New Insights into the Roles of lncRNAs as Modulators of Cytoskeleton Architecture and Their Implications in Cellular Homeostasis and in Tumorigenesis
Abstract
1. Introduction
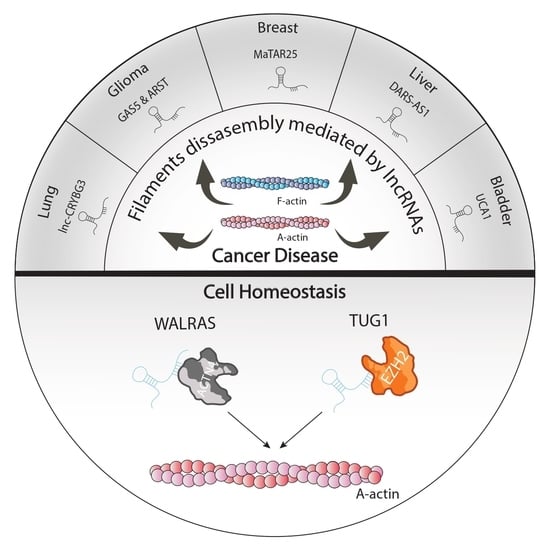
2. lncRNAs as Cytoskeletal Modulators of Cellular Homeostasis
3. lncRNAs Modulators of Actin Filaments and Accessory Proteins in Cancer Pathogenesis
4. lncRNAs as Modulators of Rho/ROCK Signaling in Tumorigenesis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elkon, R.; Agami, R. Characterization of noncoding regulatory DNA in the human genome. Nat. Biotechnol. 2017, 35, 732–746. [Google Scholar] [CrossRef] [PubMed]
- García-Padilla, C.; Dueñas, Á.; García-López, V.; Aránega, A.; Franco, D.; Garcia-Martínez, V.; López-Sánchez, C. Molecular Mechanisms of lncRNAs in the Dependent Regulation of Cancer and Their Potential Therapeutic Use. Int. J. Mol. Sci. 2022, 23, 764. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Padilla, C.; Dueñas, A.; Franco, D.; Garcia-Lopez, V.; Aranega, A.; Garcia-Martinez, V.; Lopez-Sanchez, C. Dynamic MicroRNA Expression Profiles during Embryonic Development Provide Novel Insights Into Cardiac Sinus Venosus/Inflow Tract Differentiation. Front. Cell Dev. Biol. 2022, 9, 767954. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 Catalog of Human Long Noncoding RNAs: Analysis of Their Gene Structure, Evolution, and Expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- De Hoon, M.; Shin, J.W.; Carninci, P. Paradigm shifts in genomics through the FANTOM projects. Mamm. Genome 2015, 26, 391–402. [Google Scholar] [CrossRef]
- Ramilowski, J.A.; Yip, C.W.; Agrawal, S.; Chang, J.-C.; Ciani, Y.; Kulakovskiy, I.V.; Mendez, M.; Ooi, J.L.C.; Ouyang, J.F.; Parkinson, N.; et al. Functional annotation of human long noncoding RNAs via molecular phenotyping. Genome Res. 2020, 30, 1060–1072. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Haines, J.E.; Perez, E.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Rackham, O.; Shearwood, A.-M.J.; Mercer, T.R.; Davies, S.M.; Mattick, J.S.; Filipovska, A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 2011, 17, 2085–2093. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, 202009045. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef] [PubMed]
- Guh, C.-Y.; Hsieh, Y.-H.; Chu, H.-P. Functions and properties of nuclear lncRNAs-from systematically mapping the interactomes of lncRNAs. J. Biomed. Sci. 2020, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, H.; Li, F.; Heindl, L.M.; He, X.; Yu, J.; Yang, J.; Ge, S.; Ruan, J.; Jia, R.; et al. Long Non-coding RNA LINC-PINT Suppresses Cell Proliferation and Migration of Melanoma via Recruiting EZH2. Front. Cell Dev. Biol. 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Liu, R.; Zhang, Y.-W.; Zhang, Y.; Zhou, R.; Sun, J.; Lv, X.-B.; Zhang, Z. Mesenchymal stem cell-associated lncRNA in osteogenic differentiation. Biomed. Pharmacother. 2019, 115, 108912. [Google Scholar] [CrossRef]
- Tang, Y.; He, Y.; Zhang, P.; Wang, J.; Fan, C.; Yang, L.; Xiong, F.; Zhang, S.; Gong, Z.; Nie, S.; et al. lncRNAs regulate the cytoskeleton and related Rho/ROCK signaling in cancer metastasis. Mol. Cancer 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Peng, W.-X.; Koirala, P.; Mo, Y.-Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- García-Padilla, C.; Lozano-Velasco, E.; López-Sánchez, C.; Garcia-Martínez, V.; Aranega, A.; Franco, D. Non-Coding RNAs in Retinoic Acid as Differentiation and Disease Drivers. Non-Coding RNA 2021, 7, 13. [Google Scholar] [CrossRef]
- Rotty, J.D.; Bear, J.E. Competition and collaboration between different actin assembly pathways allows for homeostatic control of the actin cytoskeleton. BioArchitecture 2015, 5, 27–34. [Google Scholar] [CrossRef]
- Hohmann, T.; Dehghani, F. The Cytoskeleton—A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Wu, X. Molecular and cytoskeletal regulations in epidermal development. Semin. Cell Dev. Biol. 2017, 69, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Stradal, T.E.B.; Pusch, R.; Kliche, S. Molecular Regulation of Cytoskeletal Rearrangements During T Cell Signalling. Results Probl. Cell Differ. 2006, 43, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2018, 19, 32–45. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef]
- Aillaud, M.; Schulte, L.N. Emerging Roles of Long Noncoding RNAs in the Cytoplasmic Milieu. Non-Coding RNA 2020, 6, 44. [Google Scholar] [CrossRef]
- Ma, X.; Dang, Y.; Shao, X.; Chen, X.; Wu, F.; Li, Y. Ubiquitination and Long Non-coding RNAs Regulate Actin Cytoskeleton Regulators in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 2997. [Google Scholar] [CrossRef]
- García-Padilla, C.; Domínguez, J.N.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Gorospe, M.; Jiménez-Sábado, V.; Ginel, A.; Hove-Madsen, L.; Aránega, A.E.; et al. Identification of atrial-enriched lncRNA Walras linked to cardiomyocyte cytoarchitecture and atrial fibrillation. FASEB J. 2021, 36, e22051. [Google Scholar] [CrossRef]
- Wen, Q.; Janmey, P.A. Polymer physics of the cytoskeleton. Curr. Opin. Solid State Mater. Sci. 2011, 15, 177–182. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Shen, H.; Hao, Q.; Fu, S.; Liu, X. Up-regulation of long non-coding RNA CYTOR induced by icariin promotes the viability and inhibits the apoptosis of chondrocytes. BMC Complement. Med. Ther. 2021, 21, 152. [Google Scholar] [CrossRef]
- Liang, J.; Wei, X.; Liu, Z.; Cao, D.; Tang, Y.; Zou, Z.; Zhou, C.; Lu, Y. Long noncoding RNA CYTOR in cancer: A TCGA data review. Clin. Chim. Acta 2018, 483, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hinds, M.; Smits, C.; Fredericks-Short, R.; Risk, J.M.; Bailey, M.; Huang, D.; Day, C. Bim, Bad and Bmf: Intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007, 14, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.T.; Navajas, P.L.; Lietha, D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules 2020, 10, 179. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, W. RAB5A promotes the formation of filopodia in pancreatic cancer cells via the activation of cdc42 and β1-integrin. Biochem. Biophys. Res. Commun. 2021, 535, 54–59. [Google Scholar] [CrossRef]
- Du, D.-S.; Yang, X.-Z.; Wang, Q.; Dai, W.-J.; Kuai, W.-X.; Liu, Y.-L.; Chu, D.; Tang, X.-J. Effects of CDC42 on the proliferation and invasion of gastric cancer cells. Mol. Med. Rep. 2016, 13, 550–554. [Google Scholar] [CrossRef]
- Newell-Litwa, K.A.; Badoual, M.; Asmussen, H.; Patel, H.; Whitmore, L.; Horwitz, A.R. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J. Cell Biol. 2015, 210, 225–242. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Blazar, B.R. ROCK2, a critical regulator of immune modulation and fibrosis has emerged as a therapeutic target in chronic graft-versus-host disease. Clin. Immunol. 2021, 230, 108823. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef]
- Tang, Y.; He, Y.; Shi, L.; Yang, L.; Wang, J.; Lian, Y.; Fan, C.; Zhang, P.; Guo, C.; Zhang, S.; et al. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget 2017, 8, 39001–39011. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Advani, T.H.T.A.A. Inflammation in Cardiovascular Disease and Regulation of the Actin Cytoskeleton in Inflammatory Cells: The Actin Cytoskeleton as a Target. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Caporizzo, M.A.; Chen, C.Y.; Prosser, B.L. Cardiac microtubules in health and heart disease. Exp. Biol. Med. 2019, 244, 1255–1272. [Google Scholar] [CrossRef] [PubMed]
- Eira, J.; Silva, C.S.; Sousa, M.; Liz, M.A. The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog. Neurobiol. 2016, 141, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, K.; Stumptner, C.; Fuchsbichler, A.; Fickert, P.; Lackner, C.; Trauner, M.; Denk, H. The keratin cytoskeleton in liver diseases. J. Pathol. 2004, 204, 367–376. [Google Scholar] [CrossRef]
- Akiyama, T.; Kawasaki, Y. Wnt signalling and the actin cytoskeleton. Oncogene 2006, 25, 7538–7544. [Google Scholar] [CrossRef]
- Galli, C.; Piemontese, M.; Lumetti, S.; Ravanetti, F.; Macaluso, G.; Passeri, G. Actin cytoskeleton controls activation of Wnt/β-catenin signaling in mesenchymal cells on implant surfaces with different topographies. Acta Biomater. 2012, 8, 2963–2968. [Google Scholar] [CrossRef]
- Wang, Q.; Symes, A.J.; Kane, C.A.; Freeman, A.; Nariculam, J.; Munson, P.; Thrasivoulou, C.; Masters, J.R.W.; Ahmed, A. A Novel Role for Wnt/Ca2+ Signaling in Actin Cytoskeleton Remodeling and Cell Motility in Prostate Cancer. PLoS ONE 2010, 5, e10456. [Google Scholar] [CrossRef]
- Lorenzon, A.; Calore, M.; Poloni, G.; De Windt, L.J.; Braghetta, P.; Rampazzo, A. Wnt/β-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 2017, 8, 60640–60655. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Garcia-Padilla, C.; Aránega, A.E.; Franco, D. Genetics of Atrial Fibrilation: In Search of Novel Therapeutic Targets. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 183–194. [Google Scholar] [CrossRef]
- Su, I.H.; Dobenecker, M.W.; Dickinson, E.; Oser, M.; Basavaraj, A.; Marqueron, R.; Viale, A.; Reinberg, D.; Wülfing, C.; Tarakhovsky, A. Polycomb Group Protein Ezh2 Controls Actin Polymerization and Cell Signaling. Cell 2005, 121, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kong, P.; Zhang, F.; Shu, Y.-N.; Nie, X.; Dong, L.-H.; Lin, Y.-L.; Xie, X.-L.; Zhao, L.-L.; Zhang, X.-J.; et al. EZH2-mediated α-actin methylation needs lncRNA TUG1, and promotes the cortex cytoskeleton formation in VSMCs. Gene 2017, 616, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sept, D.; Xu, J.; Pollard, T.D.; McCammon, J.A. Annealing Accounts for the Length of Actin Filaments Formed by Spontaneous Polymerization. Biophys. J. 1999, 77, 2911–2919. [Google Scholar] [CrossRef]
- Chánez-Paredes, S.; Montoya-García, A.; Schnoor, M. Cellular and pathophysiological consequences of Arp2/3 complex inhibition: Role of inhibitory proteins and pharmacological compounds. Cell. Mol. Life Sci. 2019, 76, 3349–3361. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular Mechanisms Controlling Actin Filament Dynamics in Nonmuscle Cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Biber, G.; Ben-Shmuel, A.; Sabag, B.; Barda-Saad, M. Actin regulators in cancer progression and metastases: From structure and function to cytoskeletal dynamics. International Review of Cell and Molecular Biology 2020, 356, 131–196. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Jones, M.C.; Zha, J.; Humphries, M.J. Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180227. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Uray, K.; Major, E.; Lontay, B. MicroRNA Regulatory Pathways in the Control of the Actin–Myosin Cytoskeleton. Cells 2020, 9, 1649. [Google Scholar] [CrossRef]
- Pei, H.; Hu, W.; Guo, Z.; Chen, H.; Ma, J.; Mao, W.; Li, B.; Wang, A.; Wan, J.; Zhang, J.; et al. Long Noncoding RNA CRYBG3 Blocks Cytokinesis by Directly Binding G-Actin. Cancer Res. 2018, 78, 4563–4572. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.L.; Kabbarah, O.; Liang, M.-C.; Ivanova, E.; Anagnostou, V.; Wu, J.; Dhakal, S.; Wu, M.; Chen, S.; Feinberg, T.; et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 2009, 459, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Bass-Zubek, A.E.; Godsel, L.M.; Delmar, M.; Green, K.J. Plakophilins: Multifunctional scaffolds for adhesion and signaling. Curr. Opin. Cell Biol. 2009, 21, 708–716. [Google Scholar] [CrossRef]
- Van Grembergen, O.; Bizet, M.; de Bony, E.J.; Calonne, E.; Putmans, P.; Brohée, S.; Olsen, C.; Guo, M.; Bontempi, G.; Sotiriou, C.; et al. Portraying breast cancers with long noncoding RNAs. Sci. Adv. 2016, 2, e1600220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, W.; Ma, D.; Cao, J.; Fu, T.; Zhang, Z.; Zhang, Y. Long non-coding RNA CYTOR regulates proliferation and metastasis of colon cancer cells through regulating miRNA-105/PTEN axis. Int. J. Clin. Experi Ment. Pathol. 2021, 14, 434–443. [Google Scholar]
- Tian, Q.; Yan, X.; Yang, L.; Liu, Z.; Yuan, Z.; Zhang, Y. lncRNA CYTOR promotes cell proliferation and tumor growth via miR-125b/SEMA4C axis in hepatocellular carcinoma. Oncol. Lett. 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Wei, F.; Wang, Y.; Zhou, Y.; Li, Y. Long noncoding RNA CYTOR triggers gastric cancer progression by targeting miR-103/RAB10. Acta Biochim. et Biophys. Sin. 2021, 53, 1044–1054. [Google Scholar] [CrossRef]
- Chen, W.; Du, M.; Hu, X.; Ma, H.; Zhang, E.; Wang, T.; Yin, L.; He, X.; Hu, Z. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma. Cancer Med. 2019, 9, 1209–1219. [Google Scholar] [CrossRef]
- Ostrowska, Z.; Moraczewska, J. Cofilin – a protein controlling dynamics of actin filaments. Postępy Higieny i Medycyny Doświadczalnej 2017, 71, 339–351. [Google Scholar] [CrossRef]
- Shishkin, S.; Eremina, L.; Pashintseva, N.; Kovalev, L.; Kovaleva, M. Cofilin-1 and Other ADF/Cofilin Superfamily Members in Human Malignant Cells. Int. J. Mol. Sci. 2016, 18, 10. [Google Scholar] [CrossRef]
- Howard, J.; Goh, C.Y.; Gorzel, K.W.; Higgins, M.; McCann, A. The potential role of cofilin-1 in promoting triple negative breast cancer (TNBC) metastasis via the extracellular vesicles (EVs). Transl. Oncol. 2021, 15, 101247. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Y.; Zhao, J.; Wu, L.; Qi, Q.; Liu, Y.; Li, G.; Li, J.; Liu, H.; Wu, H. Cofilin: A Promising Protein Implicated in Cancer Metastasis and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tao, L.; Jin, F.; Gu, H.; Dai, X.; Ni, T.; Feng, J.; Ding, Y.; Xiao, W.; Qian, Y.; et al. Cofilin 1 induces the epithelial-mesenchymal transition of gastric cancer cells by promoting cytoskeletal rearrangement. Oncotarget 2017, 8, 39131–39142. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Ng, O.; Saunders, J.H.; Acheson, A.G.; Parsons, S.L. Study of cofilin 1 gene expression in colorectal cancer. J. Gastrointest. Oncol. 2018, 9, 791–796. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, P.; Liu, J.; Zheng, J.; Liu, Y.; Chen, J.; Xue, Y. Gas5 Exerts Tumor-suppressive Functions in Human Glioma Cells by Targeting miR-222. Mol. Ther. 2015, 23, 1899–1911. [Google Scholar] [CrossRef]
- Sun, J.; He, D.; Fu, Y.; Zhang, R.; Guo, H.; Wang, Z.; Wang, Y.; Gao, T.; Wei, Y.; Guo, Y.; et al. A novel lncRNA ARST represses glioma progression by inhibiting ALDOA-mediated actin cytoskeleton integrity. J. Exp. Clin. Cancer Res. 2021, 40, 187. [Google Scholar] [CrossRef]
- Cartron, P.-F.; Loussouarn, D.; Campone, M.; Martin, S.A.; Vallette, F. Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis. 2012, 3, e421. [Google Scholar] [CrossRef]
- Scott, G.A.; McClelland, L.A.; Fricke, A.F.; Fender, A. Plexin C1, A Receptor for Semaphorin 7A, Inactivates Cofilin and Is a Potential Tumor Suppressor for Melanoma Progression. J. Investig. Dermatol. 2009, 129, 954–963. [Google Scholar] [CrossRef]
- Fuse, M.; Kojima, S.; Enokida, H.; Chiyomaru, T.; Yoshino, H.; Nohata, N.; Kinoshita, T.; Sakamoto, S.; Naya, Y.; Nakagawa, M.; et al. Tumor suppressive microRNAs (miR-222 and miR-31) regulate molecular pathways based on microRNA expression signature in prostate cancer. J. Hum. Genet. 2012, 57, 691–699. [Google Scholar] [CrossRef]
- Garofalo, M.; Quintavalle, C.; Romano, G.; Croce, C.M.; Condorelli, G. miR221/222 in Cancer: Their Role in Tumor Progression and Response to Therapy. Curr. Mol. Med. 2012, 12, 27–33. [Google Scholar] [CrossRef]
- Quintavalle, C.; Garofalo, M.; Zanca, C.; Romano, G.; Iaboni, M.; Caro, M.D.B.D.; Martinez-Montero, J.C.; Incoronato, M.; Nuovo, G.; Croce, C.M.; et al. miR-221/222 overexpession in human glioblastoma increases invasiveness by targeting the protein phosphate PTPμ. Oncogene 2011, 31, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.K.; Aspalter, I.M.; Sixt, M. Focal Adhesion–Independent Cell Migration. Annu. Rev. Cell Dev. Biol. 2016, 32, 469–490. [Google Scholar] [CrossRef]
- Shih, Y.-P.; Sun, P.; Wang, A.; Lo, S.H. Tensin1 positively regulates RhoA activity through its interaction with DLC1. Biochim. et Biophys. Acta 2015, 1853, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.-Y.E.; Lim, S.-T.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Diermeier, S.D.; Yu, A.T.; Brine, L.D.; Russo, S.; Bhatia, S.; Alsudani, H.; Kostroff, K.; Bhuiya, T.; Brogi, E.; et al. MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression. Nat. Commun. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kelm, R.J.; Lamba, G.S.; Levis, J.E.; Holmes, C.E. Characterization of purine-rich element binding protein B as a novel biomarker in acute myelogenous leukemia prognostication. J. Cell. Biochem. 2018, 119, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.H.; Daugherty, A.E.; Choi, C.K.; Horwitz, A.F.; Brautigan, D.L. Tensin1 Requires Protein Phosphatase-1α in Addition to RhoGAP DLC-1 to Control Cell Polarization, Migration, and Invasion. J. Biol. Chem. 2009, 284, 34713–34722. [Google Scholar] [CrossRef]
- Chen, H.; Duncan, I.C.; Bozorgchami, H.; Lo, S.H. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc. Natl. Acad. Sci. USA 2002, 99, 733–738. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 1–11. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, G.; Zhang, L.; Zhou, H.; Wang, W.; Guo, P.; Cheng, C.; Ji, L.; Cai, Q.; Feng, Y.; et al. LncRNA DARS-AS1 aggravates the growth and metastasis of hepatocellular carcinoma via regulating the miR-3200-5p-Cytoskeleton associated protein 2 (CKAP2) axis. Bioeng. 2021, 12, 8217–8232. [Google Scholar] [CrossRef] [PubMed]
- Machesky, L.M. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008, 582, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145– ZEB 1/2– FSCN 1 pathway. Cancer Sci. 2015, 107, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Li, L.; Cao, J.; Guo, Y.; Wu, Y.; Gao, W. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol. Ther. Oncolytics 2021, 20, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, K.; O’Neill, A.; Prencipe, M.; Bugler, J.; Murphy, L.; Fabre, A.; Puhr, M.; Culig, Z.; Murphy, K.; Watson, R.W. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol. Oncol. 2017, 11, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Symons, M. Rho family GTPases: The cytoskeleton and beyond. Trends Biochem. Sci. 1996, 21, 178–181. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. RHO GTPASES: Biochemistry and Biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in cancer: Friend or foe? Oncogene 2019, 38, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Bement, W.M. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 2009, 11, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Strugnell, S.S.; Goetz, J.; Kojic, L.D.; Cox, M.E.; Griffith, O.; Chan, S.K.; Jones, S.; Leung, S.-P.; Masoudi, H.; et al. Phosphorylated Caveolin-1 Regulates Rho/ROCK-Dependent Focal Adhesion Dynamics and Tumor Cell Migration and Invasion. Cancer Res. 2008, 68, 8210–8220. [Google Scholar] [CrossRef]
- García-Mariscal, A.; Li, H.; Pedersen, E.; Peyrollier, K.; Ryan, K.M.; Stanley, A.; Quondamatteo, F.; Brakebusch, C. Loss of RhoA promotes skin tumor formation and invasion by upregulation of RhoB. Oncogene 2018, 37, 847–860. [Google Scholar] [CrossRef]
- Gallo, G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell Sci. 2006, 119, 3413–3423. [Google Scholar] [CrossRef]
- Heasman, S.J.; Ridley, A.J. Multiple roles for RhoA during T cell transendothelial migration. Small GTPases 2010, 1, 174–179. [Google Scholar] [CrossRef]
- Fernandez-Borja, M.; Janssen, L.; Verwoerd, D.; Hordijk, P.; Neefjes, J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J. Cell Sci. 2005, 118, 2661–2670. [Google Scholar] [CrossRef]
- Egami, Y.; Kawai, K.; Araki, N. RhoC regulates actin remodeling to form phagosomes during FcγR-mediated phagocytosis. J. Cell Sci. 2017, 130, 4168–4179. [Google Scholar] [CrossRef]
- Bompard, G.; Sharp, S.J.; Freiss, G.; Machesky, L. Involvement of Rac in actin cytoskeleton rearrangements induced by MIM-B. J. Cell Sci. 2005, 118, 5393–5403. [Google Scholar] [CrossRef][Green Version]
- Kurokawa, K.; Itoh, R.; Yoshizaki, H.; Nakamura, Y.O.T.; Matsuda, M. Coactivation of Rac1 and Cdc42 at Lamellipodia and Membrane Ruffles Induced by Epidermal Growth Factor. Mol. Biol. Cell 2004, 15, 1003–1010. [Google Scholar] [CrossRef]
- Mehidi, A.; Rossier, O.; Schaks, M.; Chazeau, A.; Binamé, F.; Remorino, A.; Coppey, M.; Karatas, Z.; Sibarita, J.-B.; Rottner, K.; et al. Transient Activations of Rac1 at the Lamellipodium Tip Trigger Membrane Protrusion. Curr. Biol. 2019, 29, 2852–2866.e5. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.K.; Cabrera, R.; Mao, S.P.; Christin, J.R.; Wu, B.; Guo, W.; Bravo-Cordero, J.J.; Condeelis, J.S.; Segall, J.E.; Hodgson, L. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J. Cell Biol. 2017, 216, 4331–4349. [Google Scholar] [CrossRef] [PubMed]
- Masi, I.; Caprara, V.; Spadaro, F.; Chellini, L.; Sestito, R.; Zancla, A.; Rainer, A.; Bagnato, A.; Rosanò, L. Endothelin-1 drives invadopodia and interaction with mesothelial cells through ILK. Cell Rep. 2021, 34, 108800. [Google Scholar] [CrossRef] [PubMed]
- Arana, E.; Vehlow, A.; Harwood, N.E.; Vigorito, E.; Henderson, R.; Turner, M.; Tybulewicz, V.; Batista, F.D. Activation of the Small GTPase Rac2 via the B Cell Receptor Regulates B Cell Adhesion and Immunological-Synapse Formation. Immun. 2008, 28, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Krugmann, S.; Jordens, I.; Gevaert, K.; Driessens, M.; Vandekerckhove, J.; Hall, A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 2001, 11, 1645–1655. [Google Scholar] [CrossRef]
- Aikemu, B.; Shao, Y.; Yang, G.; Ma, J.; Zhang, S.; Yang, X.; Hong, H.; Yesseyeva, G.; Huang, L.; Jia, H.; et al. NDRG1 regulates Filopodia-induced Colorectal Cancer invasiveness via modulating CDC42 activity. Int. J. Biol. Sci. 2021, 17, 1716–1730. [Google Scholar] [CrossRef]
- Xiao, X.-H.; Lv, L.-C.; Duan, J.; Wu, Y.-M.; He, S.-J.; Hu, Z.-Z.; Xiong, L.-X. Regulating Cdc42 and Its Signaling Pathways in Cancer: Small Molecules and MicroRNA as New Treatment Candidates. Molecules 2018, 23, 787. [Google Scholar] [CrossRef]
- Jerrell, R.J.; Leih, M.J.; Parekh, A. The ROCK isoforms differentially regulate the morphological characteristics of carcinoma cells. Small GTPases 2020, 11, 131–137. [Google Scholar] [CrossRef][Green Version]
- Darenfed, H.; Dayanandan, B.; Zhang, T.; Hsieh, S.H.-K.; Fournier, A.E.; Mandato, C.A. Molecular characterization of the effects of Y-27632. Cell Motil. Cytoskelet. 2007, 64, 97–109. [Google Scholar] [CrossRef]
- Chin, V.T.; Nagrial, A.M.; Chou, A.; Biankin, A.V.; Gill, A.J.; Timpson, P.; Pajic, M. Rho-associated kinase signalling and the cancer microenvironment: Novel biological implications and therapeutic opportunities. Expert Rev. Mol. Med. 2015, 17, e17. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-M.; Wei, L.; Au, S.L.-K.; Fan, D.N.-Y.; Zhou, Y.; Tsang, F.H.-C.; Law, C.-T.; Lee, J.M.-F.; He, X.; Shi, J.; et al. MiR-200b/200c/429 subfamily negatively regulates Rho/ROCK signaling pathway to suppress hepatocellular carcinoma metastasis. Oncotarget 2015, 6, 13658–13670. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, Y.; Yang, W.; Xia, Y.; Yang, C.; Yang, S.; Liu, X. Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J. Orthop. Res. 2016, 34, 932–941. [Google Scholar] [CrossRef]
- Chou, J.; Wang, B.; Zheng, T.; Li, X.; Zheng, L.; Hu, J.; Zhang, Y.; Xing, Y.; Xi, T. MALAT1 induced migration and invasion of human breast cancer cells by competitively binding miR-1 with cdc42. Biochem. Biophys. Res. Commun. 2016, 472, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Rozenchan, P.B.; Pasini, F.S.; Roela, R.A.; Katayama, M.L.H.; Mundim, F.G.L.; Brentani, H.; Lyra, E.C.; Brentani, M.M. Specific upregulation of RHOA and RAC1 in cancer-associated fibroblasts found at primary tumor and lymph node metastatic sites in breast cancer. Tumor Biol. 2015, 36, 9589–9597. [Google Scholar] [CrossRef]
- Ge, Z.; Cheng, Z.; Yang, X.; Huo, X.; Wang, N.; Wang, H.; Wang, C.; Gu, D.; Zhao, F.; Yao, M.; et al. Long noncoding RNASchLAHsuppresses metastasis of hepatocellular carcinoma through interacting with fused in sarcoma. Cancer Sci. 2017, 108, 653–662. [Google Scholar] [CrossRef]
- Wang, C.; Yan, G.; Zhang, Y.; Jia, X.; Bu, P. Long non-coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma 2015, 62, 541–549. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Surma, M.; Vemula, S.; Zhang, L.; Yang, Y.; Kapur, R.; Wei, L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013, 4, e483. [Google Scholar] [CrossRef]
- Shen, D.; Liu, Y.; Liu, Y.; Wang, T.; Yuan, L.; Huang, X.; Wang, Y. Long non-coding RNA EWSAT1 promoted metastasis and actin cytoskeleton changes via miR-24-3p sponging in osteosarcoma. J. Cell. Mol. Med. 2021, 25, 716–728. [Google Scholar] [CrossRef]




| Cytoskeletal-lncRNAs Related to Tumorigenesis | ||||
|---|---|---|---|---|
| lncRNA | Target Molecule | Function | Tissue or Cell Line | Reference |
| LNC-CRYBG3 | G-actin | Inhibition of F-actin polymerization avoiding G-actin phosphorylation | Lung cancer | [28] |
| CYTOR | Golph3, Rhobtb3 and PKP4 | Cytoskeletal homeostasis and cell cycle progression | Breast cancer cell line | [29] |
| Gas5 | miR-222 | Enhance Bmf and PLXN1 expression reducing aggressiveness tumour | U87 and U251 glioma cell line | [30] |
| ARST | ALDOA | Mediate actin fibers integrity avoiding that ALDOA can attach to F-actin binding sites increasing F-actin depolymeration | U87 and U251 glioma cell line | [31] |
| MaTaR25 | PURB and Tensin1 | Enhance PURB dependent genes remodelling cytoskeleton architecture and increasing migration and spread out of maligned cells | Breast cancer cell line | [32] |
| DARS-AS1 | miR-3002 | Sponge miR-3002 enhancing CKAP2 translation and aggravating the growth and metastasis of tumor | Hepatocellular carcinoma | [33] |
| UCA1 | ZEB1/2 and FSCN1 | Increase formation of actin-dependent cell filopodia enhancing metastasis | Bladder carcinoma | [34] |
| Malat1 | RhoA, ROCK1 and ROCK2 | Increasing RhoA, ROCK1, and ROCK2 translation required to migration and cytoskeletal homeostasis | Osteosarcoma | [35] |
| Malat1 | miR-1 | Sponge miR-1 enhancing Cdc42 translation required to migration and cytoskeletal homeostasis | Breast carcinoma | [36] |
| AFAP1-AS1 | RhoA and Rac1 | Enhancing progression and poor prognosis of nasopharyngeal carcinoma increasing capacity of spreading out | nasopharyngeal carcinoma | [37] |
| SchLAH | FUS/TLS | Repressing cellular migration and therein metastasis triggering downregulation of RhoA/Rac2 signalling | Lung carcinoma | [38] |
| EWAST1 | miR-24-3p | Sponge miR-24-3p enhancing expression of ROCK1 and promoting actin stress fiber formation and migration | Osteosarcoma | [39] |
| Walras | ACTN4 | Required to actin cytoskeleton integrity | Cardiomyocites | [40] |
| TUG1 | EZH2 and actin | Methylation of α-actin by EZH2 | Vascular smooth muscle cells | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Padilla, C.; Muñoz-Gallardo, M.d.M.; Lozano-Velasco, E.; Castillo-Casas, J.M.; Caño-Carrillo, S.; García-López, V.; Aránega, A.; Franco, D.; García-Martínez, V.; López-Sánchez, C. New Insights into the Roles of lncRNAs as Modulators of Cytoskeleton Architecture and Their Implications in Cellular Homeostasis and in Tumorigenesis. Non-Coding RNA 2022, 8, 28. https://doi.org/10.3390/ncrna8020028
García-Padilla C, Muñoz-Gallardo MdM, Lozano-Velasco E, Castillo-Casas JM, Caño-Carrillo S, García-López V, Aránega A, Franco D, García-Martínez V, López-Sánchez C. New Insights into the Roles of lncRNAs as Modulators of Cytoskeleton Architecture and Their Implications in Cellular Homeostasis and in Tumorigenesis. Non-Coding RNA. 2022; 8(2):28. https://doi.org/10.3390/ncrna8020028
Chicago/Turabian StyleGarcía-Padilla, Carlos, María del Mar Muñoz-Gallardo, Estefanía Lozano-Velasco, Juan Manuel Castillo-Casas, Sheila Caño-Carrillo, Virginio García-López, Amelia Aránega, Diego Franco, Virginio García-Martínez, and Carmen López-Sánchez. 2022. "New Insights into the Roles of lncRNAs as Modulators of Cytoskeleton Architecture and Their Implications in Cellular Homeostasis and in Tumorigenesis" Non-Coding RNA 8, no. 2: 28. https://doi.org/10.3390/ncrna8020028
APA StyleGarcía-Padilla, C., Muñoz-Gallardo, M. d. M., Lozano-Velasco, E., Castillo-Casas, J. M., Caño-Carrillo, S., García-López, V., Aránega, A., Franco, D., García-Martínez, V., & López-Sánchez, C. (2022). New Insights into the Roles of lncRNAs as Modulators of Cytoskeleton Architecture and Their Implications in Cellular Homeostasis and in Tumorigenesis. Non-Coding RNA, 8(2), 28. https://doi.org/10.3390/ncrna8020028