Impacts of MicroRNA-483 on Human Diseases
Abstract
1. Introduction
2. MiR-483 Biogenesis
3. Role of MiR-483-5p in Diabetes
3.1. MiR-483-5p Protects Pancreatic β-Cells Function and Identity
3.2. MiR-483-5p Mitigates Hyperlipidemia-Associated Fatty Liver Disease
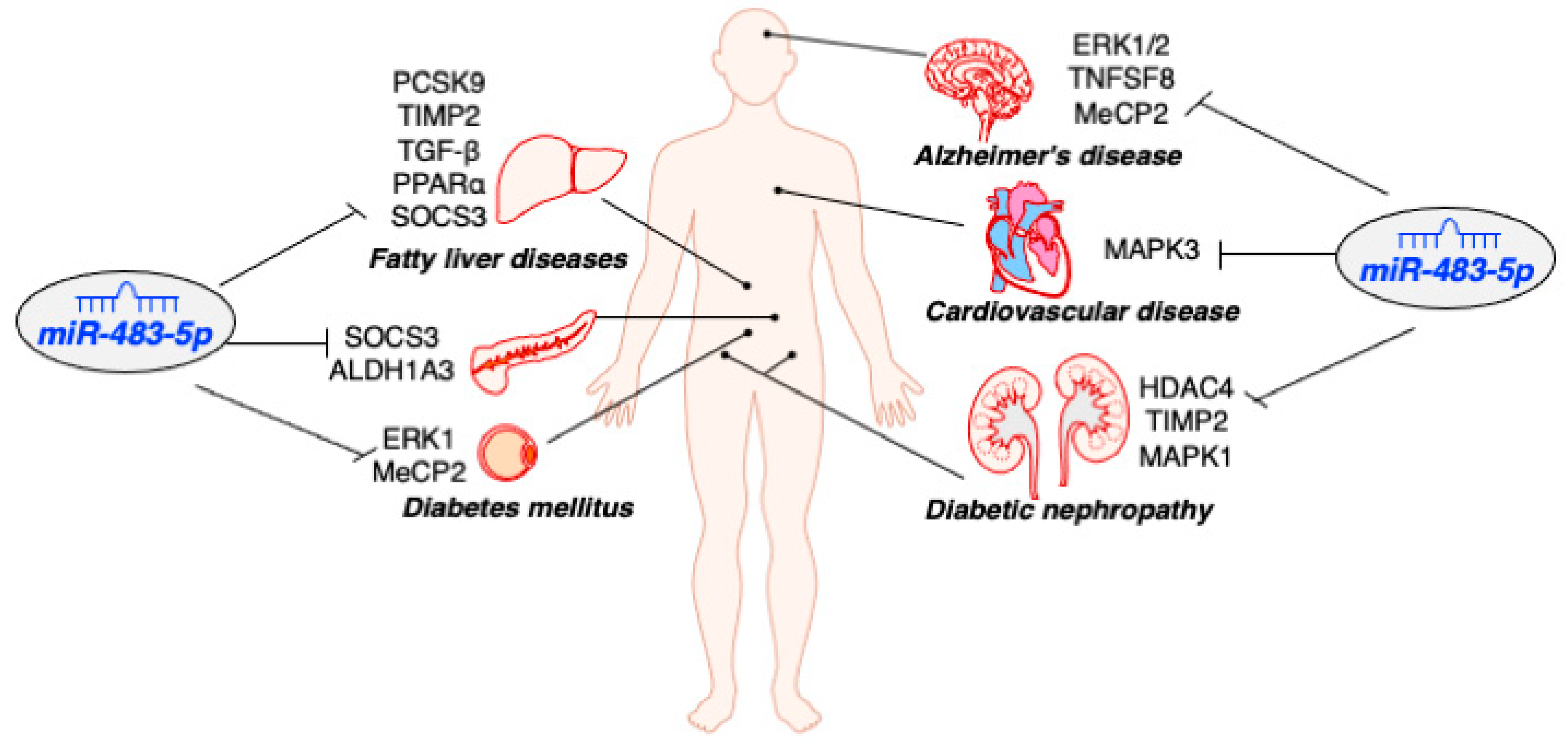
| Type of Disease | Expression Pattern | Tissue | Cell Lines | Targets | Function | References |
|---|---|---|---|---|---|---|
| Type 2 Diabetes | down | pancreatic islets | MIN6 | SOCS3 ALDH1A3 | Induce insulin secretion, inhibit glucagon secretion, and maintain β-cell identity | [16,24] |
| Obesity/ Diabetes | down | adipose | 3T3-L1 | ERK1 MeCP2 | Promote adipogenesis | [41,42,43] |
| Fatty liver disease (NAFLD/ AFLD) | down | liver | HepG2 | PCSK9 TIMP2 TGF-β PPARα SOCS3 | Reduce lipid deposition and inhibit liver fibrosis | [32,33,38,39] |
| Diabetic nephropathy | down | Kidney tubule | HK2 TCMK-1 | HDAC4 TIMP2 MAPK1 | Prevent renal tubular damage and renal fibrosis | [44,45] |
| Alzheimer, Brain injury after cardiac arrest | down | neuron | Neonatal Fibroblasts, PC12 | ERK1/2 TNFSF8 MeCP2 | Promote mitochondrial biogenesis, inhibit ROS generation, protect neurological function, and regulate fetal brain development | [46] |
| Cardiovascular disease | up | serum, carotid bulb | AC16 | MAPK3 | Induced cell apoptosis and oxidative stress | [18,47,48,49] |
3.3. MiR-483-5p Promotes Adipogenesis in the Adipose
4. Role of MiR-483-5p in Other Human Diseases
4.1. MiR-483-5p Downregulation in Patients with Diabetic Nephropathy
4.2. MiR-483-5p Protects Neurological Function against Oxidative Stress
4.3. Elevation of Circulating MiR-483-5p as a Biomarker for Cardiovascular Disease
5. Implication of MiR-483-3p in Human Disease
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Handy, R.M.; Holloway, G.P. Insights into the development of insulin resistance: Unraveling the interaction of physical inactivity, lipid metabolism and mitochondrial biology. Front. Physiol. 2023, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Dinić, S.; Jovanović, J.A.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H. Metabolic Impact of Frailty Changes Diabetes Trajectory. Metabolites 2023, 13, 295. [Google Scholar] [CrossRef]
- Yoshida, T.; Asano, Y.; Ui-Tei, K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Non Coding RNA 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- LaPierre, M.P.; Stoffel, M. MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol. Metab. 2017, 6, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef]
- Guay, C.; Menoud, V.; Rome, S.; Regazzi, R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun. Signal. 2015, 13, 17. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Polychronakos, C.; Kukuvitis, A.; Giannoukakis, N.; Colle, E. Parental imprinting effect at the INS-IGF2 diabetes susceptibility locus. Diabetologia 1995, 38, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Tabano, S.; Colapietro, P.; Cetin, I.; Grati, F.R.; Zanutto, S.; Mandò, C.; Antonazzo, P.; Pileri, P.; Rossella, F.; Larizza, L. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics 2010, 5, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C.; Borai, A. Insulin-like growth factor-II: Its role in metabolic and endocrine dis-ease. Clin. Endocrinol. 2014, 80, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wang, X.; Qiao, Y.; Li, F.; Hui, Y.; Zou, C.; Jin, J.; Lv, G.; Peng, Y.; Wang, L.; et al. Coexpression of an intronic microRNA and its host gene reveals a potential role for miR-483-5p as an IGF2 partner. Mol. Cell. Endocrinol. 2011, 333, 96–101. [Google Scholar] [CrossRef]
- Le, F.; Wang, L.Y.; Wang, N.; Li, L.; Li, L.J.; Zheng, Y.M.; Lou, H.Y.; Liu, X.Z.; Xu, X.R.; Sheng, J.Z. In vitro fertilization alters growth and expression of Igf2/H19 and their epigenetic mechanisms in the liver and skeletal muscle of newborn and elder mice. Biol. Reprod. 2013, 88, 75. [Google Scholar] [CrossRef]
- Mohan, R.; Mao, Y.; Zhang, S.; Zhang, Y.W.; Xu, C.R.; Gradwohl, G.; Tang, X. Differentially Expressed MicroRNA-483 Confers Distinct Functions in Pancreatic beta- and alpha-Cells. J. Biol. Chem. 2015, 290, 19955–19966. [Google Scholar] [CrossRef]
- Emmerling, V.V.; Fischer, S.; Stiefel, F.; Holzmann, K.; Handrick, R.; Hesse, F.; Hörer, M.; Kochanek, S.; Otte, K. Temperature-sensitive miR-483 is a conserved regulator of recombinant protein and viral vector production in mammalian cells. Biotechnol. Bioeng. 2015, 113, 830–841. [Google Scholar] [CrossRef]
- Gallo, W.; Esguerra JL, S.; Eliasson, L.; Melander, O. miR-483-5p associates with obesity and insulin resistance and independently asso-ciates with new onset diabetes mellitus and cardiovascular disease. PLoS ONE 2018, 13, e0206974. [Google Scholar] [CrossRef]
- Veronese, A.; Visone, R.; Consiglio, J.; Acunzo, M.; Lupini, L.; Kim, T.; Ferracin, M.; Lovat, F.; Miotto, E.; Balatti, V.; et al. Mutated beta-catenin evades a microRNA-dependent regulatory loop. Proc. Natl. Acad. Sci. USA 2011, 108, 4840–4845. [Google Scholar] [CrossRef]
- Kuschnerus, K.; Straessler, E.T.; Müller, M.F.; Lüscher, T.F.; Landmesser, U.; Kränkel, N. Increased Expression of miR-483-3p Impairs the Vascular Response to Injury in Type 2 Diabetes. Diabetes 2018, 68, 349–360. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Gregg, B.; Blandino-Rosano, M.; Cras-Méneur, C.; Bernal-Mizrachi, E. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol. Aspects Med. 2015, 42, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Landsman, L.; Parent, A.; Hebrok, M. Elevated Hedgehog/Gli signaling causes beta-cell dedif-ferentiation in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 17010–17015. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell fail-ure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mohan, R.; Chen, X.; Matson, K.; Waugh, J.; Mao, Y.; Li, W.; Tang, X.; Satin, L.S. microRNA-483 Protects Pancreatic beta-Cells by Targeting ALDH1A3. Endocrinology 2021, 162, bqab031. [Google Scholar] [CrossRef] [PubMed]
- Kim-Muller, J.Y.; Fan, J.; Kim YJ, R.; Lee, S.A.; Ishida, E.; Blaner, W.S.; Accili, D. Aldehyde dehydrogenase 1a3 defines a subset of failing pancreatic beta cells in diabetic mice. Nat. Commun. 2016, 7, 12631. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Rotella, C.M.; Giannini, S. Dyslipidemia and Diabetes: Reciprocal Impact of Impaired Lipid Metabolism and Beta-Cell Dysfunction on Micro- and Macrovascular Complications. Rev. Diabet. Stud. 2012, 9, 82–93. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabès, J.-P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Lopez, Y.O.N.; Retnakaran, R.; Zinman, B.; Pratley, R.E.; Seyhan, A.A. Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol. Metab. 2018, 20, 63–78. [Google Scholar] [CrossRef]
- Bourbon, M.; Alves, A.C.; Sijbrands, E.J. Low-density lipoprotein receptor mutational analysis in diagnosis of familial hypercholesterolemia. Curr. Opin. Infect. Dis. 2017, 28, 120–129. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.-W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Mao, L.; Zuo, M.L.; Song, G.L.; Tan, L.M.; Yang, Z.B. The Role of MicroRNAs in Hyperlipidemia: From Pathogenesis to Therapeutical Ap-plication. Mediators Inflamm. 2022, 2022, 3101900. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; He, M.; Li, J.; Pessentheiner, A.; Wang, C.; Zhang, J.; Sun, Y.; Wang, W.-T.; Zhang, Y.; Liu, J.; et al. microRNA-483 ameliorates hypercholesterolemia by inhibiting PCSK9 production. J. Clin. Investig. 2020, 5, e143812. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Lagace, T.A.; McNutt, M.C.; Horton, J.D.; Deisenhofer, J. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA 2008, 105, 1820–1825. [Google Scholar] [CrossRef]
- Zhang, P.-Y. PCSK9 as a therapeutic target for cardiovascular disease. Exp. Ther. Med. 2017, 13, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Zimmers, T.A.; Koniaris, L.G.; Furlanetto, R.W.; Mooney, R.A. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleu-kin-6-dependent insulin resistance in hepatocytes. J. Biol. Chem. 2003, 278, 13740–13746. [Google Scholar] [CrossRef]
- Bjørbæk, C.; Elmquist, J.K.; Frantz, J.D.; Shoelson, S.E.; Flier, J.S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1998, 1, 619–625. [Google Scholar] [CrossRef]
- Li, F.; Ma, N.; Zhao, R.; Wu, G.; Zhang, Y.; Qiao, Y.; Han, D.; Xu, Y.; Xiang, Y.; Yan, B. Overexpression of miR-483-5p/3p cooperate to inhibit mouse liver fibrosis by sup-pressing the TGF-beta stimulated HSCs in transgenic mice. J. Cell Mol. Med. 2014, 18, 966–974. [Google Scholar] [CrossRef]
- Niture, S.; Gadi, S.; Qi, Q.; Gyamfi, M.A.; Varghese, R.S.; Rios-Colon, L.; Chimeh, U.; Vandana; Ressom, H.W.; Kumar, D. MicroRNA-483-5p Inhibits Hepatocellular Carcinoma Cell Proliferation, Cell Steatosis, and Fibrosis by Targeting PPARα and TIMP2. Cancers 2023, 15, 1715. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Lacedonia, D.; Tartaglia, N.; Scioscia, G.; Soccio, P.; Pavone, G.; Moriondo, G.; Gallo, C.; Barbaro, M.P.F.; Ambrosi, A. Different Expression of Micro-RNA in the Subcutaneous and Visceral Adipose Tissue of Obese Subjects. Rejuvenation Res. 2022, 25, 89–94. [Google Scholar] [CrossRef]
- Chen, K.; He, H.; Xie, Y.; Zhao, L.; Zhao, S.; Wan, X.; Yang, W. miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci. Rep. 2015, 5, 11909. [Google Scholar] [CrossRef]
- Giuliani, A.; Sabbatinelli, J.; Amatori, S.; Graciotti, L.; Silvestrini, A.; Matacchione, G.; Ramini, D.; Mensà, E.; Prattichizzo, F.; Babini, L.; et al. MiR-422a promotes adipogenesis via MeCP2 downregulation in human bone marrow mesenchymal stem cells. Cell. Mol. Life Sci. 2023, 80, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, H.; Yun, J.; Song, L.; Ma, X.; Luo, S.; Song, Y. miRNA-483-5p Targets HDCA4 to Regulate Renal Tubular Damage in Diabetic Nephropathy. Horm. Metab. Res. 2021, 53, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, F.; Li, Z.; Pan, S.; Xie, J.; Zhao, Z.; Liu, Z.; Zhang, J.; Liu, Z. HNRNPA1-mediated exosomal sorting of miR-483-5p out of renal tubular epithelial cells promotes the progression of diabetic nephropathy-induced renal interstitial fibrosis. Cell Death Dis. 2021, 12, 255. [Google Scholar] [CrossRef]
- Han, K.; Gennarino, V.A.; Lee, Y.; Pang, K.; Hashimoto-Torii, K.; Choufani, S.; Raju, C.S.; Oldham, M.C.; Weksberg, R.; Rakic, P.; et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes Dev. 2013, 27, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.; Wu, M.; Chi, C.; Hu, D.; Cui, Y.; Song, J.; Lee, C.; Chen, H. Early diagnostic value of circulating microRNAs in patients with suspected acute myo-cardial infarction. J. Cell. Physiol. 2019, 234, 13649–13658. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, H.; Yu, H. Retracted article: Downregulation of miR-483-5p decreases hypoxia-induced injury in human cardiomyocytes by targeting MAPK3. Cell. Mol. Biol. Lett. 2020, 25, 20. [Google Scholar] [CrossRef]
- Harling, L.; Lambert, J.; Ashrafian, H.; Darzi, A.; Gooderham, N.J.; Athanasiou, T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardio Thoracic Surg. 2017, 51, 73–78. [Google Scholar] [CrossRef]
- Artasensi, A.; Mazzolari, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Thera-peutic Options. Molecules 2023, 28, 3094. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2016, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting perox-isome proliferator-activated receptor gamma with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, J.; Wei, Y.; Zhang, W.; Geng, M.; Yuan, Y.; Chen, Y.; Sun, Y.; Chen, H.; Zhang, Y.; et al. Fat-Specific Knockout of Mecp2 Upregulates Slpi to Reduce Obesity by Enhancing Browning. Diabetes 2019, 69, 35–47. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Luo, Z.-W.; Li, F.-X.; Cao, J.; Rao, S.-S.; Liu, Y.-W.; Wang, Y.-Y.; Zhu, G.-Q.; Gong, J.-S.; Zou, J.-T.; et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat. Commun. 2022, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Sun, Q.; Wang, Y.; Yang, J.; Yang, J.; Zhang, T.; Luo, S.; Wang, L.; Jiang, Y. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 2017, 25, 715–727. [Google Scholar] [CrossRef]
- Usui, I. Common metabolic features of hypertension and type 2 diabetes. Hypertens. Res. 2023, 46, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Schanz, M.; Kimmel, M.; Alscher, M.D.; Amann, K.; Daniel, C. TIMP-2 and IGFBP7 in human kidney biopsies in renal disease. Clin. Kidney J. 2023, 16, sfad010. [Google Scholar] [CrossRef]
- Sonoda, H.; Lee, B.R.; Park, K.-H.; Nihalani, D.; Yoon, J.-H.; Ikeda, M.; Kwon, S.-H. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019, 9, 4692. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, H.; Liu, C.; Zhang, C.; Wei, H.; Li, B.; Zhou, D.; Lu, Y.; Huang, S.; Cheng, J. Neuroprotective Effect of miR-483-5p Against Cardiac Arrest-Induced Mitochon-drial Dysfunction Mediated Through the TNFSF8/AMPK/JNK Signaling Pathway. Cell. Mol. Neurobiol. 2022, 43, 2179–2202. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Want, A.; Laskowska-Kaszub, K.; Fesiuk, A.; Vaz, S.; Logarinho, E.; Wojda, U. Candidate Alzheimer’s Disease Biomarker miR-483-5p Lowers TAU Phosphoryla-tion by Direct ERK1/2 Repression. Int. J. Mol. Sci. 2021, 22, 3653. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McMurray, J.; Boren, J.; Rawshani, A.; Omerovic, E.; Berg, N.; Holminen, J.; Skoglund, K.; Eliasson, B.; Gerstein, H.C.; et al. Twenty Years of Cardiovascular Complications and Risk Factors in Patients with Type 2 Diabetes: A Nationwide Swedish Cohort Study. Circulation 2023, 147, 1872–1886. [Google Scholar] [CrossRef]
- Li, R.; Jiang, L.; Wang, X. Aberrant expression of miR-483-5p in patients with asymptomatic carotid artery stenosis and its predictive value for cerebrovascular event occurrence. Exp. Ther. Med. 2021, 22, 1101. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lee, C.; Song, J.; Lu, C.; Liu, J.; Cui, Y.; Liang, H.; Cao, C.; Zhang, F.; Chen, H. Circulating microRNAs as potential biomarkers for coronary plaque rupture. Oncotarget 2017, 8, 48145–48156. [Google Scholar] [CrossRef] [PubMed]
- Gallo, W.; Ottosson, F.; Kennbäck, C.; Jujic, A.; Esguerra, J.L.S.; Eliasson, L.; Melander, O. Replication study reveals miR-483-5p as an important target in prevention of car-diometabolic disease. BMC Cardiovasc. Disord. 2021, 21, 162. [Google Scholar] [CrossRef]
- Pepe, F.; Visone, R.; Veronese, A. The Glucose-Regulated MiR-483-3p Influences Key Signaling Pathways in Cancer. Cancers 2018, 10, 181. [Google Scholar] [CrossRef]
- Bork-Jensen, J.; Thuesen, A.C.B.; Bang-Bertelsen, C.H.; Grunnet, L.G.; Pociot, F.; Beck-Nielsen, H.; Ozanne, S.; Poulsen, P.; Vaag, A. Genetic versus Non-Genetic Regulation of miR-103, miR-143 and miR-483-3p Expression in Adipose Tissue and Their Metabolic Implications-A Twin Study. Genes 2014, 5, 508–517. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Twinn, D.; Cannell, I.; David, H.; Warner, M.; Vaag, A.; Bork-Jensen, J.; Brøns, C.; Gant, T.; Willis, A.; et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2017, 19, 1003–1012. [Google Scholar] [CrossRef]
- Kong, L.; Hu, N.; Du, X.; Wang, W.; Chen, H.; Li, W.; Wei, S.; Zhuang, H.; Li, X.; Li, C. Upregulation of miR-483-3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J. Transl. Med. 2016, 14, 23. [Google Scholar] [CrossRef]
- Fleischhacker, S.N.; Bauersachs, S.; Wehner, A.; Hartmann, K.; Weber, K. Differential expression of circulating microRNAs in diabetic and healthy lean cats. Veter J. 2013, 197, 688–693. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhao, Y.; Liu, Y.; Ma, N.; Wang, C.; Zou, J.; Liu, Z.; Zhou, Z.; Han, D.; He, J. miR-483-3p regulates hyperglycaemia-induced cardiomyocyte apoptosis in trans-genic mice. Biochem. Biophys. Res. Commun. 2016, 477, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Guo, X.; Chen, Y.; Wang, C.; Gao, J.; Wen, E.; Lai, B.; Bai, L. Endothelial MicroRNA-483-3p Is Hypertension-Protective. Oxidative Med. Cell. Longev. 2022, 2022, 3698219. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Esmerats, J.; Villa-Roel, N.; Kumar, S.; Gu, L.; Salim, M.T.; Ohh, M.; Taylor, W.R.; Nerem, R.M.; Yoganathan, A.P.; Jo, H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1alpha (Hypoxia-Inducible Factor-1alpha) Pathway in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 467–481. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, Q.; Guo, Z.; Wu, L.; Chen, Y.; Chen, Z.; Yang, K.; Cao, J. Therapeutic Hypothermia Inhibits Hypoxia-Induced Cardiomyocyte Apoptosis Via the MiR-483-3p/Cdk9 Axis. J. Am. Heart Assoc. 2023, 12, e026160. [Google Scholar] [CrossRef] [PubMed]

| Type of Disease | Expression Pattern | Tissue | Cell Lines | Targets | Function | Refs |
|---|---|---|---|---|---|---|
| prediabetes/ type 2 diabetes | up | adipose | 3T3-L1 | GDF3 | Induce lipotoxicity and insulin resistance | [69] |
| diabetic vascular disease | up | vascular endothelium, endothelial progenitor cells, cardiomyocytes | HAEC, H9C2 | VEZF1 SRF1 IGF1 | Induce apoptosis in cardiomyocytes and endothelial cells | [20,70,71,72] |
| cardiovascular disease, hypertension, aortic valve calcification | down | serum, heart/aortic valve endothelial cells, cardiomyocytes | TGF-β CTGF ACE1 ET1 UBE2C CDK9 | Inhibit apoptosis, Protects endothelial function against inflammation | [73,74,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matson, K.; Macleod, A.; Mehta, N.; Sempek, E.; Tang, X. Impacts of MicroRNA-483 on Human Diseases. Non-Coding RNA 2023, 9, 37. https://doi.org/10.3390/ncrna9040037
Matson K, Macleod A, Mehta N, Sempek E, Tang X. Impacts of MicroRNA-483 on Human Diseases. Non-Coding RNA. 2023; 9(4):37. https://doi.org/10.3390/ncrna9040037
Chicago/Turabian StyleMatson, Katy, Aaron Macleod, Nirali Mehta, Ellie Sempek, and Xiaoqing Tang. 2023. "Impacts of MicroRNA-483 on Human Diseases" Non-Coding RNA 9, no. 4: 37. https://doi.org/10.3390/ncrna9040037
APA StyleMatson, K., Macleod, A., Mehta, N., Sempek, E., & Tang, X. (2023). Impacts of MicroRNA-483 on Human Diseases. Non-Coding RNA, 9(4), 37. https://doi.org/10.3390/ncrna9040037







