1. Introduction
Graphene oxide (GO) [
1] has emerged as a key material in the field of materials science [
2], owing to its exceptional properties such as high mechanical strength, electrical conductivity, and optical transparency [
3]. These features position GO as an ideal candidate for numerous advanced applications [
2], including optoelectronics, energy storage, transparent conductive films (TCFs), and membrane applications [
4,
5]. Consequently, there has been extensive research into the synthesis, deposition, and characterization of GO thin films to optimize their performance for these applications [
6]. The author has also contributed significantly to this field, with several published studies on GO films [
7].
Among the various techniques used for characterizing the optical properties of GO thin films, Variable Angle Spectroscopic Ellipsometry (VASE) [
8] stands out due to its precision and ability to provide comprehensive data.
This study shifts the focus to a more accessible yet effective method of depositing GO films—drop casting [
9]—while maintaining reference standards through the study of graphene produced by Chemical Vapor Deposition (CVD) [
10] and GO films deposited by electrophoretic deposition (EPD) [
11]. The decision to compare these methods by VASE arises from the need to understand the practical and performance trade-offs between a simple, widely accessible technique like drop casting and more advanced methods such as EPD and CVD.
Despite its ease of use, the drop casting method for GO films has not been thoroughly explored, particularly in terms of its impact on the optical properties of the films when characterized using VASE. Additionally, isopropanol and water were selected as solvents for the drop casting process due to their different evaporation rates and interactions with GO, which will have significant impacts on film formation. This comparison aims to thoroughly examine how solvent choice and deposition methods impact the final film quality, addressing a significant gap in the existing literature. By exploring these factors, the study intends to deepen the understanding of GO films and expand their potential applications across various technological fields.
2. Experimental Methods
A monolayer of graphene was purchased from Graphenea on 300 nm SiO2 (San Sebastián, Spain) to establish a reliable reference for the optical characterization of GO films. This graphene monolayer was produced by CVD on a copper catalyst and subsequently transferred to a SiO2/Si substrate. The physical and electrical properties of this reference film included a transparency greater than 97%, a coverage exceeding 95%, a theoretical thickness of 0.345 nm, and a sheet resistance of 450 ± 40 Ohms per square for a 1 cm × 1 cm area.
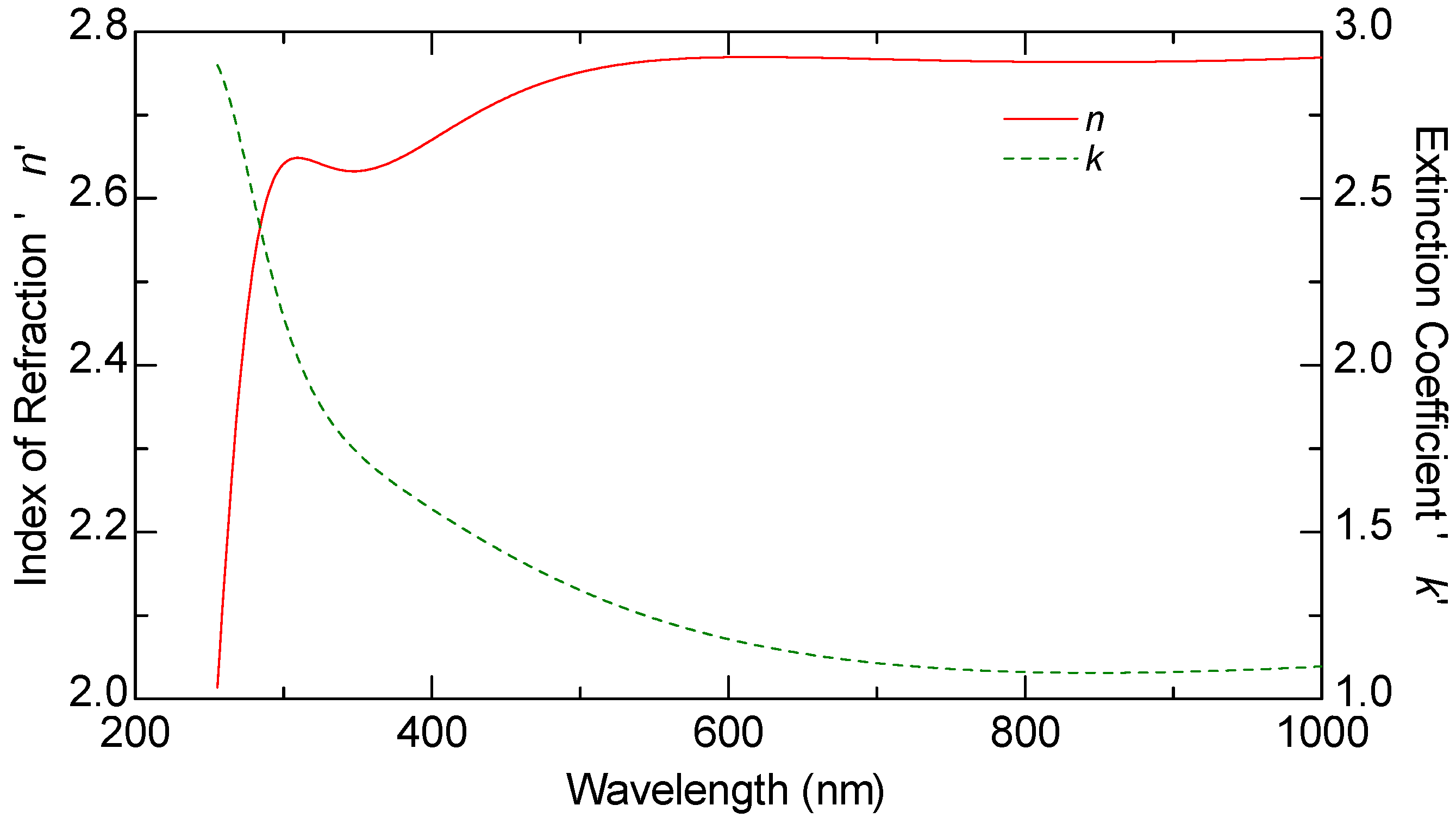
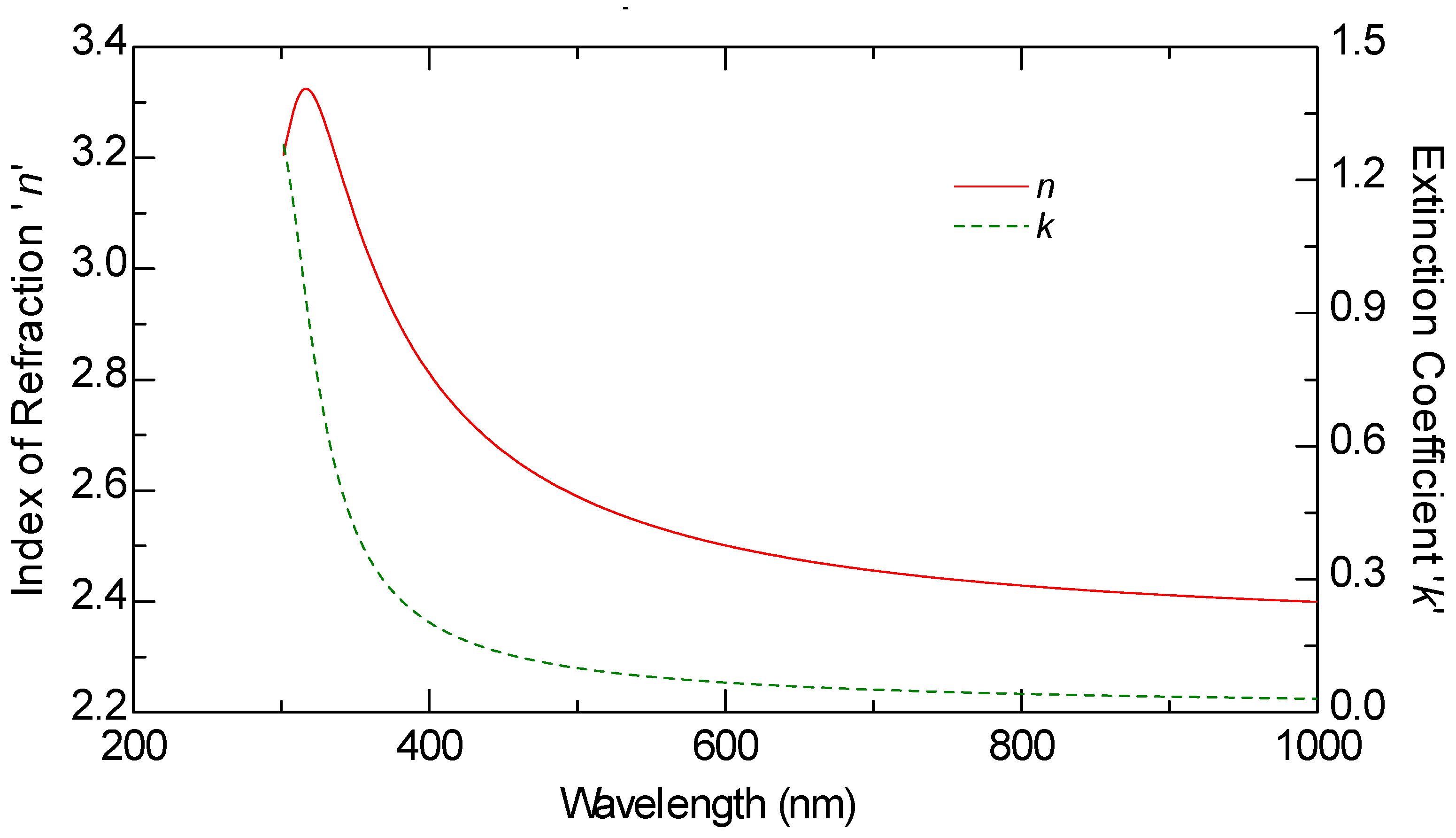
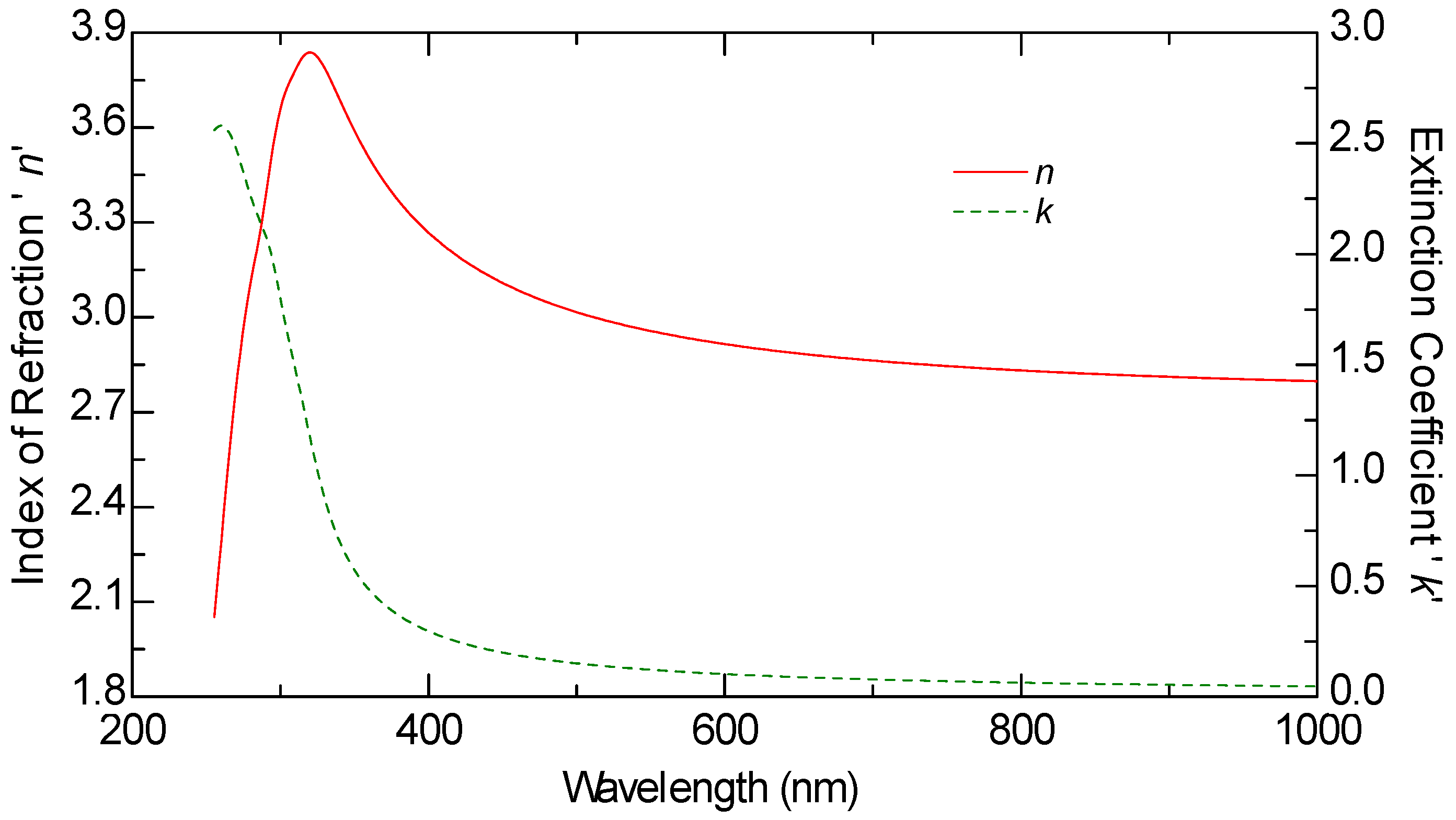
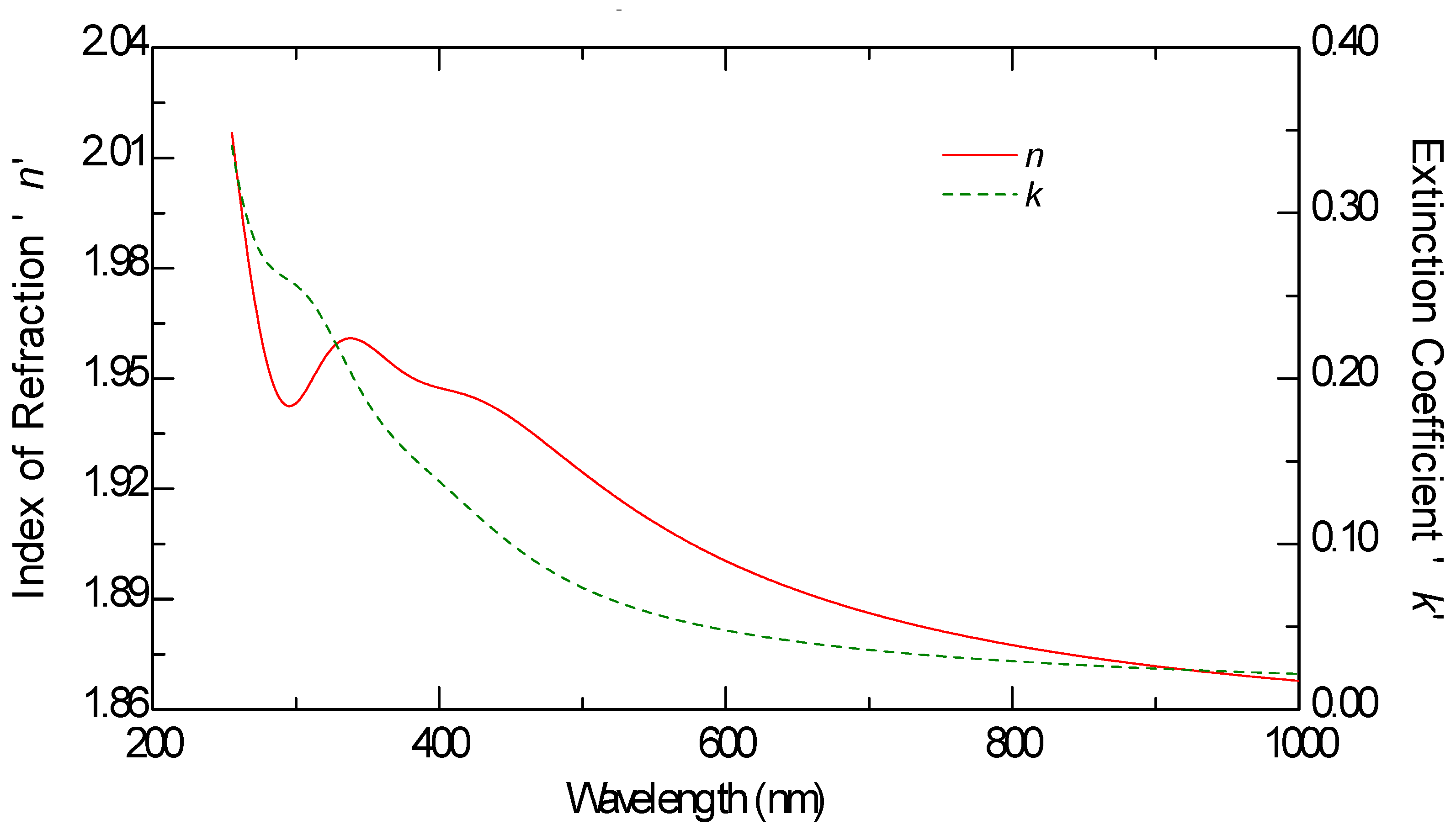
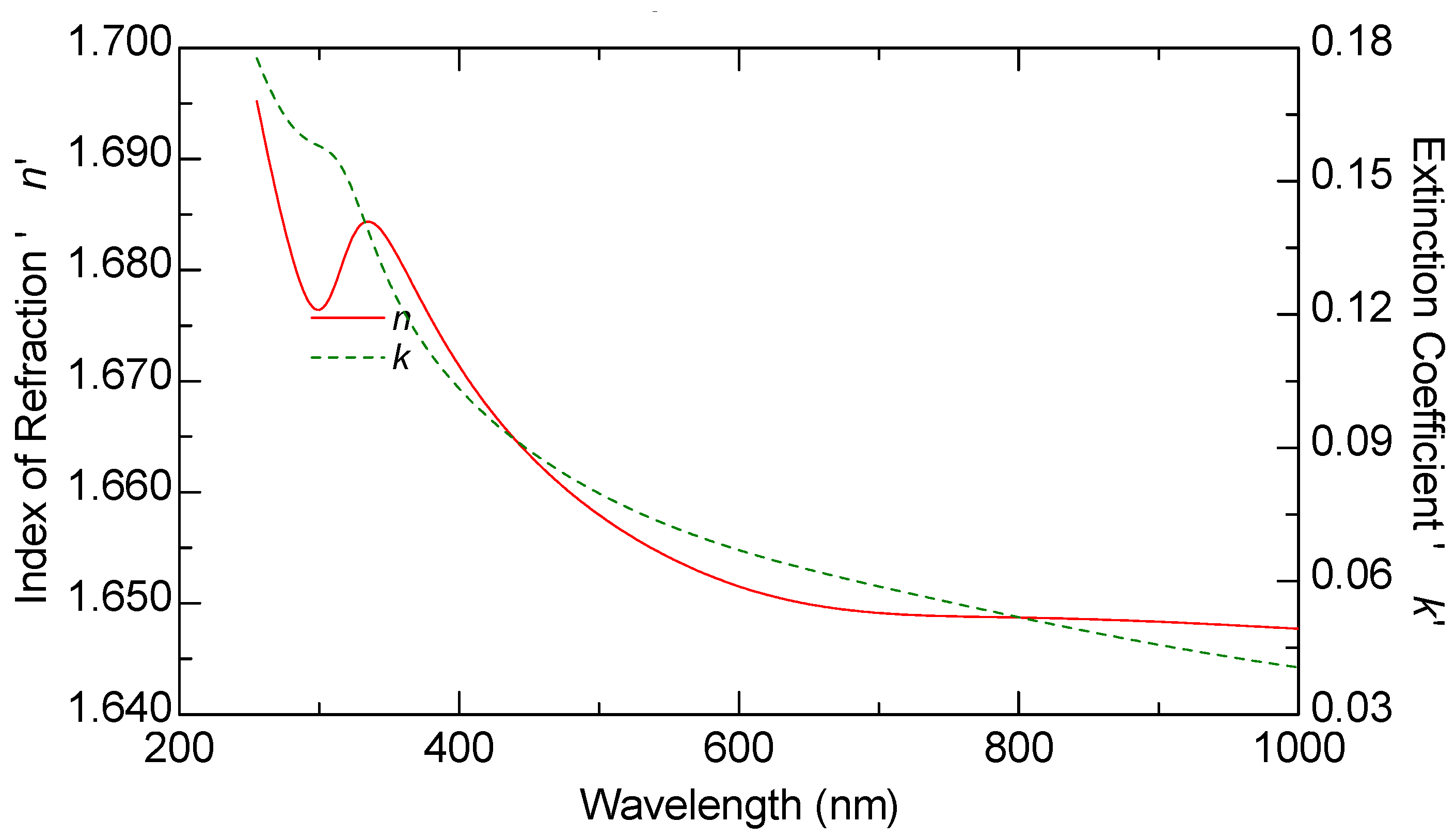
Ellipsometric measurements were conducted on all samples, including the graphene monolayer, to obtain their optical constants. The ellipsometric angles, denoted as
ψ and Δ, were measured using a J.A. Woollam M2000 F (Woollam Co., Lincoln, NE, USA) rotating compensator ellipsometer (RCE) over the wavelength range of 250 nm to 1000 nm. These measurements were carried out at varying angles of incidence between 60 and 70 degrees, in increments of 5 degrees. The optical constants n and k of graphene, as well as those of the other samples, were derived from multiple measurements taken at these different angles, rather than a single fixed incidence, allowing for more reliable modeling results.
The thickness of all samples, including the graphene layer, was estimated through optical modeling using the VASE model. The ellipsometric spectra were analyzed by modeling each sample as a stack of layers, with each layer characterized by its thickness, complex refractive index, and composition. These parameters were determined by fitting the experimental data using the Levenberg–Marquardt algorithm [
12].
The mathematical model used in this study, implemented via the WVASE32
® software (version 3.690) [
13], employs a collection of Lorentz oscillators [
8] to represent the optical behavior of the material. The complex dielectric function
ε is expressed as
In the Lorentz model,
is the energy of the incident photons,
is the real part of the dielectric function when
,
is the strength expressed in
,
is the broadening in
, and
is the central energy of the
k-th oscillator.
also represents the percentage contribution of oscillator
to the whole system. In this model, unknown parameters of film thickness and Lorentz parameters for GO are defined as fitting variables. The thickness of all other reference samples was GO films made by electrophoretic deposition (EPD) [
14]. EPD is a two-step process where charged particles in a liquid are moved towards an electrode by applying an electric field, followed by the deposition of these particles on the electrode. EPD was only applicable to electrically conductive substrates such as copper, nickel, aluminum, stainless steel, and titanium.
The cathode used in this experiment was platinum and the anode was titanium sputtered using DC magnetron sputtering with a power of 100 W.
GO concentrations of 0.25 mg/mL were used, with a direct current (DC) voltage of 10 V applied for deposition times ranging from 1 to 10 min. The optimal deposition time was found to be between 1 and 2 min, as longer deposition times led to randomly stacked GO sheets. Ellipsometry was performed on the EPD samples in the wavelength range of 250–1000 nm.
The drop casting method [
15] produced noteworthy results in the preparation of GO thin films. In this method, glass surfaces were used as substrates. To enhance the hydrophilicity of the glass, the slides were immersed in a “Piranha solution”, which typically consists of a 3:1 mixture of concentrated sulfuric acid and 30% hydrogen peroxide. After treatment with the Piranha solution for a day, the glass substrates were thoroughly dried using argon gas. GO multilayer films were then prepared by pipetting the GO solution onto the treated glass substrates and heating them at 80 °C until the film achieved a relatively homogeneous appearance, spreading uniformly across the surface. Several concentrations of GO solutions, ranging from 0.017 mg/mL to 0.5 mg/mL, were tested in different solvents, including water and isopropanol. The concentration of 0.1 mg/mL was chosen for reporting because, at this concentration, the resulting GO films were the most uniform. A significant challenge encountered during this process was the exfoliation of GO in high boiling point solvents, which posed difficulties in the deposition of individual flakes. The slow evaporation rate of these solvents allowed sufficient time for GO flakes to aggregate, leading to non-uniform films.
To mitigate this challenge, the exfoliation of GO at relatively high concentrations in low boiling point solvents, such as isopropanol (boiling point: 82.6 °C), was investigated. These solvents facilitated more effective exfoliation and deposition, reducing the aggregation of GO flakes and resulting in more uniform films. Dynamic Light Scattering (DLS) measurements were performed to further characterize the size distribution of the GO particles. The results indicated an average particle size distribution ranging between 500 and 700 nm, as shown in
Figure S1 (Supplementary Materials). This analysis supports the discussion by providing insights into the particle dimensions, which influence the uniformity and deposition quality of the GO films. Additionally, the energy band gap of GO films was calculated from the transmittance spectra, with representative results presented in
Figure S2 (Supplementary Materials). These calculations provide critical information about the optical properties of GO films in different solvents (water and isopropanol).
4. Conclusions
This study provides a comparative optical analysis of graphene oxide (GO) films prepared through drop casting, with reference samples of GO films deposited by electrophoretic deposition (EPD) and graphene produced by chemical vapor deposition (CVD). The analysis using Variable Angle Spectroscopic Ellipsometry (VASE) highlighted significant differences in the uniformity and optical properties of the films, largely influenced by the deposition method and solvent choice.
CVD graphene exhibited the highest uniformity in optical properties, setting the standard for film quality. GO films prepared via EPD, particularly after annealing, also demonstrated consistent optical characteristics, closely aligning with the quality seen in CVD graphene. In contrast, GO films produced through drop casting displayed greater variability in uniformity, which was notably dependent on the solvent used. Lower boiling point solvents, such as isopropanol, resulted in films with lower refractive indices, suggesting a less dense structure compared to films created using water-based solutions. The lower refractive index in GO films cast from isopropanol films can be advantageous in certain applications because it allows for greater optical transparency, which is particularly important in devices where minimizing light reflection is critical. For example, in anti-reflective coatings or transparent conductive films (TCFs), a lower refractive index reduces the mismatch between the film and air, thereby minimizing light reflection and enhancing the transmission of light through the material.
The non-uniformity observed in drop-cast films at elevated temperatures can be attributed to the rapid evaporation of solvents like isopropanol and water, which reduces the time available for GO flakes to self-assemble into a uniform layer. At higher temperatures, this process is further accelerated, leading to the formation of films with varying thicknesses and densities. The solvent choice therefore plays a crucial role here; isopropanol, with its lower boiling point, evaporates more quickly than water, leading to less time for the flakes to align properly, which may result in a less dense film structure.
Water, with its higher boiling point, allows for a slower evaporation process, which can improve uniformity but also introduces the risk of solvent trapping if the temperature is not sufficiently high to drive off all residual water.
The findings presented here highlight the importance of optimizing both the solvent and the deposition technique to achieve the desired GO film quality.
While drop casting is a simple and accessible technique, its application in large-scale production requires careful consideration, especially at higher temperatures. Future studies could investigate how to scale up this process without compromising GO film quality, potentially by integrating automated deposition systems or by combining drop casting with other deposition methods to achieve better control over film thickness and uniformity at elevated temperatures.