Advanced Graphene-Based Technologies for Antibiotic Removal from Wastewater: A Review (2016–2024)
Abstract
1. Introduction
1.1. Graphene-Based Materials
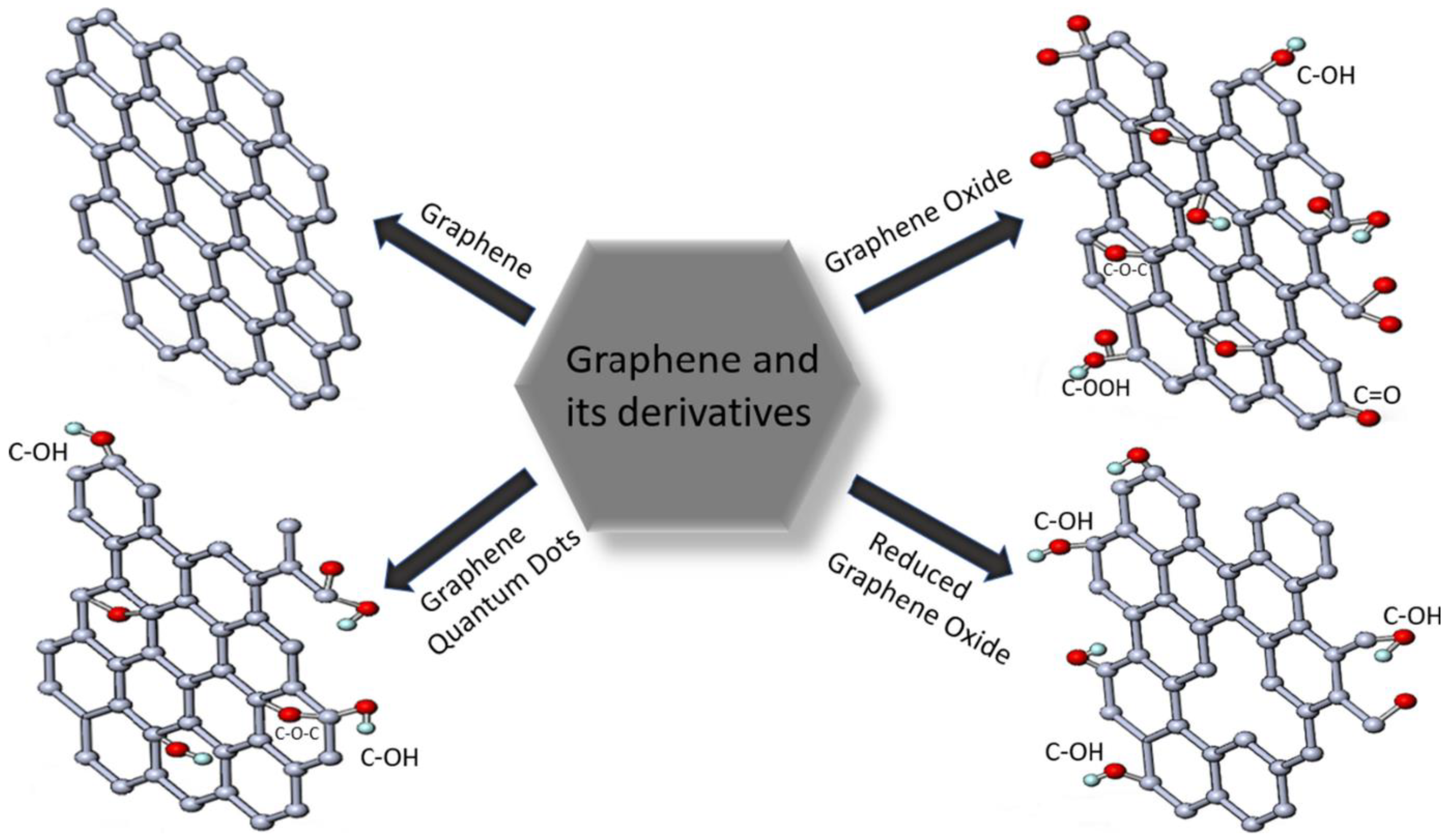
1.1.1. Types of Graphene-Based Materials
Pristine Graphene
Graphene Oxide
Reduced Graphene Oxide
Graphene Quantum Dots (GQDs)
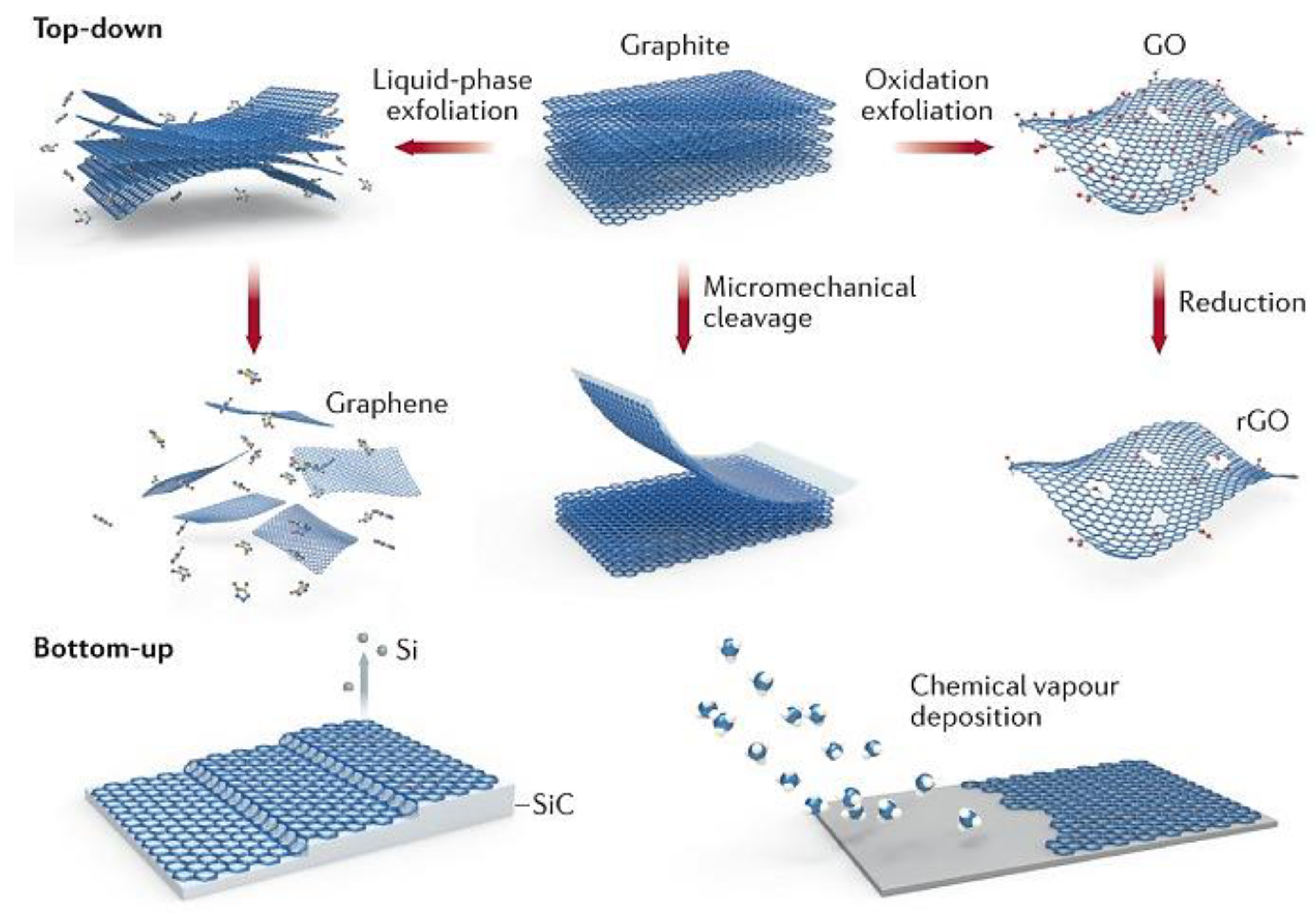
1.1.2. Synthesis Methods for the Graphene-Based Materials
Liquid-Phase Exfoliation
Oxidation Exfoliation for Graphene Oxide (GO) and Reduction for Reduced Graphene Oxide (rGO)
Micromechanical Cleavage
Silicon Carbide (SiC) to Graphene
Chemical Vapor Deposition (CVD)
2. Antibiotics in Wastewater: A Growing Environmental Concern
2.1. Sources of Antibiotics in Wastewater
2.1.1. Human Medicine
2.1.2. Veterinary Medicine and Agriculture
2.1.3. Pharmaceutical Manufacturing
2.2. Properties of Graphene-Based Materials for the Treatment of Wastewater with Antibiotics
3. Research Methods
3.1. Systematic Literature Review
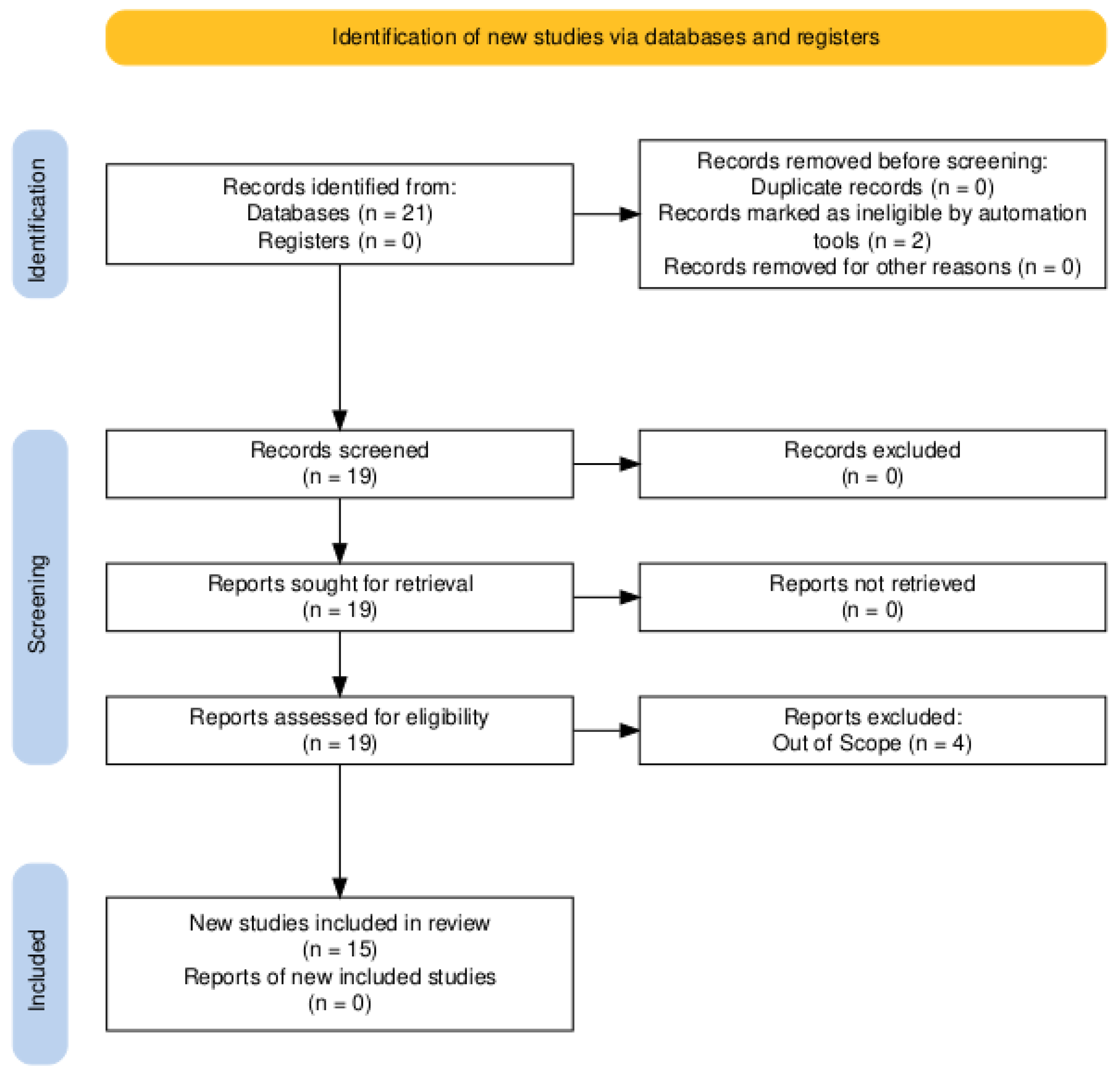
3.1.1. Identification
3.1.2. Screening
3.1.3. Eligibility
3.1.4. Included Articles and Quality Assessment
4. Removal of Antibiotics from Wastewater via Graphene-Based Materials
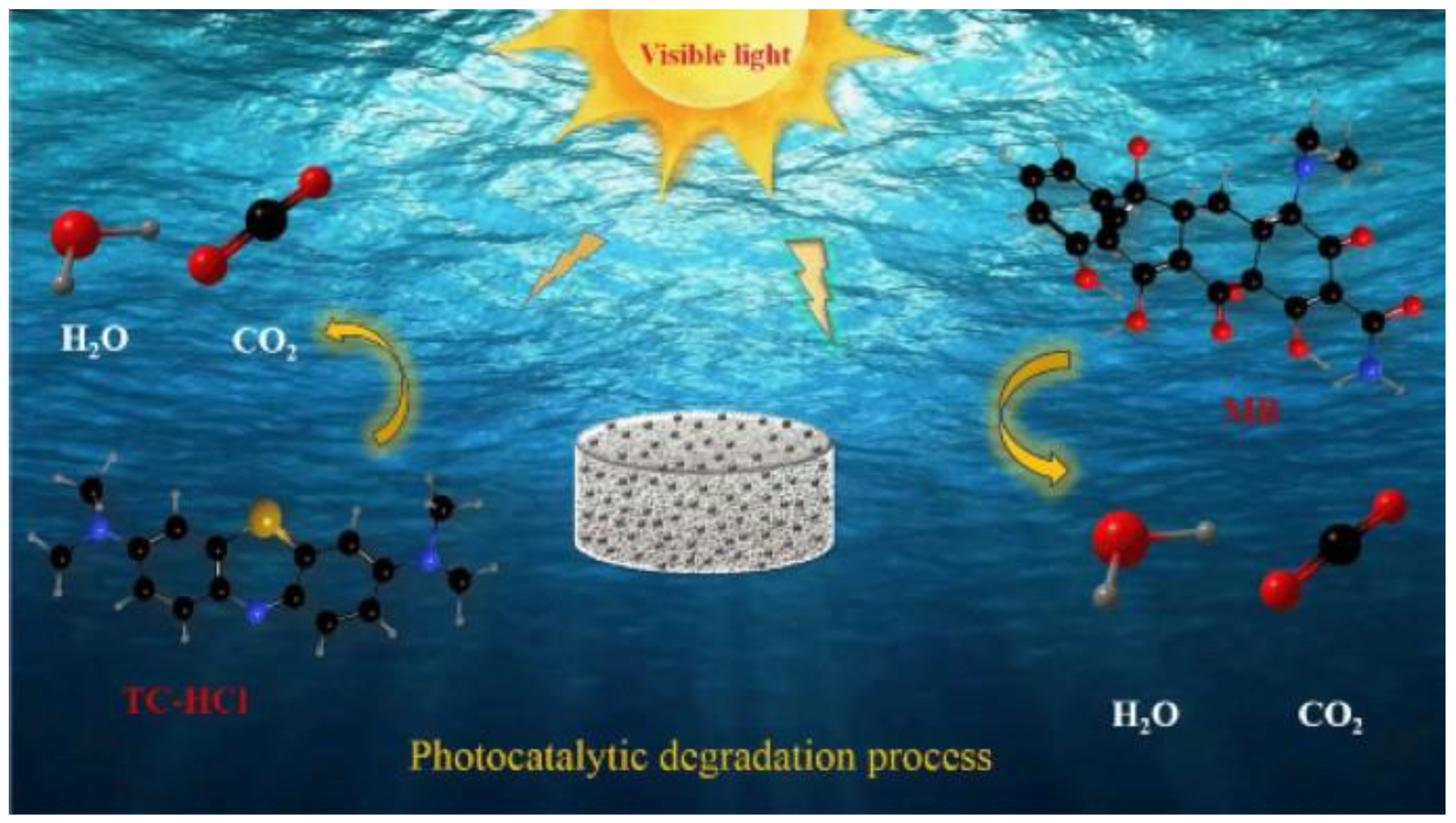
4.1. Removal of Antibiotics from Wastewater via Photocatalytic Degradation via Graphene-Based Materials
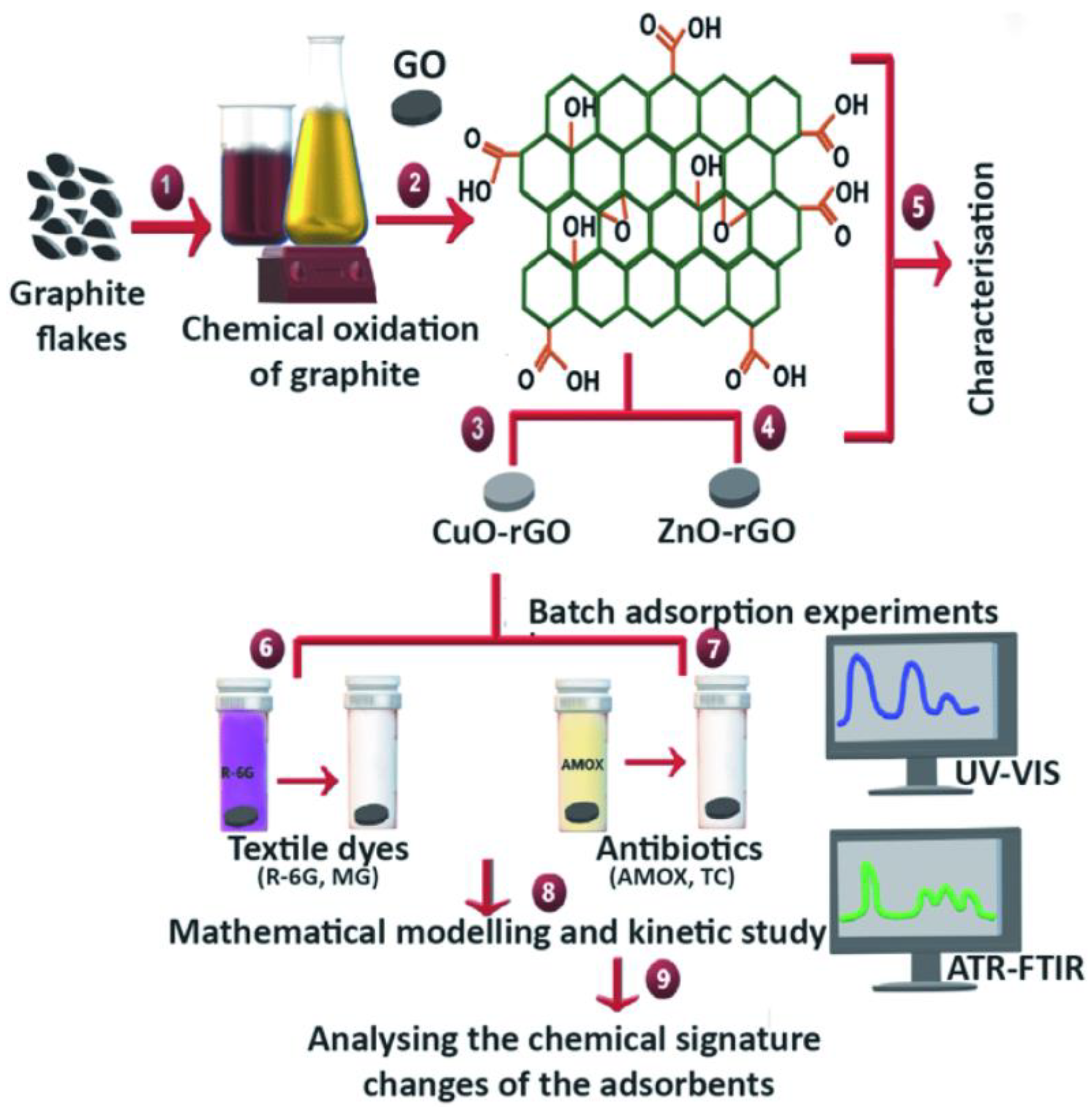
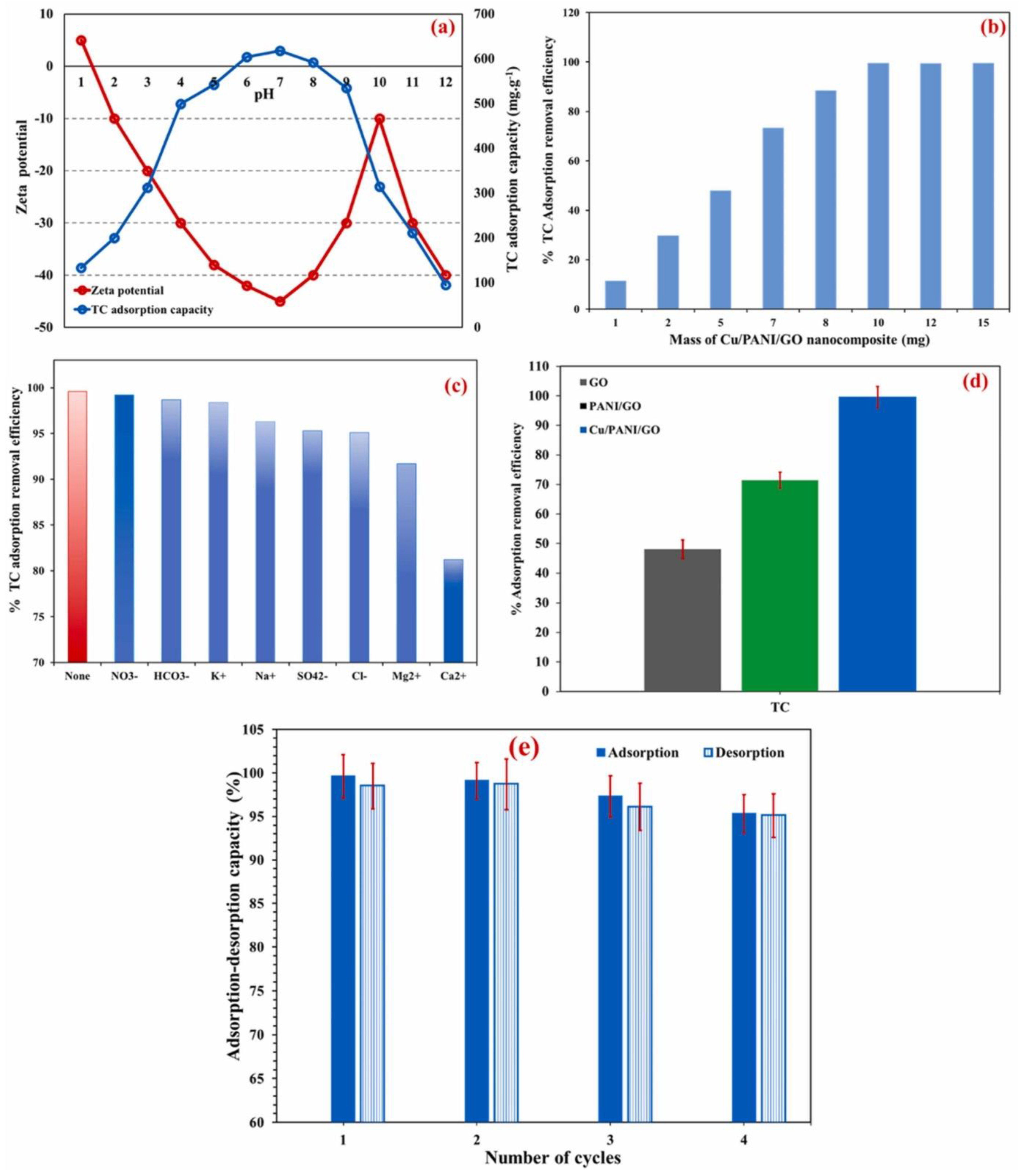
4.2. Removal of Antibiotics from Wastewater via Adsorption via Graphene-Based Materials
4.3. Removal of Antibiotics from Wastewater via Other Methods via Graphene-Based Materials
5. Challenges, Potential Solutions and Mitigation Strategies
6. Conclusions
Funding
Conflicts of Interest
References
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Naicker, N. Contribution of Wastewater to Antimicrobial Resistance: A Review Article. J. Glob. Antimicrob. Resist. 2023, 34, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of Pharmaceutical Effluents on Aquatic Ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in Food Chain: The Consequences for Antibiotic Resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Zinicovscaia, I., Cepoi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–25. ISBN 978-3-319-26751-7. [Google Scholar]
- Sengupta, J.; Hussain, C.M. Sensitive and Selective Detection of Heavy Metal Ions and Organic Pollutants with Graphene-Integrated Sensing Platforms. Nanoscale 2024, 16, 14195–14212. [Google Scholar] [CrossRef]
- Avornyo, A.; Chrysikopoulos, C.V. Applications of Graphene Oxide (GO) in Oily Wastewater Treatment: Recent Developments, Challenges, and Opportunities. J. Environ. Manag. 2024, 353, 120178. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A Review of Graphene-Based Nanomaterials for Removal of Antibiotics from Aqueous Environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of Graphene in Energy Storage Device—A Review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.Ö.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Tarhini, A.; Tehrani-Bagha, A.R. Advances in Preparation Methods and Conductivity Properties of Graphene-Based Polymer Composites. Appl. Compos. Mater. 2023, 30, 1737–1762. [Google Scholar] [CrossRef]
- Mazilova, T.I.; Sadanov, E.V.; Mikhailovskij, I.M. Tensile Strength of Graphene Nanoribbons: An Experimental Approach. Mater. Lett. 2019, 242, 17–19. [Google Scholar] [CrossRef]
- Liu, D.; Fu, H.; Yang, T.; Wang, W.; Zhao, J.; Wu, K.; Wu, C.; Yong, Z.; Zhang, Y. A Modified Spin-Casting Approach for Scalable Preparation of Ultra-Thick Reduced Graphene Oxide Films with High Thermal Conductivity. Mater. Res. Express 2022, 9, 036405. [Google Scholar] [CrossRef]
- Sun, B.; Pang, J.; Cheng, Q.; Zhang, S.; Li, Y.; Zhang, C.; Sun, D.; Ibarlucea, B.; Li, Y.; Chen, D.; et al. Synthesis of Wafer-Scale Graphene with Chemical Vapour Deposition for Electronic Device Applications. Adv. Mater. Technol. 2021, 6, 2000744. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. Tensile, Flexural and Interlaminar Shear Strength of Carbon Fibre Reinforced Epoxy Composites Modified by Graphene. Polym. Bull. 2023, 80, 7469–7490. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L. Chapter 1—Carbon Nanomaterials. In Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Li, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–38. ISBN 978-0-12-815053-5. [Google Scholar]
- Belyaeva, L.A.; Schneider, G.F. Wettability of Graphene. Surf. Sci. Rep. 2020, 75, 100482. [Google Scholar] [CrossRef]
- Liu, Z.; Navik, R.; Tan, H.; Xiang, Q.; Wahyudiono; Goto, M.; Ibarra, R.M.; Zhao, Y. Graphene-Based Materials Prepared by Supercritical Fluid Technology and Its Application in Energy Storage. J. Supercrit. Fluids 2022, 188, 105672. [Google Scholar] [CrossRef]
- Ivan, R.; Popescu, C.; Antohe, V.A.; Antohe, S.; Negrila, C.; Logofatu, C.; del Pino, A.P.; György, E. Iron Oxide/Hydroxide–Nitrogen Doped Graphene-like Visible-Light Active Photocatalytic Layers for Antibiotics Removal from Wastewater. Sci. Rep. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Utkan, G.; Yumusak, G.; Tunali, B.C.; Ozturk, T.; Turk, M. Production of Reduced Graphene Oxide by Using Three Different Microorganisms and Investigation of Their Cell Interactions. ACS Omega 2023, 8, 31188–31200. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Akbar Shah, S.R.; Khan, A.; Ahmad, N.; Ahmed, S.; Ain, N.; Mehmood, A.; Salah, B.; Ullah, S.S.; Khan, R. Synthesis of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) and Their Application as Nano-Fillers to Improve the Physical and Mechanical Properties of Medium Density Fiberboard. Front. Mater. 2023, 10, 1206918. [Google Scholar] [CrossRef]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the Oxygen Content of Graphite and Reduced Graphene Oxide for Specific Applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef]
- Ashan, M.; Alharbi, F.F.; Aman, S.; Al-Sehemi, A.G.; Ahmad, K.; Aslam, M.; Saleem, M.I. Elevating Capacitive Features of Hydrothermally Produced CeVS3 Utilizing rGO for Supercapacitor Applications. Diam. Relat. Mater. 2024, 147, 111319. [Google Scholar] [CrossRef]
- Tan, B.; Han, S.; Luo, W.; Chao, Z.; Fan, J.; Wang, M. Synthesis of RGO-Supported Layered MoS2 with Enhanced Electrochemical Performance for Aluminum Ion Batteries. J. Alloys Compd. 2020, 841, 155732. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic Bandgap Engineering of Graphene Quantum Dots and Applications for Photocatalytic Water Splitting and CO2 Reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef]
- Rai, D.; Jaiswal, Y.; Sinha, S. Graphene Quantum Dots Synthesis Using Waste Unburnt Carbon: Implications for Optoelectronics. Appl. Surf. Sci. 2024, 653, 159386. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Ankitha, M.; Pillai, V.K.; Alwarappan, S. Graphene Quantum Dots for Biosensing and Bioimaging. RSC Adv. 2024, 14, 16001–16023. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Kurniawan, D.; Tsai, M.-D.; Chang, J.-W.; Chang, Y.-N.; Yang, S.-C.; Chiang, W.-H.; Kung, C.-W. Two-Dimensional Metal–Organic Framework for Post-Synthetic Immobilization of Graphene Quantum Dots for Photoluminescent Sensing. Commun. Chem. 2024, 7, 108. [Google Scholar] [CrossRef]
- El-Mahalawy, A.M.; Abbas, W.; Mostafa, O.; Zidan, N.A.; Issa, H.H.; Fedawy, M.; Wassel, A.R. Integrative Role of PEDOT: PSS in Adjusting the Photoresponse Efficiency of Novel Reduced Graphene Quantum Dots/Silicon Heterojunction for Optoelectronics and Solar Energy Conversion Applications. Surf. Interfaces 2024, 46, 103946. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Shi, M.; Wang, Y.; Meng, X.; Chen, Y.; Huang, Q.; Liu, C. A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application. C 2024, 10, 7. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision Synthesis versus Bulk-Scale Fabrication of Graphenes. Nat. Rev. Chem. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Sohrabi, B.; Poorsargol, M.; Ingram, S.; Roudsari, G. Surfactants-Emerging Amphiphiles in Liquid Phase Exfoliation Method for Dispersing Carbon Nanotubes and Graphene: Experimental and Molecular Dynamics Simulation Studies. J. Mol. Liq. 2024, 409, 125493. [Google Scholar] [CrossRef]
- Gharib, D.H.; Gietman, S.; Malherbe, F.; Moulton, S.E. High Yield, Solid Exfoliation and Liquid Dispersion of Graphite Driven by a Donor-Acceptor Interaction. Carbon 2017, 123, 695–707. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W.S.W. Ultrasonication an Intensifying Tool for Preparation of Stable Nanofluids and Study the Time Influence on Distinct Properties of Graphene Nanofluids—A Systematic Overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Biranje, P.M.; Patwardhan, A.W.; Joshi, J.B.; Dasgupta, K. Exfoliated Graphene and Its Derivatives from Liquid Phase and Their Role in Performance Enhancement of Epoxy Matrix Composite. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106886. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic Exfoliation of Graphene in Water: A Key Parameter Study. Carbon 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Z.; Liu, Z.; Ren, G. Mechanism of Oxidization of Graphite to Graphene Oxide by the Hummers Method. ACS Omega 2022, 7, 23503–23510. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Shen, L.; Bao, N. Revisiting the Oxidation of Graphite: Reaction Mechanism, Chemical Stability, and Structure Self-Regulation. ACS Omega 2020, 5, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (rGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, R.; Wahyuningsih, S.; Ramelan, A.H. Simple Synthesis of rGO (Reduced Graphene Oxide) by Thermal Reduction of GO (Graphene Oxide). IOP Conf. Ser. Mater. Sci. Eng. 2020, 858, 012009. [Google Scholar] [CrossRef]
- Zhou, A.; Bai, J.; Hong, W.; Bai, H. Electrochemically Reduced Graphene Oxide: Preparation, Composites, and Applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Ahmad, R.T.M.; Hong, S.-H.; Shen, T.-Z.; Song, J.-K. Water-Assisted Stable Dispersal of Graphene Oxide in Non-Dispersible Solvents and Skin Formation on the GO Dispersion. Carbon 2016, 98, 188–194. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Phiri, J.; Johansson, L.-S.; Gane, P.; Maloney, T. A Comparative Study of Mechanical, Thermal and Electrical Properties of Graphene-, Graphene Oxide- and Reduced Graphene Oxide-Doped Microfibrillated Cellulose Nanocomposites. Compos. Part B Eng. 2018, 147, 104–113. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, C.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent Mechanical Processing Techniques of Two-Dimensional Layered Materials: A Review. J. Sci. Adv. Mater. Devices 2021, 6, 135–152. [Google Scholar] [CrossRef]
- Madurani, K.A.; Suprapto, S.; Machrita, N.I.; Bahar, S.L.; Illiya, W.; Kurniawan, F. Progress in Graphene Synthesis and Its Application: History, Challenge and the Future Outlook for Research and Industry. ECS J. Solid. State Sci. Technol. 2020, 9, 093013. [Google Scholar] [CrossRef]
- Norimatsu, W. A Review on Carrier Mobilities of Epitaxial Graphene on Silicon Carbide. Materials 2023, 16, 7668. [Google Scholar] [CrossRef]
- Han, D.; Wang, X.; Zhao, Y.; Chen, Y.; Tang, M.; Zhao, Z. High-Quality Graphene Synthesis on Amorphous SiC through a Rapid Thermal Treatment. Carbon 2017, 124, 105–110. [Google Scholar] [CrossRef]
- Huang, H.; Chen, S.; Wee, A.T.S.; Chen, W. 1—Epitaxial Growth of Graphene on Silicon Carbide (SiC). In Graphene; Skákalová, V., Kaiser, A.B., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–26. ISBN 978-0-85709-508-4. [Google Scholar]
- Zhao, J.; Ji, P.; Li, Y.; Li, R.; Zhang, K.; Tian, H.; Yu, K.; Bian, B.; Hao, L.; Xiao, X.; et al. Ultrahigh-Mobility Semiconducting Epitaxial Graphene on Silicon Carbide. Nature 2024, 625, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Endoh, N.; Akiyama, S.; Tashima, K.; Suwa, K.; Kamogawa, T.; Kohama, R.; Funakubo, K.; Konishi, S.; Mogi, H.; Kawahara, M.; et al. High-Quality Few-Layer Graphene on Single-Crystalline SiC Thin Film Grown on Affordable Wafer for Device Applications. Nanomaterials 2021, 11, 392. [Google Scholar] [CrossRef]
- Pedrazzetti, L.; Gibertini, E.; Bizzoni, F.; Russo, V.; Lucotti, A.; Nobili, L.; Magagnin, L. Graphene Growth on Electroformed Copper Substrates by Atmospheric Pressure CVD. Materials 2022, 15, 1572. [Google Scholar] [CrossRef]
- Lee, D.Y.; Nam, J.; Lee, G.Y.; Lee, I.; Jang, A.-R.; Kim, K.S. Conveyor CVD to High-Quality and Productivity of Large-Area Graphene and Its Potentiality. Nano Converg. 2024, 11, 32. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterization, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Dhiman, G.; Kumar, S.; Kumar, R.; Brajpuriya, R. An Improved CVD Design for Graphene Growth and Transfer Improvements. J. Electron. Mater. 2024, 53, 5916–5925. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, S.; Chen, X.; Poduval, G.K.; Stride, J.A. Optimizing the Seeded CVD-Growth of Uniform Graphene Films on Silicon. J. Mater. Sci. 2023, 58, 9434–9445. [Google Scholar] [CrossRef]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene Transfer Methods: A Review. Nano Res. 2021, 14, 3756–3772. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.A.; Wright, G.D. The Past, Present, and Future of Antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Batuman, O.; Britt-Ugartemendia, K.; Kunwar, S.; Yilmaz, S.; Fessler, L.; Redondo, A.; Chumachenko, K.; Chakravarty, S.; Wade, T. The Use and Impact of Antibiotics in Plant Agriculture: A Review. Phytopathology 2024, 114, 885–909. [Google Scholar] [CrossRef]
- Tian, L.; Fang, G.; Li, G.; Li, L.; Zhang, T.; Mao, Y. Metagenomic Approach Revealed the Mobility and Co-Occurrence of Antibiotic Resistomes between Non-Intensive Aquaculture Environment and Human. Microbiome 2024, 12, 107. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, Y.; Gao, Y.; Lin, Z.; Lin, Y.; Lu, Y.; Zhang, L. Antibiotics and Antimycotics in Waste Water Treatment Plants: Concentrations, Removal Efficiency, Spatial and Temporal Variations, Prediction, and Ecological Risk Assessment. Environ. Res. 2022, 215, 114135. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic Removal Processes from Water & Wastewater for the Protection of the Aquatic Environment—A Review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Samrot, A.V.; Wilson, S.; Sanjay Preeth, R.S.; Prakash, P.; Sathiyasree, M.; Saigeetha, S.; Shobana, N.; Pachiyappan, S.; Rajesh, V.V. Sources of Antibiotic Contamination in Wastewater and Approaches to Their Removal—An Overview. Sustainability 2023, 15, 12639. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide Traceability of Antibiotic Residues from Livestock in Wastewater and Soil: A Systematic Review. Animals 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Catalán, N.; Mas-Pla, J.; Čelić, M.; Petrović, M.; Farré, M.J. Groundwater Antibiotic Pollution and Its Relationship with Dissolved Organic Matter: Identification and Environmental Implications. Environ. Pollut. 2021, 289, 117927. [Google Scholar] [CrossRef]
- Suyamud, B.; Chen, Y.; Quyen, D.T.T.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial Resistance in Aquaculture: Occurrence and Strategies in Southeast Asia. Sci. Total Environ. 2024, 907, 167942. [Google Scholar] [CrossRef] [PubMed]
- Rayan, R.A. Pharmaceutical Effluent Evokes Superbugs in the Environment: A Call to Action. Biosaf. Health 2023, 5, 363–371. [Google Scholar] [CrossRef]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of Antibiotic Residues and Antibiotic-Resistant Bacteria in Effluents of Pharmaceutical Manufacturers and Other Sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A Systematic Review of Industrial Wastewater Management: Evaluating Challenges and Enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Pal, N.; Sarma, D.K.; Kumar, M. Antibiotic Disposal Challenges in India: Investigating Causes and Effects. Environ. Monit. Assess. 2024, 196, 325. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Radmehr, S.; Hosseini Sabzevari, M.; Ghaedi, M.; Ahmadi Azqhandi, M.H.; Marahel, F. Adsorption of Nalidixic Acid Antibiotic Using a Renewable Adsorbent Based on Graphene Oxide from Simulated Wastewater. J. Environ. Chem. Eng. 2021, 9, 105975. [Google Scholar] [CrossRef]
- Kumar, R.; Samykano, M.; Ngui, W.K.; Pandey, A.K.; Kalidasan, B.; Kadirgama, K.; Tyagi, V.V. Investigation of Thermal Performance and Chemical Stability of Graphene Enhanced Phase Change Material for Thermal Energy Storage. Phys. Chem. Earth Parts A/B/C 2022, 128, 103250. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Ma, X.; Jiang, B.; Chen, R.; Feng, H.; Xia, Y. Efficient Electrochemical Oxidation of Antibiotic Wastewater Using a Graphene-Loaded PbO2 Membrane Anode: Mechanisms and Applications. Environ. Res. 2024, 259, 119517. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of Antibiotics, Antibiotic-Resistant Bacteria and Their Associated Genes by Graphene-Based TiO2 Composite Photocatalysts under Solar Radiation in Urban Wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Solís, R.R.; Dinc, Ö.; Fang, G.; Nadagouda, M.N.; Dionysiou, D.D. Activation of Inorganic Peroxides with Magnetic Graphene for the Removal of Antibiotics from Wastewater. Environ. Sci. Nano 2021, 8, 960–977. [Google Scholar] [CrossRef]
- Dickson, K.; Yeung, C.A. PRISMA 2020 Updated Guideline. Br Dent J 2022, 232, 760–761. [Google Scholar] [CrossRef]
- López-Sánchez, J.A.; Patiño-Vanegas, J.C.; Valencia-Arias, A.; Valencia, J. Use and Adoption of ICTs Oriented to University Student Learning: Systematic Review Using PRISMA Methodology. Cogent Educ. 2023, 10, 2288490. [Google Scholar] [CrossRef]
- Scopus|Abstract and Citation Database|Elsevier. Available online: https://www.elsevier.com/en-in/products/scopus (accessed on 24 September 2024).
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimized Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai-Yazdi, F.-S.; Ebrahimian Pirbazari, A.; Esmaeili Khalilsaraei, F.; Asasian Kolur, N.; Gilani, N. Photocatalytic Treatment of Tetracycline Antibiotic Wastewater by Silver/TiO2 Nanosheets/Reduced Graphene Oxide and Artificial Neural Network Modelling. Water Environ. Res. 2020, 92, 662–676. [Google Scholar] [CrossRef]
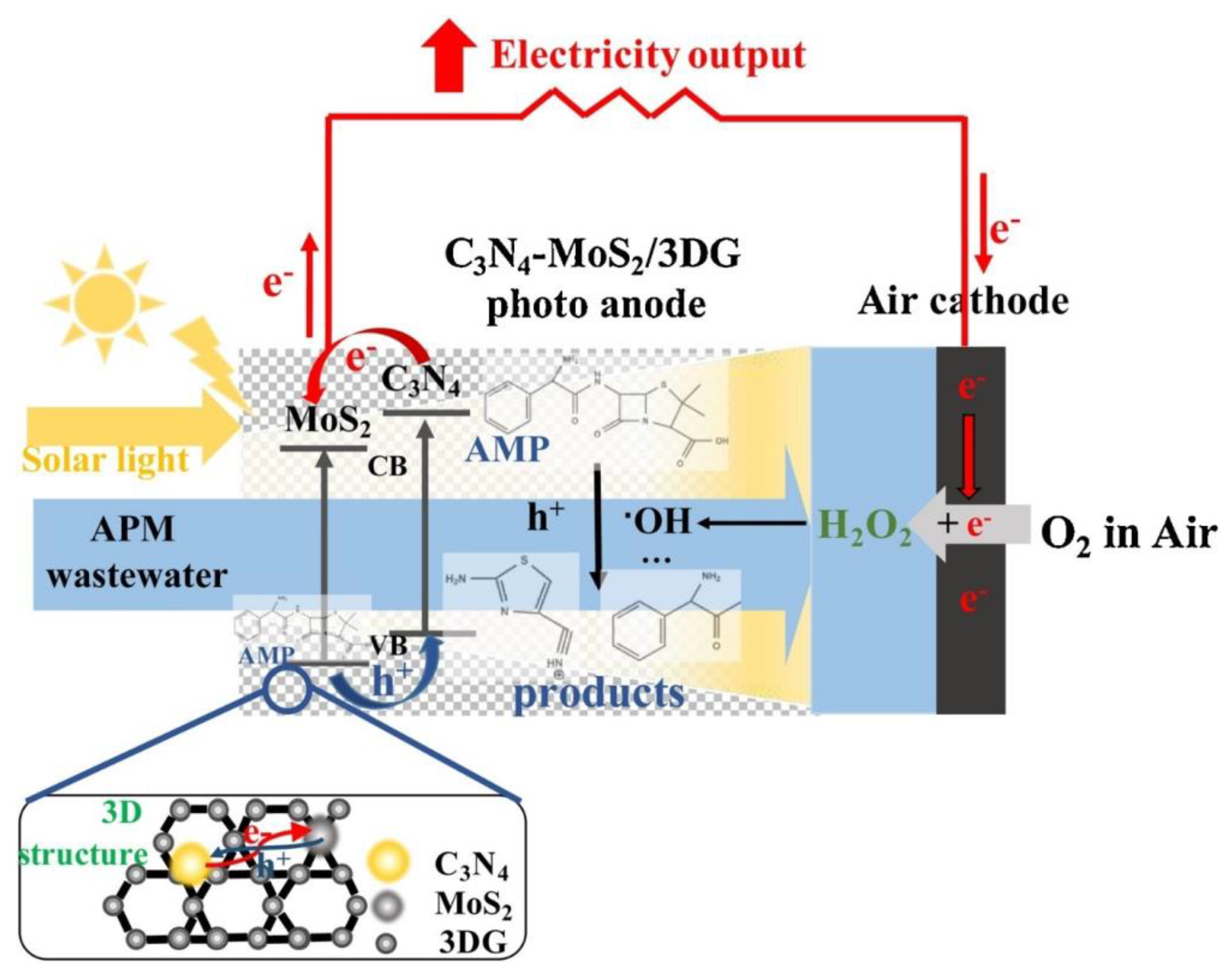
- Yang, W.; Wang, Y. Enhanced Electron and Mass Transfer Flow-through Cell with C3N4-MoS2 Supported on Three-Dimensional Graphene Photoanode for the Removal of Antibiotic and Antibacterial Potencies in Ampicillin Wastewater. Appl. Catal. B Environ. 2021, 282, 119574. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, J.; Zhang, Y.; Li, Y.; Liao, Y.; Zhang, H. Graphene Oxide Structure-Oriented NM88B/GO/SA Aerogel for Highly Efficient Degradation of Dye and Antibiotic Wastewater. J. Polym. Environ. 2024, 32, 2091–2104. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Chen, R.; Pham-Huy, C.; Hui, X.; He, H. Adsorptive Removal of Trace Sulfonamide Antibiotics by Water-Dispersible Magnetic Reduced Graphene Oxide-Ferrite Hybrids from Wastewater. J. Chromatogr. B 2016, 1029–1030, 106–112. [Google Scholar] [CrossRef]
- Behzadi, A.; Yazdanbakhsh, A. Synthesis and Characterization of Modified Resorcinol Formaldehyde Aerogel by Graphene/m-Phenylenediamine as a Novel Adsorbent to Remove Tetracycline Antibiotics from Wastewater. J. Water Environ. Nanotechnol. 2022, 7, 44–54. [Google Scholar] [CrossRef]
- Kogut, I.; Armbruster, F.; Polak, D.; Kaur, S.; Hussy, S.; Thiem, T.; Gerhardts, A.; Szwast, M. Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants. Processes 2022, 10, 2051. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Orrell-Trigg, R.; Truong, Y.B.; Cozzolino, D.; Truong, V.K.; Chapman, J. Wastewater Depollution of Textile Dyes and Antibiotics Using Unmodified and Copper Oxide/Zinc Oxide Nanofunctionalised Graphene Oxide Materials. Environ. Sci. Adv. 2022, 1, 456–469. [Google Scholar] [CrossRef]
- Taleb, M.A.; Kumar, R.; Barakat, M.A. Multifunctional Carboxymethyl Cellulose/Graphene Oxide/Polyaniline Hybrid Thin Film for Adsorptive Removal of Cu(II) and Oxytetracycline Antibiotic from Wastewater. Int. J. Biol. Macromol. 2023, 253, 126699. [Google Scholar] [CrossRef] [PubMed]
- AbuZaid, M.; Pandey, R.P.; Hasan, S.W. Efficient Antibiotic Remediation from Wastewater Using a Lamellar Free-Standing 2D Graphene Oxide/Ti3C2Tx Hybrid Membrane. Surf. Interfaces 2024, 49, 104431. [Google Scholar] [CrossRef]
- Shaker, M.A.; Alshitari, W.H.; Basha, M.T.; Aly, N.A.; Asim, M.; Albishri, H.M.; Bhawani, S.A.; Yakout, A.A. Synergetic Impact of Copper Nanoparticles and Polyaniline Reinforced Graphene Oxide Nanocomposite on the Sequestration of Tetracycline Antibiotic from Milk and Wastewaters Samples. Mater. Today Commun. 2024, 38, 107869. [Google Scholar] [CrossRef]
- Safian, M.T.; Umar, K.; Mohamad Ibrahim, M.N. Synthesis and Scalability of Graphene and Its Derivatives: A Journey towards Sustainable and Commercial Material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Lv, H.; Du, M.; Li, Z.; Xiao, L.; Zhou, S. Cost Optimization of Graphene Oxide-Modified Ultra-High-Performance Concrete Based on Machine Learning Methods. Inorganics 2024, 12, 181. [Google Scholar] [CrossRef]
- Xiao, Y.; Pang, Y.X.; Yan, Y.; Qian, P.; Zhao, H.; Manickam, S.; Wu, T.; Pang, C.H. Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, Challenges, and Perspectives. Adv. Sci. 2023, 10, 2205292. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Costinas, C.; Salagean, C.A.; Cotet, L.C.; Baia, M.; Todea, M.; Magyari, K.; Baia, L. Insights into the Stability of Graphene Oxide Aqueous Dispersions. Nanomaterials 2022, 12, 4489. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Liu, C.; Zhang, J.; Wang, L.; Luo, J.-L.; Fu, X.-Z. Roll-to-Roll Scale Fabrication of High-Performance Graphene-Assembled Film Cathode Current Collectors for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2023, 11, 13483–13491. [Google Scholar] [CrossRef]
- Su, L.; Ning, Y.; Ma, Z.; Zhang, Y.; Liu, C.; Zhang, Y.; Miao, L.; Zhou, J.; Wu, B.; Qian, J. Polypyrrole-Reinforced N,S-Doping Graphene Foam for Efficient Solar Purification of Wastewater. Sol. RRL 2021, 5, 2100210. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.Q.; Drmosh, Q.A.; Onaizi, S.A. Enhancement of Bisphenol a Removal from Wastewater via the Covalent Functionalization of Graphene Oxide with Short Amine Molecules. Case Stud. Chem. Environ. Eng. 2022, 6, 100233. [Google Scholar] [CrossRef]
- Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research Progress of Graphene-Based Nanomaterials for the Environmental Remediation. Chin. Chem. Lett. 2020, 31, 1462–1473. [Google Scholar] [CrossRef]
- Rana, K.; Kaur, H.; Singh, N.; Sithole, T.; Siwal, S.S. Graphene-Based Materials: Unravelling Its Impact in Wastewater Treatment for Sustainable Environments. Next Mater. 2024, 3, 100107. [Google Scholar] [CrossRef]
- Ågerstrand, M.; Josefsson, H.; Wernersson, A.-S.; Larsson, D.G.J. Opportunities to Tackle Antibiotic Resistance Development in the Aquatic Environment through the Water Framework Directive. Ambio 2023, 52, 941–951. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Ma, J.; Sun, Y. Study on the Application of Shell-Activated Carbon for the Adsorption of Dyes and Antibiotics. Water 2022, 14, 3752. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, G.; Xie, H.; Zhai, Y.; Li, X. Advances and Solutions in Biological Treatment for Antibiotic Wastewater with Resistance Genes: A Review. J. Environ. Manag. 2024, 368, 122115. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to Improve the Adsorption Properties of Graphene-Based Adsorbent towards Heavy Metal Ions and Their Compound Pollutants: A Review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef] [PubMed]
- What Factors Impact Graphene Cost? (Updated 2024) | INN. Available online: https://investingnews.com/daily/tech-investing/nanoscience-investing/graphene-investing/graphene-cost/ (accessed on 28 September 2024).
- Ding, X.; Pu, Y.; Tang, M.; Zhang, T. Environmental and Health Effects of Graphene-Family Nanomaterials: Potential Release Pathways, Transformation, Environmental Fate and Health Risks. Nano Today 2022, 42, 101379. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. π-π Stacking Derived from Graphene-like Biochar/g-C3N4 with Tunable Band Structure for Photocatalytic Antibiotics Degradation via Peroxymonosulfate Activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Škulj, S.; Rožman, M. Adsorption of a Wide Variety of Antibiotics on Graphene-Based Nanomaterials: A Modelling Study. Chemosphere 2022, 296, 134010. [Google Scholar] [CrossRef]
- Yasmin, S.; Azam, M.G.; Hossain, M.S.; Akhtar, U.S.; Kabir, M.H. Efficient Removal of Ciprofloxacin from Aqueous Solution Using Zn–C Battery Derived Graphene Oxide Enhanced by Hydrogen Bonding, Electrostatic and π-π Interaction. Heliyon 2024, 10, e33317. [Google Scholar] [CrossRef]
- Liu, Y.; Biswas, B.; Hassan, M.; Naidu, R. Green Adsorbents for Environmental Remediation: Synthesis Methods, Ecotoxicity, and Reusability Prospects. Processes 2024, 12, 1195. [Google Scholar] [CrossRef]



| 1. | Are the aims and objectives explicitly articulated? |
| 2. | Is the reporting structured in a logical, cohesive, and coherent manner? |
| 3. | Is the proposed technique described in sufficient detail? |
| 4. | Is the research methodology aligned with the study’s objectives? |
| 5. | Are the methods for data collection clearly and thoroughly explained? |
| 6. | Do the explanations and conclusions rely appropriately on the data presented? |
| 7. | Does the study make a meaningful contribution to the body of knowledge? |
| 8. | Has the stated aims and objectives been achieved? |
| 9. | Is the research process clearly and comprehensively documented? |
| 10. | Can the study be reproduced based on the information provided? |
| Graphene Type | Target | Type of Treatment | Results Achieved | Reference |
|---|---|---|---|---|
| Reduced Graphene Oxide | sulfamethoxazole (SMX), erythromycin (ERY), and clarithromycin (CLA) | Photocatalytic degradation | Degradation efficiency SMX (87 ± 4%), ERY (84 ± 2%), CLA (86 ± 5%) | Karaolia et al. [87] |
| Reduced Graphene Oxide | Tetracycline | Photocatalytic degradation | Degradation efficiency 52.56% | Yazdi et al. [93] |
| Graphene | Ampicillin | Photocatalytic degradation | Removal efficiency 74.6% | Yang et al. [94] |
| Reduced Graphene Oxide | Chloramphenicol sodium succinate | Photocatalytic degradation | Removal efficiency 80% | Ivan et al. [22] |
| Graphene Oxide | Tetracycline | Photocatalytic degradation | Removal efficiency > 99% | Lin et al. [95] |
| Reduced Graphene Oxide | Sulfonamide | Adsorption | Recoveries range from 89.1 and 101.7% | Wu et al. [96] |
| Graphene Oxide | Nalidixic acid | Adsorption | Adsorption capacity 277.79 mg/g (Removal efficiency 92%) | Radmehr et al. [84] |
| Graphene | Tetracycline | Adsorption | Removal efficiency 93.3% | Behzadi et al. [97] |
| Graphene | Tetracycline | Adsorption | NA | Kogut et al. [98] |
| Graphene Oxide | Amoxicillin and Tetracycline | Adsorption | Adsorption capacity 405 mg/g and 552 mg/g for Amoxicillin and Tetracycline, respectively | Rajapaksha et al. [99] |
| Graphene Oxide | Oxytetracycline | Adsorption | Adsorption capacity 180.240 mg/g (Removal efficiency > 90%) | Taleb et al. [100] |
| Graphene Oxide | Tetracycline | Adsorption | Removal efficiency 99.8% | AbuZaid et al. [101] |
| Graphene Oxide | Tetracycline | Adsorption | Adsorption capacity 434.78 mg/g (Removal efficiency 99.6%) | Shaker et al. [102] |
| Graphene | Sulfamethoxazole, Norfloxacin, Tetracycline, Flumequine | Catalytic activation of inorganic peroxides | NA | Solís et al. [88] |
| Graphene | Benzylpenicillin sodium | Electrochemical oxidation | Removal efficiency 99.34% | Peng et al. [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, J.; Hussain, C.M. Advanced Graphene-Based Technologies for Antibiotic Removal from Wastewater: A Review (2016–2024). C 2024, 10, 92. https://doi.org/10.3390/c10040092
Sengupta J, Hussain CM. Advanced Graphene-Based Technologies for Antibiotic Removal from Wastewater: A Review (2016–2024). C. 2024; 10(4):92. https://doi.org/10.3390/c10040092
Chicago/Turabian StyleSengupta, Joydip, and Chaudhery Mustansar Hussain. 2024. "Advanced Graphene-Based Technologies for Antibiotic Removal from Wastewater: A Review (2016–2024)" C 10, no. 4: 92. https://doi.org/10.3390/c10040092
APA StyleSengupta, J., & Hussain, C. M. (2024). Advanced Graphene-Based Technologies for Antibiotic Removal from Wastewater: A Review (2016–2024). C, 10(4), 92. https://doi.org/10.3390/c10040092









