LncRNA-Dependent Mechanisms of Transforming Growth Factor-β: From Tissue Fibrosis to Cancer Progression
Abstract
1. Introduction
2. TGF-β1 Signaling Pathways
3. TGF-β1 Signaling in Kidney Diseases
3.1. TGF-β1-Associated lncRNAs in Kidney Diseases
3.1.1. lncRNAs in TGF-β1 Induced EMT
| LncRNA | Biological Process | Model | Species | Mechanism | Year | Ref. |
|---|---|---|---|---|---|---|
| lnc453774.1 | anti-fibrosis | HK-2 cells | Human | associated with ceRNAs targeting FBN1, IGF1R, KLF7 PPI networks | 2021 | [61] |
| ATB | pro-inflammation | HK-2 cells | Human | promotes apoptosis, senescence, inflammatory cytokines (TNF-α, IL-1β, and IL-6), and adhesion molecules (VCAM-1 and sE-selectin) expression | 2020 | [62] |
| HOTAIR | pro-fibrosis | UUO, TECs-HK-2 | Human | promotes EMT via Notch1 and miR-124 | 2019 | [58] |
| ENST00000453774.1 | anti-fibrosis | Renal biopsy, UUO, TECs-HK-2 | Human | promotes autophagy (Atg5/7) and Nrf2-driven HO-1 expression and suppresses ECM synthesis (Fn, Col-I) | 2019 | [63] |
| MEG3 | anti-fibrosis | HK-2 cells | Human | suppresses EMT of HK2 cells and is regulated by miR-185/DNMT1/MEG3 pathway | 2019 | [59] |
| TCONS_00088786 | pro-fibrosis | UUO, NRK52E cells | Rat | promotes collagen I, III, and miR-132 expression | 2018 | [60] |
| pro-fibrosis | RNA-seq of rat UUO, NRK52E cells | Rat | promotes Col1a1 and Col3a1 expression | 2017 | [64] | |
| TCONS_01496394 | promotes Ctgf and Fn1 expression | |||||
| ASncmtRNA-2 | pro-fibrosis | HRMC, DN | Human, mouse | promotes TGF-β and Fn1 expression | 2017 | [65] |
| lnc-MGC | pro-fibrosis | STZ-DN, MMC, MCs | Human, mouse | host of miRNA mega-clusters regulating profibrotic genes expression | 2016 | [57] |
| PVT1 | pro-fibrotic | MC, RPTEC, podocytes | Human | PVT1-derived miR-1207-5p-induced TGF-β1, PAI-1, and FN1 | 2013 | [56] |
| pro-fibrotic | ESRD-T2D GWAS | Human | 23 SNPs associated with ESRD | 2007 | [55] |
3.1.2. lncRNAs Associated with Reactive Oxygen Species
3.2. Smad3-Dependent lncRNAs in Kidney Diseases
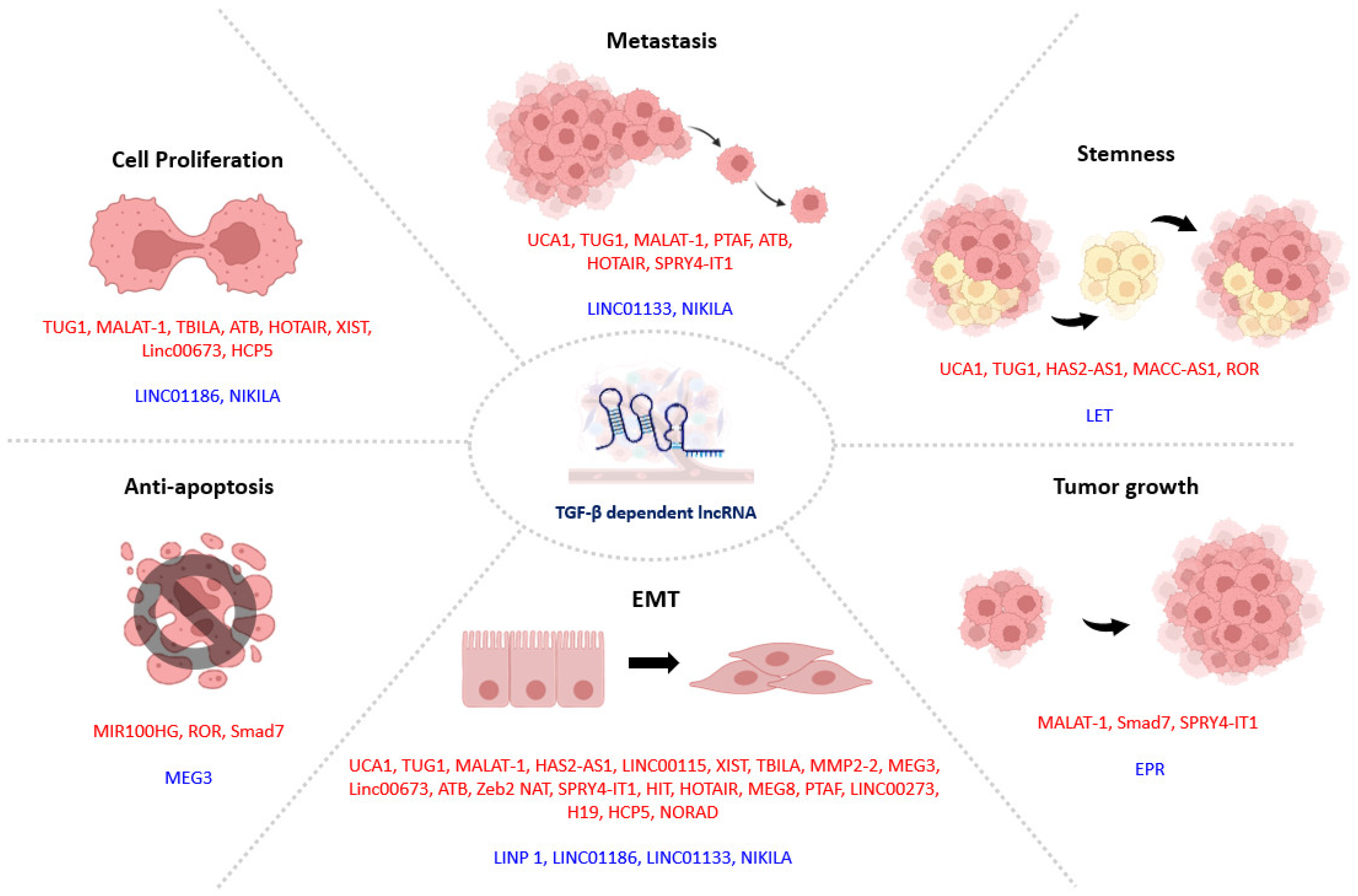
4. TGF-β1 Signaling in Tumor Progression
4.1. TGF-β1-Dependent lncRNAs in Tumor Progression
4.1.1. lncRNAs in TGF-β1 Induced EMT
4.1.2. lncRNAs Associated with TGF-β1-Induced Drug Resistance
4.2. Smad3-Associated lncRNAs in Tumor Progression
5. Therapeutic Strategies Targeting lncRNAs
6. Conclusions
Funding
Conflicts of Interest
References
- Tang, P.M.; Zhang, Y.Y.; Lan, H.Y. LncRNAs in TGF-beta-Driven Tissue Fibrosis. Noncoding RNA 2018, 4, 26. [Google Scholar] [CrossRef]
- Wei, L.H.; Guo, J.U. Coding functions of “noncoding” RNAs. Science 2020, 367, 1074–1075. [Google Scholar] [CrossRef]
- Van der Hauwaert, C.; Glowacki, F.; Pottier, N.; Cauffiez, C. Non-Coding RNAs as New Therapeutic Targets in the Context of Renal Fibrosis. Int. J. Mol. Sci. 2019, 20, 1977. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Yang, F.; Wang, F.; Ma, J.Z.; Guo, Y.J.; Tao, Q.F.; Liu, F.; Pan, W.; Wang, T.T.; Zhou, C.C.; et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014, 25, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Chan, M.K.; Li, J.S.; Chan, A.S.; Tang, P.C.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M. TGF-beta Signaling: From Tissue Fibrosis to Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 7575. [Google Scholar] [CrossRef]
- Gil, N.; Ulitsky, I. Regulation of gene expression by cis-acting long non-coding RNAs. Nat. Rev. Genet. 2020, 21, 102–117. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Lan, H.-Y. MicroRNAs in TGF-β/Smad-mediated Tissue Fibrosis. Curr. Pathobiol. Rep. 2014, 2, 235–243. [Google Scholar] [CrossRef][Green Version]
- Tang, P.C.; Zhang, Y.Y.; Chan, M.K.; Lam, W.W.; Chung, J.Y.; Kang, W.; To, K.F.; Lan, H.Y.; Tang, P.M. The Emerging Role of Innate Immunity in Chronic Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 4018. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Ten Dijke, P. Targeting TGF-beta Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Xue, V.W.; Chung, J.Y.; Cordoba, C.A.G.; Cheung, A.H.; Kang, W.; Lam, E.W.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M. Transforming Growth Factor-beta: A Multifunctional Regulator of Cancer Immunity. Cancers 2020, 12, 3099. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhang, Y.Y.; Mak, T.S.; Tang, P.C.; Huang, X.R.; Lan, H.Y. Transforming growth factor-beta signalling in renal fibrosis: From Smads to non-coding RNAs. J. Physiol. 2018, 596, 3493–3503. [Google Scholar] [CrossRef]
- Tang, P.M.; Tang, P.C.; Chung, J.Y.; Lan, H.Y. TGF-beta1 signaling in kidney disease: From Smads to long non-coding RNAs. Noncoding RNA Res. 2017, 2, 68–73. [Google Scholar] [CrossRef]
- Roberts, A.B.; Kim, S.J.; Noma, T.; Glick, A.B.; Lafyatis, R.; Lechleider, R.; Jakowlew, S.B.; Geiser, A.; O’Reilly, M.A.; Danielpour, D.; et al. Multiple forms of TGF-beta: Distinct promoters and differential expression. Ciba Found Symp. 1991, 157, 7–15. [Google Scholar] [CrossRef]
- Burt, D.W. Evolutionary grouping of the transforming growth factor-beta superfamily. Biochem. Biophys. Res. Commun. 1992, 184, 590–595. [Google Scholar] [CrossRef]
- Chung, A.C.; Zhang, H.; Kong, Y.Z.; Tan, J.J.; Huang, X.R.; Kopp, J.B.; Lan, H.Y. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J. Am. Soc. Nephrol. 2010, 21, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Chung, J.Y.; Xue, V.W.; Xiao, J.; Meng, X.M.; Huang, X.R.; Zhou, S.; Chan, A.S.; Tsang, A.C.; Cheng, A.S.; et al. Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition. Adv. Sci. 2022, 9, e2101235. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Chan, A.S.; Zhang, C.B.; Garcia Cordoba, C.A.; Zhang, Y.Y.; To, K.F.; Leung, K.T.; Lan, H.Y.; Tang, P.M. TGF-beta1 Signaling: Immune Dynamics of Chronic Kidney Diseases. Front. Med. 2021, 8, 628519. [Google Scholar] [CrossRef] [PubMed]
- Piek, E.; Ju, W.J.; Heyer, J.; Escalante-Alcalde, D.; Stewart, C.L.; Weinstein, M.; Deng, C.; Kucherlapati, R.; Bottinger, E.P.; Roberts, A.B. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 2001, 276, 19945–19953. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.G. Smad7: Not only a regulator, but also a cross-talk mediator of TGF-beta signalling. Biochem. J. 2011, 434, 1–10. [Google Scholar] [CrossRef]
- Lan, H.Y.; Chung, A.C. TGF-beta/Smad signaling in kidney disease. Semin. Nephrol. 2012, 32, 236–243. [Google Scholar] [CrossRef]
- Ma, T.T.; Meng, X.M. TGF-beta/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 347–364. [Google Scholar] [CrossRef]
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative regulators of TGF-beta1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin. Sci. 2021, 135, 275–303. [Google Scholar] [CrossRef]
- Robertson, I.B.; Horiguchi, M.; Zilberberg, L.; Dabovic, B.; Hadjiolova, K.; Rifkin, D.B. Latent TGF-beta-binding proteins. Matrix Biol. 2015, 47, 44–53. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhang, Y.Y.; Xiao, J.; Tang, P.C.; Chung, J.Y.; Li, J.; Xue, V.W.; Huang, X.R.; Chong, C.C.; Ng, C.F.; et al. Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc. Natl. Acad. Sci. USA 2020, 117, 20741–20752. [Google Scholar] [CrossRef]
- Pan, B.; Liu, G.; Jiang, Z.; Zheng, D. Regulation of renal fibrosis by macrophage polarization. Cell Physiol. Biochem. 2015, 35, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Hernandez, F.J.; Lopez-Novoa, J.M. Role of TGF-beta in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012, 347, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yu, L.; Zhang, T.; Qi, H.; Xavier, S.; Ju, W.; Bottinger, E. Smad2-dependent downregulation of miR-30 is required for TGF-beta-induced apoptosis in podocytes. PLoS ONE 2013, 8, e75572. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Thomas, M.C.; Thallas-Bonke, V.; Saleem, M.; Cooper, M.E.; Kantharidis, P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: A model for diabetic podocytopathy. Diabetes 2011, 60, 1779–1788. [Google Scholar] [CrossRef]
- Lee, H.S.; Song, C.Y. Differential role of mesangial cells and podocytes in TGF-beta-induced mesangial matrix synthesis in chronic glomerular disease. Histol. Histopathol. 2009, 24, 901–908. [Google Scholar] [CrossRef]
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016, 92, 102–107. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Lan, H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr. Opin. Nephrol. Hypertens. 2003, 12, 25–29. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, X.; Tan, T.K.; Zhao, H.; Zhang, Y.; Liu, L.; Zhang, J.; Wang, L.; Cao, Q.; Wang, Y.; et al. Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial-mesenchymal transition. Nephrol. Dial. Transplant. 2017, 32, 781–791. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhou, S.; Li, C.J.; Liao, J.; Xiao, J.; Wang, Q.M.; Lian, G.Y.; Li, J.; Huang, X.R.; To, K.F.; et al. The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int. 2018, 93, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Maezawa, Y.; Yokote, K.; Joh, K.; Kobayashi, K.; Kawamura, H.; Nishimura, M.; Roberts, A.B.; Saito, Y.; Mori, S. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem. Biophys. Res. Commun. 2003, 305, 1002–1007. [Google Scholar] [CrossRef]
- Moon, J.A.; Kim, H.T.; Cho, I.S.; Sheen, Y.Y.; Kim, D.K. IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 2006, 70, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Wang, L.; Tang, P.M.; Wang, H.L.; Li, J.C.; Xu, B.H.; Xue, V.W.; Tan, R.Z.; Jin, N.; Chan, T.F.; et al. Smad3 deficiency promotes beta cell proliferation and function in db/db mice via restoring Pax6 expression. Theranostics 2021, 11, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. Macrophage Phenotype in Kidney Injury and Repair. Kidney Dis. 2015, 1, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Tang, P.; You, Y.; Huang, X.; Liu, B.-C.; Lan, H. Long Noncoding RNA-7949 Regulates Macrophage Activation in Renal Inflammation via the TLR4/NF-KB Pathway. Hong Kong J. Nephrol. 2015, 17, S76. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.M.; Ng, Y.Y.; Ma, F.Y.; Zhou, S.; Zhang, Y.; Yang, C.; Huang, X.R.; Xiao, J.; Wang, Y.Y.; et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 2016, 7, 8809–8822. [Google Scholar] [CrossRef]
- Yang, X.; Letterio, J.J.; Lechleider, R.J.; Chen, L.; Hayman, R.; Gu, H.; Roberts, A.B.; Deng, C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999, 18, 1280–1291. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Osielska, M.A.; Jagodzinski, P.P. Long non-coding RNA as potential biomarkers in non-small-cell lung cancer: What do we know so far? Biomed. Pharmacother 2018, 101, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.S.; Ebrahimi, R.; Al, E.A.A.; Panahi, G.; Meshkani, R.; Younesi, S.; Saadat, P.; Parsian, H. Competing Endogenous RNAs (CeRNAs): Novel Network in Neurological Disorders. Curr. Med. Chem. 2021, 28, 5983–6010. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.L.; Craig, D.W.; Millis, M.P.; Yeatts, K.A.; Kobes, S.; Pearson, J.V.; Lee, A.M.; Knowler, W.C.; Nelson, R.G.; Wolford, J.K. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes 2007, 56, 975–983. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Kiefer, J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: Implications for diabetic nephropathy. PLoS ONE 2013, 8, e77468. [Google Scholar] [CrossRef]
- Kato, M.; Wang, M.; Chen, Z.; Bhatt, K.; Oh, H.J.; Lanting, L.; Deshpande, S.; Jia, Y.; Lai, J.Y.; O’Connor, C.L.; et al. An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat. Commun. 2016, 7, 12864. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, L.; Yu, Z.H.; Hong, S.J.; Zhang, Z.W.; Qiu, Z.Z. LncRNA HOTAIR promotes renal interstitial fibrosis by regulating Notch1 pathway via the modulation of miR-124. Nephrology 2019, 24, 472–480. [Google Scholar] [CrossRef]
- Xue, R.; Li, Y.; Li, X.; Ma, J.; An, C.; Ma, Z. miR-185 affected the EMT, cell viability, and proliferation via DNMT1/MEG3 pathway in TGF-beta1-induced renal fibrosis. Cell Biol. Int. 2019, 43, 1152–1162. [Google Scholar] [CrossRef]
- Zhou, S.G.; Zhang, W.; Ma, H.J.; Guo, Z.Y.; Xu, Y. Silencing of LncRNA TCONS_00088786 reduces renal fibrosis through miR-132. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 166–173. [Google Scholar] [CrossRef]
- Yuan, X.; Tang, W.B.; Peng, L.; Chen, Y.; Tang, S.; Ge, H.; Wang, X.; Xiao, X. Elevation of LncRNA ENST00000453774.1 Prevents Renal Fibrosis by Upregulating FBN1, IGF1R, and KLF7. Kidney Blood Press. Res. 2021, 46, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ke, C.; Zhang, L.; Tian, C.; Zhang, Z.; Wu, S. Long Non-Coding RNA (LncRNA)-ATB Promotes Inflammation, Cell Apoptosis and Senescence in Transforming Growth Factor-beta1 (TGF-beta1) Induced Human Kidney 2 (HK-2) Cells via TGFbeta/SMAD2/3 Signaling Pathway. Med. Sci. Monit. 2020, 26, e922029. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yuan, Q.; Chen, Y.; Huang, Z.; Fang, X.; Zhang, H.; Peng, L.; Xiao, P. LncRNA ENST00000453774.1 contributes to oxidative stress defense dependent on autophagy mediation to reduce extracellular matrix and alleviate renal fibrosis. J. Cell Physiol. 2019, 234, 9130–9143. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, S.; Shi, B.; Zheng, D.; Shi, J. Transcriptome Identified lncRNAs Associated with Renal Fibrosis in UUO Rat Model. Front. Physiol. 2017, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Z.Y.; Wang, Y.; Liu, Y.; Ma, J.X.; Li, Y.K. Long non-coding RNA ASncmtRNA-2 is upregulated in diabetic kidneys and high glucose-treated mesangial cells. Exp. Ther. Med. 2017, 13, 581–587. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Lv, L.L.; Tang, P.M.; Li, C.J.; You, Y.K.; Li, J.; Huang, X.R.; Ni, J.; Feng, M.; Liu, B.C.; Lan, H.Y. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017, 91, 587–602. [Google Scholar] [CrossRef]
- Tang, P.M.; Zhang, Y.Y.; Hung, J.S.; Chung, J.Y.; Huang, X.R.; To, K.F.; Lan, H.Y. DPP4/CD32b/NF-kappaB Circuit: A Novel Druggable Target for Inhibiting CRP-Driven Diabetic Nephropathy. Mol. Ther. 2021, 29, 365–375. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Lai, W.; Tang, Y.; Huang, X.R.; Ming-Kuen Tang, P.; Xu, A.; Szalai, A.J.; Lou, T.Q.; Lan, H.Y. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int. 2016, 90, 610–626. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiong, Y.; Huang, X.R.; Tang, P.; Yu, X.; Lan, H.Y. Identification of Genes Associated with Smad3-dependent Renal Injury by RNA-seq-based Transcriptome Analysis. Sci. Rep. 2015, 5, 17901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Tan, R.Z.; Yu, Y.; Niu, Y.Y.; Yu, C. LncRNA GAS5 protects against TGF-beta-induced renal fibrosis via the Smad3/miRNA-142-5p axis. Am. J. Physiol. Renal. Physiol. 2021, 321, F517–F526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Tang, P.M.; Tang, P.C.; Xiao, J.; Huang, X.R.; Yu, C.; Ma, R.C.W.; Lan, H.Y. LRNA9884, a Novel Smad3-Dependent Long Noncoding RNA, Promotes Diabetic Kidney Injury in db/db Mice via Enhancing MCP-1-Dependent Renal Inflammation. Diabetes 2019, 68, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, M.L.; Song, E.; Zhou, Z.; Ma, T.; Wang, J.; Jia, N.; Wang, G.; Nie, S.; Liu, Y.; et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-beta/Smad3 pathway. Sci. Transl. Med. 2018, 10, eaat2039. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, P.M.; Niu, Y.; Garcia Cordoba, C.A.; Huang, X.R.; Yu, C.; Lan, H.Y. Long Non-coding RNA LRNA9884 Promotes Acute Kidney Injury via Regulating NF-kB-Mediated Transcriptional Activation of MIF. Front. Physiol. 2020, 11, 590027. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, H.; Wu, X.; Mei, M.; Shen, B. The long noncoding RNA Ptprd-IR is a novel molecular target for TGF-beta1-mediated nephritis. Int. J. Biochem. Cell Biol. 2020, 122, 105742. [Google Scholar] [CrossRef]
- Xu, B.H.; Sheng, J.; You, Y.K.; Huang, X.R.; Ma, R.C.W.; Wang, Q.; Lan, H.Y. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism 2020, 103, 154013. [Google Scholar] [CrossRef]
- Lu, J.; Miao, J.; Sun, J. LncRNA np_5318 promotes renal ischemia-reperfusion injury through the TGF-beta/Smad signaling pathway. Exp. Ther. Med. 2020, 19, 2833–2840. [Google Scholar] [CrossRef]
- Feng, M.; Tang, P.M.; Huang, X.R.; Sun, S.F.; You, Y.K.; Xiao, J.; Lv, L.L.; Xu, A.P.; Lan, H.Y. TGF-beta Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol. Ther. 2018, 26, 148–161. [Google Scholar] [CrossRef]
- Sun, S.F.; Tang, P.M.K.; Feng, M.; Xiao, J.; Huang, X.R.; Li, P.; Ma, R.C.W.; Lan, H.Y. Novel lncRNA Erbb4-IR Promotes Diabetic Kidney Injury in db/db Mice by Targeting miR-29b. Diabetes 2018, 67, 731–744. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, X.R.; Yu, J.; Yu, X.; Lan, H.Y. Long Noncoding RNA Arid2-IR Is a Novel Therapeutic Target for Renal Inflammation. Mol. Ther. 2015, 23, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, W.; Yang, Z.J.; Chi, J.R.; Li, Y.R.; Ding, Y.; Ge, J.; Wang, X.; Cao, X.C. miR-190 suppresses breast cancer metastasis by regulation of TGF-beta-induced epithelial-mesenchymal transition. Mol. Cancer 2018, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Oristian, D.; Fuchs, E. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 2015, 160, 963–976. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Huang, Y.H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massague, J. TGF-beta Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFbeta in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Schober, M.; Fuchs, E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 10544–10549. [Google Scholar] [CrossRef]
- Wildey, G.M.; Patil, S.; Howe, P.H. Smad3 potentiates transforming growth factor beta (TGFbeta )-induced apoptosis and expression of the BH3-only protein Bim in WEHI 231 B lymphocytes. J. Biol. Chem. 2003, 278, 18069–18077. [Google Scholar] [CrossRef]
- Schrantz, N.; Bourgeade, M.F.; Mouhamad, S.; Leca, G.; Sharma, S.; Vazquez, A. p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFbeta-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol. Biol. Cell 2001, 12, 3139–3151. [Google Scholar] [CrossRef]
- Hou, Z.H.; Xu, X.W.; Fu, X.Y.; Zhou, L.D.; Liu, S.P.; Tan, D.M. Long non-coding RNA MALAT1 promotes angiogenesis and immunosuppressive properties of HCC cells by sponging miR-140. Am. J. Physiol. Cell Physiol. 2020, 318, C649–C663. [Google Scholar] [CrossRef]
- Xiao, H.; Tang, K.; Liu, P.; Chen, K.; Hu, J.; Zeng, J.; Xiao, W.; Yu, G.; Yao, W.; Zhou, H.; et al. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget 2015, 6, 38005–38015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Yang, F.Q.; Chen, S.J.; Che, J.; Zheng, J.H. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015, 36, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Nakajima, K.; Tabatabai, Z.L.; Ishii, N.; Dahiya, R. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205. Cancer Res. 2015, 75, 1322–1331. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, G.; Yuan, B.; Yang, C.; Gao, R.; Zhou, X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015, 36, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014, 20, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, P.; Qu, J.; Shi, L.; Zhuang, W.; Fu, J.; Li, J.; Zhang, X.; Sun, Y.; Zhuang, W. Activation of LTBP3 gene by a long noncoding RNA (lncRNA) MALAT1 transcript in mesenchymal stem cells from multiple myeloma. J. Biol. Chem. 2014, 289, 29365–29375. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Diederichs, S.; Wang, W.; Boing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zheng, J.; Yao, C.; Lu, X. LncRNA UCA1 attenuated the killing effect of cytotoxic CD8 + T cells on anaplastic thyroid carcinoma via miR-148a/PD-L1 pathway. Cancer Immunol. Immunother. 2021, 70, 2235–2245. [Google Scholar] [CrossRef]
- Hu, M.L.; Wang, X.Y.; Chen, W.M. TGF-beta1 upregulates the expression of lncRNA UCA1 and its downstream HXK2 to promote the growth of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4846–4854. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhong, Q.; Wu, J.; Tang, Z. LncRNA UCA1 is necessary for TGF-beta-induced epithelial-mesenchymal transition and stemness via acting as a ceRNA for Slug in glioma cells. FEBS Open Bio 2018, 8, 1855–1865. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Ungerleider, N.; Chen, W.; Song, K.; Wang, Y.; Kwon, H.; Ma, W.; Wu, T. A Transforming Growth Factor-beta and H19 Signaling Axis in Tumor-Initiating Hepatocytes That Regulates Hepatic Carcinogenesis. Hepatology 2019, 69, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Matouk, I.J.; Raveh, E.; Abu-lail, R.; Mezan, S.; Gilon, M.; Gershtain, E.; Birman, T.; Gallula, J.; Schneider, T.; Barkali, M.; et al. Oncofetal H19 RNA promotes tumor metastasis. Biochim. Biophys. Acta 2014, 1843, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Rahaman, A.; Biswas, I.; Mukherjee, G.; Chatterjee, S.; Bhattacharjee, S.; Mandal, D.P. TGFbeta mediated LINC00273 upregulation sponges mir200a-3p and promotes invasion and metastasis by activating ZEB1. J. Cell Physiol. 2020, 235, 7159–7172. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hu, X.; Mao, J.; Wu, Y.; Liu, H.; Shen, J.; Yu, J.; Chen, W. The long noncoding RNA TUG1 is required for TGF-beta/TWIST1/EMT-mediated metastasis in colorectal cancer cells. Cell Death Dis. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.F.; Zhao, F.L. Long non-coding RNA TUG1 can promote proliferation and migration of pancreatic cancer via EMT pathway. Eur Rev. Med. Pharmacol. Sci. 2017, 21, 2377–2384. [Google Scholar] [PubMed]
- Fu, Y.; Zhang, P.; Nan, H.; Lu, Y.; Zhao, J.; Yang, M.; Song, Q. LncRNA CASC11 promotes TGF-beta1, increases cancer cell stemness and predicts postoperative survival in small cell lung cancer. Gene 2019, 704, 91–96. [Google Scholar] [CrossRef]
- Miao, F.; Chen, J.; Shi, M.; Song, Y.; Chen, Z.; Pang, L. LncRNA HAND2-AS1 inhibits non-small cell lung cancer migration, invasion and maintains cell stemness through the interactions with TGF-beta1. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Kolliopoulos, C.; Lin, C.Y.; Heldin, C.H.; Moustakas, A.; Heldin, P. Has2 natural antisense RNA and Hmga2 promote Has2 expression during TGFbeta-induced EMT in breast cancer. Matrix Biol. 2019, 80, 29–45. [Google Scholar] [CrossRef]
- Tang, J.; Yu, B.; Li, Y.; Zhang, W.; Alvarez, A.A.; Hu, B.; Cheng, S.Y.; Feng, H. TGF-beta-activated lncRNA LINC00115 is a critical regulator of glioma stem-like cell tumorigenicity. EMBO Rep. 2019, 20, e48170. [Google Scholar] [CrossRef]
- He, W.; Liang, B.; Wang, C.; Li, S.; Zhao, Y.; Huang, Q.; Liu, Z.; Yao, Z.; Wu, Q.; Liao, W.; et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene 2019, 38, 4637–4654. [Google Scholar] [CrossRef]
- Cui, W.; Meng, W.; Zhao, L.; Cao, H.; Chi, W.; Wang, B. TGF-beta-induced long non-coding RNA MIR155HG promotes the progression and EMT of laryngeal squamous cell carcinoma by regulating the miR-155-5p/SOX10 axis. Int. J. Oncol. 2019, 54, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, L.; Liu, Z.; Xu, G.; Wang, S.; Su, Z.; Zhang, Y.; Zhang, C.; Liu, X.; Lei, Z.; et al. Long non-coding RNA XIST promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018, 418, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G.; Cheng, Z.; Dai, L.; Jia, L.; Jing, X.; Wang, H.; Zhang, R.; Liu, M.; Jiang, T.; et al. Knockdown of LncRNA-XIST Suppresses Proliferation and TGF-beta1-Induced EMT in NSCLC Through the Notch-1 Pathway by Regulation of miR-137. Genet. Test. Mol. Biomarkers 2018, 22, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Deng, S.H.; Liu, T.; Han, R.; Zhang, T.; Xu, Y. TGF-beta-mediated exosomal lnc-MMP2-2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Med. 2018, 7, 5118–5129. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, X.; Wang, C.; Sun, J.; Chen, Y.; Wang, G.; Fang, L.; Yang, R.; Yu, M.; Gu, Y.; et al. Systematic analyses reveal long non-coding RNA (PTAF)-mediated promotion of EMT and invasion-metastasis in serous ovarian cancer. Mol. Cancer 2018, 17, 96. [Google Scholar] [CrossRef]
- Terashima, M.; Ishimura, A.; Wanna-Udom, S.; Suzuki, T. MEG8 long noncoding RNA contributes to epigenetic progression of the epithelial-mesenchymal transition of lung and pancreatic cancer cells. J. Biol. Chem. 2018, 293, 18016–18030. [Google Scholar] [CrossRef]
- Terashima, M.; Tange, S.; Ishimura, A.; Suzuki, T. MEG3 Long Noncoding RNA Contributes to the Epigenetic Regulation of Epithelial-Mesenchymal Transition in Lung Cancer Cell Lines. J. Biol. Chem. 2017, 292, 82–99. [Google Scholar] [CrossRef]
- Wang, M.; Huang, T.; Luo, G.; Huang, C.; Xiao, X.Y.; Wang, L.; Jiang, G.S.; Zeng, F.Q. Long non-coding RNA MEG3 induces renal cell carcinoma cells apoptosis by activating the mitochondrial pathway. J. Huazhong Univ. Sci. Technol. 2015, 35, 541–545. [Google Scholar] [CrossRef]
- Mondal, T.; Subhash, S.; Vaid, R.; Enroth, S.; Uday, S.; Reinius, B.; Mitra, S.; Mohammed, A.; James, A.R.; Hoberg, E.; et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015, 6, 7743. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, H.; Niu, Y.; Wu, Y.; Sun, W.; Li, H.; Kong, J.; Ding, K.; Shen, H.M.; Wu, H.; et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol. Cancer 2017, 16, 118. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, X.; Zhang, D.; Luo, J.; Chen, R. Long noncoding RNA LINC01186, regulated by TGF-beta/SMAD3, inhibits migration and invasion through Epithelial-Mesenchymal-Transition in lung cancer. Gene 2017, 608, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Shen, L.; Yang, L.; Huang, X.; Lu, Q.; Cui, Y.; Zheng, X.; Zhao, X.; Zhang, D.; Huang, R.; et al. TGFbeta1 Promotes Gemcitabine Resistance through Regulating the LncRNA-LET/NF90/miR-145 Signaling Axis in Bladder Cancer. Theranostics 2017, 7, 3053–3067. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Sun, W.; Li, C.; Wan, L.; Wang, S.; Wu, Y.; Xu, E.; Zhang, H.; Lai, M. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett. 2016, 380, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Qiu, S.; Zhao, S.; Liu, C.; Zhang, D.; Yu, F.; Peng, Z.; Yan, D. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J. Gastroenterol. Hepatol. 2016, 31, 595–603. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, L.J.; Yu, B.; Li, Y.H.; Jin, Y.; Bai, X.Z. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget 2015, 6, 11652–11663. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kurashige, J.; Nambara, S.; Komatsu, H.; Hirata, H.; Ueda, M.; Sakimura, S.; Uchi, R.; Takano, Y.; Shinden, Y.; et al. A Long Non-coding RNA Activated by Transforming Growth Factor-beta is an Independent Prognostic Marker of Gastric Cancer. Ann. Surg. Oncol. 2015, 22, S915–S922. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Uchi, R.; Nambara, S.; Saito, T.; Komatsu, H.; Hirata, H.; Ueda, M.; Sakimura, S.; Takano, Y.; Kurashige, J.; et al. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015, 35, 1385–1388. [Google Scholar]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep. 2015, 5, 11924. [Google Scholar] [CrossRef]
- Beltran, M.; Puig, I.; Pena, C.; Garcia, J.M.; Alvarez, A.B.; Pena, R.; Bonilla, F.; de Herreros, A.G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008, 22, 756–769. [Google Scholar] [CrossRef]
- Takahashi, K.; Yan, I.K.; Kogure, T.; Haga, H.; Patel, T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 2014, 4, 458–467. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Zheng, Y.; You, L.; Kuang, D.; Liu, T. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol. 2014, 35, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Fukuhara, S.; Saini, S.; Majid, S.; Deng, G.; Shahryari, V.; Chang, I.; Tanaka, Y.; Enokida, H.; Nakagawa, M.; et al. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J. Biol. Chem. 2014, 289, 12550–12565. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Endo, H.; Yokoyama, M.; Abe, J.; Tamai, K.; Tanaka, N.; Sato, I.; Takahashi, S.; Kondo, T.; Satoh, K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 436, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Padua Alves, C.; Fonseca, A.S.; Muys, B.R.; de Barros, E.L.B.R.; Burger, M.C.; de Souza, J.E.; Valente, V.; Zago, M.A.; Silva, W.A., Jr. Brief report: The lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells 2013, 31, 2827–2832. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, Z.; Li, S.; Wang, X.; Zhou, X. LncRNA SPRY4-IT was concerned with the poor prognosis and contributed to the progression of thyroid cancer. Cancer Gene Ther. 2018, 25, 39–46. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Li, R.K.; Qi, Y.; Li, X.N.; Yang, Y.; Liu, D.L.; Zhao, J.; Zhu, D.Y.; Wu, K.; Zhou, X.D.; et al. Upregulation of long noncoding RNA SPRY4-IT1 promotes metastasis of esophageal squamous cell carcinoma via induction of epithelial-mesenchymal transition. Cell Biol. Toxicol. 2016, 32, 391–401. [Google Scholar] [CrossRef]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Aghdassi, A.; Sendler, M.; Guenther, A.; Mayerle, J.; Behn, C.O.; Heidecke, C.D.; Friess, H.; Buchler, M.; Evert, M.; Lerch, M.M.; et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012, 61, 439–448. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Z.; Li, Y.; Wang, J.; Zhang, Z.; Che, Y.; Huang, J.; Sun, S.; Mao, S.; Lei, Y.; et al. TGF-beta-induced NKILA inhibits ESCC cell migration and invasion through NF-kappaB/MMP14 signaling. J. Mol. Med. 2018, 96, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.Y.; Wang, R.; Chen, L.B. MicroRNA-145: A potent tumour suppressor that regulates multiple cellular pathways. J. Cell Mol. Med. 2014, 18, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Zhou, S.; Meng, X.M.; Wang, Q.M.; Li, C.J.; Lian, G.Y.; Huang, X.R.; Tang, Y.J.; Guan, X.Y.; Yan, B.P.; et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat. Commun. 2017, 8, 14677. [Google Scholar] [CrossRef]
- Tang, P.M.; Tang, P.C.; Chung, J.Y.; Hung, J.S.C.; Wang, Q.M.; Lian, G.Y.; Sheng, J.; Huang, X.R.; To, K.F.; Lan, H.Y. A Novel Feeder-free System for Mass Production of Murine Natural Killer Cells In Vitro. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Wang, Q.M.; Tang, P.M.; Lian, G.Y.; Li, C.; Li, J.; Huang, X.R.; To, K.F.; Lan, H.Y. Enhanced Cancer Immunotherapy with Smad3-Silenced NK-92 Cells. Cancer Immunol. Res. 2018, 6, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Miwa, T.; Hokari, S.; Sakurai, T.; Ohmori, K.; Miyauchi, K.; Miyazono, K.; Koinuma, D. Long noncoding RNA NORAD regulates transforming growth factor-beta signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 2018, 109, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Bucci, G.; Rizzotto, D.; Bordo, D.; Marzi, M.J.; Puppo, M.; Flinois, A.; Spadaro, D.; Citi, S.; Emionite, L.; et al. LncRNA EPR controls epithelial proliferation by coordinating Cdkn1a transcription and mRNA decay response to TGF-beta. Nat. Commun. 2019, 10, 1969. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Che, Y.; Huang, J.; Sun, S.; Mao, S.; Lei, Y.; Li, N.; Sun, N.; He, J. The TGFbeta-induced lncRNA TBILA promotes non-small cell lung cancer progression in vitro and in vivo via cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer Lett. 2018, 432, 156–168. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Wang, J.; Che, Y.; Sun, S.; Huang, J.; Chen, Z.; He, J. Long non-coding RNA NKILA inhibits migration and invasion of non-small cell lung cancer via NF-kappaB/Snail pathway. J. Exp. Clin. Cancer Res. 2017, 36, 54. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, R.; Fang, L.; Ge, X.; Chen, L.; Zhou, M.; Zhou, Y.; Xiong, W.; Hu, Y.; Tang, X.; et al. HCP5 is a SMAD3-responsive long non-coding RNA that promotes lung adenocarcinoma metastasis via miR-203/SNAI axis. Theranostics 2019, 9, 2460–2474. [Google Scholar] [CrossRef]
- Ottaviani, S.; Stebbing, J.; Frampton, A.E.; Zagorac, S.; Krell, J.; de Giorgio, A.; Trabulo, S.M.; Nguyen, V.T.M.; Magnani, L.; Feng, H.; et al. TGF-beta induces miR-100 and miR-125b but blocks let-7a through LIN28B controlling PDAC progression. Nat. Commun. 2018, 9, 1845. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Chan, M.K.; Tang, P.C.; Chan, A.S.; Chung, J.S.; Meng, X.M.; To, K.F.; Lan, H.Y.; Leung, K.T.; Tang, P.M. AANG: A natural compound formula for overcoming multidrug resistance via synergistic rebalancing the TGF-beta/Smad signalling in hepatocellular carcinoma. J. Cell Mol. Med. 2021, 25, 9805–9813. [Google Scholar] [CrossRef] [PubMed]
- Arase, M.; Horiguchi, K.; Ehata, S.; Morikawa, M.; Tsutsumi, S.; Aburatani, H.; Miyazono, K.; Koinuma, D. Transforming growth factor-beta-induced lncRNA-Smad7 inhibits apoptosis of mouse breast cancer JygMC(A) cells. Cancer Sci. 2014, 105, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hao, Y.; Wang, Y.; Xu, J.; Teng, Y.; Yang, X. TGF-beta/SMAD4-Regulated LncRNA-LINP1 Inhibits Epithelial-Mesenchymal Transition in Lung Cancer. Int. J. Biol. Sci. 2018, 14, 1715–1723. [Google Scholar] [CrossRef]
- Bommireddy, R.; Engle, S.J.; Ormsby, I.; Boivin, G.P.; Babcock, G.F.; Doetschman, T. Elimination of both CD4+ and CD8+ T cells but not B cells eliminates inflammation and prolongs the survival of TGFbeta1-deficient mice. Cell Immunol. 2004, 232, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Fervenza, F.C.; Campbell, K.N.; Diaz, M.; Gesualdo, L.; Nelson, P.; Praga, M.; Radhakrishnan, J.; Sellin, L.; Singh, A.; et al. A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients With Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2017, 2, 800–810. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Morris, J.C.; Lawrence, D.P.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Berzofsky, J.A.; Hsu, F.J.; Guitart, J. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor beta by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 2015, 64, 437–446. [Google Scholar] [CrossRef]
- Sun, S.F.; Tang, P.M.K.; Huang, X.R.; Lan, H.Y. Response letter: “Novel lncRNA Erbb4-IR promotes diabetic kidney injury in db/db mice by targeting miR-29b”. Transl. Cancer Res. 2018, 67, S629–S631. [Google Scholar] [CrossRef]
- Feng, M.; Tang, P.M.-K.; You, Y.-K.; Lv, L.-L.; Huang, X.-R.; Xu, A.; Lan, H. Long Non-coding RNA_5318 is a Novel Therapeutic Target for Renal Fibrosis in Obstructive Nephropathy. Hong Kong J. Nephrol. 2015, 17, S62–S63. [Google Scholar] [CrossRef][Green Version]
- Shen, S.; Wang, J.; Zheng, B.; Tao, Y.; Li, M.; Wang, Y.; Ni, X.; Suo, T.; Liu, H.; Liu, H.; et al. LINC01714 Enhances Gemcitabine Sensitivity by Modulating FOXO3 Phosphorylation in Cholangiocarcinoma. Mol. Ther. Nucleic Acids 2020, 19, 446–457. [Google Scholar] [CrossRef]
- Maruyama, R.; Yokota, T. Knocking Down Long Noncoding RNAs Using Antisense Oligonucleotide Gapmers. Methods Mol. Biol. 2020, 2176, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.F.; Vickers, T.A.; Nichols, J.; Li, C.; Crooke, S.T. Defining the factors that contribute to on-target specificity of antisense oligonucleotides. PLoS ONE 2014, 9, e101752. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, T.A.; Kim, T.W.; Shen, L.; Serota, D.; Papagiannis, C.; Park, S.Y.; Kim, Y.; Henry, S.P. Chronic Toxicity Assessment of 2′-O-Methoxyethyl Antisense Oligonucleotides in Mice. Nucleic Acid Ther. 2018, 28, 233–241. [Google Scholar] [CrossRef]
- Sennoga, C.A.; Kanbar, E.; Auboire, L.; Dujardin, P.A.; Fouan, D.; Escoffre, J.M.; Bouakaz, A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Exp. Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Xue, V.W.; Chung, J.Y.; Tang, P.C.; Chan, A.S.; To, T.H.; Chung, J.S.; Mussal, F.; Lam, E.W.; Li, C.; To, K.F.; et al. USMB-shMincle: A virus-free gene therapy for blocking M1/M2 polarization of tumor-associated macrophages. Mol. Ther. Oncolytics 2021, 23, 26–37. [Google Scholar] [CrossRef]
- Chen, Q.; Su, Y.; He, X.; Zhao, W.; Wu, C.; Zhang, W.; Si, X.; Dong, B.; Zhao, L.; Gao, Y.; et al. Plasma long non-coding RNA MALAT1 is associated with distant metastasis in patients with epithelial ovarian cancer. Oncol. Lett. 2016, 12, 1361–1366. [Google Scholar] [CrossRef]
- Noviello, T.M.R.; Di Liddo, A.; Ventola, G.M.; Spagnuolo, A.; D’Aniello, S.; Ceccarelli, M.; Cerulo, L. Detection of long non-coding RNA homology, a comparative study on alignment and alignment-free metrics. BMC Bioinform. 2018, 19, 407. [Google Scholar] [CrossRef]
- Xue, W.J.; Ying, X.L.; Jiang, J.H.; Xu, Y.H. Prostate cancer antigen 3 as a biomarker in the urine for prostate cancer diagnosis: A meta-analysis. J. Cancer Res. Ther. 2014, 10, C218–C221. [Google Scholar] [CrossRef]
- Luan, Y.; Li, X.; Luan, Y.; Zhao, R.; Li, Y.; Liu, L.; Hao, Y.; Oleg Vladimir, B.; Jia, L. Circulating lncRNA UCA1 Promotes Malignancy of Colorectal Cancer via the miR-143/MYO6 Axis. Mol. Ther. Nucleic Acids 2020, 19, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.; Habbout, K.; Shimauchi, T.; Wu, W.H.; Breuils-Bonnet, S.; Tremblay, E.; Martineau, S.; Nadeau, V.; Gagnon, K.; Mazoyer, F.; et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation 2020, 142, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, M.; Ali Beg, M.M.; Alshahrani, M.Y.; Ahmad, I.; Alkhathami, A.G.; Joshi, P.C.; Alshehri, O.M.; Alamri, A.M.; Verma, A.K. Circulating long non-coding RNAs NKILA, NEAT1, MALAT1, and MIAT expression and their association in type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2021, 9, e001821. [Google Scholar] [CrossRef] [PubMed]
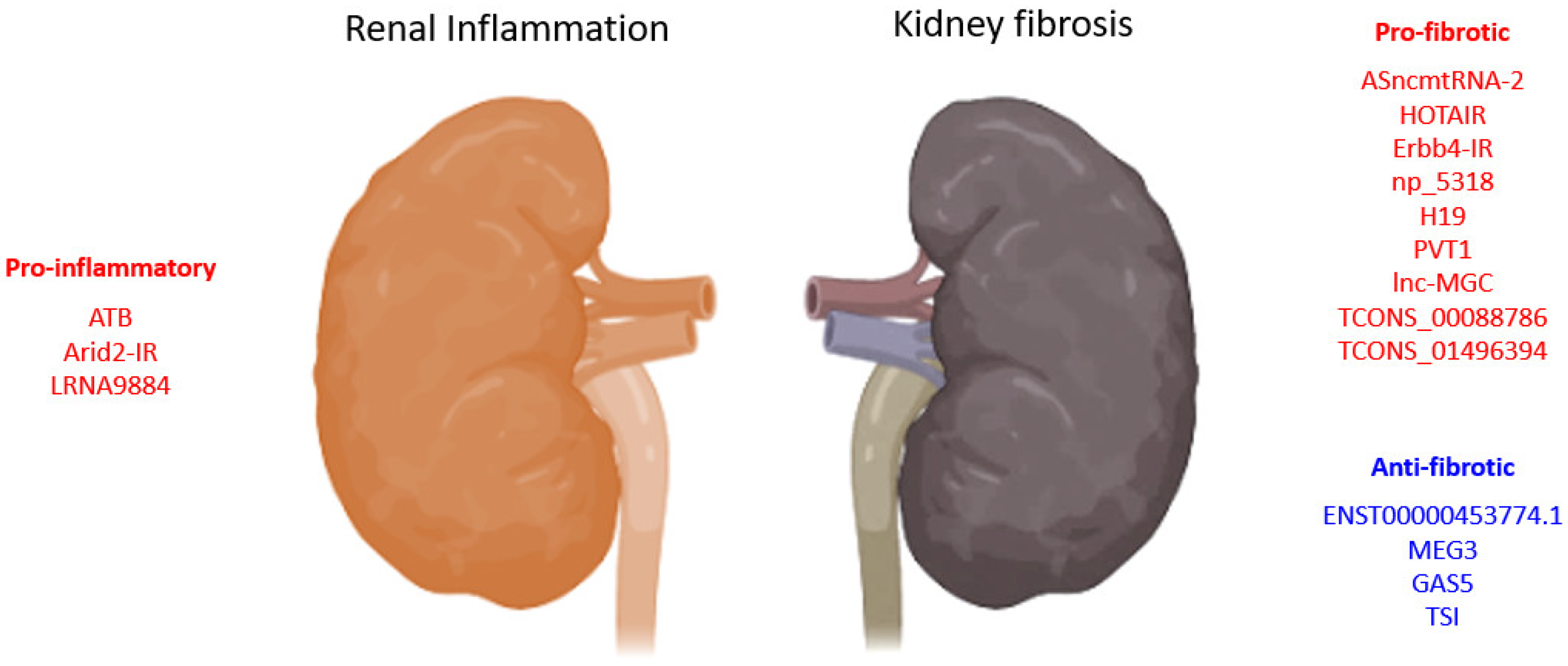
| LncRNA | Biological Process | Model | Species | Mechanism | Year | Ref. |
|---|---|---|---|---|---|---|
| GAS5 | anti-fibrosis | Smad3-WT/KO UUO, mTECs, MEFs | Mouse | suppresses TGF-β1-induced Col-I/Fn expression and apoptosis, promotes miR-142-5p expression | 2021 | [72] |
| LRNA9884 | pro-inflammation | Cisplatin-AKI, mTECs | Mouse | promotes IL-1β-induced p-p65,TNF-α, MCP-1, and IL-6, binds directly to MIF promoter | 2020 | [75] |
| Smad3-WT/KO-DN, mTECs, SV40 MES 13 | Mouse | Smad3 dependently induced, suppresses IL-1β, TNF-α, and MCP-1, binds directly to the promoter of MCP-1 | 2019 | [73] | ||
| Ptprd-IR (np_4334) | pro-inflammation | mTECs, HEK293T, UUO mice | Human, mouse | Smad3 direct target; promotes inflammatory response and macrophage and T-cell infiltration | 2020 | [76] |
| Erbb4-IR (np_5318) | pro-fibrotic | Smad3-WT/KO-DN, TECs, MCs | Mouse | Smad3 deletion suppressed Erbb4-IR and restored miR-29b expression | 2020 | [77] |
| AKI, PCS-400-012 cells | Human, mouse | promotes I/R-induced renal cell death, further enhances TGF-β1/Smad3 signaling | 2020 | [78] | ||
| UUO, TEC, MEF | Mouse | suppresses Smad7 via promoter binding, enhances Smad3-driven Col-I α-SMA expression | 2018 | [79] | ||
| Smad3-WT/KO-DN, TECs, MCs, MEF | Mouse | enhances Smad3-driven Col-I/IV expression, suppress protective miR-29b via 3’UTR binding | 2018 | [80] | ||
| TSI | anti-fibrosis | UUO, HK2, TECs, MC, HL-7702, LX-2, IMR-90, 16HBE, HKC8 cells | Human, mouse | inhibits Smad3 by direct binding to MH2 domain | 2018 | [74] |
| Arid2-IR | pro-inflammation | UUO, TEC | Mouse | Smad3 direct target; promote fibrotic and inflammatory response, macrophage and T-cell infiltration | 2015 | [81] |
| RNA-seq | pro-fibrotic | UUO /anti-GBM GN of Smad3-WT/KO mice | Mouse | 21 TGF-β/Smad3 dependent lncRNAs | 2014 | [71] |
| LncRNA | Cancer Type | Model | Species | Mechanism | Year | Ref. |
|---|---|---|---|---|---|---|
| UCA1 | Thyroid carcinoma | Nthy-ori 3-1 and Hth74 cell, 8505C cell and xenograft, thyroid carcinoma biopsy, mouse isolated CD8+ T cell | Human, mouse | promotes PD-L1-dependent CD8 + T cell suppression via miR-148a | 2021 | [98] |
| Hepatocellular carcinoma | HepG2 and Huh7 cells, HCC cohort | Human | associated with lower OS; promote proliferation via HXK2 | 2018 | [99] | |
| Glioma | U87 and U251 cells, glioma and adjacent tissues | Human | promotes EMT (E-cad, Slug) and stemness (Aldh1, Nanog) via sponging miR-1 and miR-203a | 2018 | [100] | |
| H19 | Liver | CCl4 induced tumor, primary hepatocytes | Human, mouse | promotes survival of tumor-initiating cells in vitro and tumorigenicity in vivo | 2019 | [101] |
| Breast, lung | Hep3B, UMUC3 and H358 cells | Human, mouse | promotes EMT via Slug | 2014 | [102] | |
| LINC00273 | Lung | A549 cells and metastasis model | Human, mouse | promotes ZEB-1-mediated EMT via sponging miR200a-3p | 2020 | [103] |
| TUG1 | Colorectal | CRCs (LoVo, HT-29, HCT116) | Human, mouse | promotes EMT via Twist1 in vitro and metastasis in vivo | 2020 | [104] |
| Pancreatic | BxPC3, PaTu8988, Sw1990 | Human | promotes cell proliferation and TGF-β/Smad3 induced EMT and MMP2/9 expression | 2017 | [105] | |
| MALAT-1 | Hepatocellular carcinoma | LO2,THP-1, HUVECs cells, HepG2 and Huh-7 cells and xenograft | Human, mouse | promotes cancer cell secretome-induced M2 polarization and VEGF-A expression via suppressing miR-140 | 2020 | [90] |
| Clear cell renal cell carcinoma | ccRCC biopsy, ACHN cells and xenograft, 786-O, SN12-PM6, HK-2, CAKI-1, and OS RC-2 cells | Human, mouse | promotes proliferation and metastasis of cancer cell via ZEB2 | 2015 | [91] | |
| ccRCC biopsy, HK-2, 786-O, ACHN, Caki-1, and Caki-2 cells | Human | associated with poorer overall survival of ccRCC patients; promotes proliferation, migration, and invasion of cancer cells | 2015 | [92] | ||
| Renal cell carcinoma | Human tissue biopsy, 786-O, A-498, Caki-1/-2 HK-2 | Human | promotes EMT via Ezh2, β-catenin nuclear localization, and miR-205 | 2015 | [93] | |
| Osteosarcoma | SaOs, U-2 OS cells | Human | promotes cell growth, invasion, and metastasis | 2015 | [94] | |
| Bladder cancer | MB49 syngeneic tumor, T24 cells and xenograft, RT4 cells | Human | promotes TGF-β-induced EMT, migration, and metastasis via suz12 | 2014 | [95] | |
| Multiple myeloma | MSCs from MM patients | Human | cooperates with Sp1 to regulate LTBP3 expression via promoter binding site | 2014 | [96] | |
| Non-small cell lung cancer | NSCLC cohort | Human | significantly associated with metastasis | 2003 | [97] | |
| CASC11 | Small cell lung cancer | SCLC cohort, SHP-77 and DMS79 cells | Human | associated with TGF-β1 abundance and poorer OS; promotes TGF-β1 and subsequent CD133 expression | 2019 | [106] |
| HAND2-AS1 | Non-small cell lung cancer | NCI-H1581 and NCI-H1993 cells, NSCLC and adjacent tissues | Human | negatively associated with TGF-β1 abundance; suppresses TGF-β1-induced migration, invasion, and CD133 expression | 2019 | [107] |
| HAS2-AS1 | Breast cancer | NMuMG, Py2T, 4 T1, and EpRas cells, breast cancer cohort | Mouse | associated with poorer OS; promotes HAS2 expression, CD44-dependent EMT, and stemness (Sox2, Nanog) | 2019 | [108] |
| LINC00115 | Glioblastoma | Public GBM cohort, U87, LN229, LN18, T98G, Patient-derived GSCs | Human | associated with poorer survival; promotes ZEB1-EMT and ZNF596/EZH2/STAT3-neuro-like sphere formation via sponging miR-200b/c | 2019 | [109] |
| MACC1-AS1 | Gastric cancer | AGS cell, MKN45 cell and xenograft, GC cohort | Human | promotes FAO-dependent stemness and sponging of miR-145-5p | 2019 | [110] |
| MIR155HG | laryngeal squamous cell carcinoma | TU686, AMC-HN-8, and 293T cells TU177 cell and xenograft | Human, mouse | promotes EMT by suppressing SOX10 via miR-155-5p upregulation | 2019 | [111] |
| XIST | Non-small cell lung cancer | NSCLC tissues, A549 and H226 | Human | promotes EMT and is associated with invasion and metastasis via the miR-367/miR-141-ZEB2 axis | 2018 | [112] |
| A549, H358, H460, H1299, 16HBE and PC9, NSCLC tissues | Human | promotes EMT and proliferation via sponging miR-137 | 2018 | [113] | ||
| MMP2-2 | Lung | A549, HMVECs | Human | associated with MMP-2 expression, promotes EMT and vascular permeability | 2018 | [114] |
| PTAF | Ovarian cancer | SKOV3, A2780 and OVCAR-3, OvCa tissue samples | Human | promotes EMT and invasion by SNAI2 via sponging miR-25; promotes growth and metastasis of orthotopic tumor | 2018 | [115] |
| MEG8 | Lung and pancreatic cancer | A549, LC-2/ad, and Panc1 cells | Human | promotes EMT by suppressing E-cadherin expression via regulating miR-34a/-203 and SNAI1/2 | 2018 | [116] |
| MEG3 | Lung cancer | LC-2/ad, A549 cells | Human | promotes EMT via transcriptional regulation of CDH1 and miR-200 family by JARID2 and EZH2 recruitment to their promoter region | 2017 | [117] |
| Renal cancer | 786-0 and SN12 cells, ccRcc samples | Human | promotes apoptosis via mitochondrial pathway | 2015 | [118] | |
| Breast cancer | BT-549, MDA-MB-231, HF cells | Human | facilitates recruitment by encoding interacting sequences for both PRC2 and GA rich regulatory element | 2015 | [119] | |
| LINC00673 | Non-small cell lung cancer | A549 cell and xenograft, H1975, H596, H520, H1650, H1703 and HEK-293T cells, TCGA cohort | Human, mouse | associated with poorer survival; promotes ZEB-1-EMT and proliferation via sponging miR-150-5p | 2017 | [120] |
| LINC01186 | Non-small cell lung cancer | NSCLC and adjacent tissues, A549, H1299 and 293T cells | Human | regulated by Smad3, inhibits EMT and proliferation | 2017 | [121] |
| LET | Urinary bladder cancers | T24, 5637 cells and xenografts J82, SW780, BIU87, ScaBER and UMUC3 cells, UBC tumor tissues | Human, mouse | suppresses cancer cell stemness for drug resistance via regulating NF90/miR-145 axis | 2017 | [122] |
| LINC01133 | Colorectal cancer | HT29, HCT8, LS513, SW620, HCT116, and HEK293FT, CRC cohort | Human | associated with increase OS; suppresses EMT and metastasis via SRSF6 | 2016 | [123] |
| ATB | Colon | Colon cancer cohort, NCM460, SW480, HCT116, Caco2, Caco205, SW620, and Lovo | Human | associated with metastasis and poorer OS and DFS; promotes EMT and proliferation | 2016 | [124] |
| Breast | SKBR-3 cells, breast cancer cohort | Human | promotes proliferation and EMT via ZEB1, ceRNA of miR-200c | 2015 | [125] | |
| Gastric | Gastric cancer cohort, MKN1, MKN7, MKN28, MKN45, MKN74, KATO III, AGS, and NUGC4 cells | Human | associated with poorer survival, TGF-β(+), ZEB1(+), and miR-200c(−) expression | 2015 | [126] | |
| Colorectal | CRC cohort | Human | associated with metastasis and lower DFS | 2015 | [127] | |
| Liver | QSG-7701 cells, MMC-7721 cells and xenograft, HCC cohort | Human, mouse | promotes EMT by stabilizing IL-11 mRNA via direct interaction | 2014 | [4] | |
| Zeb2 NAT | Urinary bladder cancer | T24, 5637 and J82 cells, Human bladder cancer specimens | Human | promotes EMT via enhancing ZEB2 expression | 2015 | [128] |
| Colon adenocarcinoma | HT-29 M6, RWP-1, SW-480, NMuMG and LS-174T,colon adenocarcinomas tissues | Human | promotes EMT via suppressing E-cadherin by preventing splicing of Zeb2 5-UTR | 2008 | [129] | |
| ROR | Hepatocellular carcinoma | HepG2 and PLC-PRF5 cells | Human | promotes stemness and suppresses apoptosis of HCC cells to reduce chemosensitivity | 2014 | [130] |
| HOTAIR | Renal | A-498 cells, OS-RC-2 cells and xenografts | Human, mouse | promotes cancer cell proliferation by modulating binding between EZH2/ H3K27me3 and p53/21/16 genes | 2014 | [131] |
| Renal | 786-O, ACHN, DU145, HT-29, and HK-2 cells | Human | promotes cancer cell proliferation and invasion via ZEB1 expression | 2014 | [132] | |
| Lung cancer | NSCLC cohort | Human | associated with poorer survival and metastasis | 2013 | [133] | |
| Breast and Colon cancer | MCF10a, HCC1954, DLD1, and HT29 cells | Human, mouse | promotes TGF-β1-induced EMT (E-Cad, Vim, Fn, β-Cat), CD133+/CD44+ cancer stem cell populations | 2013 | [134] | |
| Breast cancer | MDA-MB-231 cells and xenografts, SK-BR-3, MCF-10A, MCF-7, HCC1954, T47D, MDA -MB-453, H16N2 cells | Human, mouse | promotes invasion and metastasis and associated genes via PRC2 | 2010 | [135] | |
| SPRY4-IT1 | Thyroid cancer | thyroid cancer cohort, SW579, K1, TPC-1 and Nthy-ori 3–1 cells | Human | associated with poorer survival; promotes growth and metastasis via reinforcing TGF-β/Smad3 activation | 2018 | [136] |
| Esophageal squamous cell carcinoma | Eca109, KYSE150, Eca9706, EC18, EC1 and HEEC cells | Human | promotes EMT via Snail | 2016 | [137] |
| LncRNA | Cancer Type | Model | Species | Mechanism | Year | Ref. |
|---|---|---|---|---|---|---|
| HCP5 | Lung AD | LUAD cohort and GEO datasets; A549 (xenograft) PC9, H1975, Calu3, HBE, HEK293 and HEK293T cells | Human, mouse | associated with poorer survival; Smad3 direct target; promotes proliferation, invasion, and EMT (miR-203/SNAI) | 2019 | [150] |
| EPR | Breast cancer | NMuMG, MDA-MB-231, HEK-293 cells, 4T1 cell and sygeneic tumor | Human, mouse | enhances Smad3 and CDKN1A promoter binding to induce CDKN1A for cell cycle arrest and suppresses tumor growth | 2019 | [147] |
| LINP 1 | NSCLC | A549, H1299, H358, H441 cells | Human | regulated by Smad4 to suppress EMT | 2018 | [154] |
| TBILA | NSCLC | H226 cells, A549 cells and xenografts, NSCLC tissues | Human, mouse | promotes EMT, proliferation, and motility of cancer cells by direct binding to S100A7 | 2018 | [148] |
| MIR100HG | Pancreatic ductal AD | BxPC-3, PANC-1, COLO357, S2-007 and S2-028 cells | Human, mouse | encodes miR-100, miR-125b to inhibit p53, apoptosis, and cell–cell junctions for tumor growth | 2018 | [151] |
| NORAD | Lung | A549 | Human | promotes EMT via enhancing nuclear localization of activated Smad3 (p-Smad3) | 2018 | [146] |
| NKILA | Esophageal squamous cell carcinoma | KYSE30, KYSE70, KYSE150, KYSE180, KYSE450, KYSE510, Het-1a, ESCC biopsies | Human | Smad3 direct target; suppresses invasion, metastasis, and p-IκBα/p-p65/MMP14 signaling | 2018 | [141] |
| Non-small cell lung cancer | H226, H292, H460, A549, ANP973, H1299 and BEAS-2B, NSCLC biopsies | Human | Smad3 direct target; suppresses EMT, proliferation via p-IκBα/p-p65, Snail | 2017 | [149] | |
| Smad7 | Breast cancer | 4T1 cell, JygMC(A) cell and xenograft | Human, mouse | suppresses TGF-β-induced apoptosis; promotes growth of xenograft | 2014 | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.C.-T.; Zhang, Y.-Y.; Li, J.S.-F.; Chan, M.K.-K.; Chen, J.; Tang, Y.; Zhou, Y.; Zhang, D.; Leung, K.-T.; To, K.-F.; et al. LncRNA-Dependent Mechanisms of Transforming Growth Factor-β: From Tissue Fibrosis to Cancer Progression. Non-Coding RNA 2022, 8, 36. https://doi.org/10.3390/ncrna8030036
Tang PC-T, Zhang Y-Y, Li JS-F, Chan MK-K, Chen J, Tang Y, Zhou Y, Zhang D, Leung K-T, To K-F, et al. LncRNA-Dependent Mechanisms of Transforming Growth Factor-β: From Tissue Fibrosis to Cancer Progression. Non-Coding RNA. 2022; 8(3):36. https://doi.org/10.3390/ncrna8030036
Chicago/Turabian StyleTang, Philip Chiu-Tsun, Ying-Ying Zhang, Jane Siu-Fan Li, Max Kam-Kwan Chan, Jiaoyi Chen, Ying Tang, Yiming Zhou, Dongmei Zhang, Kam-Tong Leung, Ka-Fai To, and et al. 2022. "LncRNA-Dependent Mechanisms of Transforming Growth Factor-β: From Tissue Fibrosis to Cancer Progression" Non-Coding RNA 8, no. 3: 36. https://doi.org/10.3390/ncrna8030036
APA StyleTang, P. C.-T., Zhang, Y.-Y., Li, J. S.-F., Chan, M. K.-K., Chen, J., Tang, Y., Zhou, Y., Zhang, D., Leung, K.-T., To, K.-F., Tang, S. C.-W., Lan, H.-Y., & Tang, P. M.-K. (2022). LncRNA-Dependent Mechanisms of Transforming Growth Factor-β: From Tissue Fibrosis to Cancer Progression. Non-Coding RNA, 8(3), 36. https://doi.org/10.3390/ncrna8030036









