Pulsed Laser Annealing of Deposited Amorphous Carbon Films
Abstract
1. Introduction
2. Materials and Methods
3. Results
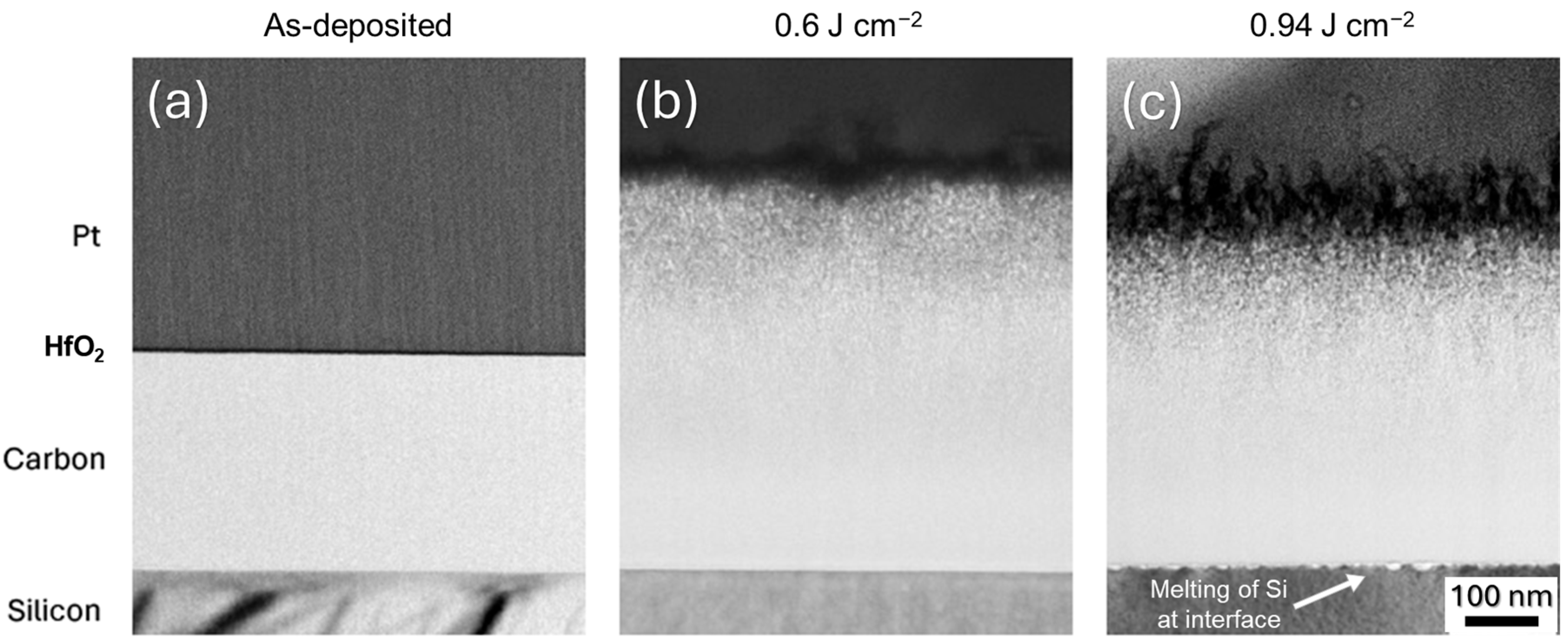
3.1. Inspection of Carbon Film Delamination
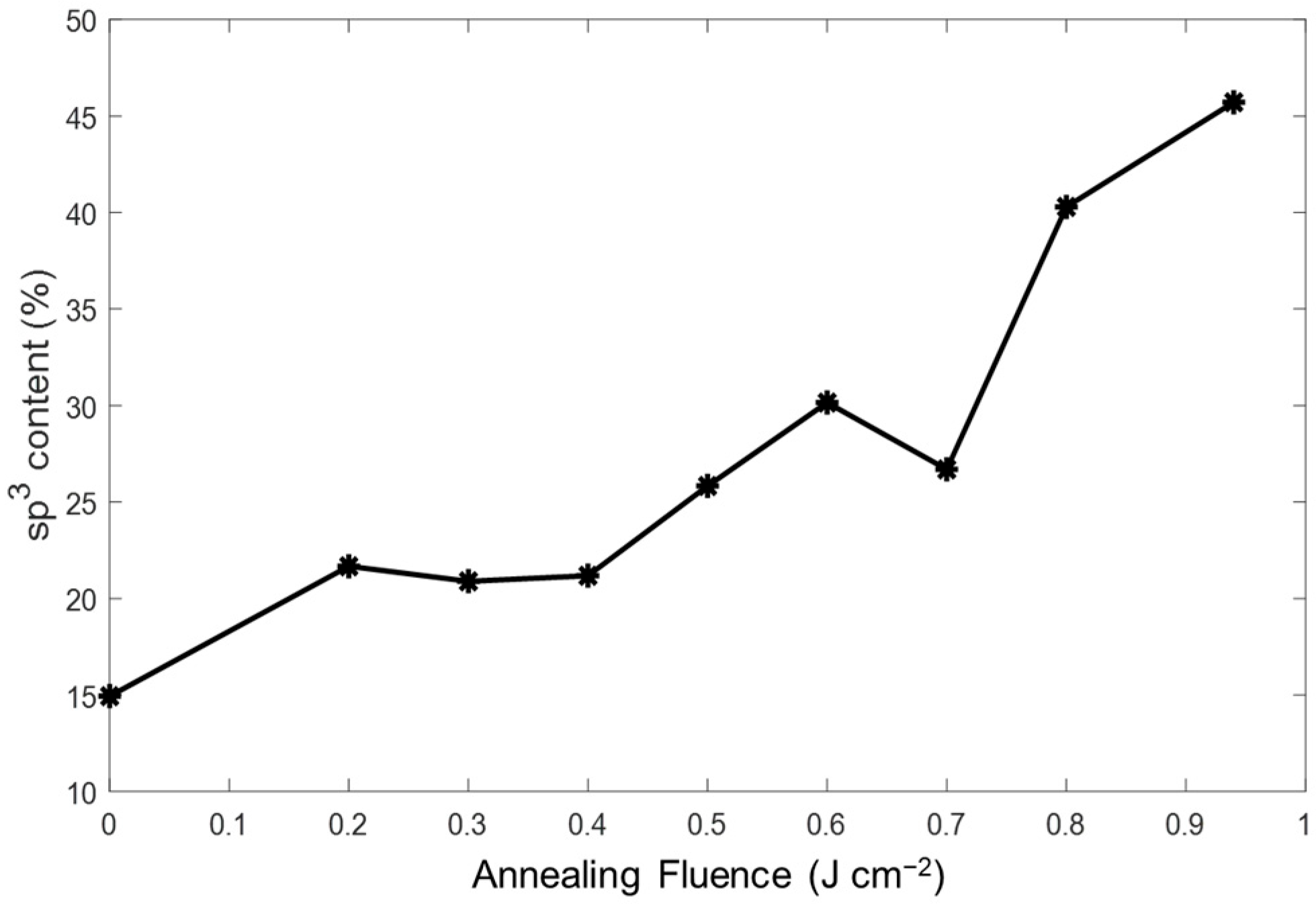
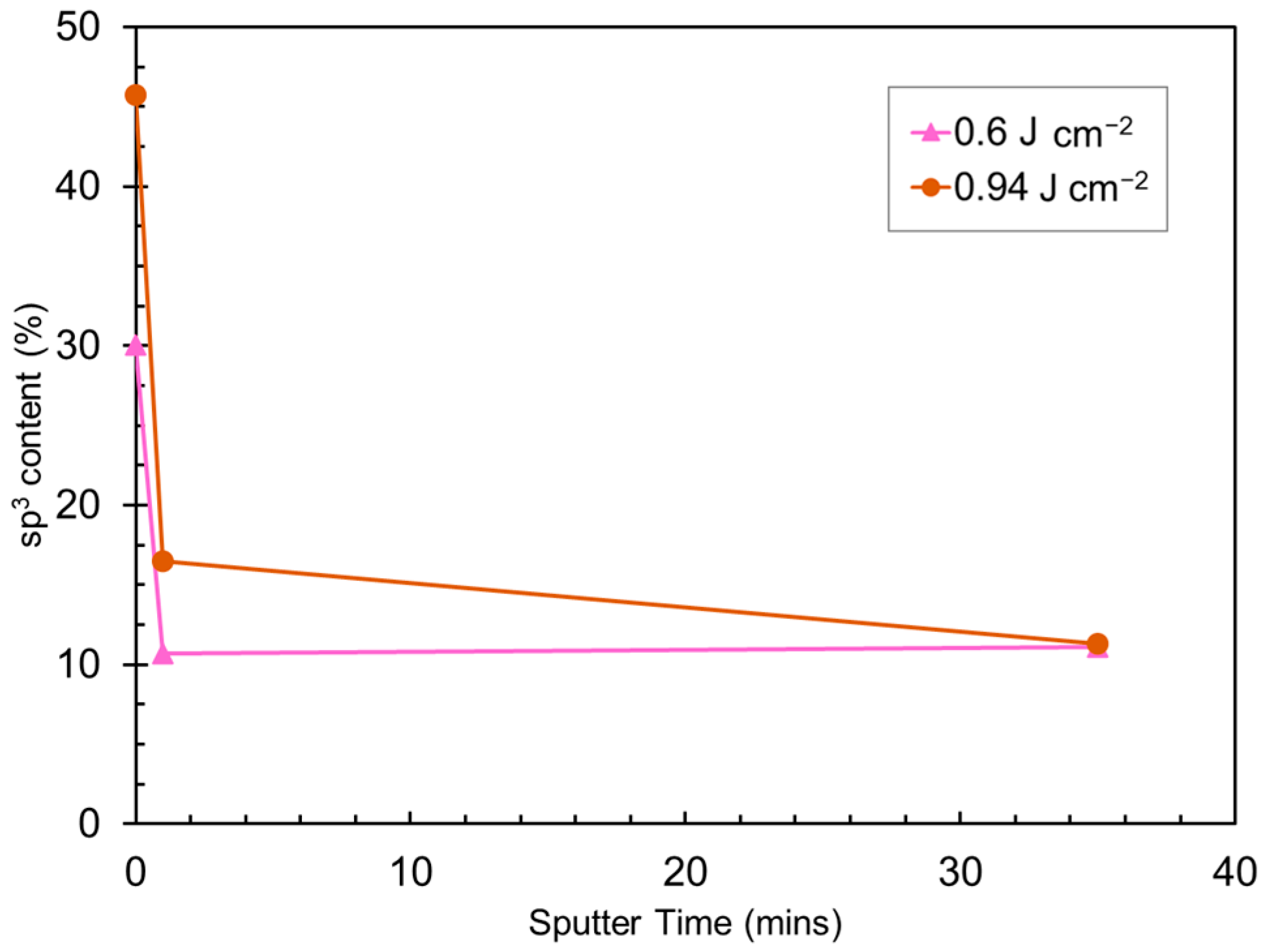
3.2. Chemical Bonding States of a-C:H Film After PLA
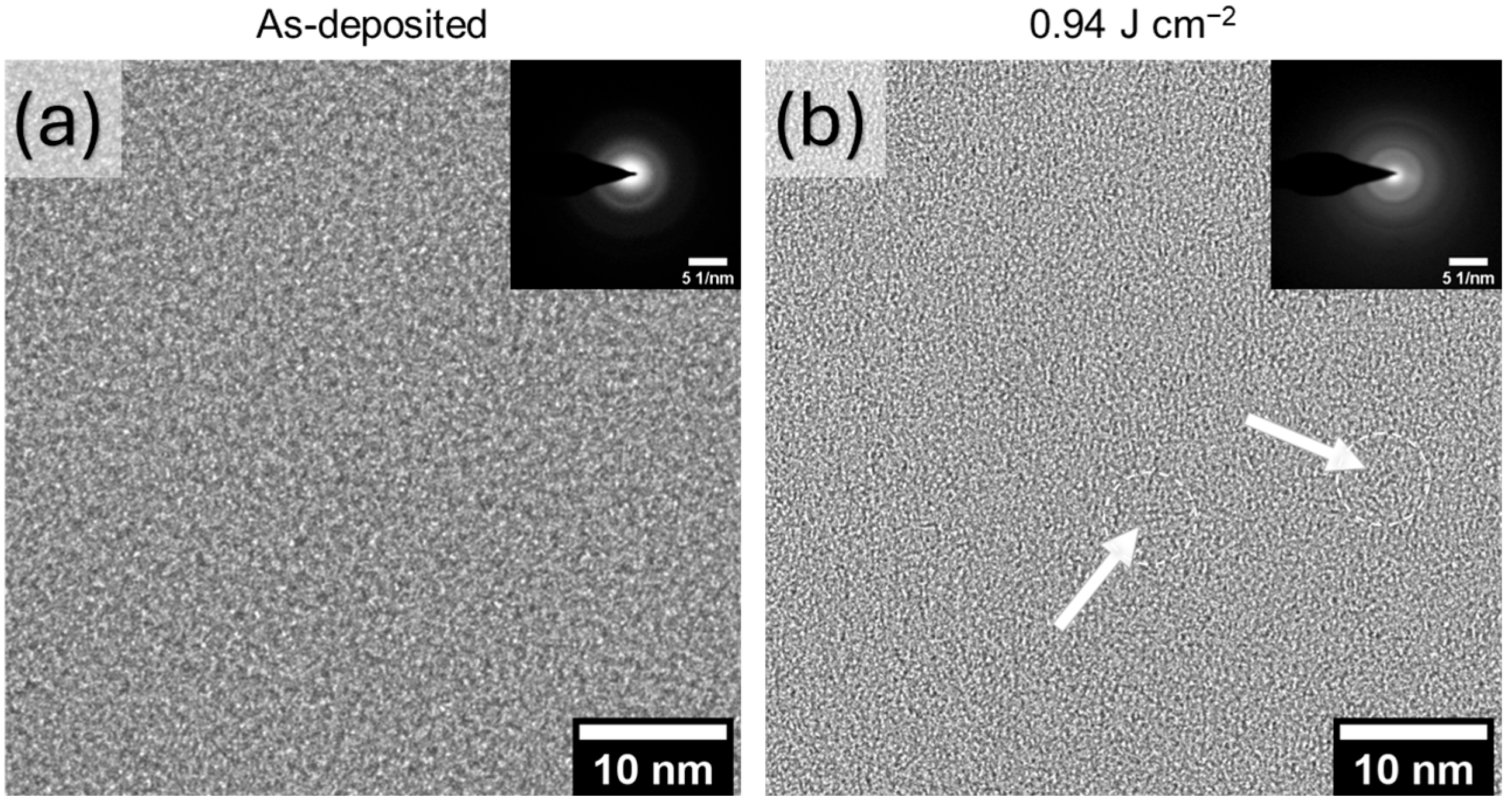
3.3. Microstructural Characterization of PLA a-C:H Film
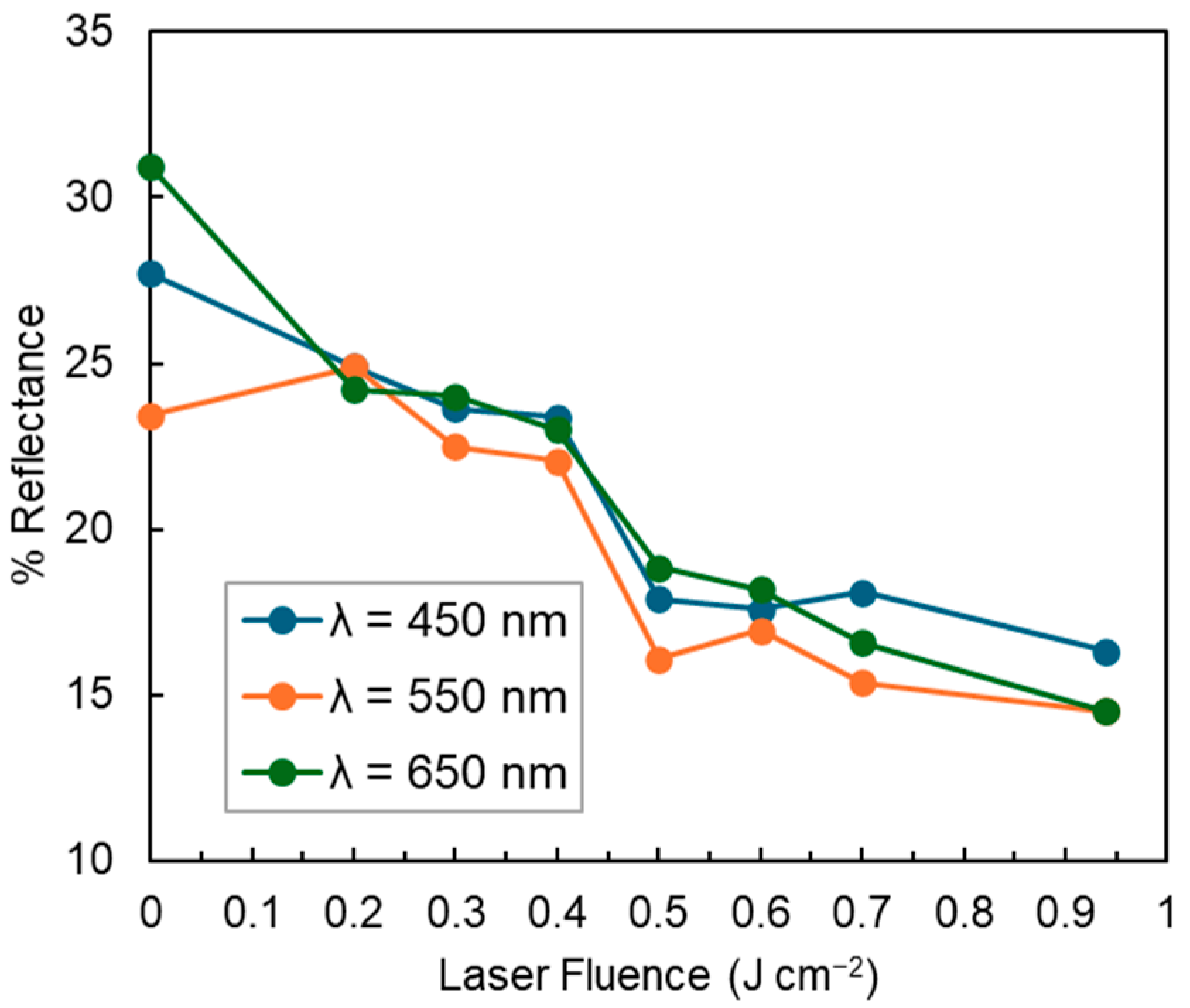
3.4. Optical Properties in the Visible Light Spectrum of PLA a-C:H Films
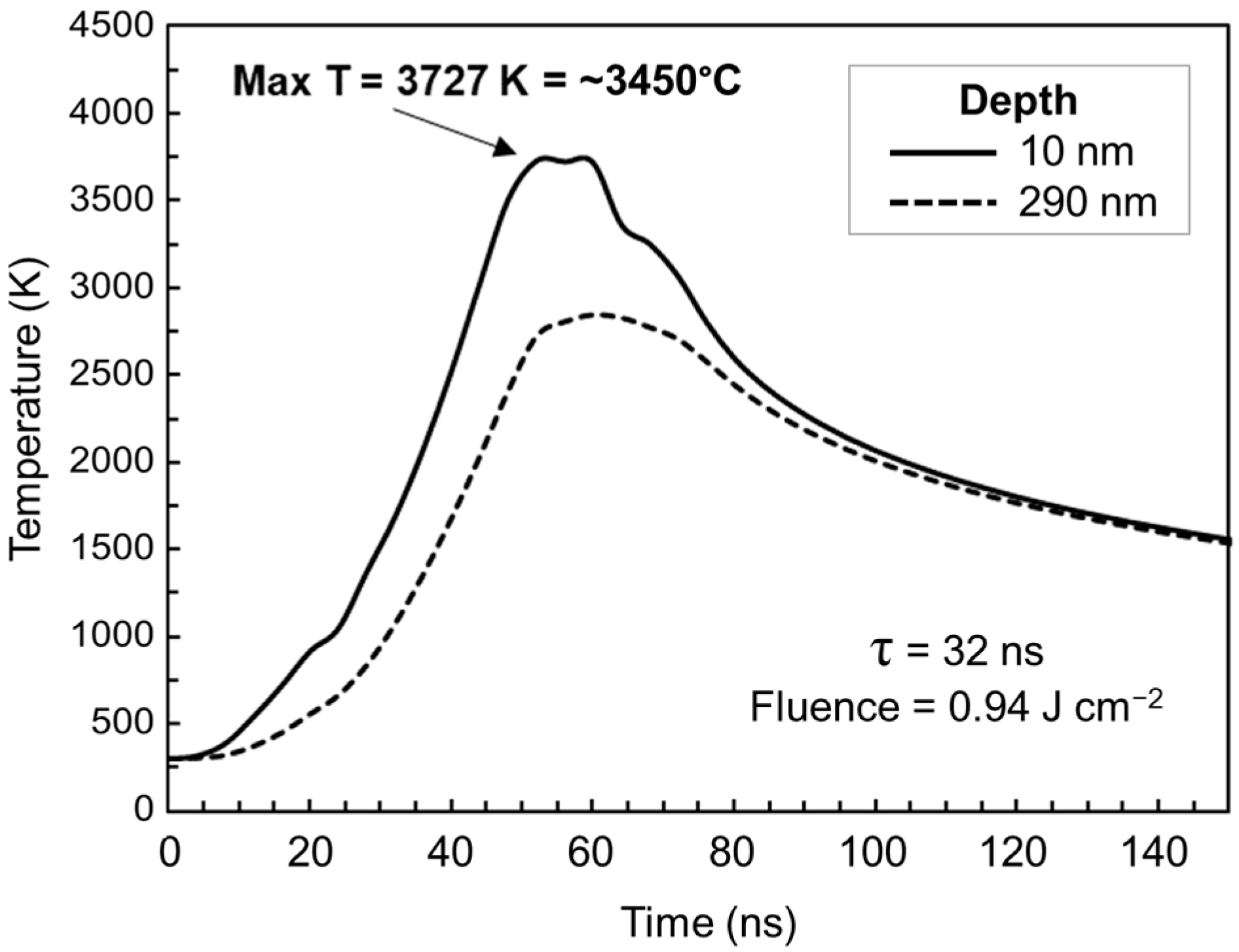
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DLC | Diamond-like carbon |
| a-C:H | Hydrogenated amorphous carbon |
| ta-C | Tetrahedral amorphous carbon |
| UV | Ultraviolet |
| PLA | Pulsed laser annealing |
| PECVD | Plasma-enhanced chemical vapor deposition |
| PLD | Pulsed laser deposition |
| XPS | X-ray photoelectron spectroscopy |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| ALD | Atomic layer deposition |
| EDS | Energy-dispersive spectroscopy |
| LIMPS | Laser induced melting prediction of one-dimensional heat flow during pulsed laser melting of thin films |
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R. Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Kim, I.-S.; Shim, C.-E.; Kim, S.W.; Lee, C.-S.; Kwon, J.; Byun, K.-E.; Jeong, U. Amorphous Carbon Films for Electronic Applications. Adv. Mater. 2022, 35, 2204912. [Google Scholar] [CrossRef]
- Dychalska, A.; Popielarski, P.; Franków, W.; Fabisiak, K.; Paprocki, K.; Szybowicz, M. Study of CVD diamond layers with amorphous carbon admixture by Raman scattering spectroscopy. Mater. Sci. Pol. 2015, 33, 799–805. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond—Like carbon, and nanodiamond. Philos. Trans. A Math. Phys. Eng. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Park, M.; Sakhrani, V.; Maria, J.-P.; Cuomo, J.J.; Teng, C.W.; Muth, J.F.; Ware, M.E.; Rodriguez, B.J.; Nemanich, R.J. Wavelength-dependent Raman scattering of hydrogenated amorphous silicon carbon with red, green, and blue light excitation. J. Mater. Res. 2003, 18, 768–771. [Google Scholar] [CrossRef][Green Version]
- Filik, J.; May, P.W.; Pearce, S.R.J.; Wild, R.K.; Hallam, K.R. XPS and laser Raman analysis of hydrogenated amorphous carbon films. Diam. Relat. Mater. 2003, 12, 974–978. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.J.; Han, S.; Chae, H. Characterization of sp2/sp3 hybridization ratios of hydrogenated amorphous carbon films deposited in C2H2 inductively coupled plasmas. Surf. Coat. Technol. 2021, 422, 127514. [Google Scholar] [CrossRef]
- Chen, J.-L.; Ji, P.-Y.; Yang, Y.; Jin, C.-G.; Zhuge, L.-J.; Wu, X.-M. The structure and properties of amorphous diamond-like carbon films deposited by helicon wave plasma chemical vapor deposition. Thin Solid Films 2020, 709, 138167. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Tang, X.-M.; Weber, J.; Baer, Y.; Müller, C.; Hänni, W.; Hintermann, H.E. Influence of hydrogen on the structure of amorphous carbon. Phys. Rev. B 1993, 48, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, H.; Sun, Q. Process Optimization of Amorphous Carbon Hard Mask in Advanced 3D-NAND Flash Memory Applications. Electronics 2021, 10, 1374. [Google Scholar] [CrossRef]
- Lai, C.C.; Chang, Y.H.; Chien, H.J.; Lu, M.C. Hard mask and lithographic capabilities improvement by amorphous carbon step coverage optimization in high aspect ratio device pattern. Vacuum 2018, 153, 267–273. [Google Scholar] [CrossRef]
- Pauliac-Vaujour, S.; Brianceau, P.; Comboroure, C.; Faynot, O. Improvement of high resolution lithography by using amorphous carbon hard mask. Microelectron. Eng. 2008, 85, 800–804. [Google Scholar] [CrossRef]
- Jaoul, C.; Jarry, O.; Tristant, P.; Merle-Méjean, T.; Colas, M.; Dublanche-Tixier, C.; Jacquet, J.-M. Raman analysis of DLC coated engine components with complex shape: Understanding wear mechanisms. Thin Solid Films 2009, 518, 1475–1479. [Google Scholar] [CrossRef]
- Krishna, K.M.; Umeno, M.; Nukaya, Y.; Soga, T.; Jimbo, T. Photovoltaic and spectral photoresponse characteristics of n-C/p-C solar cell on a p-silicon substrate. Appl. Phys. Lett. 2000, 77, 1472–1474. [Google Scholar] [CrossRef]
- Grigonis, A.; Rutkunienė, Ž.; Medvids, A. The influence of nanosecond pulse laser irradiation on the properties of a-C:H films. Vacuum 2008, 82, 1212–1215. [Google Scholar] [CrossRef]
- Cappelli, E.; Scilletta, C.; Orlando, S.; Valentini, V.; Servidori, M. Laser annealing of amorphous carbon films. Appl. Surf. Sci. 2009, 255, 5620–5625. [Google Scholar] [CrossRef]
- Ibenskas, A.; Galdikas, A.; Grigonis, A. Kinetic modeling of laser annealing processes in a-C:H films. Vacuum 2011, 86, 124–130. [Google Scholar] [CrossRef]
- Kononenko, T.V.; Kononenko, V.V.; Pimenov, S.M.; Zavedeev, E.; Konov, V.I.; Romano, V.; Dumitru, G. Effects of pulse duration in laser processing of diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 1368–1376. [Google Scholar] [CrossRef]
- Narayan, J.; Gupta, S.; Sachan, R.; Bhaumik, A.; Cellini, F.; Riedo, E. Q-carbon harder than diamond. MRS Commun. 2018, 8, 428–436. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Annealing effect on the structure, mechanical and tribological properties of hydrogenated diamond-like carbon films. Thin Solid Films 2006, 515, 2153–2160. [Google Scholar] [CrossRef]
- Choi, W.S.; Hong, B. The effect of annealing on the properties of diamond-like carbon protective antireflection coatings. Renew Energy 2008, 33, 226–231. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, L.; Hu, L.; Hu, T.; Wang, Y.; Zhang, Y. An explanation for laser-induced spallation effect in a-C:H films: Altered phase evolution route caused by hydrogen doping. J. Appl. Phys. 2011, 109, 013501. [Google Scholar] [CrossRef]
- Grigonis, A.; Rutkunienė, Ž.; Manikowski, H.; Šilinskas, M. Laser-induced transformation of a-C:H thin films. Vacuum 2009, 83, S152–S154. [Google Scholar] [CrossRef]
- Nandihalli, N. Microwave-driven synthesis and modification of nanocarbons and hybrids in liquid and solid phases. J. Energy Storage 2025, 111, 115315. [Google Scholar]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Gupta, S.; Sachan, R.; Bhaumik, A.; Pant, P.; Narayan, J. Undercooling driven growth of Q-carbon, diamond, and graphite. MRS Commun. 2018, 8, 533–540. [Google Scholar] [CrossRef]
- Haque, A.; Pant, P.; Narayan, J. Large-area diamond thin film on Q-carbon coated crystalline sapphire by HFCVD. J. Cryst. Growth 2018, 504, 17–25. [Google Scholar] [CrossRef]
- Narayan, J.; Khosla, N. Self-organization of amorphous Q-carbon and Q-BN nanoballs. Carbon 2022, 192, 301–307. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A.; Gupta, S.; Joshi, P.; Riley, P.; Narayan, R. Role of Q-carbon in nucleation and formation of continuous diamond film. Carbon 2021, 176, 558–568. [Google Scholar] [CrossRef]
- Nistor, L.C.; Van Landuyt, J.; Ralchenko, V.G.; Kononenko, T.V.; Obraztsova, E.D.; Strelnitsky, V.E. Direct observation of laser-induced crystallization of a-C:H films. Appl. Phys. A 1994, 58, 137–144. [Google Scholar] [CrossRef]
- Xu, N.; Tsang, S.H.; Teo, E.H.T.; Wang, X.; Ng, C.M.; Tay, B.K. Effect of initial sp3 content on bonding structure evolution of amorphous carbon upon pulsed laser annealing. Diam. Relat. Mater. 2012, 30, 48–52. [Google Scholar] [CrossRef]
- Tomasella, E.; Meunier, C.; Mikhailov, S. a-C:H thin films deposited by radio-frequency plasma: Influence of gas composition on structure, optical properties and stress levels. Surf. Coat. Technol. 2001, 141, 289–296. [Google Scholar] [CrossRef]
- Viana, W.E.S.S.; Elzubair, A.E.; Wysard, M.M.; Franeschini, D.F.; Camargo, S.S. Comparison of the properties of a-C:H films deposited from methane and heptane precursors: Study of the mechanical, chemical and structural properties. Thin Solid Films 2020, 695, 137733. [Google Scholar] [CrossRef]
- Adams, B.E.; Howells, S.C.; Jennings, D.; Li, J.; Thomas, T.N.; Moffatt, S. Apparatus and Method of Improving Beam Shaping and Beam Homogenization. U.S. Patent 9908200 B2, 06 March 2018. [Google Scholar]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Proctor, A.; Sherwood, P.M.A. Data analysis techniques in x-ray photoelectron spectroscopy. Anal. Chem. 1982, 54, 13–19. [Google Scholar] [CrossRef]
- Ba, E.C.T.; Dumont, M.R.; Martins, P.S.; Da Silva Pinheiro, B.; da Cruz, M.P.M.; Barbosa, J.W. Deconvolution process approach in Raman spectra of DLC coating to determine the sp3 hybridization content using the ID/IG ratio in relation to the quantification determined by X-ray photoelectron spectroscopy. Diam. Relat. Mater. 2022, 122, 108818. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Bîru, E.I.; Iovu, H. Graphene nanocomposites studied by Raman spectroscopy. In Raman Spectroscopy; Do Nascimento, G.M., Ed.; InTechOpen: London, UK, 2018; Volume 1, pp. 179–201. [Google Scholar]
- Petrov, D.V.; Matrosov, I.I.; Sedinkin, D.O.; Zaripov, A.R. Raman Spectra of Nitrogen, Carbon Dioxide, and Hydrogen in a Methane Environment. Opt. Spectrosc. 2018, 124, 8–12. [Google Scholar] [CrossRef]
- Jorio, A.; Ferreira, E.H.M.; Moutinho, M.V.O.; Stavale, F.; Achete, C.A.; Capaz, R.B. Measuring disorder in graphene with the G and D bands. Phys. Status Solidi B 2010, 247, 2980–2982. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Park, C.K.; Chang, S.M.; Uhm, H.S.; Seo, S.H.; Park, J.S. XPS and XRR studies on microstructures and interfaces of DLC films deposited by FCVA method. Thin Solid Films 2002, 420–421, 235–240. [Google Scholar] [CrossRef]
- Johnson, J.A. Pulsed Laser Melting of Silicon-Germanium/Silicon Heterostructures. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2021. [Google Scholar]
- Jiang, X.; Beyer, W.; Reichelt, K. Gas evolution from hydrogenated amorphous carbon films. J. Appl. Phys. 1990, 68, 1378–1380. [Google Scholar] [CrossRef]
- Park, H.; Woo, D.; Lee, J.M.; Park, S.J.; Lee, S.; Kim, H.J.; Yoon, E.; Lee, G.-D. The influence of hydrogen concentration in amorphous carbon films on mechanical properties and fluorine penetration: A density functional theory and ab initio molecular dynamics study. RSC Adv. 2020, 10, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J. Fifty shades of black. Phys. World 2015, 28, 25–28. [Google Scholar] [CrossRef]
- Theocharous, E.; Chunnilall, C.J.; Mole, R.; Gibbs, D.; Fox, N.; Shang, N.; Howlett, G.; Jensen, B.; Taylor, R.; Reveles, J.R.; et al. The partial space qualification of a vertically aligned carbon nanotube coating on aluminium substrates for EO applications. Opt. Express 2014, 22, 7290–7307. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.M.; Gaspar, G.; Wijayantha, K.G.U.; Lobato, K. The impact of laser-scribing carbon-based supercapacitor electrodes. Appl. Surf. Sci. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, A.D.; Hershkovitz, E.; Panoutsopoulos, P.; de Jesus Lopez, M.X.; Simpson, B.; Kim, H.; Narayanan, R.; Johnson, J.; Jones, K.S. Pulsed Laser Annealing of Deposited Amorphous Carbon Films. C 2025, 11, 60. https://doi.org/10.3390/c11030060
Rivera AD, Hershkovitz E, Panoutsopoulos P, de Jesus Lopez MX, Simpson B, Kim H, Narayanan R, Johnson J, Jones KS. Pulsed Laser Annealing of Deposited Amorphous Carbon Films. C. 2025; 11(3):60. https://doi.org/10.3390/c11030060
Chicago/Turabian StyleRivera, Arianna D., Eitan Hershkovitz, Panagiotis Panoutsopoulos, Manny X. de Jesus Lopez, Bradley Simpson, Honggyu Kim, Rajaram Narayanan, Jesse Johnson, and Kevin S. Jones. 2025. "Pulsed Laser Annealing of Deposited Amorphous Carbon Films" C 11, no. 3: 60. https://doi.org/10.3390/c11030060
APA StyleRivera, A. D., Hershkovitz, E., Panoutsopoulos, P., de Jesus Lopez, M. X., Simpson, B., Kim, H., Narayanan, R., Johnson, J., & Jones, K. S. (2025). Pulsed Laser Annealing of Deposited Amorphous Carbon Films. C, 11(3), 60. https://doi.org/10.3390/c11030060






