Abstract
Trace elements such as cobalt (Co), molybdenum (Mo), and zinc (Zn) play necessary roles in different biological functions. Co is a microelement that influences the vascular system. Mo works as an enzymatic cofactor of three enzymes (aldehyde oxidase, sulfite oxidase, and xanthine oxidase dehydrogenase). However, these elements are difficult to detect, since the analytical methods developed have a high cost, which restrict their applicability. In this sense, fluorescent sensors are an alternative for detecting trace elements, such as Mo4+ ions. Herein, a new multichannel trace elements sensor has been proposed to detect Mo entities. In this sense, two different N-CQDs were synthesized and fully characterized. The N-CQDs presented quantum yield values of 25.93% and 6.02% and excellent solubility in water. Also, a mixture of these two carbon-based nanoparticles was used to identify and to quantify Mo in water between seven different trace elements. The method was found to reach 1.28 and 3.88 ppm for limit of detection (LOD) and quantification (LOQ), respectively. To further verify the potential of the detection platform, the multichannel sensor was applied to identify the different concentrations of metal ions (Fe2+, Co2+, Mn2+, Cu2+, Zn2+, Mg2+, and Mo4+) in water. The data matrix was treated using different algorithms, such as K-Means and Discriminant Analysis (DA). The detection strategy has successfully identified the molybdenum ions at 5 ppm. This result shows the potential application of a multichannel sensor toward the detection of Mo entities, since it is comparable with the molybdenum test already available on the market.
1. Introduction
Trace elements are essential for different physiological functions. Some examples include copper (Cu), molybdenum (Mo), and cobalt (Co). For instance, Cu is vital for many living organisms and plays an important role in physiological and pathological processes, such as bone formation and cellular respiration [1]. Mo acts as an enzymatic cofactor of several enzymes (such as aldehyde oxidase, sulfite oxidase, and xanthine oxidase dehydrogenase), which catalyzes several substrates’ hydroxylation [2]. However, abnormal levels of these elements in the human body pose a severe threat, since it may cause distinct diseases. An excessive intake of Co2+ can lead to serious health issues, including blindness and deafness [3]. So, it is clear the demand for the detection of trace elements. Molybdenum ions are easily detected due to their interactions with carbonaceous matrices. For example, Amin and collaborators developed a polymeric membrane to incorporate the metal. The authors attribute the interaction mechanism, which resulted in changes from the optical signal to electrostatic attraction [4]. Consequently, many methods have been developed to detect these kinds of chemical entities. In this sense, inductively coupled plasma mass spectroscopy (ICP-MS), atomic absorption spectroscopy/emission spectroscopy, potentiometry, and voltammetry may be mentioned. These techniques are found to be costly, technically complicated, time-consuming, and require expensive instruments. Therefore, the development of an optional method is imperative. In this context, fluorescent sensors emerged as an alternative strategy for metal detection owing to their simplicity, high sensitivity, good selectivity, and rapid response [5,6].
Carbon quantum dots (CQDs) are a new class of fluorescent nanoparticles (NPs) that have attracted the interest of many researchers worldwide. These carbon-based nanomaterials display biocompatibility, chemical stability, green synthesis, as well as surface modifications with different functional groups [7,8,9]. Furthermore, it is possible to adjust their structural composition to improve spectral properties [10]. Currently, numerous reports have been focused on nitrogen, boron, sulfur, phosphorus, or silicon doped-CQDs [11,12]. By introducing atomic impurities into CQDs, their electronic structure can be adjusted, generating n-type or p-type carriers [13]. Therefore, due to the versatility of the CQDs, their applications have broadened in the sensing field, where it was already used to detect organic molecules (e.g., quinolinic acid in human serum) [14], and inorganics (boric acid and hydroxylamine hydrochloride) [15]. Also, cationic (e.g., Fe3+ and Cu2+) [16,17] and anionic species (NO2− in meat) [18] were detected. However, identifying the target analyte is highly desired, since it would allow for a fast decision toward the best medical treatment. Therefore, in recent years, the CQDs were considered to be excellent candidates to develop an array-based strategy to discriminate distinct analytes like proteins [19,20], natural amino acids [21], pesticides [22], food additives [23], antibiotics [24], phosphate anions [25], metallic ions [26,27], and others [28].
Sensor arrays are based on the “chemical nose-tongue” system, defined as an ensemble of receptors (for instance, synthetic molecules and/or nanomaterials), to mimic mammalian olfactory systems [29]. A “nose”-based strategy developed using CQDs provides differential events to create a unique fluorescent pattern, which is further analyzed using computational tools [30,31] to discriminate/classify the target analytes. This type of strategy was developed based on electronic nose and colorimetric sensors, which presented impressive detection results [32,33]. Thereby, this kind of methodology may differentiate a broad range of analytes in a complex matrix using relatively few single receptors to generate fingerprint-like signals [34]. In addition to these advantages, the strategy may be time-consuming, which is undesired. So, a multichannel sensing [35,36,37] strategy came out to surpass this difficulty. The combination of different receptors creates a cross-reactive array able to provide multiple events by performing one single measurement. Therefore, it is possible to reduce the time of analysis, as well as the volume used. Also, it allows us to increase the sample throughput. Herein, we developed a multichannel sensing strategy using two different types of N-based CQDs to identify and quantify trace elements, such Mo4+ ions. By using our methodology, the two “nose”-based receptors were able to identify the molybdenum ions with 100% of accuracy.
2. Materials and Methods
2.1. Materials
Citric acid anhydrous (CA, 99.5%, MQuant®, Darmstadt, Germany), urea (99.5%, Aldrich, Darmstadt, Germany) and N,N-Dimethylformamide (DMF, 99.5%, MQuant®, Darmstadt, Germany), Sodium phosphate monobasic (NaH2PO4, 99%, Vetec, Darmstadt, Germany), sodium phosphate dibasic heptahydrate (Na2HPO4, 99%, Vetec, Darmstadt, Germany), ethylenediamine (98%, Vetec, Darmstadt, Germany), ultra-high Milli-Q water, Darmstadt, Germany (resistivity of 18.2 MΩ.cm−1), magnesium chloride hydrate (MgCl2.H2O, 99%), manganese chloride (MnCl2, 99%), copper (II) sulfate pentahydrate (CuSO4.5H2O, 99%), zinc sulfate heptahydrate (ZnSO4.7H2O, 99%), iron (II) chloride hexahydrate (FeCl2.6H2O, 99%), and cobalt chloride dihydrate (CoCl2.2H2O, 99%) were purchased from Vetec, Darmstadt, Germany, Molybdenum solution 1000 ppm (ACROS ORGANICS, Waltham, MA, USA).
2.2. Apparatus
Regarding CQDs characterization, the optical properties were investigated by acquiring the UV–Vis spectrum in the spectrophotometer Uv-Vis Shimadzu UV-2600, Kyoto, Japan. The fluorescence spectra were obtained in the Shimadzu RF-6000 fluorophotometer, Kyoto, Japan. The structural-functional groups on the surface were studied through X-ray photoelectron spectroscopy (XPS) (Physical Electronics Quantum 2000 spectrometer) using a monochromatic Al Kα excitation at a spot size of 10 mm with a pass energy of 46.95 eV, Turku-Finland and FT-IR VERTEX 70 V, Columbus, OH, USA, and Raman Spectroscopy investigated the carbon structure of the nanomaterials. The Raman Spectrum was recorded in a range of 1000–3000 cm−1 using a Raman HORIBA spectrometer, Columbus, OH, USA. The spectral excitation was performed in a laser using a 785 nm line. The Atomic Force Microscopy (AFM) image was obtained using an Asphalt Research microscope of the MFP-3D AFM type, Zurich, Switzerland.
2.3. Synthesis of N-CQDs
Initially, the first N-doped-CQD (labeled as CQD-NH2) was synthesized according to the well-described methodology of Wang et al. [38]. Briefly, 500 mg of citric acid (CA) with 500 μL of ethylenediamine were added into a 10 mL beaker. Then, it was led to the muffle, previously heated at 180 °C, where it remained for 30 min. After this step, the recipient was allowed to cool down naturally, and the product was solubilized in Milli-Q water. The second N doped-CQD (labeled as CQD-CONH2) was produced following the protocol previously described by Chen et al. [39]. In this regard, 1.0 g of CA and 2.0 g of urea were dissolved in 20 mL of DMF and stirred to form a transparent solution. Subsequently, the solution was transferred into a 25 mL Teflon-lined stainless autoclave. The sealed autoclave vessel was heated at 200 °C and held for 6 h. Afterward, the mixture was held at rest until it cooled to room temperature. Once both CQDs dispersions were cooled down, their purification was carried out through the dialysis membrane (cut-off tubing in the range of 1 kDa) for 48 h. Finally, each colloidal dispersion was lyophilized to obtain the powder, and was stored in the fridge at 4 °C for further characterization and application.
2.4. Quantum Yield
Quinine sulphate was used as a standard for the determination of quantum yield (QY) of the prepared CQDs. The fluorescence spectra of quinine sulphate (in 0.1 mol.L−1 H2SO4 as a solvent, QY = 0.54) and CQDs solution, as well as their absorbances, were recorded at 350 nm. The following Equation was used to calculate the QY ():
where is the standard of quinine sulfate, I is the integrated emission intensity, A is optical absorbance at 350 nm, and n is the refractive index of the solvent. Subscript ref denotes the values obtained for the standard, representing the known quantum yield [40].
2.5. Metallic Ions Sensing Study
Firstly, stock solutions of different metallic cations (Co2+, Cu2+, Mg2+, Mn2+, Fe2+, Mo4+, and Zn2+) were previously prepared in Milli-Q water. In addition, to accurately calculate the concentration of the CQDs, a calibration curve (concentration x absorbance) was previously constructed for each sample based on the mass/volume ratio. Then, for the sensing studies, the concentration of CQDs was measured at 350 nm and 400 nm for CQD-NH2 and CQD-CONH2, respectively. The selected wavelengths correspond to the absorption band maximum of the respective CQDs. This procedure was carried out separately, due to the distinct chemical structure of the two nanoparticles. So, by knowing the precise concentration of both CQDs, a dispersion containing CQD-NH2 and CQD-CONH2 was prepared. Subsequently, two emission fluorescence spectra were recorded, taking into consideration the conditions for each CQD. In this sense, the first one was taken in the range of 380–650 nm using 360 nm as the excitation wavelength, while the second one was measured from 440 to 650 nm, considering 420 nm as the excitation wavelength. Each spectrum was used as the blank. Afterward, we carried out a procedure where eight aliquots of 10 μL were removed from a metal ion stock solution and added into the N-CQDs dispersion previously prepared and measured. For each addition, two spectrum were recorded, regarding the previously reported measurement conditions. This protocol was repeated six times for each metal ion investigated (Co2+, Cu2+, Mg2+, Mn2+, Fe2+, Mo2+, and Zn2+).
2.6. Data Treatment
Taking into consideration the protocol described in Section 2.5, a raw data matrix of 2 nose-based receptors × 7 metal ions × 8 concentrations × 6 replicates were generated for each metal cation analyzed. The ratio (where I and I0 denote the final and initial intensity of the fluorescence spectrum at 450 nm and 515 nm, respectively) was the signal investigated as a function of ion concentration. The LOD and LOQ parameters were estimated according to the International Conference on Harmonization’s (ICH) guidelines, which indicates the following formulas: and . denotes the standard deviation of y-intercept, while represents the slope of the regression line. The curves were further fitted using the Stern–Volmer equation, and the n parameter was evaluated. In addition, the whole raw data matrix was evaluated using the K-Means, PCA, and LDA methods to identify/classify each target analyte [22].
3. Results and Discussion
3.1. CQDs Characterization
UV–Visible and fluorescence spectroscopies were carried out to investigate the optical properties of the CQD-NH2 and CQD-CONH2, both being prepared in an aqueous solution. Figure 1a,b displays the presence of two strong absorption bands around 350 and 335 nm, respectively. These transitions can be assigned to the n-π* transition of C-O, C=O, and N-H functional groups [41,42], which evidences the doping of N in both samples. Moreover, the absorption spectrum for CQD-CONH2 (Figure 1b) displayed additional bands centered around 430 nm, 510, and 561 nm. These transitions may be attributed to the presence of abundant N functional groups, as the N doping process is capable of inducing N-related defect states between the HOMO and LUMO [39]. In Figure 1c, the intensity of the photoluminescence emission of the CQD-NH2 at 450 nm remained unchanged when the excitation wavelength was increased from 300 to 400 nm. This clearly indicates that the CQD-NH2 maximum fluorescence emission is independent of the excitation wavelength. This behavior can be explained by surface defects, but also by the uniform size distribution of the nanoparticles. This distribution can be confirmed using AFM imaging [43,44,45]. Still, the emission of CQD-CONH2 (Figure 1d) is clearly excitation-dependent, since the fluorescence could be observed in the range 448–620 nm when excited at different wavelengths (360–520 nm). Figure S1 shows this dependence in point graph style. In this case, the electron-rich nitrogen atoms could offer more active sites, and the excitation-dependent fluorescence behavior could be related to the different surface states of the CDQ-CONH2 [46]. The quantum yield (QY) of CQD-NH2 and CQD-CONH2 in the water was measured to be 25.93% and 6.02%, respectively, using quinine sulphate as a reference.
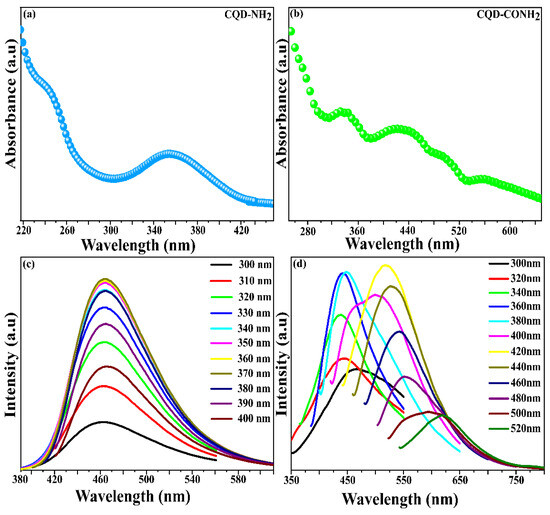
Figure 1.
(a) UV-Vis absorption spectra of CQD-NH2; (b) UV-Vis absorption spectra of CQD-CONH2; (c) Fluorescence spectra of CQD-NH2 with different excitation wavelengths; (d) Fluorescence spectra of CQD-COONH2 with different excitation wavelengths.
X-ray photoelectron spectroscopy (XPS) analyses were performed to characterize the functional groups into CQDs. The CQD-NH2 and CQD-CONH2 showed compositions of C1s, N1s, and O1s (Figure 2a,b). In CQD-NH2, the high resolution of C1s (Figure 2c) and the binding energy peak at 284.6 eV confirms the graphitic structure sp2 (C—C and C=C). The peak at 286.3 eV suggests the presence of C—N, while the peak around 288.0 eV could be assigned to C=O bonds [47,48]. The O1s spectrum exhibited two peaks at 530.8 and 532.0 eV (Figure S2), which can be assigned to the C=O and C-O-C/C-OH groups, respectively [49]. The high-resolution N1s (Figure 2e) show two peaks, 399.8 and 401.6eV, assigned to the absorption of N-H and C-N-C groups, respectively [50,51,52,53]. In CQD-CONH2, the C1s band can be mainly deconvoluted into two peaks (Figure 2d), which represent graphitic sp2 carbons (C=C/C–C, 284.6 eV) and carbonyl groups (C=O, 286.5 eV), and the peak around 288.0 eV could be attributed to C=O bonds. The O1s band can be assigned to two peaks (Figure S1b), C=O (532.3 eV) and C–O (533.4 eV). The N1s band can be fitted into one peak (Figure 2f), which corresponds to pyrrolic N (399.5 eV) [39]. However, the attribution of this peak to the N-H groups can not be discarded, due to the proximity of the bound energy values.
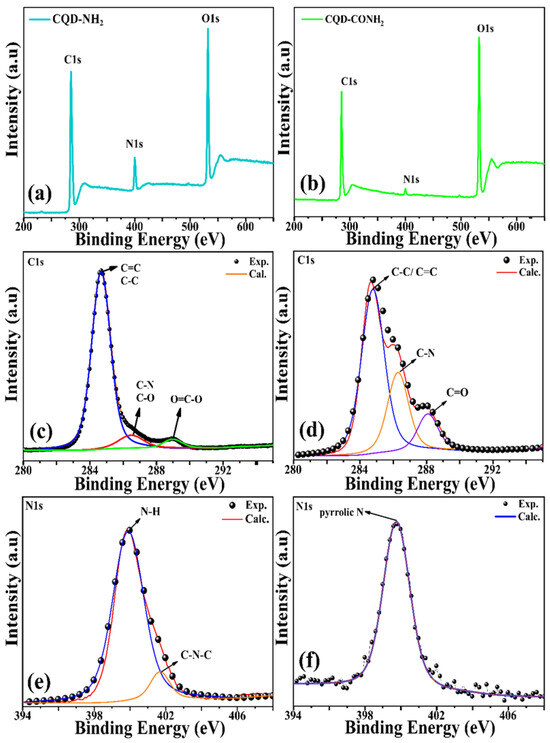
Figure 2.
(a) XPS full survey, (c,e) high-resolution XPS of C1s and N1s spectra of the CQD-NH2, respectively; (b) XPS full survey, (d,f) high-resolution XPS of C1s and N1s spectra of the CQD-CONH2, respectively.
Figure 3 shows the Raman spectra for N-CQDs samples, which was used to characterize the intrinsic structure of the particles. From the Raman spectra, we performed a deconvolution into four other spectra, using Gaussian curves, whose statistical parameters are presented in Table S1. Among these four curves, the two with the highest intensities correspond to bands D (A1g mode) and G (E2g mode) for both CQDs. In this context, the G bands were found to be at 1548 cm−1 and 1505 cm−1 for CQD-NH2 (Figure 3a) and CQD-CONH2 (Figure 3b), respectively. According to the literature [54], this band can be attributed to the vibration of sp2 carbon atoms in a two-dimensional hexagonal (2D) graphite grid. The D Band is centered at 1386 cm−1 for CQD-NH2 (Figure 3a) and at 1393 cm−1 for CQD-CONH2 (Figure 3b). This band corresponded to the vibration of the sp3 hybridized carbon atoms in the termination plane of the disordered graphite [55,56]. Furthermore, it is well-known that the ID/IG ratio can provide relevant information about the defects of the structure. So, the ratio values were calculated to be 1.12 and 0.82 for CQD-NH2 and CQD-CONH2, respectively. However, our results evidence the presence of a greater amount of amorphous nature in CQD-CONH2. From XPS results, this sample presented a greater amount of C-N and C=O bonds in the structure in comparison to the CQD-NH2. Thus, it is fair to assume that the presence of these kinds of chemical bonds in the termination plane of the carbon structure may contribute to increasing the disorder.
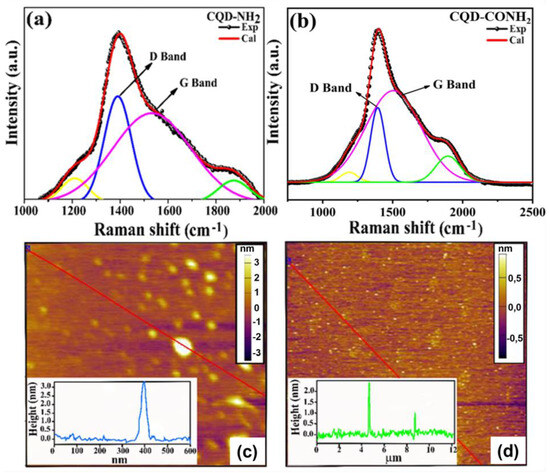
Figure 3.
Raman spectra for the different CQDs samples from (a) CQD–NH2 and (b) CQD–CONH2. (c,d) Atomic force microscopy (AFM) image CQD–NH2 and CQD–CONH2 sample’s inset height profile along the line, respectively.
The functional groups of CQD-NH2 and CQD-CONH2 were investigated through FTIR spectra (Figure S3). The typical N-H bands at 3268 for CQD-N and 3325 cm−1 for CQD-CONH2 were observed to originate from conjugated amine groups (stretching vibrations of N–H) connected to the carbon core. Furthermore, the FTIR spectrum for CQD-CONH2 displayed bands at 1665–1594 cm−1, which can be assigned to the stretching vibration of the amide linkage, indicating the incorporation of many desired amino groups in the N-doped CQD’s structure. These revealed C=O stretching, O–H bending, and N–H bending bands indicate the presence of carboxylic and amide functional groups on CQDs surface. The bands C–N 1368, 1188, and 1117 cm−1 are assigned C=C, C–N, and C–O groups [57,58,59]. The morphology of CQDs was characterized using AFM. Figure 3c,d shows the topographic images of CQD-NH2 and CQD-CONH2, respectively. It can be seen that the particle size distribution is relatively uniform for both N-CQD samples, and their height profiles are in the range of 1–4 nm (inset Figure 3c,d), which can be understood to be nanoparticles with quasi-spherical morphology [60,61].
3.2. N Doped-CQDs as “Nose”-Receptors
Once the CQDs were fully characterized and their properties were revealed, the next step was to find the “nose”-based receptors to build up a multichannel sensing methodology. Our hypothesis is that the presence of CQD-NH2 and CQD-CONH2 in a mixture can help to obtain an unique fluorescence pattern, which will further allow us to perform the analyte discrimination. In this sense, both CQDs were firstly mixed, requiring an appropriate concentration to acquire a balance between the fluorescence intensity. As a result, a unique dispersion of concentrations of 1.0 and 1.8 mg.mL−1 for CQD-NH2 and CQD-CONH2, respectively, was prepared. Figure 4a shows the emission behavior of the mixture when excited at different wavelengths (360–420 nm). Clearly, there is an emission band for each CQD, depending on the excitation wavelength used. For instance, a strong emission from CQD-NH2, centered at 450 nm, was obtained by using 360 nm as excitation wavelength. On the other hand, when excited at 420 nm, the emission from CQD-CONH2 was found to be centered at 515 nm. In addition, no significant overlap was observed, which helps it to obtain a clean emission with no interference from other CQDs. Therefore, after this optimization, the dispersion containing both CQDs was tested against seven different metal ions (Co2+, Fe2+, Mo4+, Cu2+, Mg2+, Mn2+, and Zn2+). Figure 4b–h displays the fluorescence responses of CQD mixtures against these seven metal ions at different concentrations in the range of 100–700 μmol.L−1.
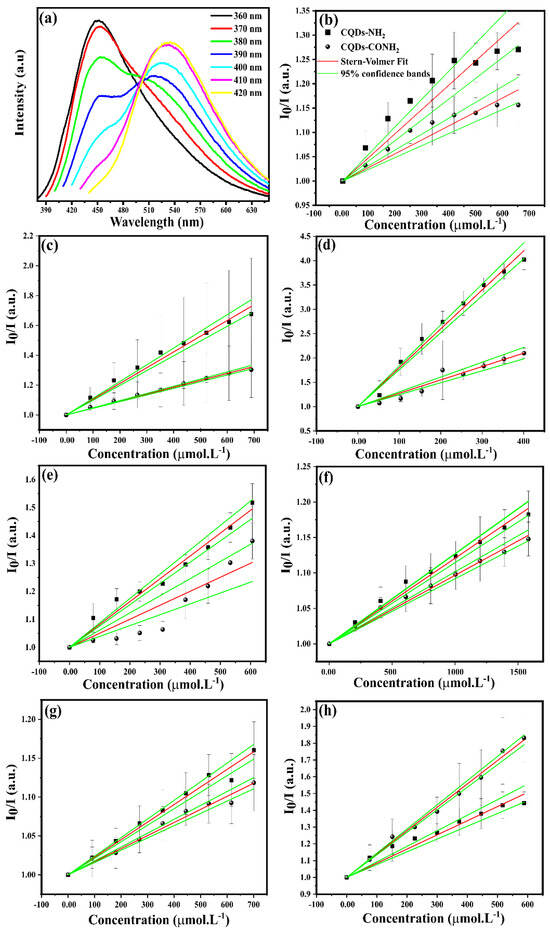
Figure 4.
(a) Emission spectra mixture CQD–NH2 and CQD–CONH2 for varying excitation wavelength multichannel sensors. Relative signal intensity ratio I/I0 in different concentrations at (b) Co2+, (c) Fe2+, (d) Mo4+, (e) Cu2+, (f) Mg2+, (g) Mn2+, and (h) Zn2+ according to classical Stern–Volmer formalism.
As can be seen, the titration experiments pointed out that each metallic cation was able to quench both CQDs in the mixture. To properly explain the interaction, as well as the quenching mechanism, it is important to understand the structure of the carbon-based NPs synthesized in this work. In this regard, according to our XPS results, the CQDs have different functional groups on their surfaces. CQD-NH2 was found to be rich in amine groups, while CQD-CONH2 contained amide groups. Therefore, the presence or absence of these groups on the surface of N doped-CQDs can strongly affect their interactions. In order to obtain a better insight, the titration curves were described using the Stern–Volmer equation [62]:
where I and I0 denote the fluorescence intensity recorded with the presence and absence of metallic cations, respectively, KSV is the Stern–Volmer quenching constant, and is the molar concentration of quencher. Herein, by assuming a linear relationship between and with an intercept of 1, can be easily obtained through graphical determination. The magnitude of this constant may help to understand why some metallic cations are more effective to quench the fluorescence intensity than others. Also, to go further, the number n of binding sites on the surface of the CQDs were calculated by utilizing a modified version of the Stern–Volmer equation [63]. The data are summarized in Table 1.

Table 1.
Values of λexc, R2, KSV, and n for each titration carried out at 298.15 K.
The R2 values for all fits were found to be in the range of 0.9129–0.9935. This evidences that more than 90% of the data variance in the y-axis (normalized fluorescence intensity) can be explained by the x-axis (quencher concentration) using a linear model. So, it is fair to assume that there is a strong linear relationship between and . However, the deviation of linearity may signal the presence of a dynamic (collisional) or static (binding-related) quenching mechanism [64]. Once it is not possible to determine which one has the quenching mechanism from a single linear Stern–Volmer plot, we carried out titrations at different temperatures (298.15 and 310.15 K) for Co2+, Cu2+, Fe2+, and Mo4+ ions. In this sense, to identify the mechanism that better defines the interaction between CQDs and metallic cations, it is fundamental to know that if the quenching is dynamic, the value of KSV should increase. On the other hand, if the mechanism is static, KSV should decrease by increasing the temperature. In this sense, the predominant quenching mechanism for Co2+ and Fe2+ ions was found to be dynamic, while for Cu2+ and Mo4+ ions, the static one was the observed one. Additionally, n values for Fe2+, Zn2+, Mg2+, Mn2+, and Co2+ are near to 1.0 for both CQDs, indicating that one empty site on the CQDs surface interacts with the mentioned metals.
However, the situation differs when Cu2+ ions are used as a quencher. In this case, the presence of amide groups on the surface of the CQDs seems to increase the number of binding sites for Cu2+ ions, since the n values were found to be 0.89 ± 0.04 and 1.51 ± 0.06 for CQD-NH2 and CQD-CONH2, respectively. Additionally, n ≈ 2 was observed for Mo4+ ions when used as quencher for both CQDs. Compared to the other metallic cations, Mo4+ ions are the ones with highest KSV values. Taking into consideration that this ion is the quencher, the values found were 61.67 × 102 ± 214.67 and 17.20 × 102 ± 115.89 M−1 for CQD-NH2 and CQD-CONH2, respectively, while the KSV values for the other ions were found to be in the range of 1.80 × 102–12.55 × 102 M−1. Once this constant signals for the interaction between the CQD’s surface and the metallic cations, it is fair to assume that the Mo4+ ions are the ones presenting the strongest interaction. This may be explained based on the electronic configuration of the cation, since the Mo4+ is the ion with the highest amount of empty d-orbitals, which may favor the interaction with an electron-rich surface.
Overall, the results demonstrated the great potential of the multichannel sensor developed for detecting Mo4+ ions. Therefore, the LOD and LOQ parameters were calculated according to the ICH guidelines to further deepen the analytical parameters regarding the sense of this cation. However, to take advantage of the multichannel sensor, the interaction of the Mo4+ ions with the two N-CQDs was used. Herein, the difference between KSV and n values calculated through the titration studies demonstrates the diversity of interactions on the CQDs surface. So, this that the sensibility of the methodology may increase, taking into consideration the interaction of the target ions with CQD-NH2 and CQD-CONH2. In this sense, Figure 5 displays a schematic illustration of the dual-response sensor array strategy. By using this strategy to obtain the data, the LOD and LOQ parameters were estimated based on the standard deviation of the response, as well as the slope of the calibration curve of the target analyte (Mo4+ ions). The response () was calculated using the formula:
where represents the initial intensity (from each N-CQD), while and denote the fluorescence intensity of CQD-NH2 and CQD-CONH2, respectively. was plotted as a function of the concentration of the Mo4+ ions, and the data were fitted using a regression line method to obtain the of the y-intercept and values. The LOD and LOQ parameters were found to be 1.28 and 3.88 ppm, respectively, which are smaller than the ones calculated using each N-CQD separately. For instance, the LOD and LOQ were estimated by utilizing the intensity from the CQD-NH2, and were calculated to be 3.25 and 9.86 ppm, respectively. This confirms our initial hypothesis that the use of both N-CQDs may increase the sensitivity of the multichannel sensor, compared to the same strategy using a single channel. Furthermore, based on the values of LOD and LOQ and the detection range used, the multichannel sensor strategy developed in this work can be easily used to detect Mo entities, since some molybdenum detection kits are already on the market with similar analytical sensibility. For instance, the MQuant®, a molybdenum test from Merck (Darmstadt, Germany), is used to sense Mo in the range of 5–250 ppm. Another example is the Macherey-Nagel™ Quantofix™ from Thermo Fischer Scientific (Waltham, MA, USA), which estimates the Mo concentration in a similar range.
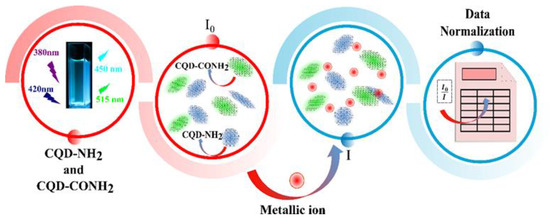
Figure 5.
Schematic illustration of dual-response sensor array based on CQD-NH2 and CQD-CONH2.
As can be seen, CQD-NH2 and CQD-CONH2 were able to produce differential binding events regarding the detection of Mo4+ ions in comparison to the other metallic cations. Therefore, in order to go further on challenging the multichannel sensor strategy, the differentiation was also tested. In this sense, the dual-response sensor array yielded a training matrix of 2 “nose”-based receptors × 7 metals ions × 8 concentrations × 6 replicates, which was firstly analyzed through K-Means and PCA. Both algorithms are unsupervised, and the whole matrix is treated to find patterns with no groups identified. Therefore, the analysis of the data matrix can confirm if the multichannel sensor strategy is able to discriminate Mo4+ ions from the other metallic cations.
Following the preprocessing and normalization of the dataset, the elbow method was applied to determine the most efficient number of clusters for dividing the dataset. The results, as plotted in Figure 6a, indicate a significant change in the slope when the number of clusters reaches three. This suggests that the algorithm can differentiate between three distinct types of metals out of the seven analyzed in the study. Based on the previous analysis, the decision was made to employ the K-means algorithm with three clusters, running the method 100 times with randomly selected centers. The distribution of cluster sizes was 6-6-30. As summarized in Figure 6b, the analysis reveals a 100% accuracy rate in detecting Mo and an 83% success rate for identifying Zn. The procedure proved to be incapable of distinguishing between the other metals. The differentiation of the metal ions was further investigated through a discriminant analysis. The results are displayed in Figure 7.
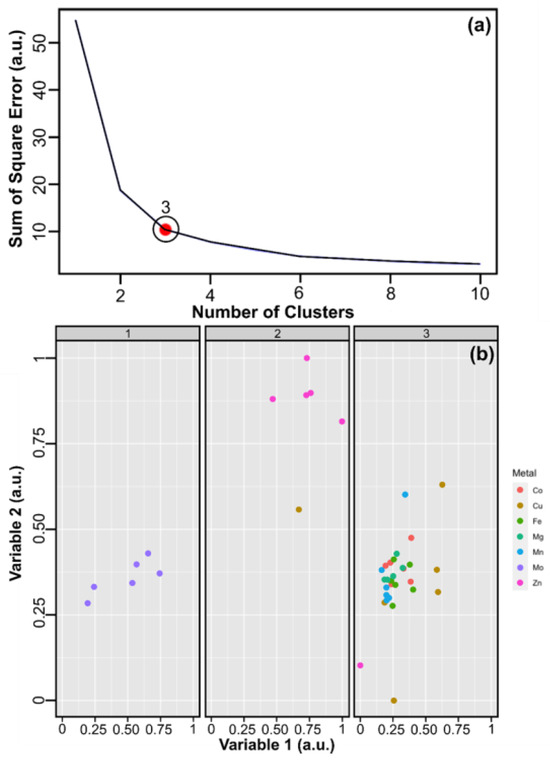
Figure 6.
Training matrix statistical treatment: (a) Elbow Method for the complete dataset, and (b) Facet wrap of the clustering process for metal detection, using the multichannel sensor strategy. The variables 1 and 2 are arbitrary and both collected from the complete dataset. Also, groups 1, 2, and 3 are associated with Mo, Zn, and remaining metals, respectively.
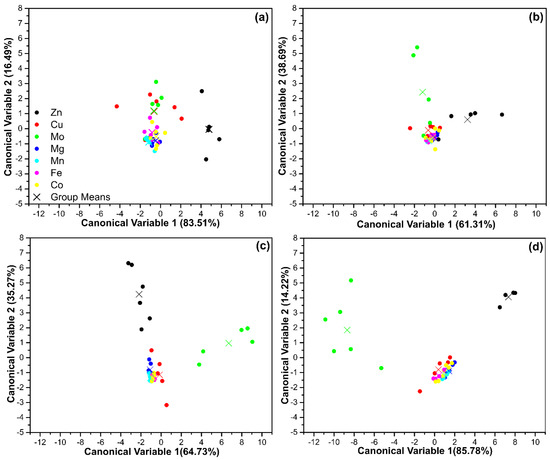
Figure 7.
Canonical score plot of CQDs–N and CQ–CONH2 for the discrimination of seven metal ions at about (a) 4.98, (b) 9.90, (c) 14.78, and (d) 19.6 ppm. Each metal group is composed of six replicates. However, a boxplot methodology was applied to exclude outliers.
The canonical score plots shown in Figure 7 evidence the ability of our multichannel sensor approach to easily discriminate metallic Mo4+ ions out of seven different metal ions. However, each ion has some specific characteristics. So, the behavior of each one on the N-CQDs surface is expected to be different, and this could influence the position of the clusters in the canonical score plots. In this regard, the position of the clusters related to the Mo4+ ions can be readily identified in Figure 7b–d. This reveals the potential of CQD-NH2 and CQD-CONH2 to create a unique fluorescent response to perform the identification of Mo species. Interestingly, Zn2+ ions were also identified. Therefore, the DA analysis corroborated the K-Means analysis, which reveals the optimization through three clusters of classification. This demonstrates the robustness of the statistical treatment used in this work and, once again, confirms that our multichannel sensor is able to differentiate Mo4+ and Zn2+ ions. This may be explained based on the titration studies, since they demonstrate that the presence of a distinct number of surface sites was found. Mo4+ ions were estimated to have 1.80 ± 0.17 and 1.96 ± 0.15 surface sites on CQD-NH2 and CQD-CONH2, respectively. For Zn2+ ions, CQD-NH2 had an 0.66 ± 0.02 surface site, while CQD-CONH2 was found to have an 0.97 ± 0.03 surface site. Both CQDs presented n values near to 1.0, which may explain the poor discrimination. According to these results, the interactions between the CQD’s surface and Fe2+, Zn2+, Mg2+, Mn2, and Co2+ ions are similar, which did not allow our multichannel sensor to differentiate between them.
The CQD-NH2 and CQD-CONH2 were able to produce three different behaviors on their surface, regarding the seven target analytes investigated. In addition, the concentration level did not increase the identification ability towards Fe2+, Zn2+, Mg2+, Mn2+, and Co2+ ions. However, the Mo4+ ions are easily identified at 9.90, 14.78, and 19.6 ppm, since the cluster from this metallic cation is separated from the other ones, as can be seen in Figure 7b–d, respectively. These results pointed out the great potential of the methodology to discriminate Mo4+ ions. The discrimination power of our “nose”-based receptors may rely on the interaction between the functional groups on the N-CQD’s surface and each ion and its ability to produce differential binding events. So, each metallic cation interacts with both CQD-NH2 and CQD-CONH2 in order to generate a fingerprint-like pattern. Once the receptors are the same, the characteristics of the ions are also important to obtain a unique interaction. In this regard, we believe that the discrimination of our methodology towards Mo4+ ions may be explained based on the electronic properties of the ion, since the electronic configuration of this cation presents a greater number of empty d-orbitals, which favors the interaction with the electron-rich surface of the N-CQDs. It is important to highlight that the proposed sensing strategy has limitations. In the future, other optimization studies should be carried out, as suggested in Figure 5. Samples containing humic, fulvic acids, or proteins require prior treatment, as such substances can interfere with the analysis process.
4. Conclusions
In summary, we present a simple and easy strategy for building up a multichannel sensor approach. In this regard, the nose-based multichannel sensor allowed for the development of a methodology to detect Mo4+ ions. Interestingly, the titration curves at different temperatures revealed a sensing mechanism through static quenching Mo4+ ions, which indicates the formation of a non-fluorescent complex on the surface of the CQD-NH2 and CQD-CONH2. Furthermore, the dataset obtained from the titration studies were used to build up a training matrix to carry out a statistical treatment through K-Means and Discriminant analysis to check the identification of the Mo species among the other ions. Mo4+ ions were easily identified at 5 ppm with the “nose”-based probes. The presence of two empty sites on the surface of the N-CQDs was a key factor to explain this result. Along with this, the presence of a greater number of empty d-orbitals in the electronic structure of the Mo4+ ions is another important feature, since it allows for a strong interaction of this cation with the N-CQDs surface. Overall, the methodology to detect Mo4+ ions based on our multichannel sensor was found to have 1.28 and 3.88 ppm for LOD and LOQ parameters, respectively. These values are comparable to the molybdenum test already available on the market, such as MQuant® (Merck) and Macherey-Nagel™ Quantofix™ (Thermo Fischer Scientific). This demonstrates the potential of the multichannel sensor strategy used in this work to carry out the detection of Mo species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/c10030057/s1, Figure S1: Graph of the emission wavelength with maximum intensity as a function of the excitation wavelength of the sample CQD-CONH2; Figure S2: High-resolution XPS of O1s spectra of the CQD-NH2 and CQD-CONH2, respectively; Figure S3: FT-IR spectra of Urea, Citric Acid (CA), CQD-N and CQD-CONH2; Table S1: Parameters of deconvolution of the Raman spectrum of the CQD-CONH2 sample.
Author Contributions
A.A.C.C.: Conceptualization, Methodology, Formal analysis, Data Curation, Writing—Original Draft, Writing—Review and Editing. N.D.G.S.: Conceptualization, Methodology, Formal analysis, Data Curation, Writing—Original Draft. J.P.B.d.S.: Conceptualization, Methodology, Formal analysis, Data Curation, Writing—Original Draft. S.V.C.: Methodology, Formal analysis, Data Curation, Writing—Original Draft, Writing—Review and Editing. C.S.C.: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Supervision. J.S.S.: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Supervision. L.M.U.D.F.: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Supervision. S.M.: Methodology, Software, Investigation, R.M.F.: Conceptualization, Methodology, Software, Writing—Review and Editing, Visualization, Supervision, Funding acquisition. P.B.A.F.: Conceptualization, Writing—Review and Editing, Visualization, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Brazilian agencies: CAPES (Finance Code 001, PROEX 23038.000509/2020-82), CNPq (308452/2022-4), Funcap (PNE-0112-00048.01.00/16). The authors also thank the Brazilian Center for Research in Energy and Materials (CNPEM) for the XPS measurements (proposal XPS-23792). Furthermore, R. M. Freire wants to thanks the support from the FONDECYT under grants 11200425, 1241178 and the Basal Program for Centers of Excellence, Grant AFB220001 CEDENNA, CONICYT.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors gratefully acknowledge the Central Analítica-UFC/CT-INFRA/MCTISISNANO/Pró-Equipamentos and the Department of Physics at UFC.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhang, X.; Tao, T.; Qiu, Y.; Guo, X.; Zhu, X.; Zhou, X. Copper-mediated novel cell death pathway in tumor cells and implications for innovative cancer therapies. Biomed. Pharmacother. 2023, 168, 115730. [Google Scholar] [CrossRef] [PubMed]
- Enemark, J.H. {Moco}n, (n = 0–8): A general formalism for describing the highly covalent molybdenum cofactor of sulfite oxidase and related Mo enzymes. J. Inorg. Biochem. 2022, 231, 111801. [Google Scholar] [CrossRef]
- Yong, W.; Huang, Q.-C.; Mu, H.-Y.; Shi, W.-X.; Dai, B.-L.; Kong, J.-J.; Chen, X.-R.; Huang, X.-C. A luminescent Zn(II) metal−organic framework assembled with a thiazolothiazole chromophore for sensing mainly cobalt(II) and nitrofuran antibiotics in aqueous solutions. J. Mol. Struct. 2024, 1301, 137424. [Google Scholar] [CrossRef]
- Amin, A.S.; El-Bahy, S.M.; Hassan, A.M.E. Construction of an optical sensor for molybdenum determination based on a new ionophore immobilized on a polymer membrane. J. King Saud Univ. Sci. 2023, 35, 102592. [Google Scholar] [CrossRef]
- Li, S.; Gao, X.; Nie, L.; Bu, L.; Dong, G.; Song, D.; Liu, W.; Meng, D.; Geng, X.; Zhou, Q. Strategy for establishing sensitive fluorescent sensor toward p-nitrophenol integrating magnetic molecularly imprinted materials and carbon dots. Talanta 2024, 272, 125749. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Ma, Y.; Zhao, D.; Luo, H.; Luo, X.; Hou, C.; Huo, D. On–Off–On fluorescent sensing platform based on nitrogen-doped carbon dots for biothiols detection. J. Photochem. Photobiol. A Chem. 2023, 439, 114595. [Google Scholar] [CrossRef]
- Saikia, M.; Hazarika, A.; Roy, K.; Khare, P.; Dihingia, A.; Konwar, R.; Saikia, B.K. Waste-derived high-yield biocompatible fluorescent carbon quantum dots for biological and nanofertiliser applications. J. Environ. Chem. Eng. 2023, 11, 111344. [Google Scholar] [CrossRef]
- Thakur, S.; Bains, A.; Sridhar, K.; Kaushik, R.; Chawla, P.; Sharma, M. Valorization of food industrial waste: Green synthesis of carbon quantum dots and novel applications. Chemosphere 2024, 347, 140656. [Google Scholar] [CrossRef] [PubMed]
- Al-Hetty, H.R.A.K.; Jalil, A.T.; Al-Tamimi, J.H.Z.; Shakier, H.G.; Kandeel, M.; Saleh, M.M.; Naderifar, M. Engineering and surface modification of carbon quantum dots for cancer bioimaging. Inorg. Chem. Commun. 2023, 149, 110433. [Google Scholar] [CrossRef]
- Benner, D.; Yadav, P.; Bhatia, D. Red emitting carbon dots: Surface modifications and bioapplications. Nanoscale Adv. 2023, 5, 4337–4353. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Liu, Q.; Chen, X. Nitrogen, sulfur-doped carbon quantum dots with large Stokes shift for real-time monitoring of pH in living cells. Talanta 2024, 269, 125479. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Zhang, Y.; Wang, X.; Xu, Q.; Li, Z.; Sheng, L.; Yan, L. Fluorescence probe based on boron-doped carbon quantum dots for high selectivity ‘on-off-on’ mercury ion sensing and cell imaging. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129150. [Google Scholar] [CrossRef]
- Miao, S.; Liang, K.; Zhu, J.; Yang, B.; Zhao, D.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Singh, R.; Kashayap, S.; Singh, V.; Kayastha, A.M.; Mishra, H.; Saxena, P.S.; Srivastava, A.; Singh, R.K. QPRTase modified N-doped carbon quantum dots: A fluorescent bioprobe for selective detection of neurotoxin quinolinic acid in human serum. Biosens. Bioelectron. 2018, 101, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, Y.; Yang, X.; Yao, C. N-doped carbon dots sensor for selective detection of hydroxylamine hydrochloride. Opt. Mater. 2019, 94, 121–129. [Google Scholar] [CrossRef]
- Elizabeth, A.T.; James, E.; Jesan, L.I.; Arockiaraj, S.D.; Vasu, A.E. Green synthesis of value-added nitrogen doped carbon quantum dots from Crescentia cujete fruit waste for selective sensing of Fe3+ ions in aqueous medium. Inorg. Chem. Commun. 2023, 149, 110427. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Zhang, Z.; Chen, J.; She, M.; Ji, Y.; Zheng, B.; Yang, Z.; Zhang, S.; Li, J. Photostable and printable fluorescence carbon quantum dots for advanced message encryption and specific reversible multiple sensing of Cu2+ and cysteine. Chem. Eng. J. 2023, 453, 139722. [Google Scholar] [CrossRef]
- Carneiro, S.V.; Oliveira, J.J.P.; Rodrigues, V.S.F.; Fechine, L.M.U.D.; Antunes, R.A.; Neto, M.L.A.; de Moura, T.A.; César, C.L.; de Carvalho, H.F.; Paschoal, A.R.; et al. Doped Carbon Quantum Dots/PVA Nanocomposite as a Platform to Sense Nitrite Ions in Meat. ACS Appl. Mater. Interfaces 2022, 14, 43597–43611. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.M.; Le, N.D.B.; Jiang, Z.; Kim, C.S.; Rotello, V.M.; Fechine, P.B.A. NH2-rich Carbon Quantum Dots: A protein-responsive probe for detection and identification. Sens. Actuators B Chem. 2018, 255, 2725–2732. [Google Scholar] [CrossRef]
- Carneiro Cruz, A.A.; Freire, R.M.; Froelich, D.B.; de Lima, A.C.A.; Muniz, A.R.; Ferreira, O.P.; Fechine, P.B.A. Fluorescence Based Platform to Discriminate Protein Using Carbon Quantum Dots. ChemistrySelect 2019, 4, 5619–5627. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, F.; Zhang, G.; Chen, L.; Wu, Q.; Liu, X. Sensor array based on single carbon quantum dot for fluorometric differentiation of all natural amino acids. Microchim. Acta 2019, 186, 858. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.V.; de Queiroz, V.; Cruz, A.; Fechine, L.; Denardin, J.; Freire, R.; Nascimento, R.D.; Fechine, P. Sensing strategy based on Carbon Quantum Dots obtained from riboflavin for the identification of pesticides. Sens. Actuators B Chem. 2019, 301, 127149. [Google Scholar] [CrossRef]
- Carneiro, S.V.; Holanda, M.; Cunha, H.; Oliveira, J.; Pontes, S.; Cruz, A.; Fechine, L.; Moura, T.; Paschoal, A.; Zambelli, R.; et al. Highly sensitive sensing of food additives based on fluorescent carbon quantum dots. J. Photochem. Photobiol. A Chem. 2021, 411, 113198. [Google Scholar] [CrossRef]
- Mercy, D.J.; Kiran, V.; Thirumalai, A.; Harini, K.; Girigoswami, K.; Girigoswami, A. Rice husk assisted carbon quantum dots synthesis for amoxicillin sensing. Results Chem. 2023, 6, 101219. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, K.; Qian, S.; Wang, Y.; Lin, H. Applying Carbon Dots-Metal Ions Ensembles as a Multichannel Fluorescent Sensor Array: Detection and Discrimination of Phosphate Anions. Anal. Chem. 2017, 89, 5542–5548. [Google Scholar] [CrossRef]
- Silva, E.C.; Gomes, C.G.; Pina, J.; Pereira, R.F.; Murtinho, D.; Fajardo, A.R.; Valente, A.J. Carbon quantum dots-containing poly(β-cyclodextrin) for simultaneous removal and detection of metal ions from water. Carbohydr. Polym. 2024, 323, 121464. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; He, S.; Liu, W.; Pei, H.; Liu, N.; Guo, R.; Mo, Z. Long wavelength emission multicolor fluorescent carbon quantum dots for the detection of pH, amino acids, and metal ions. J. Photochem. Photobiol. A Chem. 2023, 444, 114967. [Google Scholar] [CrossRef]
- Padmapriya, A.; Thiyagarajan, P.; Devendiran, M.; Kalaivani, R.A.; Shanmugharaj, A.M. Electrochemical sensor based on N,P–doped carbon quantum dots derived from the banana flower bract (Musa acuminata) biomass extract for selective and picomolar detection of dopamine. J. Electroanal. Chem. 2023, 943, 117609. [Google Scholar] [CrossRef]
- Geng, Y.; Peveler, W.J.; Rotello, V.M. Array-based ‘Chemical Nose’ Sensing in Diagnostics and Drug Discovery. Angew. Chem. Int. Ed. 2019, 58, 5190–5200. [Google Scholar] [CrossRef]
- Belhumeur, P.N.; Hespanha, J.P.; Kriegman, D.J. Eigenfaces vs. Fisherfaces: Recognition using class specific linear projection. IEEE Trans. Pattern Anal. Mach. Intell. 1997, 19, 711–720. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, Y.; Lee, S.; Kang, H.; Kim, J.; Choi, J.; Ryu, J.; Joo, H.; Jung, H.-T.; Kim, J. Finding Hidden Signals in Chemical Sensors Using Deep Learning. Anal. Chem. 2020, 92, 6529–6537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, Y.; Devaraj, V.; Nguyen, T.M.; Kim, Y.-J.; Kim, Y.H.; Kim, C.; Choi, E.J.; Han, D.-W.; Oh, J.-W. Investigation of colorimetric biosensor array based on programable surface chemistry of M13 bacteriophage towards artificial nose for volatile organic compound detection: From basic properties of the biosensor to practical application. Biosens. Bioelectron. 2021, 188, 113339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Devaraj, V.; Jeong, N.-N.; Lee, Y.; Kim, Y.-J.; Kim, T.; Yi, S.H.; Kim, W.-G.; Choi, E.J.; Kim, H.-M.; et al. Neural mechanism mimetic selective electronic nose based on programmed M13 bacteriophage. Biosens. Bioelectron. 2022, 196, 113693. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ji, H.; Guan, Y.; Ran, X.; Ren, J.; Qu, X. A graphene-based chemical nose/tongue approach for the identification of normal, cancerous and circulating tumor cells. NPG Asia Mater. 2017, 9, e356. [Google Scholar] [CrossRef]
- Sun, Z.; Xing, H.H.; Qing, M.; Shi, Y.; Ling, Y.; Li, N.B.; Luo, H.Q. From the perspective of high-throughput recognition: Sulfur quantum dots-based multi-channel sensing platform for metal ions detection. Chem. Eng. J. 2023, 452, 139594. [Google Scholar] [CrossRef]
- Fu, L.; Liu, T.; Yang, F.; Wu, M.; Yin, C.; Chen, L.; Niu, N. A multi-channel array for metal ions discrimination with animal bones derived biomass carbon dots as sensing units. J. Photochem. Photobiol. A Chem. 2022, 424, 113638. [Google Scholar] [CrossRef]
- Zhu, L.; Mei, X.; Peng, Z.; Liu, J.; Yang, J.; Li, Y. A rotating paper-based microfluidic sensor array combining Michael acceptors and carbon quantum dots for discrimination of biothiols. Chem. Eng. J. 2023, 454, 140065. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon. N. Y. 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Chen, D.; Wu, W.; Yuan, Y.; Zhou, Y.; Wan, Z.; Huang, P. Intense multi-state visible absorption and full-color luminescence of nitrogen-doped carbon quantum dots for blue-light-excitable solid-state-lighting. J. Mater. Chem. C Mater. 2016, 4, 9027–9035. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Saha, A.; Maity, A.R.; Ray, S.C.; Jana, N.R. Carbon nanoparticle-based fluorescent bioimaging probes. Sci. Rep. 2013, 3, srep01473. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Wang, H.; Song, H. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051. [Google Scholar] [CrossRef]
- Zhou, C.; He, X.; Ya, D.; Zhong, J.; Deng, B. One step hydrothermal synthesis of nitrogen-doped graphitic quantum dots as a fluorescent sensing strategy for highly sensitive detection of metacycline in mice plasma. Sens. Actuators B Chem. 2017, 249, 256–264. [Google Scholar] [CrossRef]
- Han, Z.; Nan, D.; Yang, H.; Sun, Q.; Pan, S.; Liu, H.; Hu, X. Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sens. Actuators B Chem. 2019, 298, 126842. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a ‘turn-off’ fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.K.; Sundaram, C.; Ngo, Y.-L.T.; Choi, W.M.; Chung, J.S.; Kim, E.J.; Hur, S.H. Pyromellitic acid-derived highly fluorescent N-doped carbon dots for the sensitive and selective determination of 4-nitrophenol. Dye. Pigment. 2019, 165, 327–334. [Google Scholar] [CrossRef]
- Paul, A.; Kurian, M. N-doped photoluminescent carbon dots from water hyacinth for tumour detection. Mater. Today Proc. 2020, 25, 213–217. [Google Scholar] [CrossRef]
- You, A.; Be, M.A.Y.; In, I. Fluorescently tuned nitrogen-doped carbon dots from carbon source with different content of carboxyl groups. APL Mater. 2015, 3, 086102. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B R. Soc. Chem. 2016, 4, 5772–5788. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2014, 44, 362–381. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Liu, S.; Cui, J.; Huang, J.; Tian, B.; Jia, F.; Wang, Z. Spectrochimica Acta Part A : Molecular and Biomolecular Spectroscopy Facile one-pot synthesis of highly fl uorescent nitrogen-doped carbon dots by mild hydrothermal method and their applications in detection of Cr(VI) ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Li, X.; Yang, H.; Chen, X. Nitrogen-doped carbon dots rapid and selective detection of mercury ion and biothiol and construction of an IMPLICATION logic gate. Talanta 2019, 194, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhao, X.; Liang, Y.; Peng, L.; Dong, H.; Xiao, Y.; Hu, C.; Hu, H.; Liu, Y.; Zheng, M. Journal of Colloid and Interface Science Small nitrogen-doped carbon dots as efficient nanoenhancer for boosting the electrochemical performance of three-dimensional graphene. J. Colloid Interface Sci. 2019, 536, 628–637. [Google Scholar] [CrossRef]
- Mistry, B.; Machhi, H.K.; Vithalani, R.S.; Patel, D.S.; Modi, C.K.; Prajapati, M.; Surati, K.R.; Soni, S.S.; Jha, P.K.; Kane, S.R. Harnessing the N-dopant ratio in carbon quantum dots for enhancing the power conversion efficiency of solar cells. Sustain. Energy Fuels 2019, 3, 3182–3190. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Liu, S.; Liu, Z.; Li, Y.; Hu, X. Sensors and Actuators B: Chemical Fluorescent carbon dots for auramine O determination and logic gate operation. Sens. Actuators B Chem. 2015, 219, 261–267. [Google Scholar] [CrossRef]
- Konar, S.; Kumar, B.N.P.; Kr, M.; Samanta, D. Sensors and Actuators B: Chemical N-doped carbon dot as fluorescent probe for detection of cysteamine and multicolor cell imaging. Sens. Actuators B Chem. 2019, 286, 77–85. [Google Scholar] [CrossRef]
- Wang, B. Concentration-induced multi-colored emissions in carbon dots: Origination from triple fluorescent centers. Nanoscale 2018, 10, 6734–6743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, X.; Bai, J.; Pan, G.; Zhu, Y. Emitting color tunable carbon dots by adjusting solvent towards light-emitting devices. Nanotechnology 2018, 29, 085705. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Li, X.; Zhang, H.; Ji, X.; Sun, W.; Yu, Y.; Liu, Y.; Huang, J.; Sarshar, Z.; Sain, M. High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging. Talanta 2021, 222, 121663. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z. A carbon dots e based nanoprobe for intracellular Fe 3 þ detection. Mater. Today Chem. 2019, 13, 121–127. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Barbero, N.; Barni, E.; Barolo, C.; Quagliotto, P.; Viscardi, G.; Napione, L.; Pavan, S.; Bussolino, F. A study of the interaction between fluorescein sodium salt and bovine serum albumin by steady-state fluorescence. Dye. Pigment. 2009, 80, 307–313. [Google Scholar] [CrossRef]
- Fraiji, L.K.; Hayes, D.M.; Werner, T.C. Static and dynamic fluorescence quenching experiments for the physical chemistry laboratory. J. Chem. Educ. 1992, 69, 424–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).