One-Pot Bottom-Up Synthesis of SiO2 Quantum Dots and Reduced Graphene Oxide (rGO) Nanocomposite as Anode Materials in Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
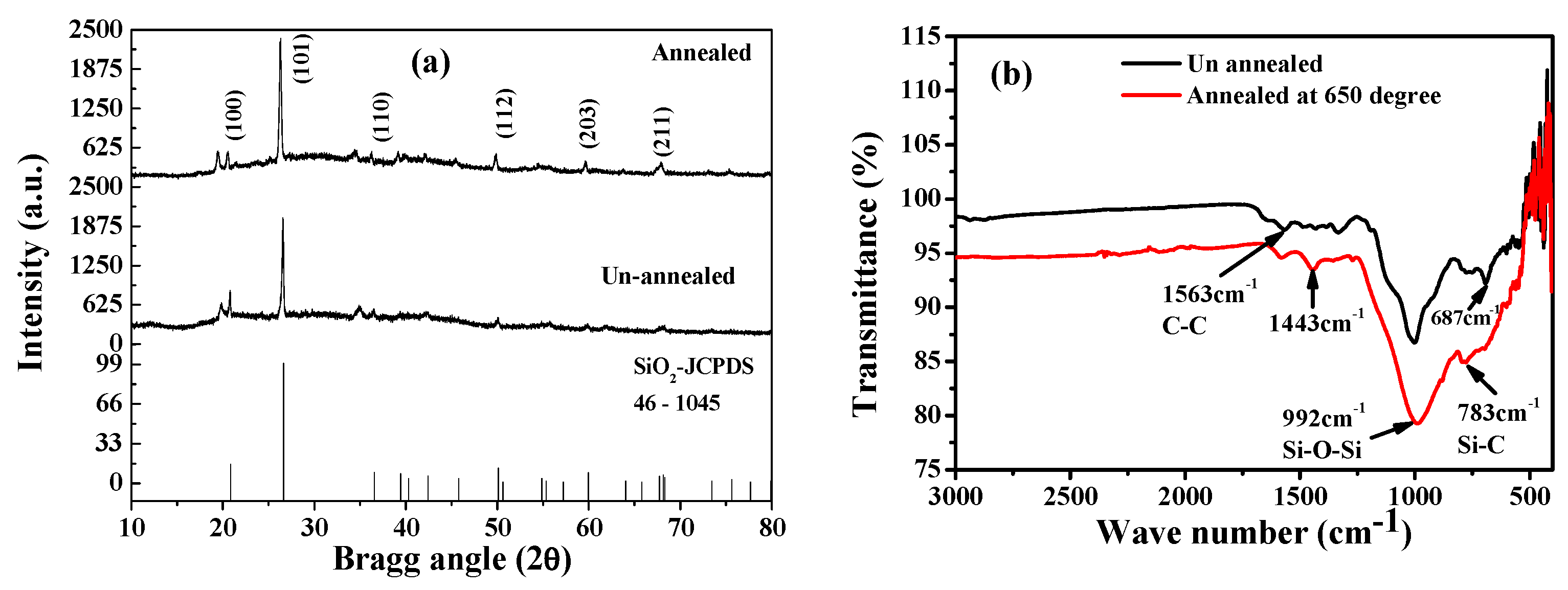
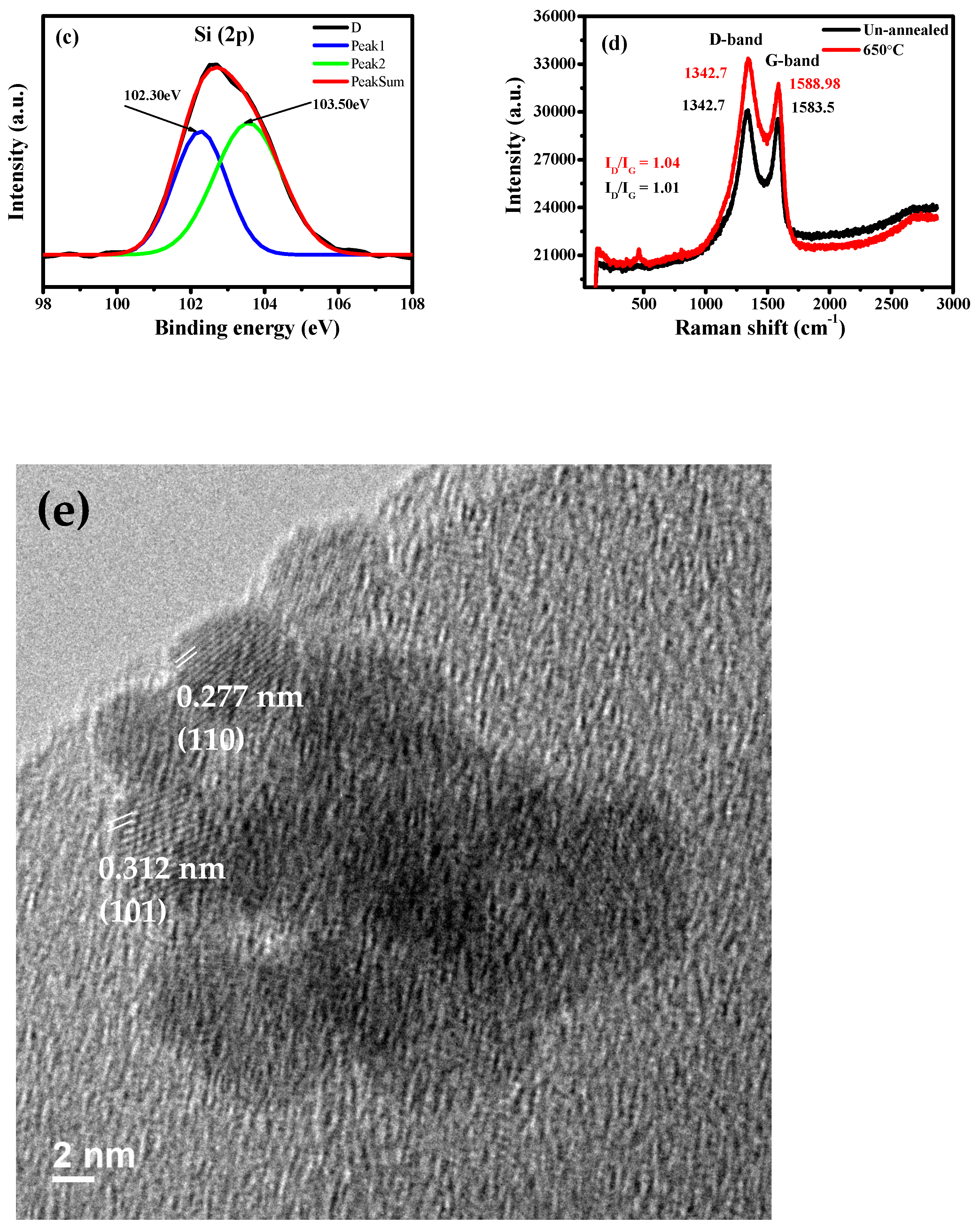
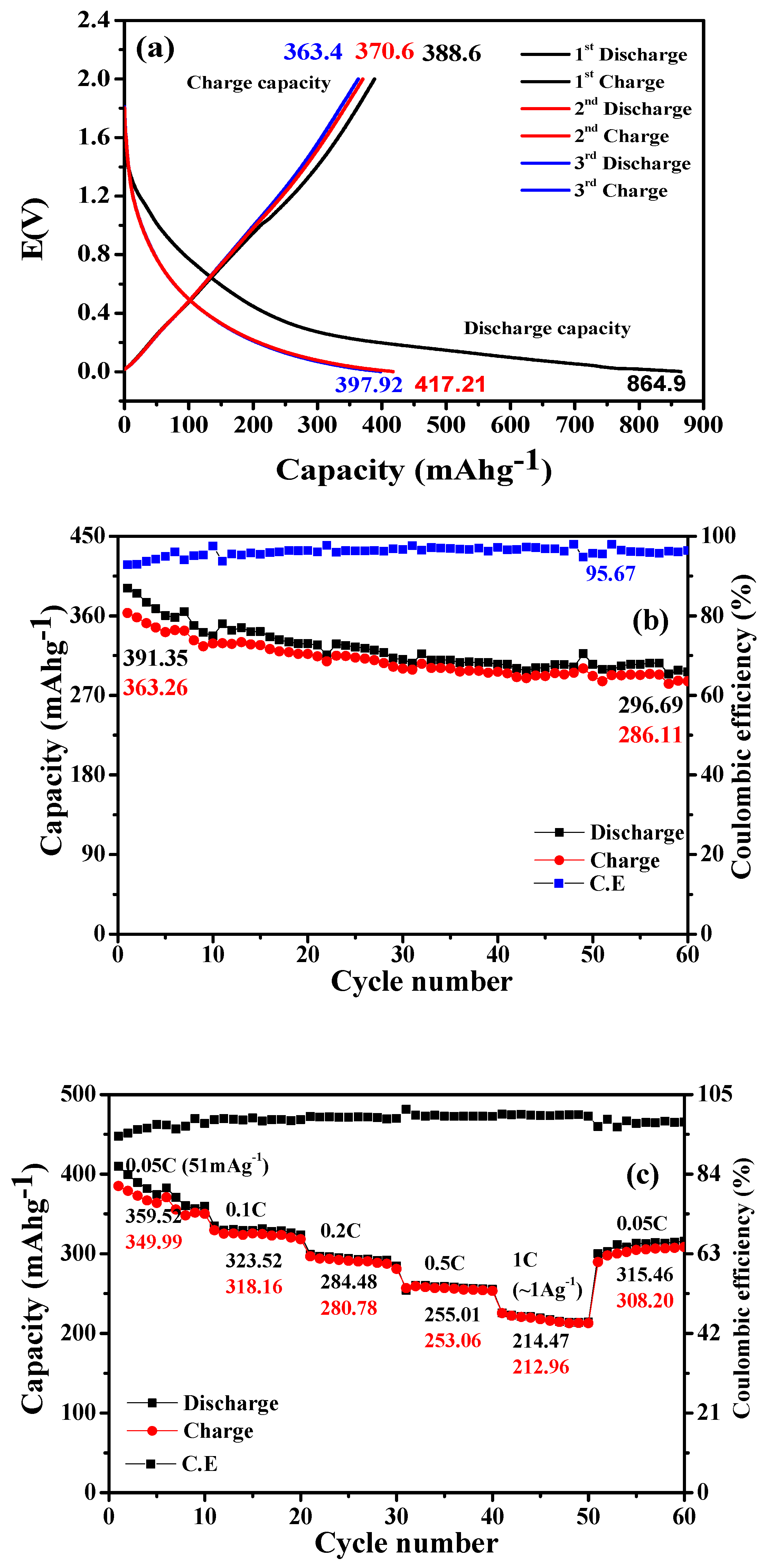
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Mateti, S.; Chen, Y.; Sharma, N.; Han, Q.; Rahman, M.M. Creating value added nano silicon anodes from end-of-life photovoltaic modules: Recovery, nano structuring, and the impact of ball milling and binder on its electrochemical performance. Energy Mater. 2024, 4, 40064. [Google Scholar] [CrossRef]
- Xie, G.; Tan, X.; Shi, Z.; Peng, Y.; Ma, Y.; Zhong, Y.; Wang, F.; He, J.; Zhu, Z.; Cheng, X.B. SiOx Based Anodes for Advanced Li-Ion Batteries: Recent Progress and Perspectives. Adv. Funct. Mater. 2025, 35, 2414714. [Google Scholar] [CrossRef]
- Ma, X.; Wei, Z.; Han, H.; Wang, X.; Cui, K.; Yang, L. Tunable construction of multi-shell hollow SiO2 microspheres with hierarchically porous structure as high-performance anodes for lithium-ion batteries. Chem. Eng. J. 2017, 323, 252–259. [Google Scholar] [CrossRef]
- Xue, M.; Zhou, Y.; Geng, J.; Zeng, P.; Xu, Y.; Wang, Y.; Tang, W.; Wu, P.; Wei, S.; Zhou, Y. Hollow porous SiO2 nanobelts containing sulfur for long-life lithium–sulfur batteries. RSC Adv. 2016, 6, 91179–91184. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, L.; Zhou, C.; Huang, H.; Liang, S.; Xia, Y.; Gan, Y.; Tao, X.; Zhang, J.; Zhang, W. Submicron silica as high− capacity lithium storage material with superior cycling performance. Mater. Res. Bull. 2017, 96, 347–353. [Google Scholar] [CrossRef]
- Tu, J.; Yuan, Y.; Zhan, P.; Jiao, H.; Wang, X.; Zhu, H.; Jiao, S. Straightforward approach toward SiO2 nanospheres and their superior lithium storage performance. J. Phys. Chem. C 2014, 118, 7357–7362. [Google Scholar] [CrossRef]
- Yan, N.; Wang, F.; Zhong, H.; Li, Y.; Wang, Y.; Hu, L.; Chen, Q. Hollow porous SiO2 nanocubes towards high-performance anodes for lithium-ion batteries. Sci. Rep. 2013, 3, 1568. [Google Scholar] [CrossRef]
- Li, X.; Dhanabalan, A.; Meng, X.; Gu, L.; Sun, X.; Wang, C. Nanoporous tree-like SiO2 films fabricated by sol–gel assisted electrostatic spray deposition. Microporous Mesoporous Mater. 2012, 151, 488–494. [Google Scholar] [CrossRef]
- Si, L.; Yan, K.; Li, C.; Huang, Y.; Pang, X.; Yang, X.; Sui, D.; Zhang, Y.; Wang, J.; Xu, C.C. Binder-free SiO2 nanotubes/carbon nanofibers mat as superior anode for lithium-ion batteries. Electrochim. Acta 2022, 404, 139747. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, Y.; Liu, J.; Dai, J.; Li, Y.; Ai, F. Facile fabrication of SiO2 nanotubes coated with nitrogen-doped carbon layers as high-performance anodes for lithium-ion batteries. Ceram. Int. 2021, 47, 1373–1380. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, J.; Ding, Z.; Ren, Y.; Liu, Z.; Chen, X.; Zhou, X. Li+ storage properties of SiO2@C core-shell submicrosphere and its hollow counterpart synthesized by molecular self-assembly in wet-chemistry condition as anodes for LIBs. J. Alloys Compd. 2021, 861, 157932. [Google Scholar] [CrossRef]
- An, W.; Fu, J.; Su, J.; Wang, L.; Peng, X.; Wu, K.; Chen, Q.; Bi, Y.; Gao, B.; Zhang, X. Mesoporous hollow nanospheres consisting of carbon coated silica nanoparticles for robust lithium-ion battery anodes. J. Power Sources 2017, 345, 227–236. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, B.; Wei, H.; Ding, J. Electrospun SiO2/C composite fibers as durable anode materials for lithium ion batteries. Solid State Ion. 2016, 292, 27–31. [Google Scholar] [CrossRef]
- Liang, Y.; Cai, L.; Chen, L.; Lin, X.; Fu, R.; Zhang, M.; Wu, D. Silica nanonetwork confined in nitrogen-doped ordered mesoporous carbon framework for high-performance lithium-ion battery anodes. Nanoscale 2015, 7, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, Z.-q.; Wang, C.-y.; Jin, J. Nanostructured SiO2/C composites prepared via electrospinning and their electrochemical properties for lithium ion batteries. J. Electroanal. Chem. 2015, 746, 62–67. [Google Scholar] [CrossRef]
- Li, M.; Yu, Y.; Li, J.; Chen, B.; Wu, X.; Tian, Y.; Chen, P. Nanosilica/carbon composite spheres as anodes in Li-ion batteries with excellent cycle stability. J. Mater. Chem. A 2015, 3, 1476–1482. [Google Scholar] [CrossRef]
- Meng, J.; Cao, Y.; Suo, Y.; Liu, Y.; Zhang, J.; Zheng, X. Facile fabrication of 3D SiO2@ graphene aerogel composites as anode material for lithium ion batteries. Electrochimica Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Li, J.; Xia, X.; He, Y.; Liu, H. Submicro-sized porous SiO2/C and SiO2/C/graphene spheres for lithium ion batteries. J. Solid State Electrochem. 2017, 21, 2425–2432. [Google Scholar] [CrossRef]
- Yubero, F.; Barranco, A.; Mejías, J.; Espinos, J.; González-Elipe, A. Spectroscopic characterisation and chemical reactivity of silicon monoxide layers deposited on Cu (100). Surf. Sci. 2000, 458, 229–238. [Google Scholar] [CrossRef]
- Liang, J.; Wei, W.; Zhong, D.; Yang, Q.; Li, L.; Guo, L. One-step in situ synthesis of SnO2/graphene nanocomposites and its application as an anode material for Li-ion batteries. ACS Appl. Mater. Inter. 2012, 4, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, X.; Lu, J.; Li, J. Preparation of SnO2-nanocrystal/graphene-nanosheets composites and their lithium storage ability. J. Phys. Chem. C 2010, 114, 21770–21774. [Google Scholar] [CrossRef]
- Park, C.-M.; Choi, W.; Hwa, Y.; Kim, J.-H.; Jeong, G.; Sohn, H.-J. Characterizations and electrochemical behaviors of disproportionated SiO and its composite for rechargeable Li-ion batteries. J. Mater. Chem. 2010, 20, 4854–4860. [Google Scholar] [CrossRef]
- Hwa, Y.; Park, C.-M.; Sohn, H.-J. Modified SiO as a high performance anode for Li-ion batteries. J. Power Sources 2013, 222, 129–134. [Google Scholar] [CrossRef]
- Yu, B.-C.; Hwa, Y.; Kim, J.-H.; Sohn, H.-J. A new approach to synthesis of porous SiOx anode for Li-ion batteries via chemical etching of Si crystallites. Electrochim. Acta 2014, 117, 426–430. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahma, S.; Wang, C.-Y.; Huang, Y.-H.; Lin, W.-F.; Huang, J.-L. One-Pot Bottom-Up Synthesis of SiO2 Quantum Dots and Reduced Graphene Oxide (rGO) Nanocomposite as Anode Materials in Lithium-Ion Batteries. C 2025, 11, 23. https://doi.org/10.3390/c11010023
Brahma S, Wang C-Y, Huang Y-H, Lin W-F, Huang J-L. One-Pot Bottom-Up Synthesis of SiO2 Quantum Dots and Reduced Graphene Oxide (rGO) Nanocomposite as Anode Materials in Lithium-Ion Batteries. C. 2025; 11(1):23. https://doi.org/10.3390/c11010023
Chicago/Turabian StyleBrahma, Sanjaya, Cheung-Yi Wang, Yi-Hsuan Huang, Wen-Feng Lin, and Jow-Lay Huang. 2025. "One-Pot Bottom-Up Synthesis of SiO2 Quantum Dots and Reduced Graphene Oxide (rGO) Nanocomposite as Anode Materials in Lithium-Ion Batteries" C 11, no. 1: 23. https://doi.org/10.3390/c11010023
APA StyleBrahma, S., Wang, C.-Y., Huang, Y.-H., Lin, W.-F., & Huang, J.-L. (2025). One-Pot Bottom-Up Synthesis of SiO2 Quantum Dots and Reduced Graphene Oxide (rGO) Nanocomposite as Anode Materials in Lithium-Ion Batteries. C, 11(1), 23. https://doi.org/10.3390/c11010023




