S.; Almeida, J.N.S.; Pereira, A.C.L.; Silva, N.F.I.; Raimundo, R.A.; Medeiros, E.S.; Lima, B.A.S.G.; Galvão, L.S.; Santos, A.S.F.; Silva, F.I. Evaluation of the Kinetics of Low Intensity Ultrasound-Assisted Sulfuric Acid Hydrolysis to Obtain Cellulose Nanocrystals (CNCs) from Microcrystalline Cellulose (MCC). Cellulose 2023, 30, 11455–11472. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Applying Ultrasound-Assisted Processing to Obtain Cellulose Fibres from Rice Straw to Be Used as Reinforcing Agents. Innov. Food Sci. Emerg. Technol. 2022, 76, 102932. [Google Scholar] [CrossRef]
- Perondi, D.; Bassanesi, G.R.; Manera, C.; Lazzari, L.K.; Godinho, M.; Zattera, A.J.; Dotto, G.L. From Cellulose to Graphene-like Porous Carbon Nanosheets. Microporous Mesoporous Mater. 2021, 323, 111217. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Wang, S.; Yin, Y. Effects of Ultrasonic Treatment during Acid Hydrolysis on the Yield, Particle Size and Structure of Cellulose Nanocrystals. Carbohydr. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef]
- Liu, C.F.; Ren, J.L.; Xu, F.; Liu, J.J.; Sun, J.X.; Sun, R.C. Isolation and Characterization of Cellulose Obtained from Ultrasonic Irradiated Sugarcane Bagasse. J. Agric. Food Chem. 2006, 54, 5742–5748. [Google Scholar] [CrossRef]
- Camani, P.H.; Anholon, B.F.; Toder, R.R.; Rosa, D.S. Microwave-Assisted Pretreatment of Eucalyptus Waste to Obtain Cellulose Fibers. Cellulose 2020, 7, 3591–3609. [Google Scholar] [CrossRef]
- Li, T.; Ma, R.; Xu, X.; Sun, S.; Lin, J. Microwave-Induced Preparation of Porous Graphene Nanosheets Derived from Biomass for Supercapacitors. Microporous Mesoporous Mater. 2021, 324, 111277. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Mohd Johari, S.A.; Lease, J.; Ayoub, M.; Andou, Y. Catalytic Efficiency and Stability of Biomass-Derived Sulfonated Graphene Catalysts in Microwave-Enhanced Biodiesel Production. Fuel 2024, 368, 131580. [Google Scholar] [CrossRef]
- Lataf, A.; Khalil Awad, A.E.; Joos, B.; Carleer, R.; Yperman, J.; Schreurs, S.; D’Haen, J.; Cuypers, A.; Vandamme, D. Iron Oxide-Activated Carbon Composites for Enhanced Microwave-Assisted Pyrolysis of Hardwood. Environments 2024, 11, 102. [Google Scholar] [CrossRef]
- Balqis, N.; Mohamed Jan, B.; Simon Cornelis Metselaar, H.; Sidek, A.; Kenanakis, G.; Ikram, R. An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects. Materials 2023, 16, 3726. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, Y.; Huang, K. Advances in Microwave-Assisted Production of Reduced Graphene Oxide. Front. Chem. 2019, 7, 355. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmed, N.B.; Adegoke, K.A.; Bello, O.S. Sorption Studies of Methyl Red Dye Removal Using Lemon Grass (Cymbopogon citratus). Chem. Data Collect. 2019, 22, 100249. [Google Scholar] [CrossRef]
- Chaala, A.; Pakdel, H.; De Caumia, B.; Darmstadt, H.; Roy, C.; Yang, J.; Chaala, A.; Darmstadt, H.; De Caumia, B.; Pakdel, H.; et al. Conversion of Used Tires to Carbon Black and Oil by Pyrolysis. In Rubber Recycling; CRC Press: Boca Raton, FL, USA, 2005; pp. 429–468. [Google Scholar] [CrossRef]
- Qu, W.H.; Xu, Y.Y.; Lu, A.H.; Zhang, X.Q.; Li, W.C. Converting Biowaste Corncob Residue into High Value Added Porous Carbon for Supercapacitor Electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Investigation of Bifunctionality of FePc-Functionalized Graphene for Enhanced ORR/OER Activity. Mol. Catal. 2023, 545, 113213. [Google Scholar] [CrossRef]
- Nuruddin, A.; Saputro, A.G.; Maulana, A.L.; Fajrial, A.K.; Shukri, G.; Mahyuddin, M.H.; Aprilyanti, F.D.; Harimawan, A.; Dipojono, H.K. Enhancing Oxygen Reduction Reaction Activity of Pyrolyzed Fe–N–C Catalyst by the Inclusion of BN Dopant at the Graphitic Edges. Appl. Surf. Sci. 2023, 608, 155203. [Google Scholar] [CrossRef]
- Naghib, S.M.; Zare, Y.; Rhee, K.Y. A Facile and Simple Approach to Synthesis and Characterization of Methacrylated Graphene Oxide Nanostructured Polyaniline Nanocomposites. Nanotechnol. Rev. 2020, 9, 53–60. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Borugadda, V.B.; Dalai, A.K.; Zhang, L. Optimization of Carbon Nanotube Growth via Response Surface Methodology for Fischer-Tropsch Synthesis over Fe/CNT Catalyst. Catal. Today 2022, 404, 117–131. [Google Scholar] [CrossRef]
- Gürünlü, B.; Taşdelen-Yücedağ, Ç.; Bayramoğlu, M. Graphene Synthesis by Ultrasound Energy-Assisted Exfoliation of Graphite in Various Solvents. Crystals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Fahmi, F.; Dewayanti, N.A.A.; Widiyastuti, W.; Setyawan, H. Preparation of Porous Graphene-like Material from Coconut Shell Charcoals for Supercapacitors. Cogent Eng. 2020, 7, 1748962. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Meng, J.; Wang, H.; Zhao, Y.; Mai, L. Advances and Perspectives on One-Dimensional Nanostructure Electrode Materials for Potassium-Ion Batteries. Mater. Today 2022, 56, 114–134. [Google Scholar] [CrossRef]
- Tavares, M.; Westphalen, G.; de Almeida, J.M.A.R.; Romano, P.N.; Sousa-Aguiar, E.F. Modified Fischer-Tropsch Synthesis: A Review of Highly Selective Catalysts for Yielding Olefins and Higher Hydrocarbons. Front. Nanotechnol. 2022, 4, 978358. [Google Scholar] [CrossRef]
- Guo, Q.; Lv, H.; Mao, J.; Zhou, J. Selective Hydrodeoxygenation of Guaiacol to Phenol over CoFe/Reduced Graphene Oxide. Mol. Catal. 2024, 554, 113857. [Google Scholar] [CrossRef]
- Bychko, I.; Kalishyn, Y.; Strizhak, P. TPR Study of Core-Shell Fe@Fe3O4 Nanoparticles Supported on Activated Carbon and Carbon Nanotubes. Adv. Mater. Phys. Chem. 2012, 2, 17–22. [Google Scholar] [CrossRef]
- Makayeva, N.; Yergaziyeva, G.; Soloviev, S.; Kutelia, E.; Nadaria, L.; Tsurtsumia, O.; Zhuginis, B.; Annisova, M.; Mambetova, M. Dossumov Effects of Cerium Oxide on the Activity of Fe-Ni/Al2O3 Catalyst in the Decomposition of Methane. Inorg. Chem. Commun. 2024, 161, 112047. [Google Scholar] [CrossRef]
- Martín, N.; Cirujano, F.G. Multifunctional Heterogeneous Catalysts for the Tandem CO2 hydrogenation-Fischer Tropsch Synthesis of Gasoline. J. CO2 Util. 2022, 65, 102176. [Google Scholar] [CrossRef]
- Buthelezi, A.S.; Tucker, C.L.; Heeres, H.J.; Shozi, M.L.; van de Bovenkamp, H.H.; Ntola, P. Fischer-Tropsch Synthesis Using Promoted, Unsupported, Supported, Bimetallic and Spray-Dried Iron Catalysts: A Review. Results Chem. 2024, 9, 101623. [Google Scholar] [CrossRef]
- Mamani, A.; Barreda, D.; Fabiana Sardella, M.; Bavio, M.; Blanco, C.; González, Z.; Santamaría, R. Fe-Doped Biomass-Derived Activated Carbons as Sustainable Electrode Materials in Supercapacitors Using Different Electrolytes. J. Electroanal. Chem. 2024, 965, 118366. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Zheng, J.Y.; Zhao, Y.S.; Yao, J. Large-Scale Production of High-Quality Graphene Using Glucose and Ferric Chloride. Chem. Sci. 2014, 5, 4656–4660. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, J.; Chen, Y.; Tang, L.; Hou, H.; Yao, Z.; Zhang, Z.; Zhong, Q. A High-Performance Nano-Sn/G@C Composite Anode Prepared by Waste Carbon Residue from Spent Lithium-Ion Batteries. Chem. Eng. J. 2022, 450, 138113. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, Y.; Liu, Z.; Zhang, X.; Ma, W.; Wu, H.; Huang, X. Novel Synthesis Route for the Preparation of Mesoporous Nitrogen-Doped Carbons from Forestry Wastes for Supercapacitors. Surf. Interfaces 2021, 24, 101132. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.; Park, S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Mater. Rev. 2021, 14, 4590. [Google Scholar] [CrossRef]
- Martínez González, J.; Reyes Contreras, D.; Vigueras Santiago, E.; Garcia Orozco, I. Chapter 3 Determining Crystallite Size and Density of Recovered Graphite Defects from New and Used Zn-C Batteries. In Handbook T-II Ingeniería y Ciencias Aplicadas; ECORFAN-México, S.C., Ed.; ECORFAN-México, S.C.: Mexico City, Mexico, 2021; pp. 18–27. ISBN 978-607-8695-68-3. [Google Scholar]
- He, K.; Zhang, Z.Y.; Zhang, F.S. Synthesis of Graphene and Recovery of Lithium from Lithiated Graphite of Spent Li-Ion Battery. Waste Manag. 2021, 124, 283–292. [Google Scholar] [CrossRef]
- Cascales, J. Estudio de Capas Nanométricas de H-BN y Grafeno Para Su Apilamiento En Multicapas. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2016. Volume 1. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Srinivasan, S.; Jeevanantham, S.; Vishnu, M.; Amith, K.V.; Sruthi, R.; Saravanan, R.; Vo, D.V.N. Insights on Synthesis and Applications of Graphene-Based Materials in Wastewater Treatment: A Review. Chemosphere 2022, 298, 134284. [Google Scholar] [CrossRef] [PubMed]
- Bleu, Y.; Bourquard, F.; Loir, A.S.; Barnier, V.; Garrelie, F.; Donnet, C. Raman Study of the Substrate Influence on Graphene Synthesis Using a Solid Carbon Source via Rapid Thermal Annealing. J. Raman Spectrosc. 2019, 50, 1630–1641. [Google Scholar] [CrossRef]
- Hu, K.; Brambilla, L.; Sartori, P.; Moscheni, C.; Perrotta, C.; Zema, L.; Bertarelli, C.; Castiglioni, C. Development of Tailored Graphene Nanoparticles: Preparation, Sorting and Structure Assessment by Complementary Techniques. Molecules 2023, 28, 565. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From Coconut Shell to Porous Graphene-like Nanosheets for High-Power Supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N. Biomass-Based Carbon Materials for CO2 capture: A Review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]




| Code | Synthesis Description |
|---|---|
| CG-ND-ND | Carbonaceous material from non-delignified, non-deoxidized biomass grass and pyrolyzed at 1000 °C. |
| CG-ND-DW | Carbonaceous material from non-delignified biomass grass, deoxidized without catalyst and pyrolyzed at 1000 °C. |
| CG-DCAP-FeCl | Carbonaceous material from delignified biomass grass with conventional alkaline peroxide, deoxidized with FeCl3 and pyrolyzed at 1000 °C. |
| CG-DCAP-KFe | Carbonaceous material from delignified biomass grass with conventional alkaline peroxide, deoxidized with K3[Fe(CN)6] and pyrolyzed at 1000 °C. |
| CG-DUAP-FeCl | Carbonaceous material from delignified biomass grass with ultrasound alkaline peroxide, deoxidized with FeCl3 and pyrolyzed at 1000 °C. |
| CG-DUAP-KFe | Carbonaceous material from delignified biomass grass with ultrasound alkaline peroxide, deoxidized with K3[Fe(CN)6] and pyrolyzed at 1000 °C. |
| CC-FeCl | Carbonaceous material from commercial cellulose, deoxidized with FeCl3 and pyrolyzed at 1000 °C. |
| CC-KFe | Carbonaceous material from commercial cellulose, deoxidized with K3[Fe(CN)6] and pyrolyzed at 1000 °C. |
| CC | Commercial cellulose (Sigmacell Cellulose Type 101, Highly purified, Fibers Sigma Aldrich (St. Louis, MO, USA). |
| CG | Commercial graphene (Graphene nanoplatelets, Sigma Aldrich). |
| OG | Graphene oxide (Sigma Aldrich). |
| Type of Biomass | Moisture (%) | Extractives (%) | Carbohydrates (%) | ||||
|---|---|---|---|---|---|---|---|
| Water | Ethanol | Lignin | Holocellulose | Hemicellulose | Cellulose | ||
| UB-KG | 9.90 | 16.13 | 9.87 | 3.62 | 80.40 | 42.03 | 38.37 |
| DCAP | 7.65 | 0 | 0.00 | 0 | 84.81 | 39.33 | 45.48 |
| DUAP | 6.69 | 0 | 8.70 | 0 | 94.00 | 28.24 | 65.80 |
| Code | Obtaining Biomass (%) | Cellulose Extraction (%) | Cellulose Deoxidation (%) | Catalytic Pyrolysis (%) | Acid Purification (%) | Overall Yield (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | SD | CV | Yield | SD | CV | Yield | SD | CV | Yield | SD | CV | Yield | SD | CV | ||
| CG-ND-ND | 13.39 | 0.03 | 0.21 | - | - | - | - | - | - | 27.39 | 0.08 | 0.31 | - | - | - | 3.67 |
| CG-ND-DW | 13.21 | 0.08 | 0.61 | - | - | - | 84.07 | 7.48 | 8.90 | 32.58 | 0.40 | 1.22 | 84.57 | 0.21 | 0.25 | 3.06 |
| CG-DCAP-FeCl | 13.38 | 0.23 | 1.72 | 44.01 | 0.11 | 0.24 | 75.81 | 1.01 | 1.34 | 27.94 | 0.39 | 1.41 | 94.26 | 0.27 | 0.29 | 1.18 |
| CG-DCAP-KFe | 13.27 | 0.08 | 0.58 | 45.13 | 0.43 | 0.95 | 94.70 | 1.15 | 1.22 | 34.07 | 0.11 | 0.33 | 41.74 | 0.47 | 1.12 | 0.81 |
| CG-DUAP-FeCl | 13.26 | 0.07 | 0.54 | 35.15 | 0.45 | 1.27 | 75.08 | 0.76 | 1.01 | 27.26 | 0.46 | 1.69 | 74.67 | 0.50 | 0.67 | 0.71 |
| CG-DUAP-KFe | 13.17 | 0.21 | 1.57 | 34.86 | 0.33 | 0.94 | 87.02 | 5.19 | 5.96 | 31.54 | 0.11 | 0.34 | 44.82 | 3.04 | 6.79 | 0.56 |
| CC-FeCl | - | - | - | - | - | - | 79.60 | 3.09 | 3.88 | 21.96 | 0.46 | 2.10 | 73.98 | 0.43 | 0.58 | 1.71 |
| CC-KFe | - | - | - | - | - | - | 90.54 | 4.81 | 5.31 | 20.30 | 0.52 | 2.59 | 63.11 | 2.11 | 3.35 | 1.55 |
| CG | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| OG | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Code | BET Surface Area (m2/g) | Average Pore Volume BJH (cm3/g) | Average Pore Diameter (nm) | Fe (wt.%) AAS | K (wt.%) AAS | pH Dispersion | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| CG-ND-ND | 42.73 | 0.028 | 8.97 | - | - | 10.12 | −34.0 |
| CG-ND-DW | 275.89 | 0.082 | 6.75 | - | - | 9.93 | −28.8 |
| CG-DCAP-FeCl | 400.22 | 0.123 | 6.32 | 3.76 | - | 6.63 | −37.3 |
| CG-DCAP-KFe | 449.06 | 0.124 | 4.92 | 5.90 | 2.73 | 9.99 | −38.0 |
| CG-DUAP-FeCl | 388.41 | 0.123 | 5.10 | 4.22 | - | 5.51 | −24.8 |
| CG-DUAP-KFe | 414.29 | 0.110 | 4.40 | 6.02 | 2.75 | 10.20 | −37.4 |
| CC-FeCl | 314.22 | 0.098 | 3.97 | 5.90 | - | 6.59 | −31.2 |
| CC-KFe | 714.50 | 0.140 | 3.18 | 8.06 | 1.88 | 9.93 | −39.4 |
| CG | 49.03 | 0.096 | 11.97 | - | - | 8.15 | −47.9 |
| Code | Reduction Temperature (°C) | Hydrogen Consumption (mmol H2/g Material) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | ||
| CG-ND-ND | - | - | - | - | - | - | - | - | - | - | - |
| CG-ND-DW | - | - | - | - | - | - | - | - | - | - | - |
| CG-DCAP-FeCl | 526.0 | 697.2 | 610.3 | - | - | 0.005 | 0.005 | 0.005 | - | - | 0.015 |
| CG-DCAP-KFe | 337.1 | 459.6 | 576.6 | 810.6 | - | 0.005 | 0.005 | 0.005 | 0.005 | - | 0.020 |
| CG-DUAP-FeCl | 536.8 | 715.5 | - | - | - | 0.061 | 0.007 | - | - | - | 0.068 |
| CG-DUAP-KFe | 544.1 | 601.5 | 708.2 | - | - | 0.032 | 0.008 | 0.009 | - | - | 0.049 |
| CC-FeCl | 462.3 | 554.3 | 687.2 | 823.5 | - | 0.015 | 0.023 | 0.011 | 0.010 | - | 0.059 |
| CC-KFe | 470.0 | 540.7 | 576.1 | - | - | 0.005 | 0.005 | 0.005 | - | - | 0.015 |
| CG | 506.4 | 586.7 | - | - | - | 0.009 | 0.010 | - | - | - | 0.019 |
| GO | 474.5 | 542.2 | 608.7 | 705.5 | - | 0.042 | 0.027 | 0.043 | 0.023 | - | 0.134 |
| Code | XRD | Raman | |||||
|---|---|---|---|---|---|---|---|
| Angle 2θ | Crystallite Size (nm) | Crystallinity (%) | Allotropic Form | Intensity Band Ratio | Layer Number | ||
| I2D/IG | ID/IG | ||||||
| CG-ND-ND | 21.96 | 0.43 | 14.0 | Amorphous carbon | 0 | 1.12 | - |
| CG-ND-DW | 21.96 | 0.38 | 6.30 | Amorphous carbon | 0 | 1.15 | - |
| CG-DCAP-FeCl | 21.78 | 0.33 | 15.0 | Amorphous carbon | 0 | 1.13 | - |
| CG-DCAP-KFe | 26.31 | 0.11 | 24.3 | Graphene-like material | 0.80 | 0.37 | 2 |
| CG-DUAP-FeCl | 21.86 | 0.25 | 12.8 | Amorphous carbon | 0 | 1.16 | - |
| CG-DUAP-KFe | 26.43 | 0.11 | 21.3 | Graphene-like material | 0.32 | 1.11 | 3 |
| CC-FeCl | 25.94 | 0.11 | 18.8 | Amorphous carbon | 0 | 1.20 | - |
| CC-KFe | 26.41 | 0.23 | 24.0 | Graphene-like material | 0.46 | 0.88 | 3 |
| CG | 26.59 | 0.26 | 54.0 | Graphene | 0.39 | 0.09 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, A.N.A.; Arriola-Villaseñor, E.; Ortiz-Quiceno, M.; Blandón-Naranjo, L.; Hernández-Maldonado, J.A. Graphene-like Carbon Materials from King Grass Biomass via Catalytic Pyrolysis Using K3[Fe(CN)6] as a Dual Catalyst and Activator. C 2025, 11, 62. https://doi.org/10.3390/c11030062
Arias ANA, Arriola-Villaseñor E, Ortiz-Quiceno M, Blandón-Naranjo L, Hernández-Maldonado JA. Graphene-like Carbon Materials from King Grass Biomass via Catalytic Pyrolysis Using K3[Fe(CN)6] as a Dual Catalyst and Activator. C. 2025; 11(3):62. https://doi.org/10.3390/c11030062
Chicago/Turabian StyleArias, Alba N. Ardila, Erasmo Arriola-Villaseñor, Madelyn Ortiz-Quiceno, Lucas Blandón-Naranjo, and José Alfredo Hernández-Maldonado. 2025. "Graphene-like Carbon Materials from King Grass Biomass via Catalytic Pyrolysis Using K3[Fe(CN)6] as a Dual Catalyst and Activator" C 11, no. 3: 62. https://doi.org/10.3390/c11030062
APA StyleArias, A. N. A., Arriola-Villaseñor, E., Ortiz-Quiceno, M., Blandón-Naranjo, L., & Hernández-Maldonado, J. A. (2025). Graphene-like Carbon Materials from King Grass Biomass via Catalytic Pyrolysis Using K3[Fe(CN)6] as a Dual Catalyst and Activator. C, 11(3), 62. https://doi.org/10.3390/c11030062







