In Silico Exfoliation of ReaxFF Graphite—Temperature, Speed, Angle Dependence, and the Effect of Gold Overlayer
Abstract
1. Introduction
2. Materials and Methods
3. Results
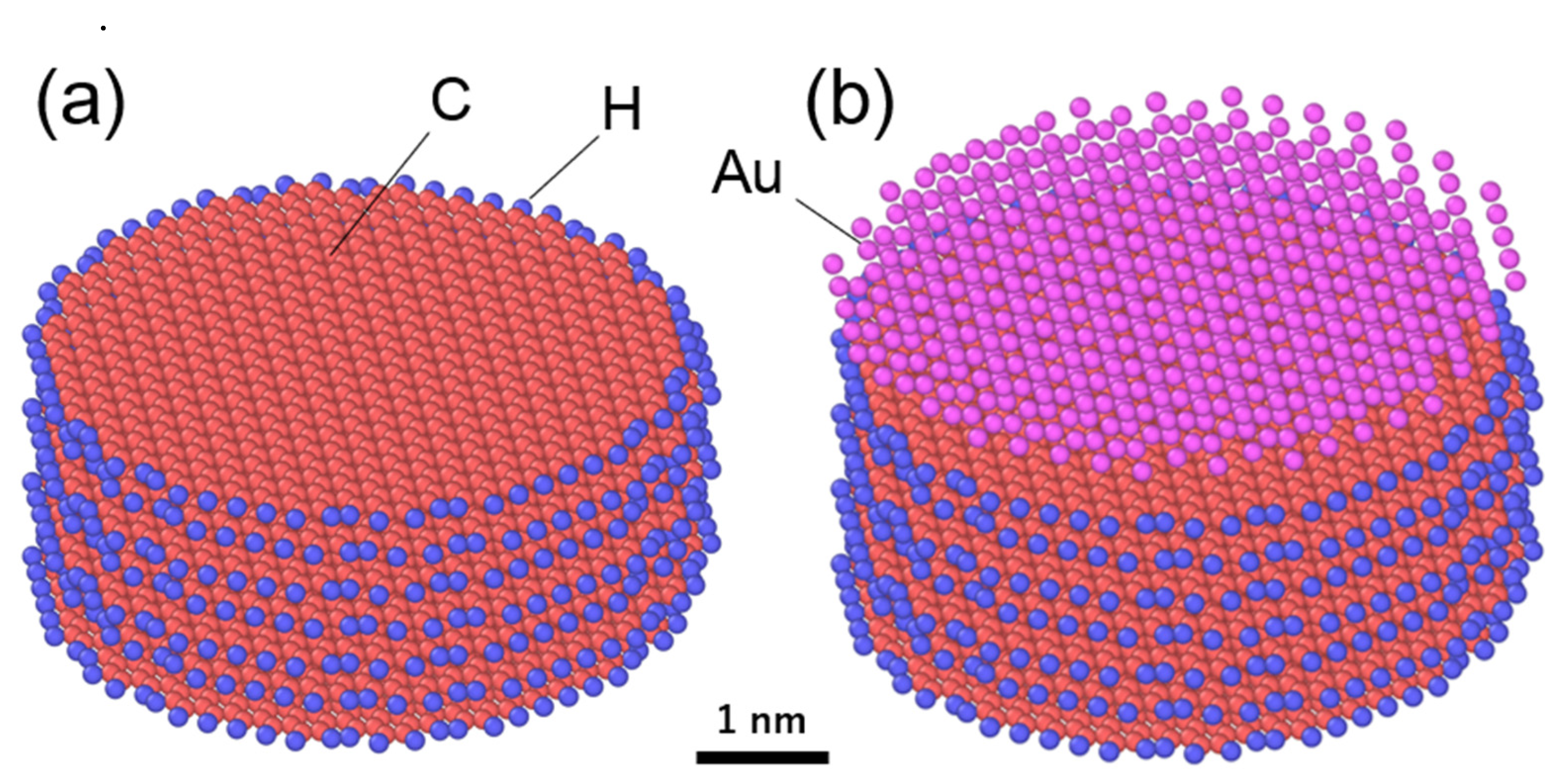
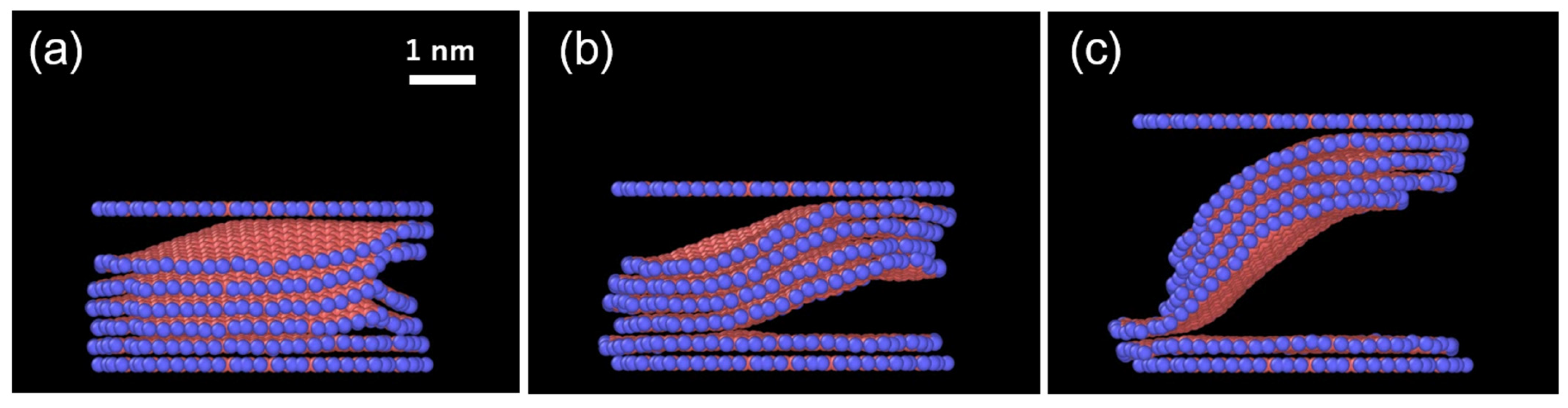
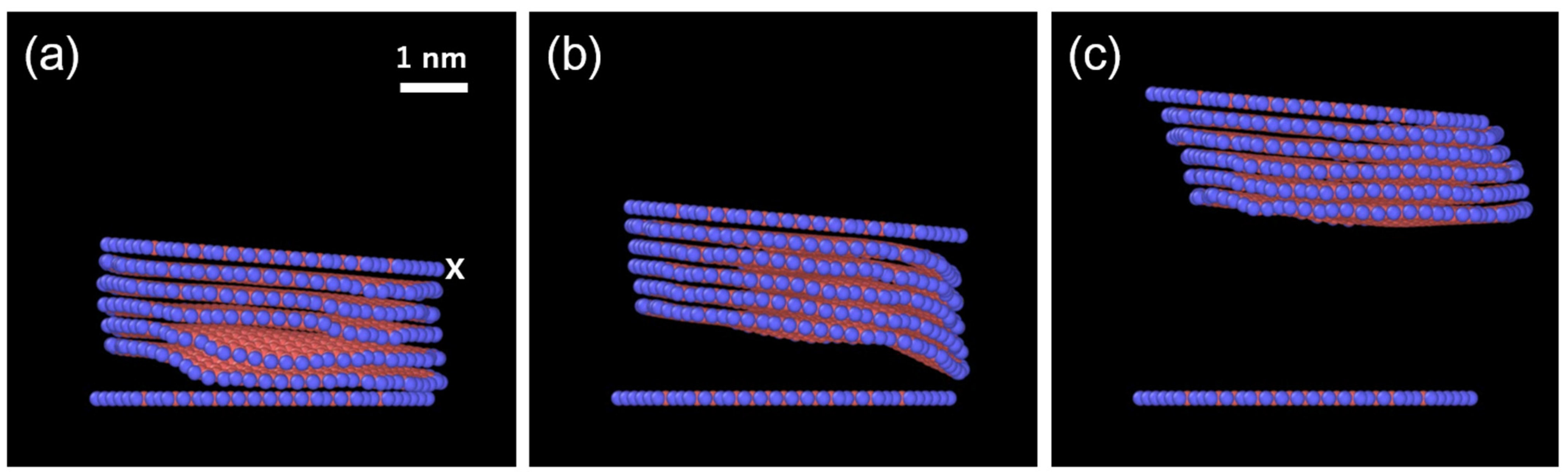
3.1. Exfoliation Behavior of ReaxFF Nanographite (Supercoronene)
- (1)
- The speed of lifting intensely affects the number of layers, and there is a certain threshold. Multiple layers are lifted with the top layers when the speed is 0.5~1 Å/ps (50~100 m/s), but the top layer is removed alone at the speed of 5 Å/ps (500 m/s). These values of lifting speed are higher than those in the real experiments, but the result can be scaled using the calculated bending rigidity of ReaxFF graphene, as explained later.
- (2)
- The effect of temperature is not straightforward. Pristine graphene exhibits a reduced number of exfoliated layers at a rate of 1 Å/ps as the temperature increases, whereas the number of exfoliated layers increases at a rate of 2 Å/ps.
- (3)
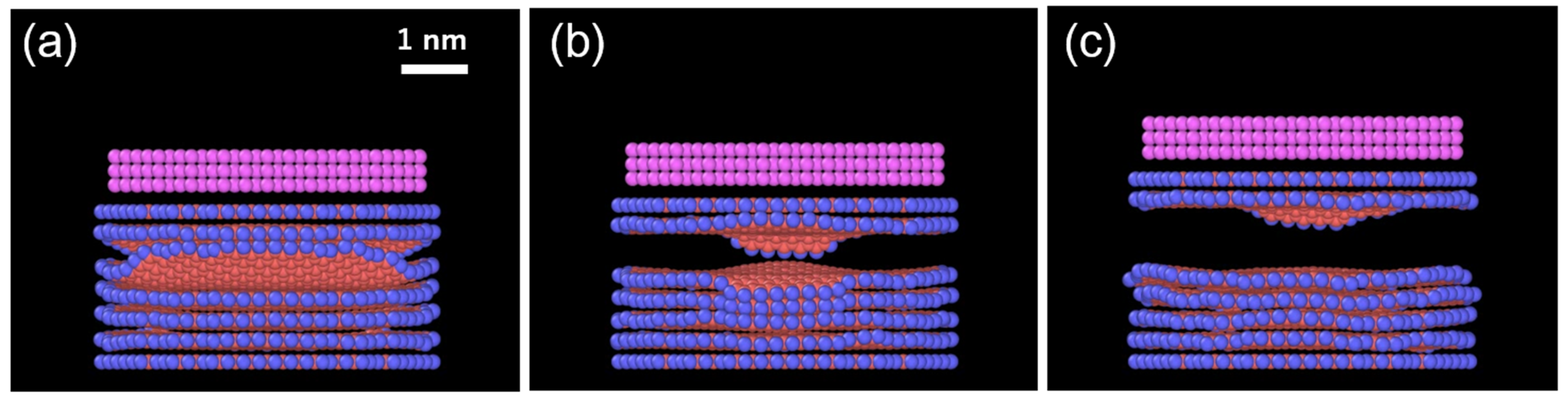
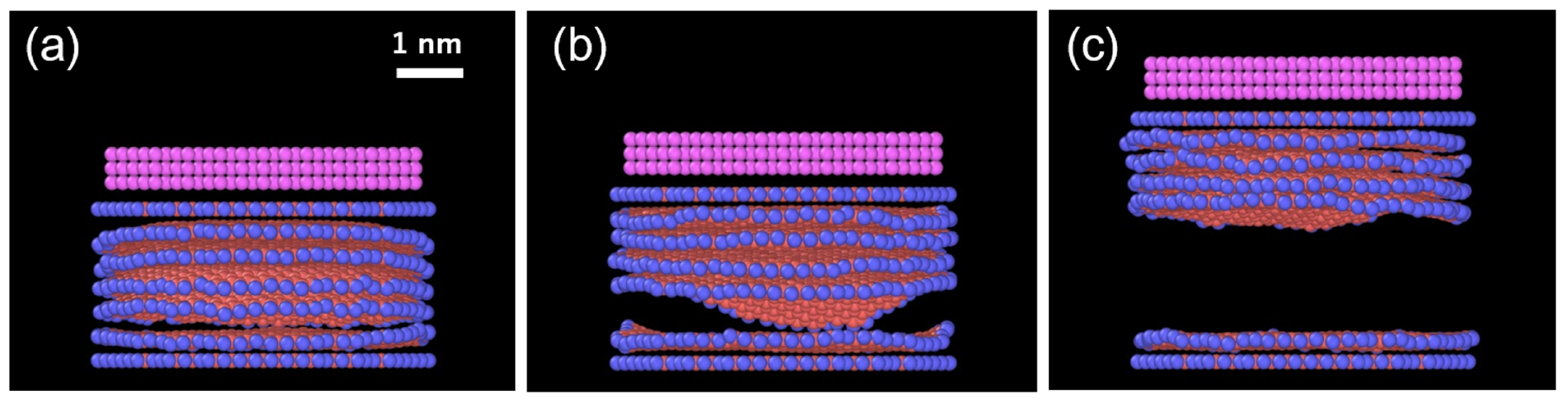
- The Au overlayer affects the result (Table 2). The deviation of the results is significantly reduced, especially in the case of 1 Å/ps, as all the replicas show identical numbers of exfoliated layers. Since the top graphene layer is lifted with the Au layers, the Au only modifies the interaction between the top layer and the second layer of the graphene sheets. It is noted that this minor sublayer modification is enough to control the results. By analyzing the total energy before and after exfoliation at 10 K, the interaction energy between the layers of ReaxFF graphene without an Au overlayer was found to be 39.8 meV/atom. This falls within the range in the literature: 30–52 meV/atom [37,38,39] from theory, and approximately 50 meV/atom from experiments using carbon nanotubes and aromatic molecules [40,41]. With an Au overlayer, our simulations yield 79.8 and 48.6 meV/atom for the first–second layers (zero-layer exfoliation) and second–third layers (one-layer exfoliation), respectively. They are substantially greater than that without an Au overlayer and steeply decrease as the layer number increases, which accounts for the present result. The critical implication of this result is that there is an appropriate speed range for the exfoliation, depending on the strength of interlayer interactions.
3.2. Impact of Pre-Tilting of the Top Layer Before Lifting
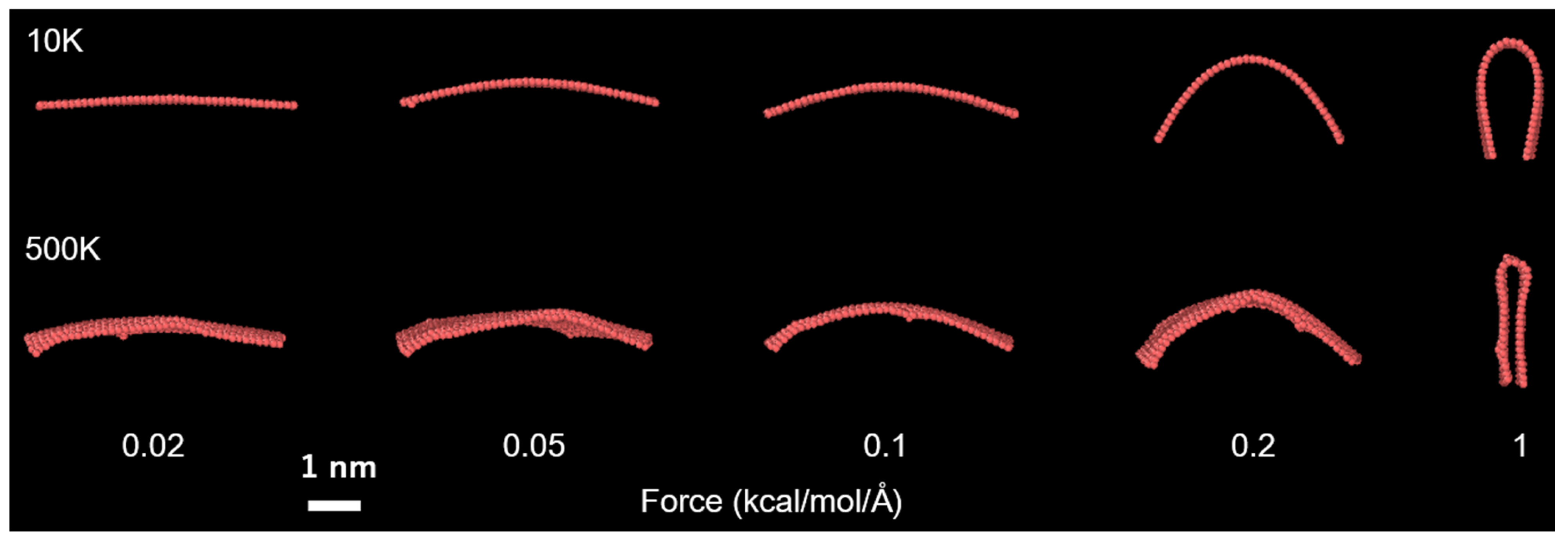
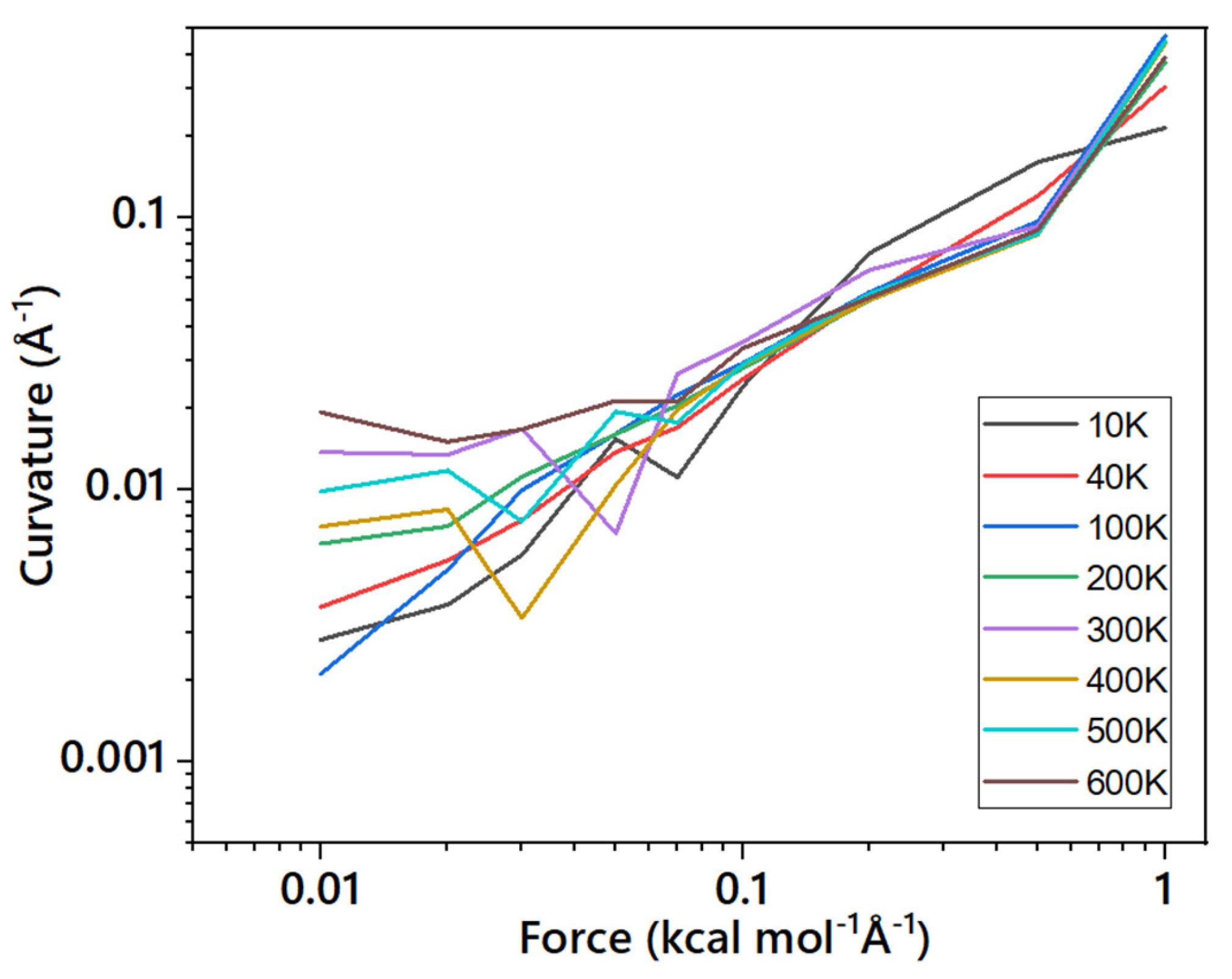
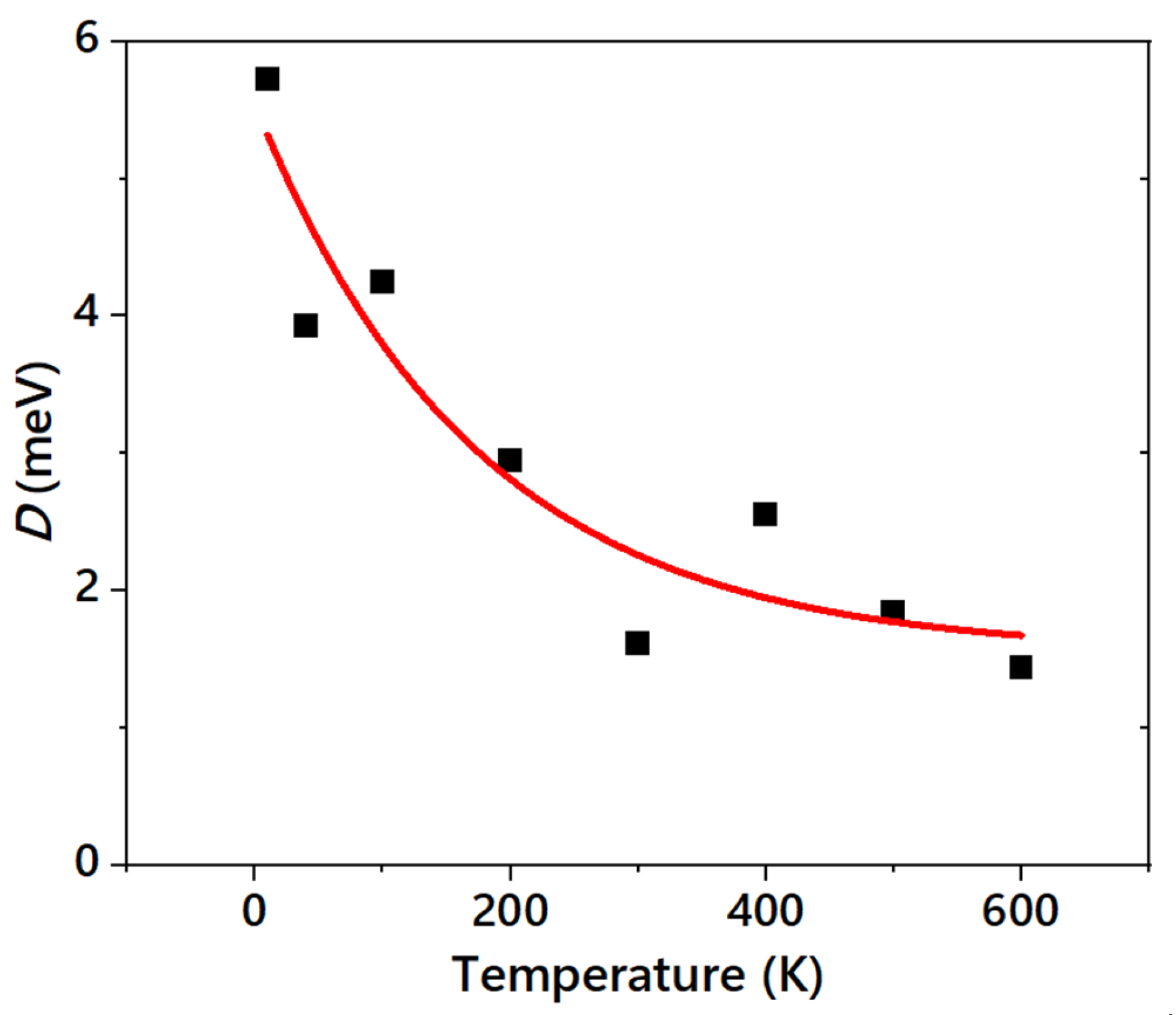
3.3. Estimation of the Bending Rigidity of ReaxFF Graphene
3.4. Implications on the Real Graphene and Other Layered Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Moriya, R.; Onodera, M.; Wakafuji, Y.; Masubuchi, S.; Watanabe, K.; Taniguchi, T.; Machida, T. Dry release transfer of graphene and few-layer h-BN by utilizing thermoplasticity of polypropylene carbonate. npj 2D Mater. Appl. 2019, 3, 22. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Munuera, J.M.; Paredes, J.I.; Enterría, M.; Pagán, A.; Villar-Rodil, S.; Pereira, M.F.R.; Martins, J.I.; Figueiredo, J.L.; Cenis, J.L.; Martínez-Alonso, A.; et al. Electrochemical Exfoliation of Graphite in Aqueous Sodium Halide Electrolytes toward Low Oxygen Content Graphene for Energy and Environmental Applications. ACS Appl. Mater. Interfaces 2017, 9, 24085–24099. [Google Scholar] [CrossRef]
- Gotzias, A.; Lazarou, Y.G. Graphene Exfoliation in Binary NMP/Water Mixtures by Molecular Dynamics Simulations. ChemPlusChem 2023, 88, e202300758. [Google Scholar] [CrossRef]
- Gotzias, A.; Tocci, E.; Sapalidis, A. Solvent-Assisted Graphene Exfoliation from Graphite Using Umbrella Sampling Simulations. Langmuir 2023, 39, 18437–18446. [Google Scholar] [CrossRef]
- Cai, L.; Hou, S.; Wei, X.; Tan, G.; Peng, Z.; Yan, Y.; Wang, L.; Lei, D.; Wu, Y.; Liu, Z. Exfoliation and stabilization mechanism of graphene in carbon dioxide expanded organic solvents: Molecular dynamics simulations. Phys. Chem. Chem. Phys. 2020, 22, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R. Molecular dynamics studies on the exfoliation of graphene in room temperature ionic liquids. J. Mol. Liq. 2021, 337, 116592. [Google Scholar] [CrossRef]
- Cai, L.; Tan, G.; Jing, X.; Wu, Y.; Liu, Z. How to obtain high monolayer content in liquid phase exfoliation of graphene: A molecular dynamic simulation. J. Mol. Liq. 2023, 382, 121873. [Google Scholar] [CrossRef]
- He, Y.; Qian, X.; Queiroz da Silva, G.C.; Gabellini, C.; Lucherelli, M.A.; Biagiotti, G.; Richichi, B.; Ménard-Moyon, C.; Gao, H.; Posocco, P.; et al. Unveiling Liquid-Phase Exfoliation of Graphite and Boron Nitride Using Fluorescent Dyes Through Combined Experiments and Simulations. Small 2024, 20, 2307817. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; He, D.; Liu, Y.; Wang, L.; Wang, M. Dynamic exfoliation of graphene in various solvents: All-atom molecular simulations. Chem. Phys. Lett. 2022, 804, 139900. [Google Scholar] [CrossRef]
- Wu, P.; Wei, T.; Wei, J.; Zhou, Q.; Zhang, W.; Liu, M. Simulation on fabricating graphene-coated nickel powders through micromechanical exfoliation. Mater. Today Commun. 2024, 38, 109853. [Google Scholar] [CrossRef]
- Nishad, W.; Subbiah, S.; Swaminathan, N. A qualitative understanding of high intensity mechanical shearing and exfoliation of graphite nanoplatelets in a three-body contact using molecular dynamic simulations. J. Manuf. Process. 2021, 71, 645–652. [Google Scholar] [CrossRef]
- Sinclair, R.C.; Suter, J.L.; Coveney, P.V. Graphene–Graphene Interactions: Friction, Superlubricity, and Exfoliation. Adv. Mater. 2017, 29, 1705791. [Google Scholar] [CrossRef]
- Prodanov, N.V.; Khomenko, A.V. Computational investigation of the temperature influence on the cleavage of a graphite surface. Surf. Sci. 2010, 604, 730–740. [Google Scholar] [CrossRef]
- Velický, M.; Donnelly, G.E.; Hendren, W.R.; McFarland, S.; Scullion, D.; DeBenedetti, W.J.I.; Calinao Correa, G.; Han, Y.; Wain, A.J.; Hines, M.A.; et al. Mechanism of Gold-Assisted Exfoliation of Centimeter-Sized Transition-Metal Dichalcogenide Monolayers. ACS Nano 2018, 12, 10463–10472. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Dai, C.; Zhu, L.; Shen, Y.; Liu, F.; Deng, S.; Ming, F. Controlling Gold-Assisted Exfoliation of Large-Area MoS2 Monolayers with External Pressure. Nanomaterials 2024, 14, 1418. [Google Scholar] [CrossRef]
- Ziewer, J.; Ghosh, A.; Hanušova, M.; Pirker, L.; Frank, O.; Velicky, M.; Gruning, M.; Huang, F. Strain-Induced Decoupling Drives Gold-Assisted Exfoliation of Large-Area Monolayer 2D Crystals. Adv. Mater. 2025, 37, 2419184. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; van Duin, A.C.T.; Ganesh, P. Development of a ReaxFF Potential for Carbon Condensed Phases and Its Application to the Thermal Fragmentation of a Large Fullerene. J. Phys. Chem. A 2015, 119, 571–580. [Google Scholar] [CrossRef]
- de Tomas, C.; Suarez-Martinez, I.; Marks, N.A. Graphitization of amorphous carbons: A comparative study of reactive force fields. Carbon 2016, 109, 681–693. [Google Scholar] [CrossRef]
- Bitzek, E.; Kermode, J.R.; Gumbsch, P. Atomistic aspects of fracture. Int. J. Fract. 2015, 191, 13–30. [Google Scholar] [CrossRef]
- Aktulga, H.M.; Fogarty, J.C.; Pandit, S.A.; Grama, A.Y. Parallel Reactive Molecular Dynamics: Numerical Methods and Algorithmic Techniques. Parallel Comput. 2012, 38, 245–259. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Nosé; S A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A. 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Järvi, T.T.; van Duin, A.C.; Nordlund, K.; Goddard, W.A. III. Development of interatomic reaxff potentials for Au−S−C−H systems. J. Phys. Chem. A 2011, 115, 10315–10322. [Google Scholar] [CrossRef]
- Bae, G.-T.; Aikens, C.M. Improved ReaxFF force field parameters for Au–S–C–H systems. J. Phys. Chem. A 2013, 117, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Carravetta, V.; Ågren, H. Simulation of gold functionalization with cysteine by reactive molecular dynamics. J. Phys. Chem. Lett. 2016, 7, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Girifalco, L.A.; Lad, R.A. Energy of cohesion, compressibility, and the potential energy functions of the graphite system. J. Chem. Phys. 1956, 25, 693–697. [Google Scholar] [CrossRef]
- Chakarova-Käck, S.D.; Schröder, E.; Lundqvist, B.I.; Langreth, D.C. Application of van der Waals density functional to an extended system: Adsorption of benzene and naphthalene on graphite. Phys. Rev. Lett. 2006, 96, 146107. [Google Scholar] [CrossRef]
- Dappe, Y.J.; Basanta, M.A.; Flores, F.; Ortega, J. Weak chemical interaction and van der Waals forces between graphene layers: A combined density functional and intermolecular perturbation theory approach. Phys. Rev. B 2006, 74, 205434. [Google Scholar] [CrossRef]
- Zacharia, R.; Ulbricht, H.; Hertel, T. Interlayer cohesive energy of graphite from thermal desorption of polyaromatic hydrocarbons. Phys. Rev. B 2004, 69, 155406. [Google Scholar] [CrossRef]
- Benedict, L.X.; Chopra, N.G.; Cohen, M.L.; Zettl, A.; Louie, S.G.; Crespi, V.H. Microscopic determination of the interlayer binding energy in graphite. Chem. Phys. Lett. 1998, 286, 490–496. [Google Scholar] [CrossRef]
- Zakharchenko, K.V.; Los, J.H.; Katsnelson, M.I.; Fasolino, A. Atomistic simulations of structural and thermodynamic properties of bilayer graphene. Phys. Rev. B 2010, 81, 235439. [Google Scholar] [CrossRef]
- Shen, Y.K.; Wu, H.A. Interlayer shear effect on multilayer graphene subjected to bending. Appl. Phys. Lett. 2012, 100, 101909. [Google Scholar] [CrossRef]
- Costamagna, S.; Neek-Amal, M.; Los, J.H.; Peeters, F.M. Thermal rippling behavior of graphane. Phys. Rev. B 2012, 86, 041408. [Google Scholar] [CrossRef]
- Ramírez, R.; Chacón, E.; Herrero, C.P. Anharmonic effects in the optical and acoustic bending modes of graphene. Phys. Rev. B 2016, 93, 235419. [Google Scholar] [CrossRef]
- Yi, L. Temperature dependence bending rigidity of 2D membranes: Graphene as an example. AIP Adv. 2018, 8, 075104. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.W. Temperature-dependent bending rigidity of graphene. Appl. Phys. Lett. 2009, 94, 231912. [Google Scholar] [CrossRef]
- Han, E.; Jaehyung, Y.; Annevelink, E.; Son, J.; Kang, D.A.; Watanabe, K.; Taniguchi, T.; Ertekin, E.; Huang, P.U.; van der Zande, A.M. Ultrasoft slip-mediated bending in few-layer graphene. Nat. Mater. 2020, 19, 305–309. [Google Scholar] [CrossRef]
- Nicklow, R.; Wakabayashi, N.; Smith, H.G. Lattice dynamics of pyrolytic graphite. Phys. Rev. B 1972, 5, 4951. [Google Scholar] [CrossRef]
- Chen, X.; Yi, C.; Ke, C. Bending stiffness and interlayer shear modulus of few-layer graphene. Appl. Phys. Lett. 2015, 106, 101907. [Google Scholar] [CrossRef]
- Taleb, A.A.; Yu, H.K.; Anemone, G.; Farias, D.; Wodtke, A.M. Helium diffraction and acoustic phonons of graphene grown on copper foil. Carbon 2015, 95, 731–737. [Google Scholar] [CrossRef]
- Tømterud, M.; Hellner, S.K.; Eder, S.D.; Forti, S.; Convertino, D.; Manson, J.R.; Coletti, C.; Frederiksen, T.; Holst, B. Observation of increasing bending rigidity of graphene with temperature. Carbon 2025, 238, 120150. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814. [Google Scholar] [CrossRef]
- Kato, M.; Ota, R.; Endo, T.; Yanase, T.; Nagahama, T.; Shimada, T. Free-Standing Nanometer-Thick Covalent Organic Framework Films for Separating CO2 and N2. ACS Appl. Nano Mater. 2022, 5, 2367. [Google Scholar] [CrossRef]
- Kato, M.; Yanase, T.; Waizumi, H.; Yokokura, S.; Shimada, T. Solvent-vapor-assisted crystallization of covalent organic framework films for CO2/CH4 separation. Chem. Lett. 2024, 53, upae191. [Google Scholar] [CrossRef]

| Lifting Speed (Å/ps) | Temperature (K) | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 100 | 200 | 300 | 400 | 500 | 600 | |
| 0.5 | 2.6 ± 0.5 | 3.8 ± 1.6 | 4.8 ± 0.4 | 3.8 ± 1.1 | 2.4 ± 0.9 | 3.8 ± 0.4 | 3.2 ± 0.8 |
| 1 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 | 2.4 ± 1.5 | 4.0 ± 0.0 | 1.6 ± 0.5 | 1.4 ± 0.5 |
| 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 2.2 | 2.6 ± 1.7 | 2.6 ± 1.7 | 2.6 ± 1.7 | 3.4 ± 0.5 |
| 5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Lifting Speed (Å/ps) | Temperature (K) | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 100 | 200 | 300 | 400 | 500 | 600 | |
| 0.5 | 1.2 ± 0.4 | 1.0 ± 0.7 | 2.4 ± 2.2 | 1.6 ± 2.2 | 2.4 ± 1.5 | 1.6 ± 1.1 | 2.0 ± 1.2 |
| 1 | 4.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 2 | 0.2 ± 0.4 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 |
| 5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Angle of Pre-Tilting (o) | Temperature (K) | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 100 | 200 | 300 | 400 | 500 | 600 | |
| 1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.2 ± 2.0 | 2.6 ± 1.7 | 2.6 ± 1.7 | 2.4 ± 1.1 |
| 2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.6 ± 1.7 | 2.2 ± 1.5 |
| 5 | 0.0 ± 0.0 | 1.0 ± 2.2 | 1.0 ± 2.2 | 1.0 ± 2.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, T.; Yokokura, S.; Waizumi, H.; Suzuki, H.; Kawashima, K.; Shimada, T. In Silico Exfoliation of ReaxFF Graphite—Temperature, Speed, Angle Dependence, and the Effect of Gold Overlayer. C 2025, 11, 59. https://doi.org/10.3390/c11030059
Ando T, Yokokura S, Waizumi H, Suzuki H, Kawashima K, Shimada T. In Silico Exfoliation of ReaxFF Graphite—Temperature, Speed, Angle Dependence, and the Effect of Gold Overlayer. C. 2025; 11(3):59. https://doi.org/10.3390/c11030059
Chicago/Turabian StyleAndo, Teruki, Seiya Yokokura, Hiroki Waizumi, Hironori Suzuki, Kenji Kawashima, and Toshihiro Shimada. 2025. "In Silico Exfoliation of ReaxFF Graphite—Temperature, Speed, Angle Dependence, and the Effect of Gold Overlayer" C 11, no. 3: 59. https://doi.org/10.3390/c11030059
APA StyleAndo, T., Yokokura, S., Waizumi, H., Suzuki, H., Kawashima, K., & Shimada, T. (2025). In Silico Exfoliation of ReaxFF Graphite—Temperature, Speed, Angle Dependence, and the Effect of Gold Overlayer. C, 11(3), 59. https://doi.org/10.3390/c11030059







