Long Non-Coding RNA TUG1 Gene Polymorphism and TUG1 Expression Level as Molecular Biomarkers of Systemic Lupus Erythematosus and Lupus Nephritis
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Subjects
2.2. LncRNA TUG 1 Genotype Distributions, Alleles and Risk of SLE
2.3. LncRNA TUG 1 Genotype, Alleles and Risk of Lupus Nephritis
2.4. Effect of Haplotypes on the Disease Risk
2.5. Relation of rs5749201 and rs886471 SNPs of the TUG 1 with Clinic Pathological Features of SLE
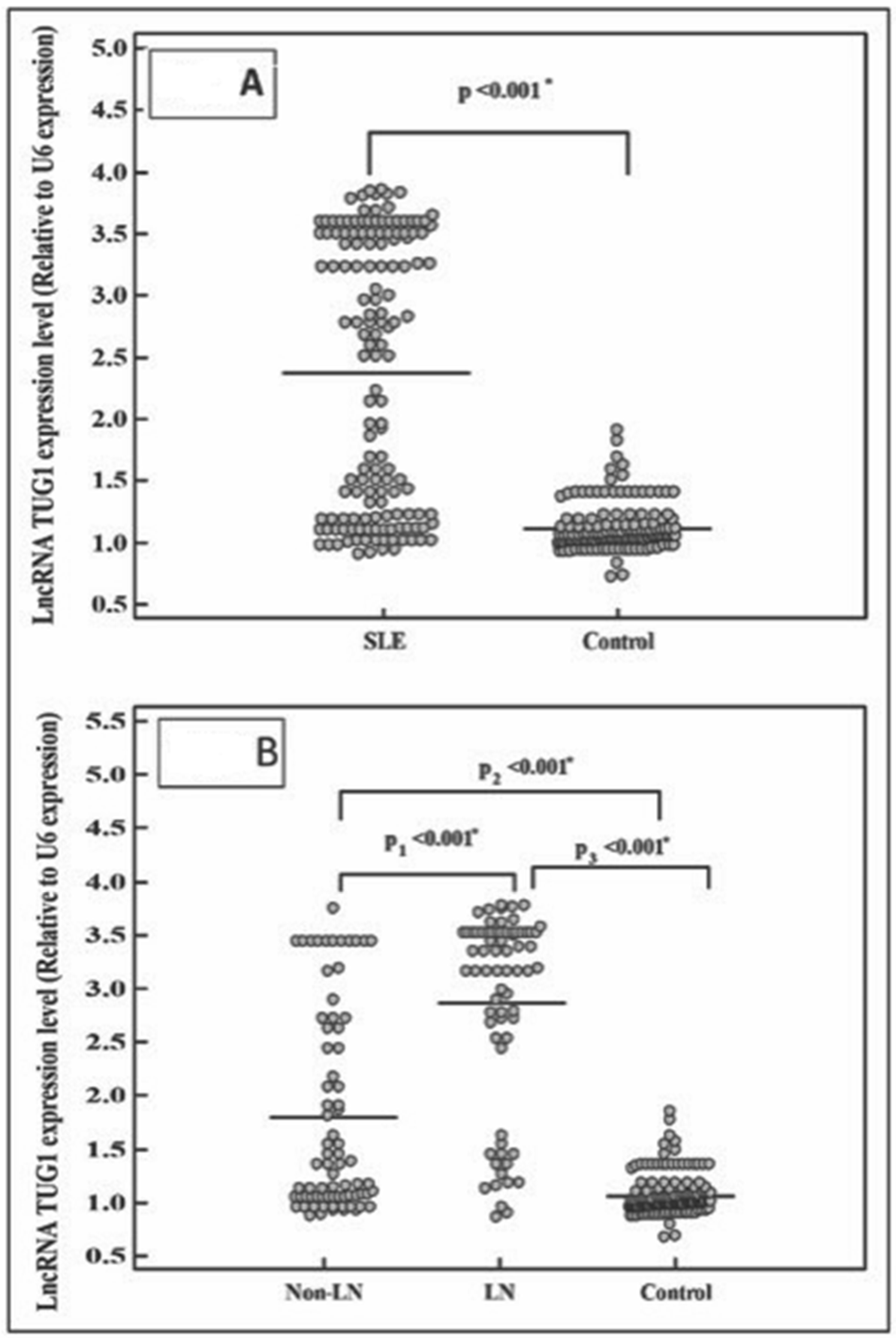
2.6. LncRNA TUG 1 Level in the Studied Groups
2.7. Relation of TUG 1 Gene Polymorphism and TUG 1 Level
2.8. Predictors of Lupus Nephritis
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Groups
4.2. Clinical Assessment
4.3. Laboratory Evaluation
4.3.1. Routine Laboratory and Autoimmune Panel
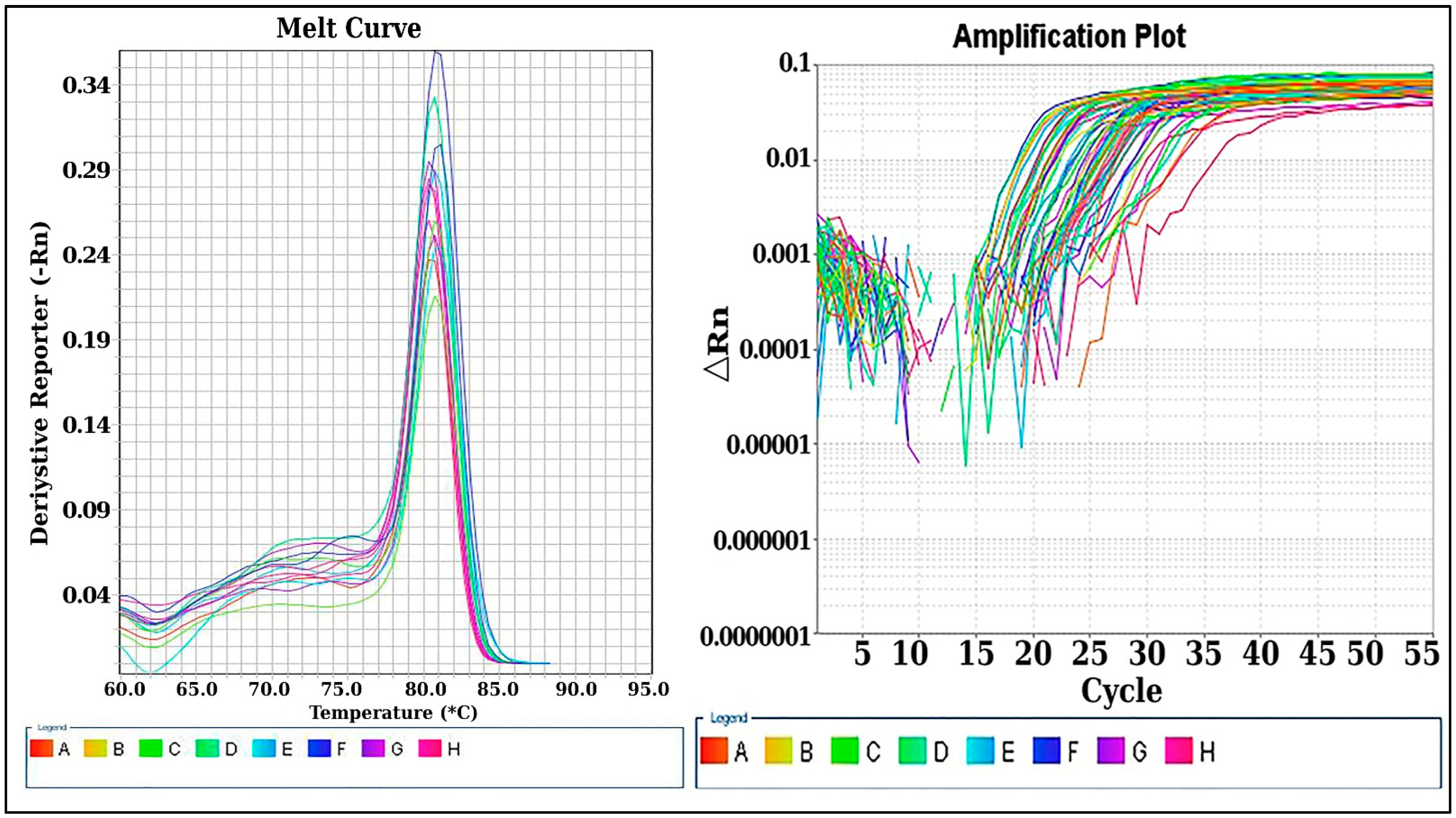
4.3.2. Quantitative Real-Time Quantitative PCR (qRT-PCR) for lncRNA TUG1 Expression Level
RNA Extraction from Plasma and Reverse Transcription
Quantification of LncRNA (TUG1) Expression by Real-Time PCR Technique
4.3.3. DNA Extraction and Detection of Genotyping
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izmirly, P.M.; Wan, I.; Sahl, S.; Buyon, J.P.; Belmont, H.M.; Salmon, J.E.; Askanase, A.; Bathon, J.M.; Geraldino-Pardilla, L.; Ali, Y.; et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol. 2017, 69, 2006–2017. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lu, M.P.; Wang, J.H.; Xu, M.; Yang, S.R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2020, 16, 19–30. [Google Scholar] [CrossRef]
- Taheri, M.; Eghtedarian, R.; Dinger, M.E.; Ghafouri-Fard, S. Exploring the Role of Non-Coding RNAs in the Pathophysiology of Systemic Lupus Erythematosus. Biomolecules 2020, 10, 937. [Google Scholar] [CrossRef]
- Tan, G.; Baby, B.; Zhou, Y.; Wu, T. Emerging Molecular Markers Towards Potential Diagnostic Panels for Lupus. Front. Immunol. 2022, 12, 808839. [Google Scholar] [CrossRef]
- Uthaya Kumar, D.B.; Williams, A. Long non-coding RNAs in immune regulation and their potential as therapeutic targets. Int. Immunopharmacol. 2020, 81, 106279. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Li, H.; Han, X.; Chen, S.; Yang, B.; Hu, Z.; Zhu, H.; Cai, C.; Chen, J.; et al. Characterization of LncRNA expression profile and identification of novel LncRNA biomarkers to diagnose coronary artery disease. Atherosclerosis 2018, 275, 359–367. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Kuo, H.C. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J. Biomed. Sci. 2020, 27, 49. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar]
- Brazão, T.F.; Johnson, J.S.; Müller, J.; Heger, A.; Ponting, C.P.; Tybulewicz, V.L.J. Long noncoding RNAs in B-cell development and activation. Blood 2016, 128, e10–e19. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Mattioli, K.; Volders, P.-J.; Gerhardinger, C.; Lee, J.C.; Maass, P.G.; Melé, M.; Rinn, J.L. High-throughput functional analysis of lncRNA core promoters elucidates rules governing tissue specificity. Genome Res. 2019, 29, 344–355. [Google Scholar] [CrossRef]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associatess with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Chan, M.T.; Wu, W.K. TUG1: A pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016, 49, 471–475. [Google Scholar] [CrossRef]
- Duan, J.; Shen, T.; Dong, H.; Han, S.; Li, G. Association of the Expression Levels of Long-Chain Noncoding RNA TUG1 and Its Gene Polymorphisms with Knee Osteoarthritis. Genet. Test. Mol. Biomarkers 2021, 25, 102–110. [Google Scholar] [CrossRef]
- Shi, H.; Dong, Z.; Gao, H. LncRNA TUG1 protects against cardiomyocyte ischaemia reperfusion injury by inhibiting HMGB1. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3511–3516. [Google Scholar] [CrossRef]
- Liu, Q.M.; Liu, L.L.; Li, X.D.; Tian, P.; Xu, H.; Li, Z.L.; Wang, L.K. Silencing lncRNA TUG1 Alleviates LPS-Induced Mouse Hepatocyte Inflammation by Targeting miR-140/TNF. Front. Cell Dev. Biol. 2021, 8, 616416. [Google Scholar] [CrossRef]
- Li, F.-P.; Lin, D.-Q.; Gao, L.-Y. LncRNA TUG1 promotes proliferation of vascular smooth muscle cell and atherosclerosis through regulating miRNA-21/ PTEN axis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7439–7447. [Google Scholar]
- Gao, Y.; Liu, C.; Wu, T.; Liu, R.; Mao, W.; Gan, X.; Lu, X.; Liu, Y.; Wan, L.; Xu, B.; et al. Current status and perspectives of non-coding RNA and phase separation interactions. Biosci. Trends 2022, 16, 330–345. [Google Scholar] [CrossRef]
- Mohammad, H.M.F.; Abdelghany, A.A.; Al Ageeli, E.; Kattan, S.W.; Hassan, R.; Toraih, E.A.; Fawzy, M.S.; Mokhtar, N. Long Non-Coding RNAs Gene Variants as Molecular Markers for Diabetic Retinopathy Risk and Response to Anti-VEGF Therapy. Pharmacogenomics Pers. Med. 2021, 14, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc. Pathol. 2018, 33, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.H.; Hendler, J.V.; Piccoli, G.F.; Gasparin, A.A.; da Silva Chakr, R.M.; Brenol, J.C.; Monticielo, O.A. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin. Rheumatol. 2017, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, Z.; Jin, Z.; Pan, W.; Liu, L.; Wu, M.; Ding, F.; Hu, H.; Ding, X.; Wie, H.; et al. Clinical and laboratorial outcome of different age-onset systemic lupus erythematosus patients in Jiangsu, China: A multicentre retrospective study. Sci. Rep. 2022, 12, 10683. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Jawad, A. Systemic lupus erythematosus: Rarer in men than women but more severe. Trends Urol. Men’s Health 2022, 13, 11–14. [Google Scholar] [CrossRef]
- Tang, N.; Dong, Y.; Xiao, T.; Zhao, H. LncRNA TUG1 promotes the intervertebral disc degeneration and nucleus pulposus cell apoptosis though modulating miR-26a/HMGB1 axis and regulating NF-κB activation. Am. J. Transl. Res. 2020, 12, 5449–5464. [Google Scholar] [CrossRef]
- Wang, H.; Liao, S.; Li, H.; Chen, Y.; Yu, J. Long non-coding RNA TUG1 sponges mir-145a-5p to regulate microglial polarization after oxygen glucose deprivation. Front. Mol. Neurosci. 2019, 12, 215. [Google Scholar] [CrossRef]

| No. (%) | |
|---|---|
| Age (years) | 40.3 ± 7.77 |
| Sex | |
| Male | 21 (14.5%) |
| Female | 124 (85.5%) |
| Disease duration (years) | 6.26 ± 5.63 |
| Malar rash | 81 (27.9%) |
| Discoid rash | 38 (13.1%) |
| Photosensitivity | 22 (7.6%) |
| Arthritis | 104 (35.9%) |
| Mucosal ulcers | 41 (14.1%) |
| Renal affection | 70 (24.1%) |
| Corticosteroid | 145 (50%) |
| Immunosuppressive | 72 (24.8%) |
| Anemic (Hb < 12.5) | 130 (89.7%) |
| WBC Leukopenia | 103 (71.0%) |
| Platelet count Thrombocytopenia | 89 (61.4%) |
| Albumin in urine | |
| Normal | 88 (60.7%) |
| Micro albuminuria | 29 (20.0%) |
| Macro albuminuria | 28 (19.3%) |
| Protein Creatinine ratio | 0.63 ± 0.83 |
| CRP | 51.5 ± 21.4 |
| ESR | 55.1 ± 31.1 |
| COOMB,S | 58 (40%) |
| Serum Creatinine (mg/dL) | 1.40 ± 0.64 |
| Serum urea | 62 ± 37.3 |
| Anti-ds-DNA | 124 (85.5%) |
| ANA | 140 (96.6%) |
| C3 (mg/dL) | 81.9 ± 33.7 |
| C4 (mg/dL) | 14.5 ± 8.79 |
| SLEDAI | 11.08 ± 6.64 |
| Mild (1–5) | 41 (28.3%) |
| Moderate (6–10) | 30 (20.7%) |
| High (11–19) | 47 (32.4%) |
| Very high (≥20) | 27 (18.6%) |
| LN | 70 (48.3%) |
| SLE (n = 145) | LN (n = 70) | Non-LN (n = 75) | Control (n = 145) | |
|---|---|---|---|---|
| rs5749201 | ||||
| HWχ2 (p) | 3.629 (0.057) | 3.926 (0.048) | 0.260 (0.610) | 3.542 (0.060) |
| rs886471 | ||||
| HWχ2 (p) | 3.256 (0.071) | 1.643 (0.200) | 0.059 (0.807 | 3.734 (0.053) |
| SLE (n = 145) | Control® (n = 145) | χ2 (p) | OR (LL–UL 95%C.I) | p0 | |
|---|---|---|---|---|---|
| rs5749201 | |||||
| Genotype | |||||
| TT® | 15 (10.3%) | (60 + 0) 60 (41.4%) | 55.696 * (<0.001 *) | 1.0 | |
| TA | 48 (33.1%) | (19 + 39) 58 (40.0%) | 3.310 (1.672–6.553) | 0.001 * | |
| AA | 82 (56.6%) | (66 − 39) 27 (18.6%) | 12.148 (5.951–24.799) | <0.001 * | |
| Dominant TA + AA vs. TT® | 130/15 | 85/60 | 36.419 * (<0.001 *) | 6.118 (3.263–11.468) | <0.001 * |
| Recessive AA vs. TT + TA® | 82/63 | 27/118 | 44.465 * (<0.001 *) | 5.688 (3.343–9.680) | <0.001 * |
| Allele | (n = 290) | (n = 290) | |||
| T® | 78 (26.9%) | 178 (61.4%) | 69.927 * (<0.001 *) | 1.0 | |
| A | 212 (73.1%) | 112 (38.6%) | 4.320 (3.041–6.136) | <0.001 * | |
| rs886471 | |||||
| Genotype | |||||
| TT® | 16 (11.0%) | (65 + 0) 65 (44.8%) | 59.351 (<0.001 *) | 1.0 | |
| TG | 50 (34.5%) | (17 + 39) 56 (38.6%) | 3.627 (1.862–7.066) | <0.001 * | |
| GG | 79 (54.5%) | (63 − 39) 24 (16.6%) | 13.372 (6.557–27.272) | <0.001 * | |
| Dominant TG + GG vs. TT® | 129/16 | 80/65 | 41.130 * (<0.001 *) | 6.551 (3.545–12.105) | <0.001 * |
| Recessive GG vs. TT + TG® | 79/66 | 24/121 | 45.545 * (<0.001 *) | 6.035 (3.545–12.105) | <0.001 * |
| Allele | (n = 290) | (n = 290) | |||
| T® | 82 (28.3%) | 186 (64.1%) | 75.025 * (<0.001 *) | 1.0 | |
| G | 208 (71.7%) | 104 (35.9%) | 4.537 (3.195–6.441) | <0.001 * | |
| Haplotype | (n = 290) | (n = 290) | |||
| TT® | 73 (25.2%) | 178 (61.4%) | 82.464 * (MC p < 0.001 *) | 1.0 | |
| TG | 5 (1.7%) | 0 (0.0%) | – | 0.999 | |
| AT | 9 (3.1%) | 8 (2.8%) | 2.743 (1.019–7.387) | 0.046 * | |
| AG | 203 (70.0%) | 104 (35.9%) | 4.759 (3.318–6.826) | <0.001 * |
| Genotype | LN (n = 70) | Non-LN® (n = 75) | χ2 (p) | p0 | OR (LL–UL 95% C.I) |
|---|---|---|---|---|---|
| rs5749201 | |||||
| Genotype | |||||
| TT® | 2 (2.9%) | 13 (17.3%) | 42.500 * (<0.001) | 1.0 | |
| TA | 9 (12.9%) | 39 (52.0%) | 0.631 | 1.50 (0.286–7.856) | |
| AA | 59 (84.3%) | 23 (30.7%) | <0.001 * | 16.67 (3.487–79.72) | |
| Dominant TA + AA vs. TT® | 68/2 | 62/13 | 8.181 * (0.004) | 0.012 * | 7.129 (1.547–32.86) |
| Recessive AA vs. TT + TA® | 59/11 | 23/52 | 42.365 * (<0.001) | <0.001 * | 12.13 (5.397–27.25) |
| Allele | (n = 140) | (n = 150) | |||
| T® | 13 (9.3%) | 65 (43.3%) | 42.693 * (<0.001) | 1.0 | |
| A | 127 (90.7%) | 85 (56.7%) | <0.001 * | 7.471 (3.878–14.393) | |
| rs886471 | |||||
| Genotype | |||||
| TT® | 2 (2.9%) | 14 (18.7%) | 36.175 * (<0.001) | 1.0 | |
| TG | 12 (17.1%) | 38 (50.7%) | 0.336 | 2.211 (0.439–11.142) | |
| GG | 56 (80.0%) | 23 (30.7%) | <0.001 * | 17.04 (3.585–81.03) | |
| Dominant TG + GG vs. TT ® | 68/2 | 61/14 | 9.218 * (0.002) | 0.008 * | 7.803 (1.704–35.73) |
| Recessive GG vs. TT + TG® | 56/14 | 23/52 | 35.533 * (<0.001) | <0.001 * | 9.043 (4.212–19.42) |
| Allele | (n = 140) | (n = 150) | |||
| T® | 16 (11.4%) | 66 (44.0%) | 37.880 * (<0.001) | 1.0 | |
| G | 124 (88.6%) | 84 (56.0%) | <0.001 * | 6.089 (3.301–11.234) | |
| Haplotype | (n = 140) | (n = 150) | |||
| TT® | 12 (8.6%) | 61 (40.7%) | 45.972 * (MC p < 0.001) | 1.0 | |
| TG | 1 (0.7%) | 4 (2.7%) | 0.837 | 1.271 (0.130–12.388) | |
| AT | 4 (2.9%) | 5 (3.3%) | 0.058 | 4.067 (0.951–17.392) | |
| AG | 123 (87.9%) | 80 (53.3%) | <0.001 * | 7.816 (3.960–15.426) |
| rs5749201 | Test of Sig. | p | TT vs. TA | TT vs. AA | TA vs. AA | |||
|---|---|---|---|---|---|---|---|---|
| TT (n = 15) | TA (n = 48) | AA (n = 82) | ||||||
| Age (years) | 36.1 ± 5.95 | 37.4 ± 8.89 | 42.8 ± 6.37 | F = 11.382 * | <0.001 * | 0.837 | 0.004 * | <0.001 * |
| Sex | ||||||||
| Male | 0 (0%) | 3 (6.3%) | 18 (22%) | χ2 = 8.860 * | 0.012 * | 1.000 a | 0.066 a | 0.019 * |
| Female | 15 (100%) | 45 (93.8%) | 64 (78%) | |||||
| Disease Duration (years) | 2.80 ± 1.07 | 4.09 ± 3.19 | 8.15 ± 6.45 | H = 16.053 * | <0.001 * | 0.466 | 0.003 * | 0.001 * |
| Malar rash | 10 (66.7%) | 21 (43.8%) | 50 (61.0%) | χ2 = 4.436 | 0.109 | – | – | – |
| Discoid rash | 3 (20.0%) | 17 (35.4%) | 18 (22.0%) | χ2 = 3.172 | 0.205 | – | – | – |
| Photosensitivity | 3 (20.0%) | 8 (16.7%) | 11 (13.4%) | χ2 = 0.552 | 0.759 | – | – | – |
| Arthritis | 4 (26.7%) | 42 (87.5%) | 58 (70.7%) | χ2 = 20.95 * | <0.001 * | <0.001 *a | 0.001 * | 0.029 * |
| Mucosal ulcers | 7 (46.7%) | 8 (16.7%) | 26 (31.7%) | χ2 = 6.167 * | 0.046 * | 0.033 *a | 0.261 | 0.060 |
| Renal affection | 2 (13.3%) | 9 (18.8%) | 59 (72.0%) | χ2 = 42.5 * | <0.001 * | 1.0 a | <0.001 * | <0.001 * |
| Anemia | 14 (93.3%) | 39 (81.3%) | 77 (93.9%) | χ2 = 4.935 | 0.067 b | – | – | – |
| WBC Leukopenia | 15 (100%) | 22 (45.8%) | 66 (80.5%) | χ2 = 24.494 * | <0.001 * | <0.001 * | 0.069 a | <0.001 * |
| thrombocytopenia | 1 (6.7%) | 23 (47.9%) | 65 (79.3%) | χ2 = 33.682 * | <0.001 * | 0.004 * | <0.001 *a | <0.001 * |
| CRP | 52.3 ± 18.0 | 45.2 ± 24.9 | 55.1 ± 19.1 | H = 11.579 * | 0.003 * | 0.587 | 0.015 * | 0.004 * |
| ESR | 35.0 ± 4.14 | 42.3 ± 30.8 | 66.2 ± 29.5 | H = 26.877 * | <0.001 * | 0.221 | <0.001 * | <0.001 * |
| Protein/Creatinine ratio | 0.22 ±0.19 | 0.42 ± 0.64 | 0.83 ± 0.93 | H = 31.173 * | <0.001 * | 0.153 | 0.038 * | <0.001 * |
| C3 (mg/dL) | 105 ±17.7 | 90.5 ± 34.9 | 72.6 ± 32.0 | H = 22.064 * | <0.001 * | 0.096 | <0.001 * | 0.001 * |
| C4 (mg/dL) | 22.3 ± 2.84 | 16.5 ± 8.10 | 11.9 ± 8.81 | H = 29.120 * | <0.001 * | 0.034 * | <0.001 * | <0.001 * |
| ANA | 15 (100%) | 44 (91.7%) | 81 (98.8%) | χ2 = 4.002 | 0.090 b | – | – | – |
| ds-DNA | 3 (20.0%) | 42 (87.5%) | 79 (96.3%) | χ2 = 59.90 * | <0.001 * | <0.001 *a | <0.001 *a | 0.075 a |
| SLEDAI | ||||||||
| High (11–19) | 0 (0.0%) | 7 (14.6%) | 40 (48.8%) | χ2 = 24.186 * | <0.001 * | 0.182 a | <0.001 * | <0.001 * |
| Very high (≥20) | 3 (20.0%) | 2 (4.2%) | 22 (26.8%) | χ2 = 10.283 * | 0.006 * | 0.083 a | 0.753 a | 0.001 * |
| rs886471 | Test of Sig. | p | TT vs. TG | TT vs. GG | TG vs. GG | |||
|---|---|---|---|---|---|---|---|---|
| TT (n = 16) | TG (n = 50) | GG (n = 79) | ||||||
| Age (years) | 37.6 ± 5.1 | 37.2 ± 9.14 | 42.9 ± 6.27 | F = 10.725 * | <0.001 * | 0.980 | 0.023 * | <0.001 * |
| Sex | ||||||||
| Male | 0 (0%) | 4 (8.0%) | 17 (21.5%) | χ2 = 7.564 * | 0.023 * | 0.565 | 0.067 a | 0.043 * |
| Female | 16 (100%) | 46 (92.0%) | 62 (78.5%) | |||||
| Disease duration (years) | 4.03 ± 3.23 | 3.73 ± 3.01 | 8.30 ± 6.43 | H = 18.365 * | <0.001 * | 0.589 | 0.031 * | <0.001 * |
| Malar rash | 10 (62.5%) | 22 (44.0%) | 49 (62.0%) | χ2 = 4.356 | 0.113 | – | – | – |
| Discoid rash | 3 (18.8%) | 17 (34.0%) | 18 (22.8%) | χ2 = 2.509 | 0.285 | – | – | – |
| Photosensitivity | 5 (31.3%) | 7 (14.0%) | 10 (12.7%) | χ2 = 3.655 | 0.161 | – | – | – |
| Arthritis | 4 (25.0%) | 43 (86.0%) | 57 (72.2%) | χ2 = 22.25 * | <0.001 * | <0.001 *a | <0.001 * | 0.066 |
| Mucosal ulcers | 8 (50.0%) | 7 (14.0%) | 26 (32.9%) | χ2 = 9.585 * | 0.008 * | 0.006 *a | 0.194 | 0.016 * |
| Renal affection | 2 (12.5%) | 12 (24.0%) | 56 (70.9%) | χ2 = 36.2 * | <0.001 * | 0.488 a | <0.001 * | <0.001 * |
| Anemia | 16 (100%) | 40 (80%) | 74 (93.7%) | χ2 = 8.245 * | 0.016 * | 0.102 a | 0.585 a | 0.018 * |
| Leucopenia | 15 (93.8%) | 24 (48.0%) | 64 (81.0%) | χ2 = 20.729 * | <0.001 * | 0.001 * | 0.293 a | <0.001 * |
| thrombocytopenia | 1 (6.3%) | 23 (46.0%) | 65 (82.3%) | χ2 = 40.059 * | <0.001 * | 0.004 * | <0.001 *a | <0.001 * |
| CRP | 46.2 ± 23.1 | 44.7 ± 24.9 | 56.9 ± 17.0 | H = 18.910 * | <0.001 * | 0.357 | 0.001 * | <0.001 * |
| ESR | 44.4 ± 29.6 | 39.2 ± 26.7 | 67.2 ± 29.0 | H = 27.795 * | <0.001 * | 0.960 | 0.001 * | <0.001 * |
| Protein/creatinine ratio | 0.43 ± 0.67 | 0.43 ± 0.64 | 0.80 ± 0.93 | H = 28.119 * | <0.001 * | 0.112 | 0.069 | <0.001 * |
| C3 (mg/dL) | 103 ± 20.4 | 92.5 ± 33.9 | 70.9 ± 31.7 | H = 24.682 * | <0.001 * | 0.238 | <0.001 * | <0.001 * |
| C4 (mg/dL) | 21.6 ± 3.76 | 17.2 ± 8.12 | 11.4 ± 8.56 | H = 35.036 * | <0.001 * | 0.084 | <0.001 * | <0.001 * |
| ANA | 16 (100%) | 46 (92.0%) | 78 (98.7%) | χ2 = 3.671 | 0.105 b | – | – | – |
| ds-DNA | 5 (31.3%) | 43 (86.0%) | 76 (96.2%) | χ2 = 45.34 * | <0.001 * | <0.001 *a | <0.001 *a | 0.046 *a |
| SLEDAI | ||||||||
| High (11–19) | 0 (0%) | 7 (14.0%) | 40 (50.6%) | χ2 = 27.382 * | <0.001 * | 0.181 a | <0.001 * | <0.001 * |
| Very high (≥20) | 2 (12.5%) | 4 (8.0%) | 21 (26.6%) | χ2 = 7.422 * | 0.024 * | 0.627 a | 0.342 a | 0.009 * |
| # Multivariate | # Univariate | |||
|---|---|---|---|---|
| OR (95% C.I = LL–UL) | p | OR (95% C.I = LL–UL) | p | |
| Disease duration | 0.990 (0.934–1.049) | 0.725 | ||
| Presence of Discoid rash | 0.331 (0.149–0.736) | 0.007 * | 0.133 (0.037–0.476) | 0.002 * |
| Presence of Arthritis | 0.488 (0.233–1.021) | 0.057 | ||
| Presence of Mucosal ulcer | 1.351 (0.654–2.789) | 0.416 | ||
| High SLEDAI (11–19) | 1.727 (0.855–3.486) | 0.128 | ||
| Very high SLEDAI (≥20) | 6.417 (2.272–18.121) | <0.001 * | 13.861 (3.093–62.113) | 0.001 * |
| rs5749201 genotypes | ||||
| TT® | 1.000 | 1.000 | ||
| TA | 1.500 (0.286–7.856) | 0.631 | 0.907 (0.095–8.673) | 0.932 |
| AA | 16.674 (3.487–79.724) | <0.001 * | 70.794 (1.369–3662.20) | 0.034 * |
| rs886471 genotypes | ||||
| TT® | 1.000 | 1.000 | ||
| TG | 2.211 (0.439–11.142) | 0.336 | 6.262 (0.448–87.503) | 0.173 |
| GG | 17.043 (3.585–81.032) | <0.001 * | 0.235 (0.008–7.130) | 0.406 |
| Lnc RNA TUG1 | 2.639 (1.887–3.689) | <0.001 * | 1.701 (0.969–2.984) | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawfeek, G.A.-E.; Kasem, H.; Abdallah, E.A.; Almulhim, M.; Almulhim, A.; Albarqi, M.; Elzorkany, K.M.A. Long Non-Coding RNA TUG1 Gene Polymorphism and TUG1 Expression Level as Molecular Biomarkers of Systemic Lupus Erythematosus and Lupus Nephritis. Non-Coding RNA 2023, 9, 56. https://doi.org/10.3390/ncrna9050056
Tawfeek GA-E, Kasem H, Abdallah EA, Almulhim M, Almulhim A, Albarqi M, Elzorkany KMA. Long Non-Coding RNA TUG1 Gene Polymorphism and TUG1 Expression Level as Molecular Biomarkers of Systemic Lupus Erythematosus and Lupus Nephritis. Non-Coding RNA. 2023; 9(5):56. https://doi.org/10.3390/ncrna9050056
Chicago/Turabian StyleTawfeek, Gehan Abd-Elfatah, Heba Kasem, Eman Ali Abdallah, Mohammed Almulhim, Abdullah Almulhim, Mohammed Albarqi, and Khaled Mohamed Amin Elzorkany. 2023. "Long Non-Coding RNA TUG1 Gene Polymorphism and TUG1 Expression Level as Molecular Biomarkers of Systemic Lupus Erythematosus and Lupus Nephritis" Non-Coding RNA 9, no. 5: 56. https://doi.org/10.3390/ncrna9050056
APA StyleTawfeek, G. A.-E., Kasem, H., Abdallah, E. A., Almulhim, M., Almulhim, A., Albarqi, M., & Elzorkany, K. M. A. (2023). Long Non-Coding RNA TUG1 Gene Polymorphism and TUG1 Expression Level as Molecular Biomarkers of Systemic Lupus Erythematosus and Lupus Nephritis. Non-Coding RNA, 9(5), 56. https://doi.org/10.3390/ncrna9050056






