Green Synthesis of a Highly Active Ag/Activated Carbon Nanocomposite from Tamarind Seeds for Methyl Orange Removal
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Making an Extract from Acacia Nilotica
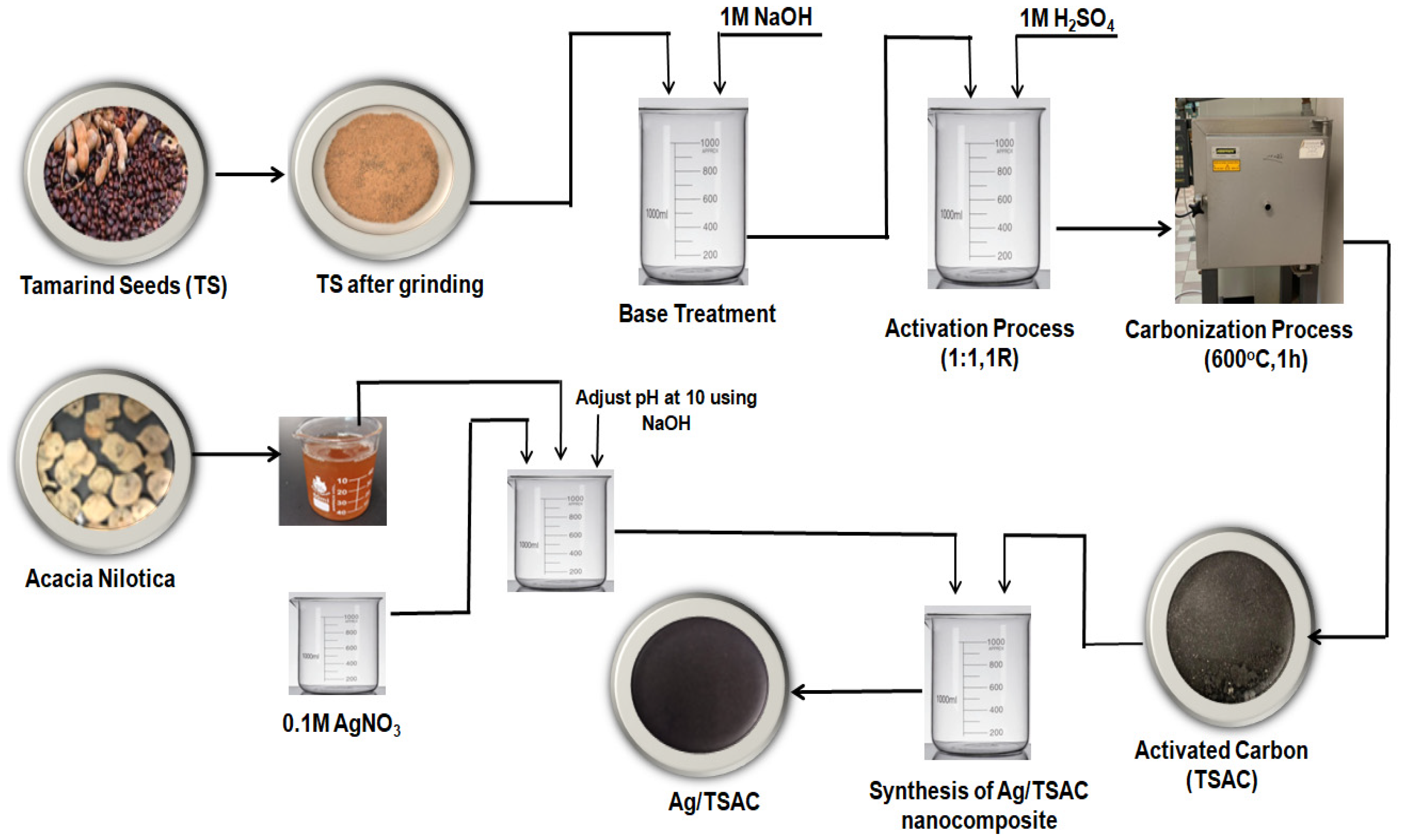
2.3. Synthesis of Activated Carbon
2.4. Synthesis of Ag/TS Activated Carbon Nanocomposite
2.5. Instrument Analysis
2.6. Experimental Procedures
3. Results and Discussions
3.1. Characterization
3.1.1. FTIR Characterization
3.1.2. SEM Characterization
3.1.3. Surface Area Analysis
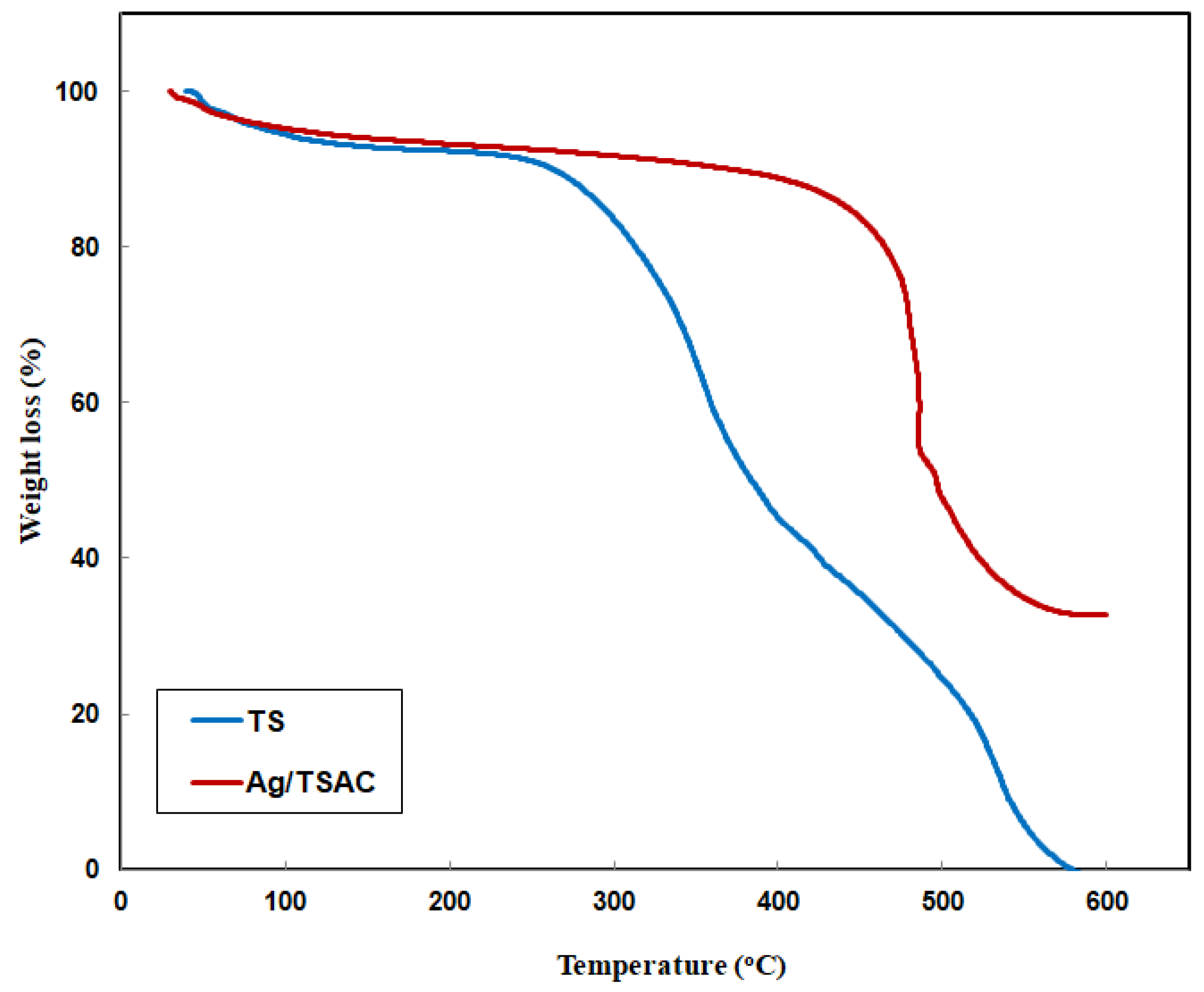
3.1.4. TGA Analysis
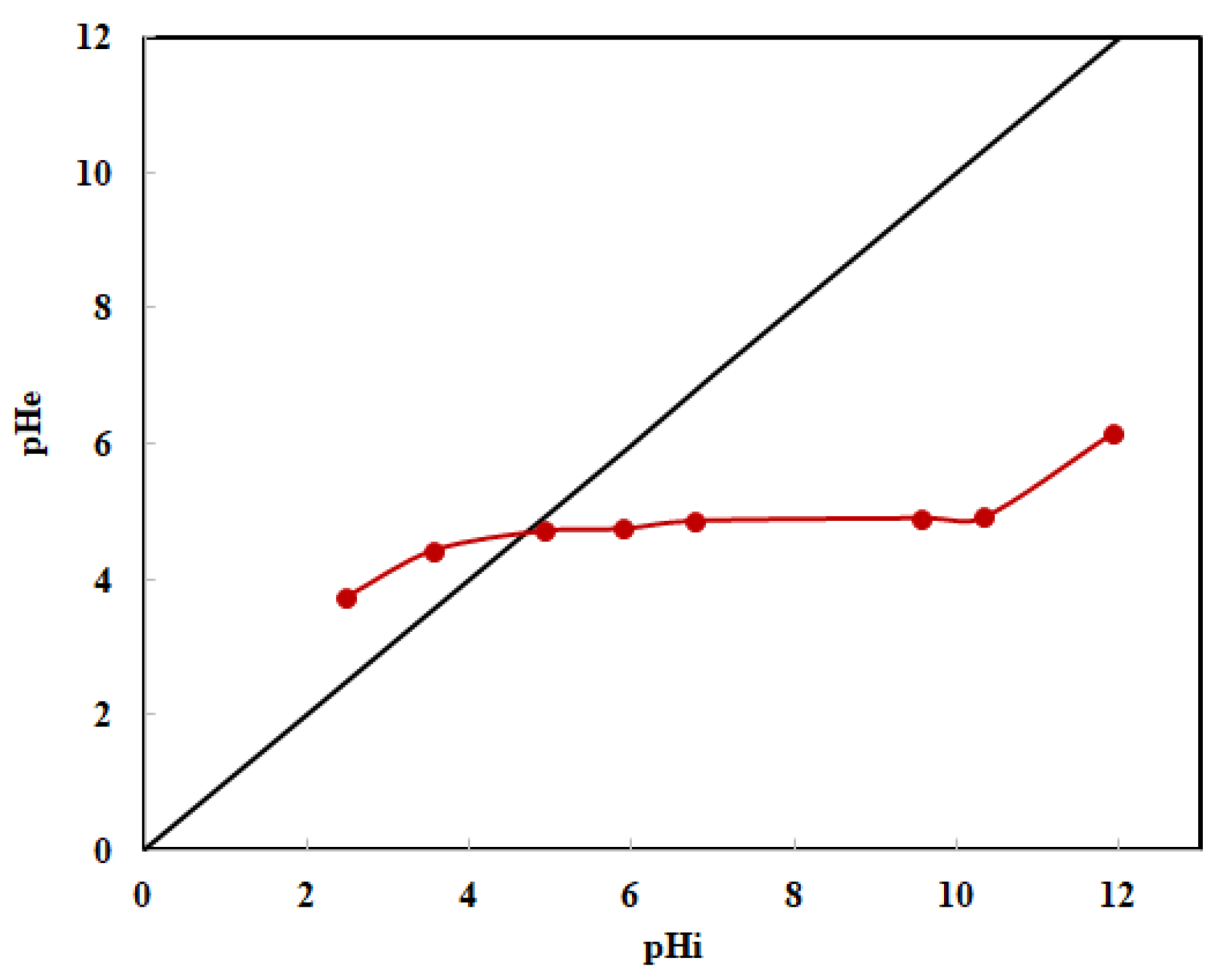
3.1.5. pH of Zero Charge Point (pHZC)
3.2. Adsorption Studies
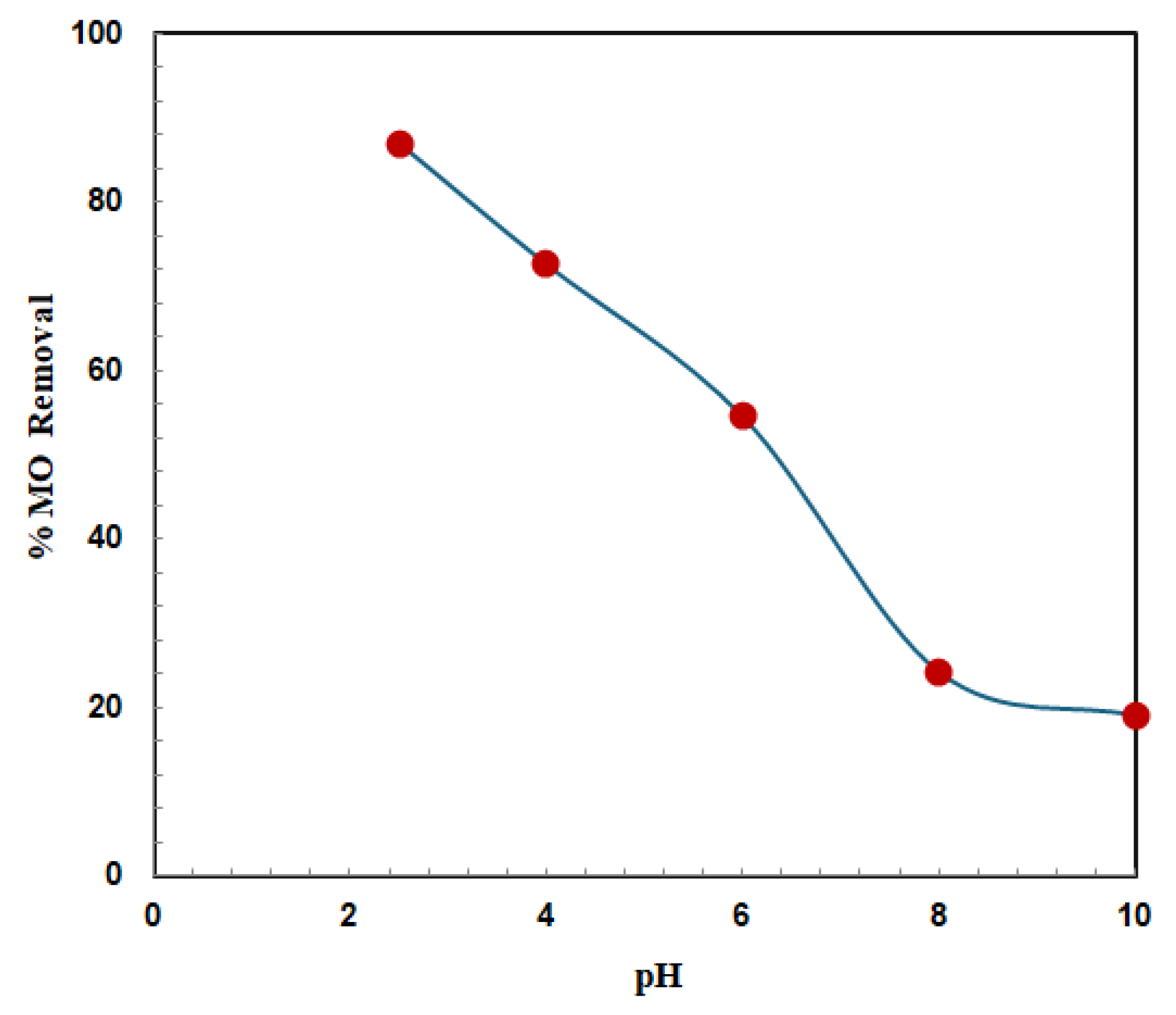
3.2.1. Effect of pH
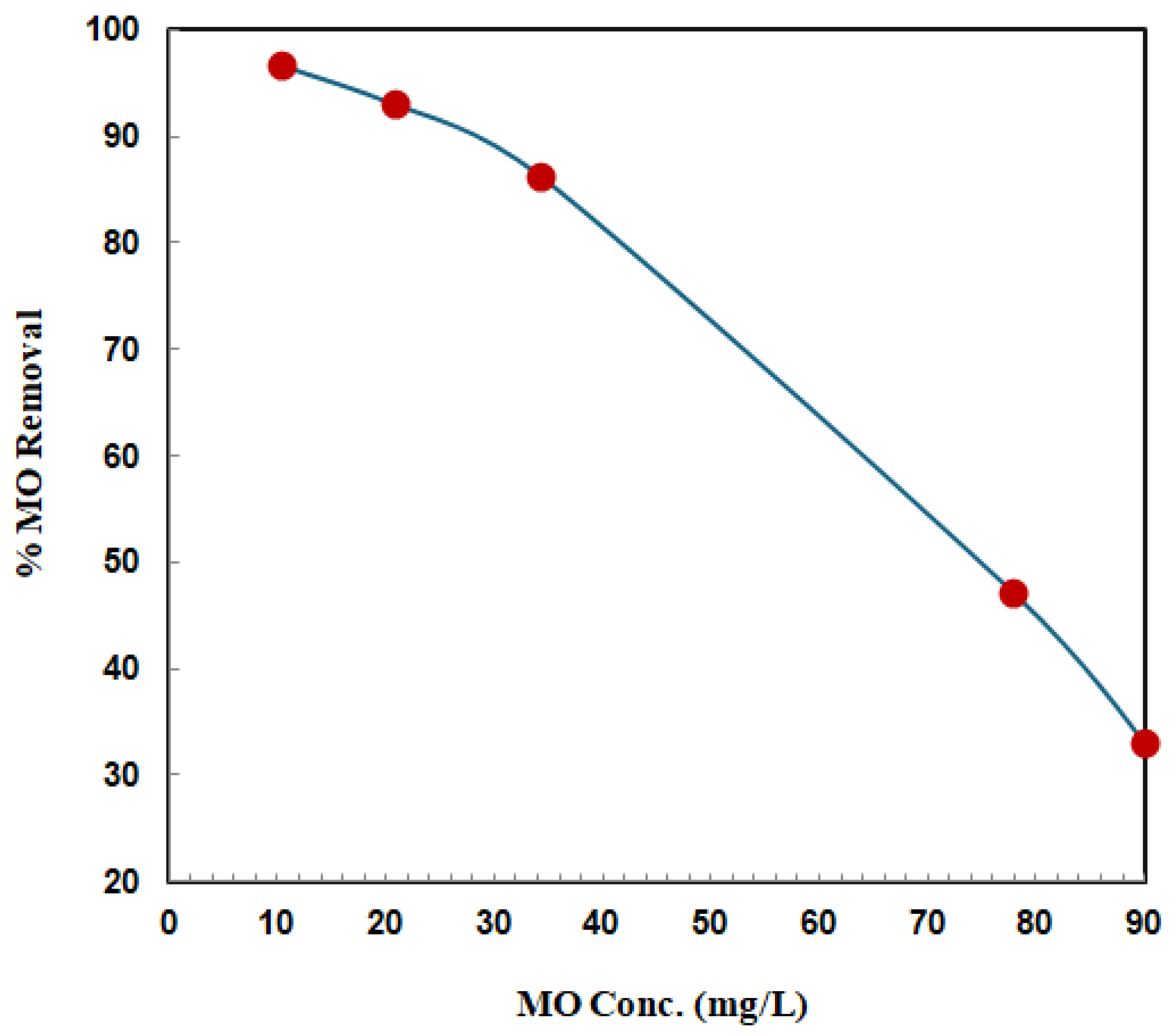
3.2.2. Effect of Initial MO Concentration
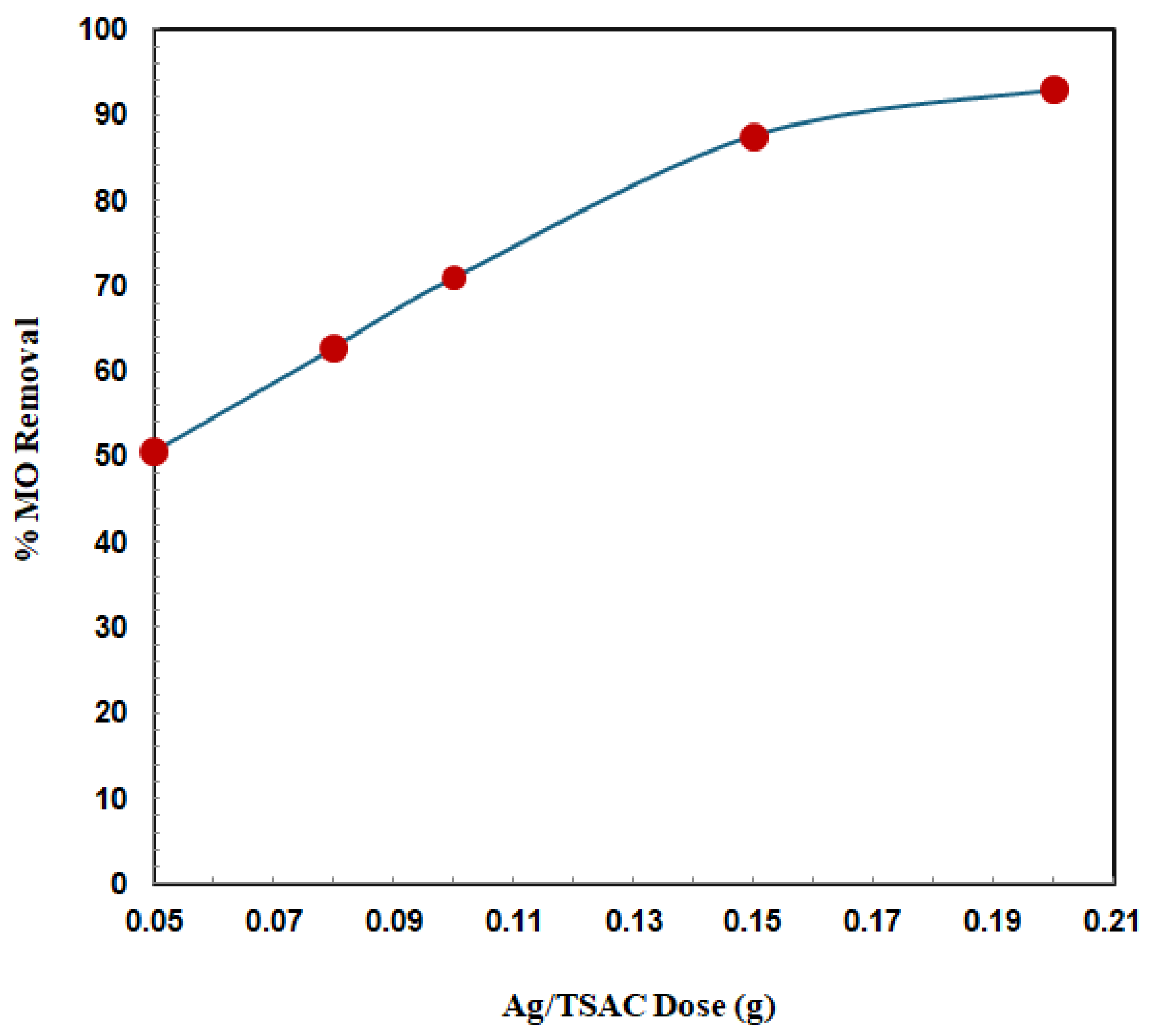
3.2.3. Effect of Adsorbent Dose
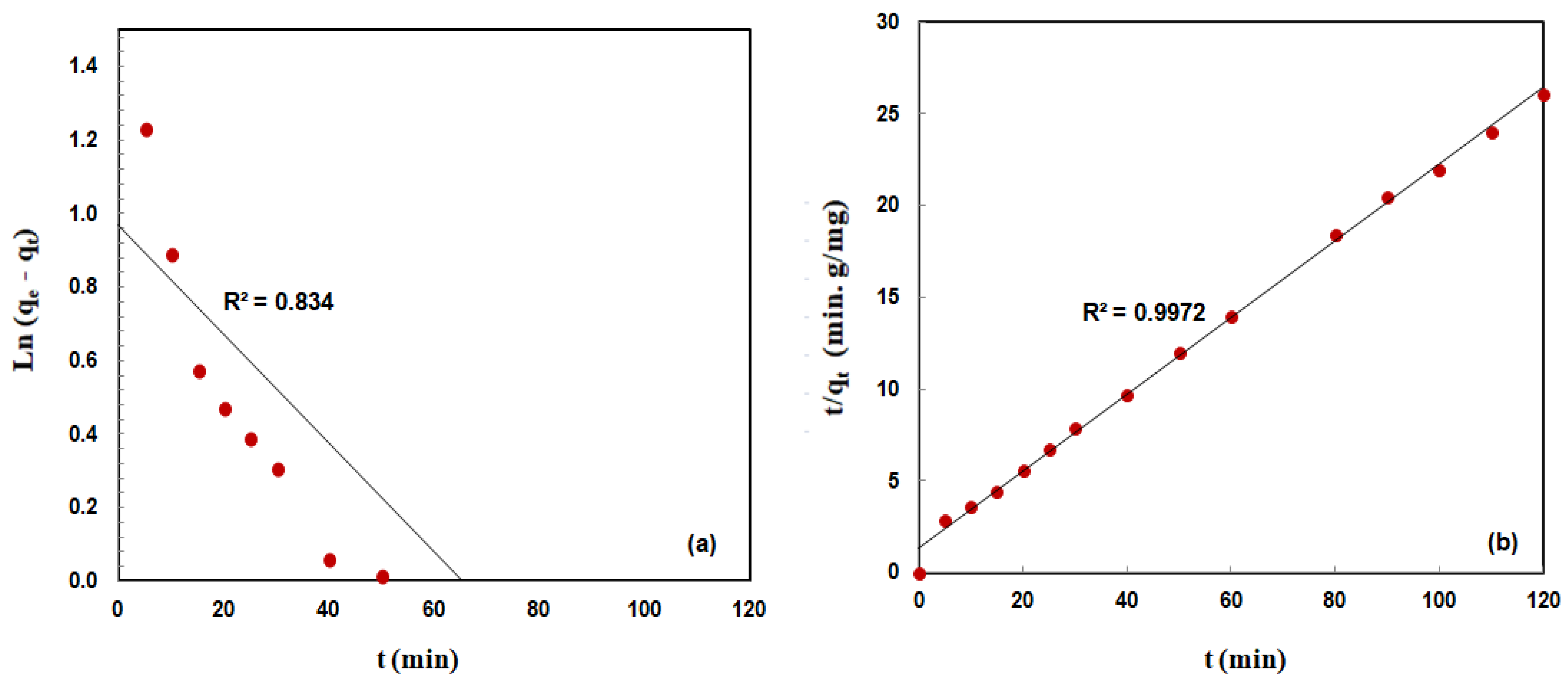
3.2.4. Adsorption Kinetics
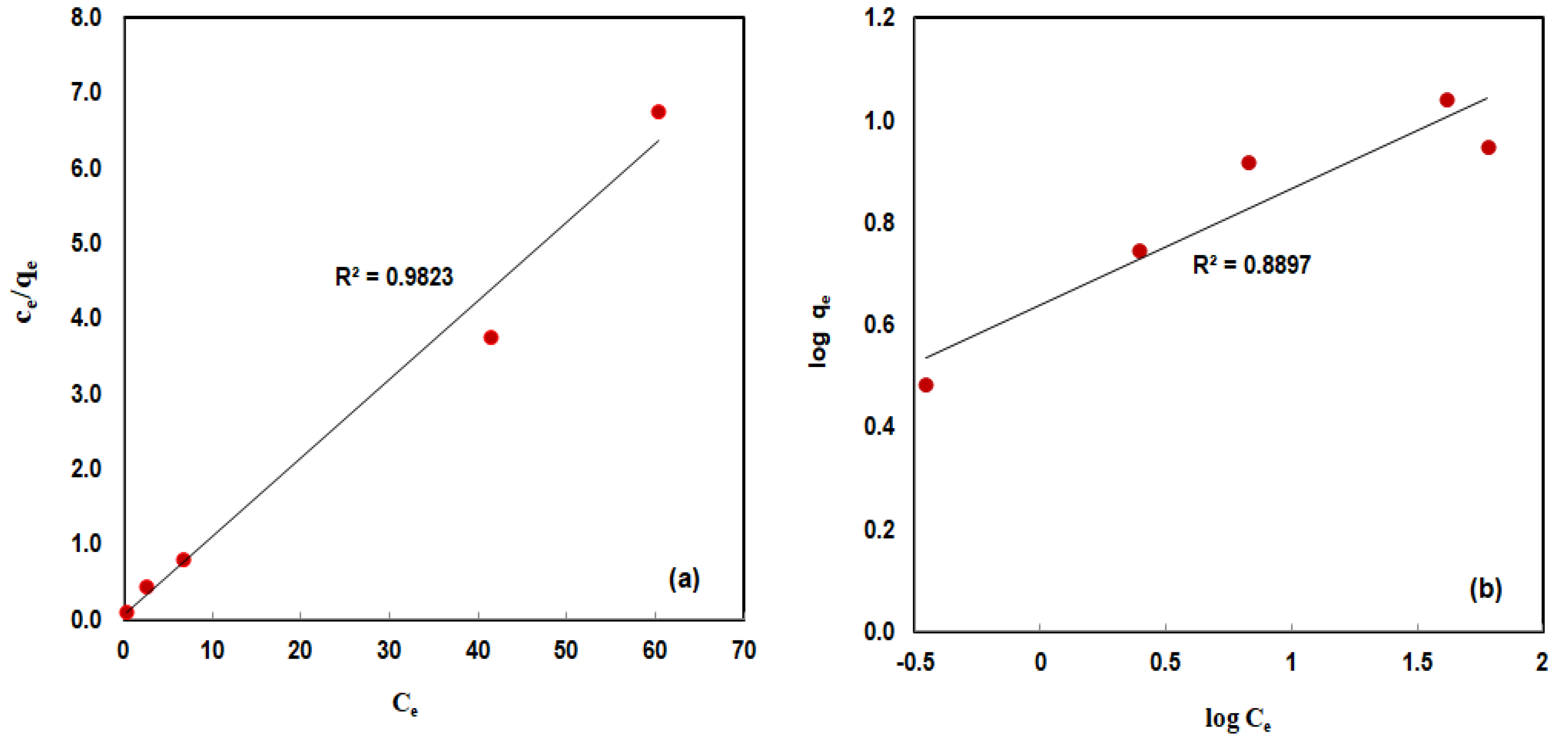
3.2.5. Isotherm Studies
3.2.6. Comparative Study of Ag/TSAC Nanocomposite with Various Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badawi, A.K.; Elkodous, M.A.; Ali, G.A. Recent advances in dye and metal ion removal using efficient adsorbents and novel nano-based materials: An overview. RSC Adv. 2021, 11, 36528–36553. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Babu, R.; Dabbawala, A.A.; Alhassan, S.M. Low-Temperature Synthesis of Magnetic Carbonaceous Materials Coated with Nanosilica for Rapid Adsorption of Methylene Blue. ACS Omega 2020, 5, 6100–6112. [Google Scholar] [CrossRef]
- Jedla, M.R.; Koneru, B.; Franco, A.; Rangappa, D.; Banerjee, P. Recent Developments in Nanomaterials Based Adsorbents for Water Purification Techniques. Biointerface Res. Appl. Chem. 2022, 12, 5821–5835. [Google Scholar]
- Si, Y.; Li, J.; Cui, B.; Tang, D.; Yang, L.; Murugadoss, V.; Maganti, S.; Huang, M.; Guo, Z. Janus phenol–formaldehyde resin and periodic mesoporous organic silica nanoadsorbent for the removal of heavy metal ions and organic dyes from polluted water. Adv. Compos. Hybrid Mater. 2022, 5, 1180–1195. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Hoijang, S.; Wangkarn, S.; Ieamviteevanich, P.; Pinitsoontorn, S.; Ananta, S.; Lee, T.R.; Srisombat, L. Silica-coated magnesium ferrite nanoadsorbent for selective removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125483. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Rani, M.; Yadav, J.; Chaudhary, S.; Shanker, U. An updated review on synthetic approaches of green nanomaterials and their application for removal of water pollutants: Current challenges, assessment and future perspectives. J. Environ. Chem. Eng. 2021, 9, 106763. [Google Scholar]
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation—A comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef]
- Hanoon, M.A.; Ahmed, M.J. Adsorption of Methyl Orange from Wastewater by using Biochar. Iraqi J. Chem. Pet. Eng. 2019, 20, 23–29. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Lemus, J.; Palomares, F.; Morales, M.; Mazarío, E. Superparamagnetic nanosorbent for water purification: Assessment of the adsorptive removal of lead and methyl orange from aqueous solutions. Sci. Total. Environ. 2020, 711, 134644. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Haque, A.; Mosharaf, K.; Marcus, P.K. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 2021, 28, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, Y.; Xu, H.; Li, H. The reuse of nano-TiO2 under different concentration of CO32– using coagulation process and its photocatalytic ability in treatment of methyl orange. Sep. Purif. Technol. 2022, 282, 120152. [Google Scholar] [CrossRef]
- Aljuaid, A.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Abdulaziz, O.; Alsharif, A.; Alsaiari, J.A.; Saih, M.; Alotaibi, R.T.; Khan, I. g-C3N4 Based Photocatalyst for the Efficient Photodegradation of Toxic Methyl Orange Dye: Recent Modifications and Future Perspectives. Molecules 2023, 28, 3199. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of photocatalytic degradation of methyl orange using immobilized scoria-Ni/TiO2 nanoparticles. J. Nanostructure Chem. 2020, 10, 143–159. [Google Scholar] [CrossRef]
- Takele, T.; Angassa, K.; Abewaa, M.; Kebede, A.M.; Tessema, I. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from H3PO4-activated khat stem waste. Biomass Convers. Biorefinery 2025, 15, 4085–4108. [Google Scholar] [CrossRef]
- Sharma, H.; Rana, N.; Sarwan, J.; Bose, J.; Devi, M.; Chugh, R. Nano-material for waste water treatment. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Vats, S.; Srivastava, S.; Maurya, N.; Saxena, S.; Mudgil, B.; Yadav, S.; Chandra, R. Advances in dye contamination: Health hazards, biodegradation, and bioremediation. In Biological Approaches to Controlling Pollutants; Woodhead Publishing: Sawston, UK, 2022; pp. 139–162. [Google Scholar]
- Ghadiri, M.; Hallajzadeh, J.; Akhghari, Z.; Nikkhah, E.; Othman, H.O. Green synthesis and antibacterial effects of silver nanoparticles on novel activated carbon. Iran J. Chem. Chem. Eng. 2023, 42, 3198–3205. [Google Scholar]
- Taha, A.; Daffalla, S. Biochar Derived from Palm Waste Supported Greenly Synthesized MnO2 Nanoparticles as a Novel Adsorbent for Wastewater Treatment. Catalysts 2023, 13, 451. [Google Scholar] [CrossRef]
- Jitjamnong, J.; Thunyaratchatanon, C.; Luengnaruemitchai, A.; Kongrit, N.; Kasetsomboon, N.; Sopajarn, A.; Chuaykarn, N.; Khantikulanon, N. Response surface optimization of biodiesel synthesis over a novel biochar-based heterogeneous catalyst from cultivated (Musa sapientum) banana peels. Biomass Convers. Biorefinery 2020, 11, 2795–2811. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab. J. Sci. Eng. 2017, 43, 5711–5722. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. (Eds.) Biochar: A Guide to Analytical Methods; Csiro Publishing: Clayton, Australia, 2017. [Google Scholar]
- Shume, W.M.; Murthy, H.C.A.; Zereffa, E.A. A Review on Synthesis and Characterization of Ag2O Nanoparticles for Photocatalytic Applications. J. Chem. 2020, 2020, 5039479. [Google Scholar] [CrossRef]
- Eletta, O.A.; Tijani, I.O.; Ighalo, J.O. Adsorption of Pb (II) and phenol from wastewater using silver nitrate modified activated carbon from groundnut (Arachis hypogaea L.) shells. West Indian J. Eng. 2020, 43, 26–35. [Google Scholar]
- Alvez-Tovar, B.; Scalize, P.S.; Angiolillo-Rodríguez, G.; Albuquerque, A.; Ebang, M.N.; de Oliveira, T.F. Agro-Industrial Waste Upcycling into Activated Carbons: A Sustainable Approach for Dye Removal and Wastewater Treatment. Sustainability 2025, 17, 2036. [Google Scholar] [CrossRef]
- Urgel, J.J.; Briones, J.M.; Diaz, E.B., Jr.; Dimaculangan, K.M.; Rangel, K.L.; Lopez, E.C. Removal of diesel oil from water using biochar derived from waste banana peels as adsorbent. Carbon Res. 2024, 3, 13. [Google Scholar] [CrossRef]
- Balavairavan, B.; Saravanakumar, S.S. Characterization of Ecofriendly Poly (Vinyl Alcohol) and Green Banana Peel Filler (GBPF) Reinforced Bio-Films. J. Polym. Environ. 2021, 29, 2756–2771. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Sun, K.; Keiluweit, M.; Kleber, M.; Pan, Z.; Xing, B. Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Bioresour. Technol. 2011, 102, 9897–9903. [Google Scholar] [CrossRef]
- Teixidó, M.; Pignatello, J.J.; Beltrán, J.L.; Granados, M.; Peccia, J. Speciation of the Ionizable Antibiotic Sulfamethazine on Black Carbon (Biochar). Environ. Sci. Technol. 2011, 45, 10020–10027. [Google Scholar] [CrossRef]
- Hameed, B.; Ahmad, A.; Aziz, N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Tapan Kumar, S. Adsorption of methyl orange onto chitosan from aqueous solution. J. Water Resour. Prot. 2010, 2, 2969. [Google Scholar]
- Zhang, L.; Tu, L.-Y.; Liang, Y.; Chen, Q.; Li, Z.-S.; Li, C.-H.; Wang, Z.-H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Dharmendirakumar, M.; Renganathan, S.; Sivanesan, S.; Baskar, G.; Elango, K.P. Removal of Congo Red from Aqueous Solutions by Perlite. Clean–Soil Air, Water 2009, 37, 355–364. [Google Scholar] [CrossRef]
- Popoola, T.J.; Okoronkwo, A.E.; Oluwasina, O.O.; Adebayo, M.A. Preparation, characterization, and application of a homemade graphene for the removal of Congo red from aqueous solutions. Environ. Sci. Pollut. Res. 2021, 28, 52174–52187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, P.; Wang, J.; Liu, Q.; Huang, R. Adsorption of methyl orange (MO) by Zr (IV)-immobilized cross-linked chitosan/bentonite composite. Int. J. Biol. Macromol. 2015, 81, 818–827. [Google Scholar] [CrossRef]
- Hambisa, A.A.; Regasa, M.B.; Ejigu, H.G.; Senbeto, C.B. Adsorption studies of methyl orange dye removal from aqueous solution using Anchote peel-based agricultural waste adsorbent. Appl. Water Sci. 2023, 13, 24. [Google Scholar] [CrossRef]
- Conde, M.A.; Liwaire, C.L.; Tchakounte, A.N.; Ntinkam, A.S.; Nzugue, D.L.; Kede, C.M. Removal of methyl orange (MO) by chitosan modified by zero valent iron. Int. J. Eng. Res. Technol. 2020, 9, 1542–1549. [Google Scholar]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, Y.; Rao, W.; Liu, T.; Liao, L. Study on the adsorption properties of methyl orange by natural one-dimensional nano-mineral materials with different structures. Sci. Rep. 2021, 11, 10640. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic Methyl Orange Removal from Aqueous Solutions by Activated Carbon Reinforced Conducting Polyaniline as Adsorbent: Synthesis, Characterization, Adsorption Behavior, Regeneration and Kinetics Study. J. Polym. Environ. 2022, 30, 886–895. [Google Scholar] [CrossRef]
- Hussain, S.; Kamran, M.; Khan, S.A.; Shaheen, K.; Shah, Z.; Suo, H.; Khan, Q.; Shah, A.B.; Rehman, W.U.; Al-Ghamdi, Y.O.; et al. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 2021, 168, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.; Lee, D. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Janani, V.A.; Gokul, D.; Dhivya, N.; Nesarani, A.; Mukilan, K.; Kumar, A.S.; Kumar, M.V. Optimization Studies on Methyl Orange (MO) Dye Adsorption using Activated Carbon Nanoadsorbent of Ocimum basilicum Linn Leaves. J. Nanomater. 2023, 2023, 7969512. [Google Scholar]
- Ghosh, G.C.; Chakraborty, T.K.; Zaman, S.; Nahar, M.N.; Kabir, A.H. Removal of methyl orange dye from aqueous solution by a low-cost activated carbon prepared from mahagoni (Swietenia mahagoni) Bark. Pollution 2020, 6, 171–184. [Google Scholar]
- Matthew, E.; Roshelle, G.M. Simultaneous adsorption of acidic and basic dyes onto magnetized polypeptidylated-hemoglobin composites. Sep. Purif. Technol. 2021, 255, 117701. [Google Scholar]
- Tran, B.L.; Chin, H.-Y.; Chang, B.K.; Chiang, A.S. Dye adsorption in ZIF-8: The importance of external surface area. Microporous Mesoporous Mater. 2019, 277, 149–153. [Google Scholar] [CrossRef]
- Alhaji, N.; Nathiya, D.; Kaviyarasu, K.; Meshram, M.; Ayeshamariam, A. A comparative study of structural and photocatalytic mechanism of AgGaO2 nanocomposites for equilibrium and kinetics evaluation of adsorption parameters. Surf. Interfaces 2019, 17, 100375. [Google Scholar] [CrossRef]
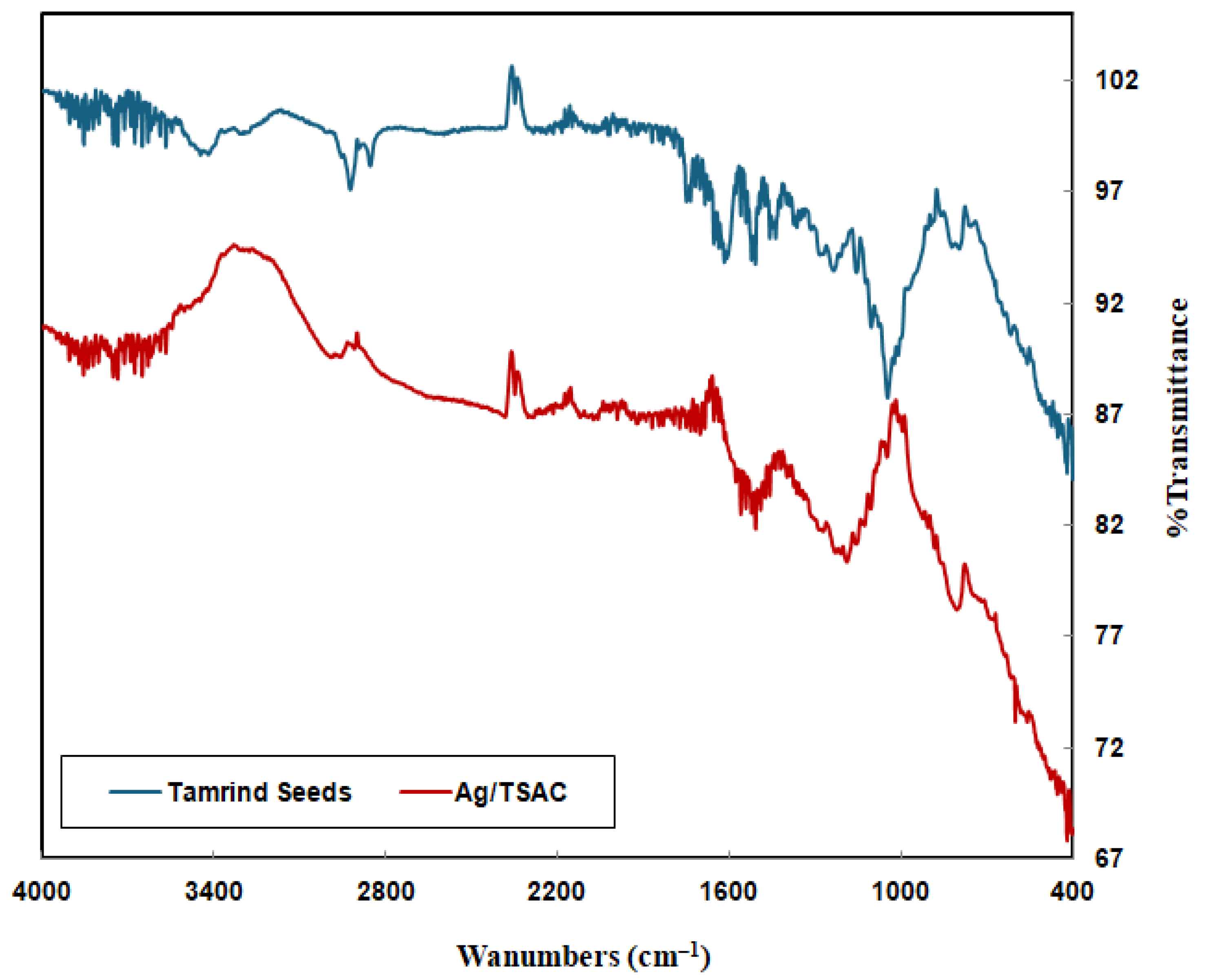

| Reference | Wavenumber (cm−1) | Functional Groups | |
|---|---|---|---|
| Ag/TSAC | TS | ||
| 3600–3400 | 3630.03 | - | O–H stretching of carboxylic and alcohol |
| 3000–2800 | 2852.72, 2924.09 | 2991.34 | C–H stretching of alkane |
| 2260–2100 | 2024.51 | 2048.9 | C=C stretching of alkyne |
| 1740–1730 | 1734.01 | 1749.44 | C–O Stretching |
| 1650–1600 | 1608.63 | 1635.06 | C=C stretching of alkene |
| 1600–1500 | 1541.12 | 1506.41 | C=C aromatic ring vibrations from lignin |
| 1480–1410 | - | 1473.62 | C–H bending |
| 1300–1000 | 1045.54, 1236.37 | 1205.51 | C–O Stretching of ether, ester |
| 1000–675 | 761.88 | 788.88 | C=C–H bending |
| 600–500 | - | 599.26 | metal–oxygen stretching |
| Sample | BET Surface Area (m2/g) | Total Pore Volume a (cm3/g) | Average Pore Diameter b (nm) |
|---|---|---|---|
| Tamarind seeds | 1.17 | 0.0287 | 41.66 |
| Ag/TSAC | 54.92 | 0.1283 | 17.75 |
| Initial MO Con. (mg/L) | Pseudo First-Order Model | Pseudo Second-Order Model | qe,exp (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (mg/g) | R2 | k2 (g/mg min) | qe (mg/g) | R2 | ||
| 40 | 0.0148 | 2.63 | 0.834 | 0.032 | 4.79 | 0.9972 | 5.16 |
| T (°C) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| 25 | b (L/mg) | Qmax (mg/g) | R2 | 1/n | KF (mg/g (L/mg) 1/n) | R2 |
| 1.875 | 9.56 | 0.982 | 0.2265 | 1.69 | 0.8897 | |
| Adsorbent. | Qmax (mg/g) | Reference |
|---|---|---|
| Activated carbon nanoadsorbent derived from Ocimum basilicum Linn leaves | 1.54 | [46] |
| Activated carbon prepared from mahagoni bark | 6.071 | [47] |
| Fe2O3/polypeptidylated hemoglobin | 15.20 | [48] |
| Surfactant-added ZIF-8 | 10.10 | [49] |
| AgGaO2 nanocomposites | 11.39 | [50] |
| Ag/TSAC nanocomposite | 9.56 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daffalla, S.; Al Mousa, N.; Ahmed, H.; Alsuwailem, J.; Almaghasla, M.I.; El-Aassar, M.R. Green Synthesis of a Highly Active Ag/Activated Carbon Nanocomposite from Tamarind Seeds for Methyl Orange Removal. C 2025, 11, 27. https://doi.org/10.3390/c11020027
Daffalla S, Al Mousa N, Ahmed H, Alsuwailem J, Almaghasla MI, El-Aassar MR. Green Synthesis of a Highly Active Ag/Activated Carbon Nanocomposite from Tamarind Seeds for Methyl Orange Removal. C. 2025; 11(2):27. https://doi.org/10.3390/c11020027
Chicago/Turabian StyleDaffalla, Samah, Nura Al Mousa, Hussain Ahmed, Jana Alsuwailem, Mustafa I. Almaghasla, and Mohamed R. El-Aassar. 2025. "Green Synthesis of a Highly Active Ag/Activated Carbon Nanocomposite from Tamarind Seeds for Methyl Orange Removal" C 11, no. 2: 27. https://doi.org/10.3390/c11020027
APA StyleDaffalla, S., Al Mousa, N., Ahmed, H., Alsuwailem, J., Almaghasla, M. I., & El-Aassar, M. R. (2025). Green Synthesis of a Highly Active Ag/Activated Carbon Nanocomposite from Tamarind Seeds for Methyl Orange Removal. C, 11(2), 27. https://doi.org/10.3390/c11020027











