Chromatin-Associated RNAs Regulate Gene Expression and Chromatin Structure
Abstract
1. Introduction
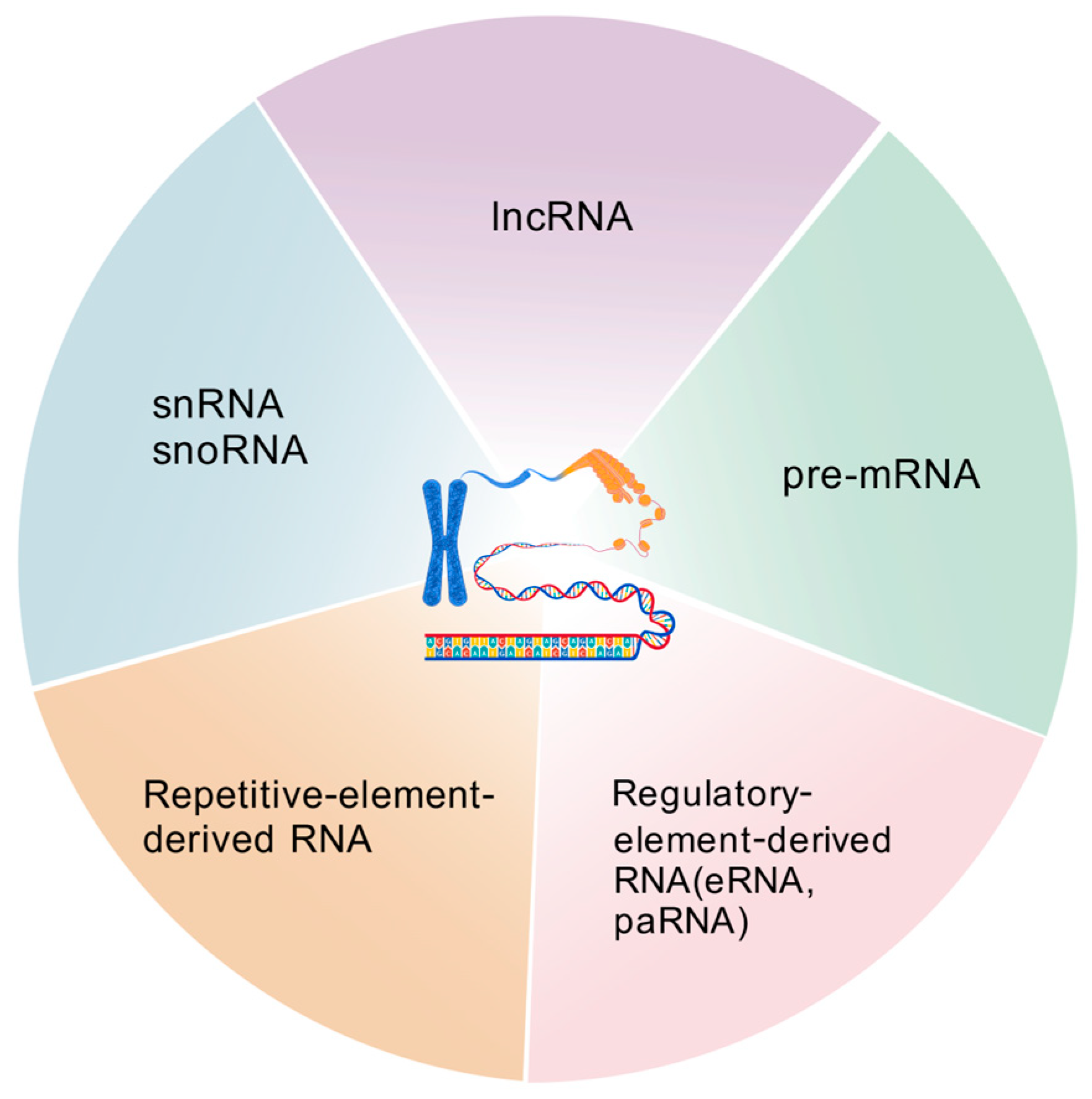
2. Chromatin-Associated RNA Species
2.1. Chromatin-Associated Protein-Coding RNAs
2.2. Chromatin-Associated ncRNAs
2.2.1. Chromatin-Associated lncRNAs
2.2.2. ncRNAs Derived from Chromatin-Associated Regulatory Elements
2.2.3. ncRNAs Transcribed from Repetitive DNA Elements
2.2.4. Chromatin-Associated snRNAs and snoRNAs
3. Chromosome-Associated RNAs During Mitosis and Meiosis
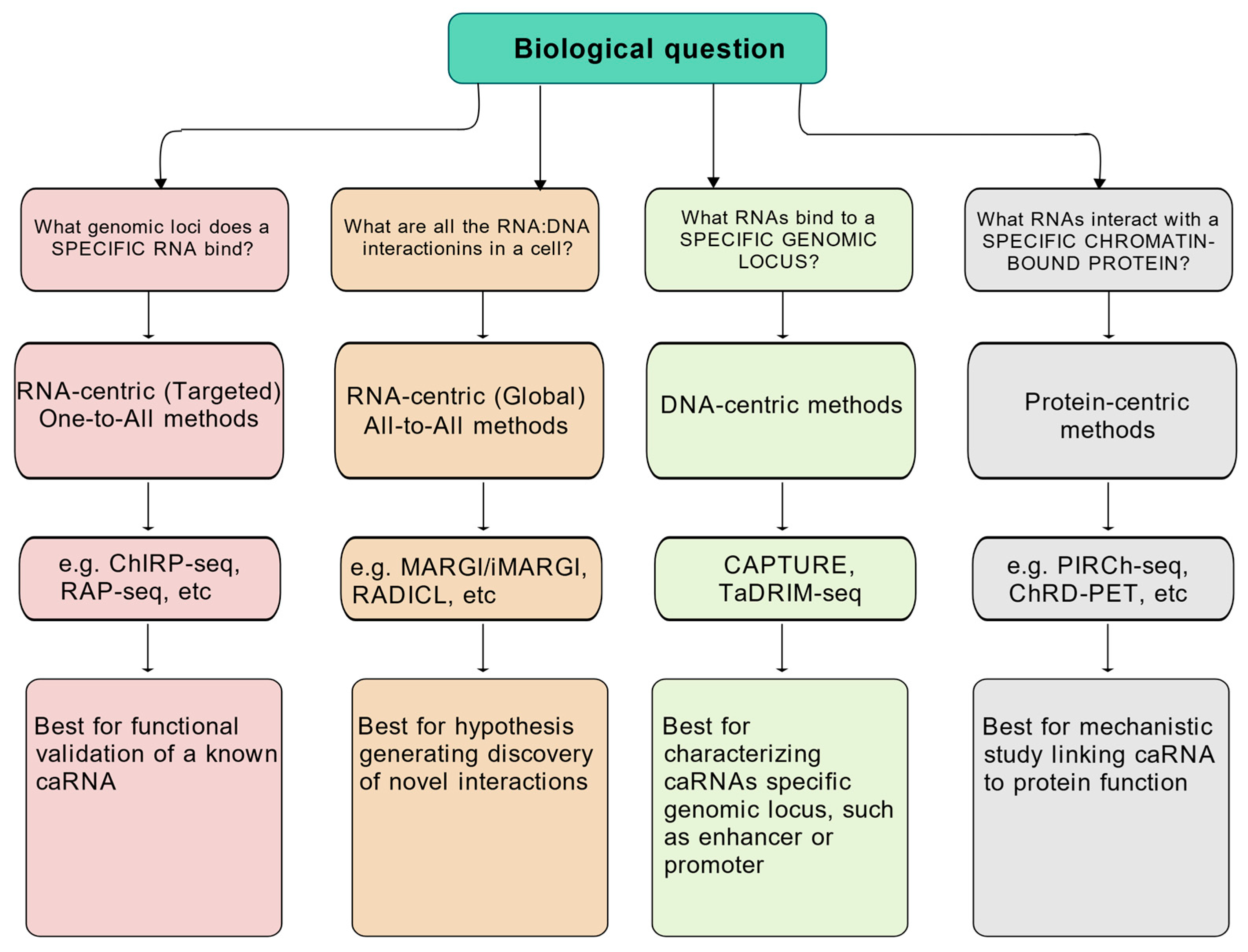
4. Methods to Study Chromatin and RNA Interactions
4.1. RNA-Centric Methods
4.1.1. One-to-All Methods
Chromatin Isolated by RNA Purification (ChIRP)
Capture Hybridization Analysis of RNA Targets (CHART)
RNA Antisense Purification (RAP)
4.1.2. All-to-All Methods
Mapping RNA–Genome Interactions (MARGI) and iMARGI
Global RNA Interaction with DNA Sequencing (GRID-seq)
Chromatin-Associated RNA Sequencing (ChAR-seq)
RNA and DNA Interacting Complexes Ligated and Sequenced (RADICL-seq)
RNA Ends on DNA Capture (Red-C)
4.2. DNA-Centric Methods
4.2.1. CRISPR Affinity Purification in Situ of Regulatory Elements (CAPTURE) Approach
4.2.2. Targeted DNA-Associated RNA and RNA-RNA Interaction Mapping by Sequencing (TaDRIM-seq)
4.3. Protein-Centric Methods
4.3.1. Chromatin-Associated RNA Immunoprecipitation Followed by Next-Generation Sequencing (CARIP-seq)
4.3.2. Profiling Interacting RNAs on Chromatin Followed by Deep Sequencing (PIRCh-seq)
4.3.3. Chromatin-Associated RNA–DNA Interactions, Followed by Paired-End-Tag Sequencing (ChRD-PET)
4.3.4. RedChIP
4.3.5. RT & Tag
4.3.6. RNA and DNA Interacting Complexes Ligated and Sequenced (RADICL-seq) with Immunoprecipitation (RADIP)
4.3.7. Chromatin Sequencing (Chrom-seq)
| Type | Method | Principle | Strengths | Limitations | Suitable for | Reference | |
|---|---|---|---|---|---|---|---|
| RNA-centric | One to all | ChIRP | Hybridization+ pull-down | High specificity with probe design; compatible with sequencing | Requires strong crosslinking; may miss transient interactions | General Individual RNA- chromatin mapping | [97] |
| CHART | Hybridization (guided by RNase H) | Rational probe design reduces background; cross-species applicability | Requires known RNA accessibility; less sensitive for poorly characterized RNAs | Structured RNAs with known access sites | [99] | ||
| RAP | Long, tiled probes | High specificity and coverage; tolerant to RNA structure | Requires complex probe synthesis | long, structured, or degraded RNAs | [104] | ||
| All to all | MARGI/ iMARGI | Proximity ligation | Genome wide and unbiased; no need for RNA specific probes | High background; needs deep sequencing | Global profiling of RNA- chromatin interactions | [84,105] | |
| GRID-seq | Proximity ligation with linker | All reads contain RNA and DNA; high interaction confidence | short reads may limit resolution | High confidence global interaction mapping | [106] | ||
| ChAR-seq | proximity ligation with sonication | Compatible with complex genomes; longer chimeric fragments | Higher background in mammalian cells | Comparative studies across species; flexible ligation fragment sizes | [108] | ||
| RADICL-seq | Proximity ligation with transcription inhibition | Reduces nascent transcription bias; high mapping efficiency | May miss transient or dynamic interactions | investigating stable RNA-chromatin contacts; transcription-dependent chromatin regulation | [109] | ||
| Red-C | Ligation of RNA 3′ends to fragmented DNA | Capture diverse coding and non-coding RNA interactions | Biased toward RNA 3′end; limited resolution | Mapping broad RNA- chromatin interactions across chromatin states | [110] | ||
| DNA-centric | CAPTURE | Locus specific targeting with dCas9-biotin complex | High sensitivity for specific loci; identifies RNA, DNA, and protein interactions | Requires gRNA design; not genome-wide | Detailed analysis of chromatin regulation at specific loci | [111] | |
| TaDRIM-seq | PG-Tn5 tethering+ in situ proximity ligation | Simultaneous detection of DNA-RNA and RNA-RNA interactions; efficient; low input | Requires targeting design; not genome-wide | Mapping complex RNA networks at specific DNA loci in animal or plant systems | [112] | ||
| Chromatin-bound protein-centric | CARIP-seq | ChIP-RIP hybrid: Crosslink +IP of chromatin proteins | Captures RNAs bound to specific chromatin proteins; good for heterochromatin | Limited to protein-specific interactions; resolution depends on antibody quality | Profiling RNAs associated with repressive chromatin marks | [113,114] | |
| PIRCh-seq | Crosslinking+IP+ caRNA profiling | Histone modification specific caRNA identification; reduced nascent RNA contamination | Cannot identify binding sites; may still co-purify mRNAs | Global profiling of caRNAs | [115] | ||
| ChRD-PET | ChIP+promximity ligation +PET sequencing | Maps specific RNA-DNA interactions with spatial context; reveals RNA-mediated chromatin looping | Technically complex; resolution depends on proximity ligation efficiency | Studying RNA’s role in 3D genome organization | [116] | ||
| RedChIP | ChIP+RNA-DNA chimera formation+sequencing | Identifies ncRNAs associated with protein defined chromatin regions | Resolution limited by proximity ligation and ChIP antibody quality | Studying ncRNAs at specific chromatin environments | [117] | ||
| RT&Tag | Antibody targeting+ in situ RT+Tn5 tagmentation | High resolution, efficient, detects RNA-chromatin/protein/modification | Depends on antibody specificity and reverse transcription efficiency | Mapping RNA-chromatin/ protein/modification in situ | [118] | ||
| RADIP | Crosslinking+RNA-DNA ligation+IP of target protein | High specificity for protein mediated RNA-DNA interactions | Requires good antibody, may miss transient or weak interactions | Studying protein specific RNA-chromatin interactions | [119] | ||
| Chrom-seq | Chromatin mark reader+ APEX2 labeling without crosslinking or antibodies | Live cell, label free mapping of RNAs near specific chromatin marks | Requires efficient expression of fusion proteins; limited by APEX2 labeling radius | In vivo detection of caRNAs at specific epigenetic states | [120] | ||
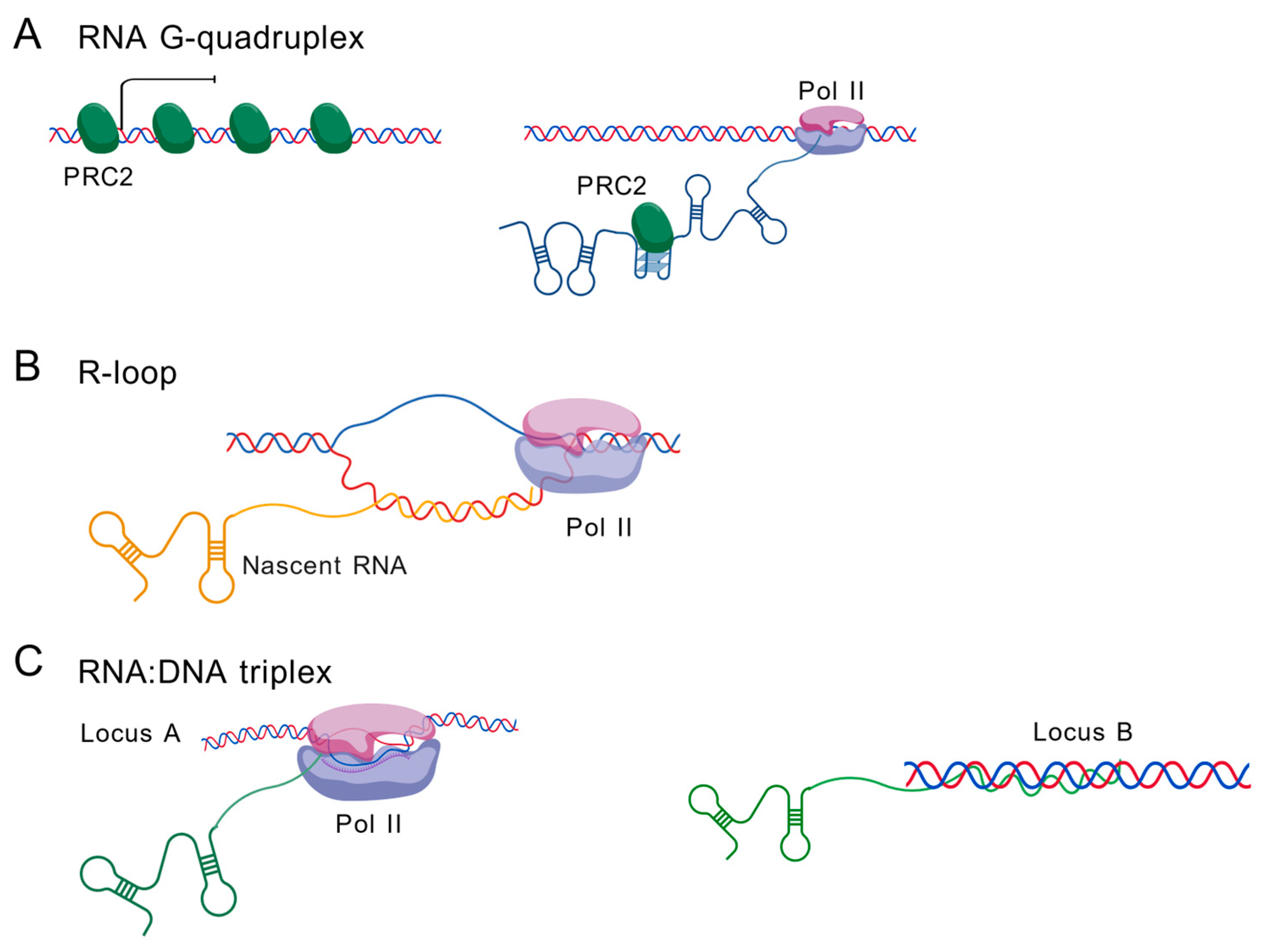
5. Non-Canonical Structures in caRNAs
5.1. RNA G-Quadruplexes (G4s)
5.2. R-Loop
5.3. RNA:DNA Triplex
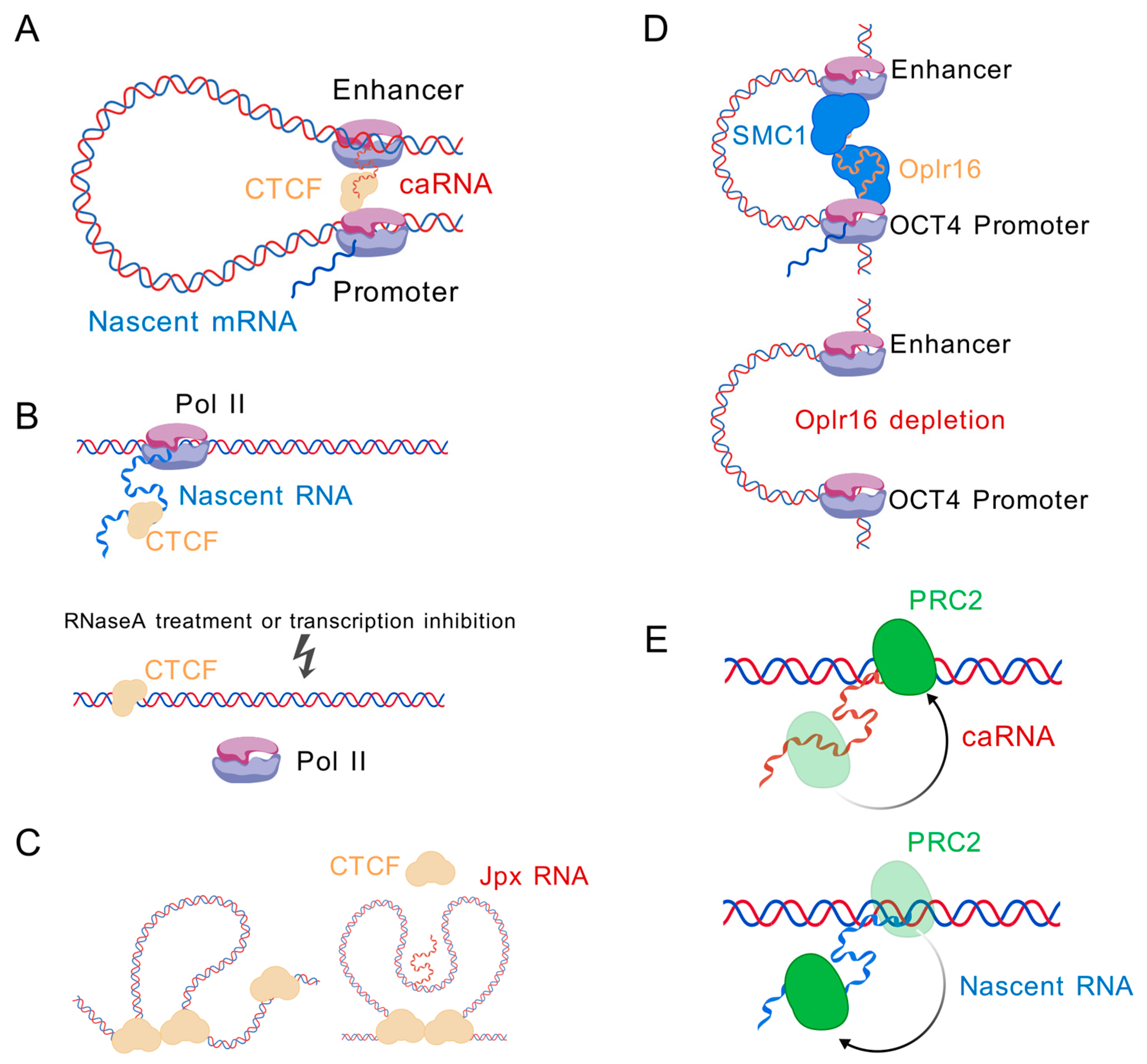
6. caRNA-Interacting Proteins
6.1. CTCF
6.2. Cohesin
6.3. PRC2
7. Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| caRNAs | Chromatin-associated RNAs |
| lncRNAs | Long non-coding RNAs |
| eRNAs | Enhancer RNAs |
| paRNAs | Promoter-associated RNAs |
| circRNAs | Circular RNAs |
| snRNAs | Small nuclear RNAs |
| snoRNAs | Small nucleolar RNAs |
| asRNAs | Antisense RNAs |
| HERVH | Human endogenous retrovirus subfamily H |
| ChIRP | Chromatin isolated by RNA purification |
| CHART | Capture hybridization analysis of RNA targets |
| RAP | RNA antisense purification |
| CARIP-seq | Chromatin-associated RNA immunoprecipitation followed by next-generation sequencing |
| PIRCh-seq | Profiling interacting RNAs on chromatin followed by deep sequencing |
| MARGI | Mapping RNA–genome interactions |
| GRID-seq | Global RNA interaction with DNA sequencing |
| ChAR-seq | Chromatin-associated RNA sequencing |
| RADICL-seq | RNA and DNA interacting complexes ligated and sequenced |
| PAS RNAs | Promoter antisense RNAs |
| Red-C | RNA ends on DNA capture |
| CAPTURE | CRISPR affinity purification in situ of regulatory elements |
| TaDRIM-seq | Targeted DNA-associated RNA and RNA-RNA interaction mapping by sequencing |
| ChRD-PET | Chromatin-associated RNA–DNA interactions, followed by paired-end-tag sequencing |
| RADIP | RNA and DNA interacting complexes ligated and sequenced (RADICL-seq) with immunoprecipitation |
| Chrom-seq | Chromatin sequencing |
| mCARs | Mitotic chromosome-associated RNAs |
References
- Palihati, M.; Saitoh, N. RNA in chromatin organization and nuclear architecture. Curr. Opin. Genet. Dev. 2024, 86, 102176. [Google Scholar] [CrossRef]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, X. Noncoding RNA-chromatin association: Functions and mechanisms. Fundam. Res. 2023, 3, 665–675. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res. 2021, 49, D165–D171. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.V.; Gavrilov, A.A. Non-coding RNAs in chromatin folding and nuclear organization. Cell Mol. Life Sci. 2021, 78, 5489–5504. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.D. Chromatin-associated RNAs as facilitators of functional genomic interactions. Nat. Rev. Genet. 2019, 20, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, X.; Xiao, D.; Liu, S.; Tao, Y. The chromatin-associated RNAs in gene regulation and cancer. Mol. Cancer 2023, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Sampath, K.; Ephrussi, A. CncRNAs: RNAs with both coding and non-coding roles in development. Development 2016, 143, 1234–1241. [Google Scholar] [CrossRef]
- Dinger, M.E.; Pang, K.C.; Mercer, T.R.; Mattick, J.S. Differentiating protein-coding and noncoding RNA: Challenges and ambiguities. PLoS Comput. Biol. 2008, 4, e1000176. [Google Scholar] [CrossRef]
- Ulveling, D.; Francastel, C.; Hube, F. When one is better than two: RNA with dual functions. Biochimie 2011, 93, 633–644. [Google Scholar] [CrossRef]
- Caudron-Herger, M.; Müller-Ott, K.; Mallm, J.-P.; Marth, C.; Schmidt, U.; Fejes-Tóth, K.; Rippe, K. Coding RNAs with a non-coding function: Maintenance of open chromatin structure. Nucleus 2014, 2, 410–424. [Google Scholar] [CrossRef]
- Xie, B.; Dean, A. Noncoding function of super enhancer derived Cpox pre-mRNA in modulating neighbouring gene expression and chromatin interactions. RNA Biol. 2025, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Langst, G. The chromatin-triple helix connection. Biol. Chem. 2023, 404, 1037–1049. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef]
- Mishra, K.; Kanduri, C. Understanding Long Noncoding RNA and Chromatin Interactions: What We Know So Far. Noncoding RNA 2019, 5, 54. [Google Scholar] [CrossRef]
- Thakur, J.; Henikoff, S. Architectural RNA in chromatin organization. Biochem. Soc. Trans. 2020, 48, 1967–1978. [Google Scholar] [CrossRef]
- Postepska-Igielska, A.; Giwojna, A.; Gasri-Plotnitsky, L.; Schmitt, N.; Dold, A.; Ginsberg, D.; Grummt, I. LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Mol. Cell 2015, 60, 626–636. [Google Scholar] [CrossRef]
- Deng, C.; Li, Y.; Zhou, L.; Cho, J.; Patel, B.; Terada, N.; Li, Y.; Bungert, J.; Qiu, Y.; Huang, S. HoxBlinc RNA Recruits Set1/MLL Complexes to Activate Hox Gene Expression Patterns and Mesoderm Lineage Development. Cell Rep. 2016, 14, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Yang, Y.W.; Liu, B.; Sanyal, A.; Corces-Zimmerman, R.; Chen, Y.; Lajoie, B.R.; Protacio, A.; Flynn, R.A.; Gupta, R.A.; et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011, 472, 120–124. [Google Scholar] [CrossRef]
- Mondal, T.; Rasmussen, M.; Pandey, G.K.; Isaksson, A.; Kanduri, C. Characterization of the RNA content of chromatin. Genome Res. 2010, 20, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cao, C.; Ji, L.; Ye, R.; Wang, D.; Xia, C.; Wang, S.; Du, Z.; Hu, N.; Yu, X.; et al. RIC-seq for global in situ profiling of RNA-RNA spatial interactions. Nature 2020, 582, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA activates Xist by evicting CTCF. Cell 2013, 153, 1537–1551. [Google Scholar] [CrossRef]
- Luo, H.; Zhu, G.; Eshelman, M.A.; Fung, T.K.; Lai, Q.; Wang, F.; Zeisig, B.B.; Lesperance, J.; Ma, X.; Chen, S.; et al. HOTTIP-dependent R-loop formation regulates CTCF boundary activity and TAD integrity in leukemia. Mol. Cell 2022, 82, 833–851.e11. [Google Scholar] [CrossRef]
- Deforzh, E.; Kharel, P.; Zhang, Y.; Karelin, A.; El Khayari, A.; Ivanov, P.; Krichevsky, A.M. HOXDeRNA activates a cancerous transcription program and super enhancers via genome-wide binding. Mol. Cell 2024, 84, 3950–3966.e6. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Guiducci, G.; Stojic, L. Long Noncoding RNAs at the Crossroads of Cell Cycle and Genome Integrity. Trends Genet. 2021, 37, 528–546. [Google Scholar] [CrossRef]
- Jegu, T.; Aeby, E.; Lee, J.T. The X chromosome in space. Nat. Rev. Genet. 2017, 18, 377–389. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Brockdorff, N.; Kawano, S.; Tsutui, K.; Tsutui, K.; Nakagawa, S. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev. Cell 2010, 19, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pullirsch, D.; Hartel, R.; Kishimoto, H.; Leeb, M.; Steiner, G.; Wutz, A. The Trithorax group protein Ash2l and Saf-A are recruited to the inactive X chromosome at the onset of stable X inactivation. Development 2010, 137, 935–943. [Google Scholar] [CrossRef]
- Henninger, J.E.; Young, R.A. An RNA-centric view of transcription and genome organization. Mol. Cell 2024, 84, 3627–3643. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Gebhard, C.; Miguel-Escalada, I.; Hoof, I.; Bornholdt, J.; Boyd, M.; Chen, Y.; Zhao, X.; Schmidl, C.; Suzuki, T.; et al. An atlas of active enhancers across human cell types and tissues. Nature 2014, 507, 455–461. [Google Scholar] [CrossRef]
- Lorenzi, L.; Chiu, H.S.; Avila Cobos, F.; Gross, S.; Volders, P.J.; Cannoodt, R.; Nuytens, J.; Vanderheyden, K.; Anckaert, J.; Lefever, S.; et al. The RNA Atlas expands the catalog of human non-coding RNAs. Nat. Biotechnol. 2021, 39, 1453–1465. [Google Scholar] [CrossRef]
- Sartorelli, V.; Lauberth, S.M. Enhancer RNAs are an important regulatory layer of the epigenome. Nat. Struct. Mol. Biol. 2020, 27, 521–528. [Google Scholar] [CrossRef]
- Schwalb, B.; Michel, M.; Zacher, B.; Frühauf, K.; Demel, C.; Tresch, A.; Gagneur, J.; Cramer, P. TT-seq maps the human transient transcriptome. Science 2016, 352, 1225–1228. [Google Scholar] [CrossRef]
- Kristjansdottir, K.; Dziubek, A.; Kang, H.M.; Kwak, H. Population-scale study of eRNA transcription reveals bipartite functional enhancer architecture. Nat. Commun. 2020, 11, 5963. [Google Scholar] [CrossRef]
- Kim, T.K.; Hemberg, M.; Gray, J.M.; Costa, A.M.; Bear, D.M.; Wu, J.; Harmin, D.A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465, 182–187. [Google Scholar] [CrossRef] [PubMed]
- De Santa, F.; Barozzi, I.; Mietton, F.; Ghisletti, S.; Polletti, S.; Tusi, B.K.; Muller, H.; Ragoussis, J.; Wei, C.L.; Natoli, G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010, 8, e1000384. [Google Scholar] [CrossRef]
- Harrison, L.J.; Bose, D. Enhancer RNAs step forward: New insights into enhancer function. Development 2022, 149, dev.200398. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Wen, J.; Chen, X.; Xie, B.; Zhao, Y. Enhancer RNAs: Mechanisms in transcriptional regulation and functions in diseases. Cell Commun. Signal 2023, 21, 191. [Google Scholar] [CrossRef]
- Schlackow, M.; Nojima, T.; Gomes, T.; Dhir, A.; Carmo-Fonseca, M.; Proudfoot, N.J. Distinctive Patterns of Transcription and RNA Processing for Human lincRNAs. Mol. Cell 2017, 65, 25–38. [Google Scholar] [CrossRef]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Kazadi, D.; Sun, J.; Federation, A.; Chao, J.; Elliott, O.; Liu, Z.P.; et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 2015, 161, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lu, J.Y.; Zhang, X.; Shao, W.; Xu, Y.; Li, P.; Hong, Y.; Cui, L.; Shan, G.; Tian, B.; et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature 2020, 580, 147–150. [Google Scholar] [CrossRef]
- Mikhaylichenko, O.; Bondarenko, V.; Harnett, D.; Schor, I.E.; Males, M.; Viales, R.R.; Furlong, E.E.M. The degree of enhancer or promoter activity is reflected by the levels and directionality of eRNA transcription. Genes Dev. 2018, 32, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Liu, Y.; Liao, X.; Zhan, H.; Liu, Y.; Huang, W. Enhancer RNAs (eRNAs): New Insights into Gene Transcription and Disease Treatment. J. Cancer 2018, 9, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Notani, D.; Ma, Q.; Tanasa, B.; Nunez, E.; Chen, A.Y.; Merkurjev, D.; Zhang, J.; Ohgi, K.; Song, X.; et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 2013, 498, 516–520. [Google Scholar] [CrossRef]
- Hah, N.; Murakami, S.; Nagari, A.; Danko, C.G.; Kraus, W.L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013, 23, 1210–1223. [Google Scholar] [CrossRef]
- Arner, E.; Daub, C.O.; Vitting-Seerup, K.; Andersson, R.; Lilje, B.; Drabløs, F.; Lennartsson, A.; Rönnerblad, M.; Hrydziuszko, O.; Vitezic, M.; et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 2015, 347, 1010–1014. [Google Scholar] [CrossRef]
- Xie, B.; Dean, A. A Super Enhancer-Derived Enhancer RNA Acts Together with CTCF/Cohesin in Trans to Regulate Erythropoiesis. Genes 2025, 16, 389. [Google Scholar] [CrossRef]
- Ivaldi, M.S.; Diaz, L.F.; Chakalova, L.; Lee, J.; Krivega, I.; Dean, A. Fetal gamma-globin genes are regulated by the BGLT3 long noncoding RNA locus. Blood 2018, 132, 1963–1973. [Google Scholar] [CrossRef]
- Mousavi, K.; Zare, H.; Dell’orso, S.; Grontved, L.; Gutierrez-Cruz, G.; Derfoul, A.; Hager, G.L.; Sartorelli, V. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 2013, 51, 606–617. [Google Scholar] [CrossRef]
- Kim, J.; Diaz, L.F.; Miller, M.J.; Leadem, B.; Krivega, I.; Dean, A. An enhancer RNA recruits KMT2A to regulate transcription of Myb. Cell Rep. 2024, 43, 114378. [Google Scholar] [CrossRef]
- Tsai, P.F.; Dell’orso, S.; Rodriguez, J.; Vivanco, K.O.; Ko, K.D.; Jiang, K.; Juan, A.H.; Sarshad, A.A.; Vian, L.; Tran, M.; et al. A Muscle-Specific Enhancer RNA Mediates Cohesin Recruitment and Regulates Transcription In trans. Mol. Cell 2018, 71, 129–141. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, J.R.; Knoll, M.; Gromatzky, A.A.; Lodish, H.F. The Super-Enhancer-Derived alncRNA-EC7/Bloodlinc Potentiates Red Blood Cell Development in trans. Cell Rep. 2017, 19, 2503–2514. [Google Scholar] [CrossRef]
- Liu, G.; Kim, J.; Nguyen, N.; Zhou, L.; Dean, A. Long noncoding RNA GATA2AS influences human erythropoiesis by transcription factor and chromatin landscape modulation. Blood 2024, 143, 2300–2313. [Google Scholar] [CrossRef]
- Core, L.J.; Waterfall, J.J.; Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 2008, 322, 1845–1848. [Google Scholar] [CrossRef]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D.A.; Duttagupta, R.; Willingham, A.T.; Stadler, P.F.; Hertel, J.; Hackermüller, J.; Hofacker, I.L.; et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007, 316, 1484–1488. [Google Scholar] [CrossRef]
- Neil, H.; Malabat, C.; d’Aubenton-Carafa, Y.; Xu, Z.; Steinmetz, L.M.; Jacquier, A. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 2009, 457, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Preker, P.; Nielsen, J.; Kammler, S.; Lykke-Andersen, S.; Christensen, M.S.; Mapendano, C.K.; Schierup, M.H.; Jensen, T.H. RNA exosome depletion reveals transcription upstream of active human promoters. Science 2008, 322, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Seila, A.C.; Calabrese, J.M.; Levine, S.S.; Yeo, G.W.; Rahl, P.B.; Flynn, R.A.; Young, R.A.; Sharp, P.A. Divergent transcription from active promoters. Science 2008, 322, 1849–1851. [Google Scholar] [CrossRef]
- Taft, R.J.; Glazov, E.A.; Cloonan, N.; Simons, C.; Stephen, S.; Faulkner, G.J.; Lassmann, T.; Forrest, A.R.; Grimmond, S.M.; Schroder, K.; et al. Tiny RNAs associated with transcription start sites in animals. Nat. Genet. 2009, 41, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Sigova, A.A.; Mullen, A.C.; Molinie, B.; Gupta, S.; Orlando, D.A.; Guenther, M.G.; Almada, A.E.; Lin, C.; Sharp, P.A.; Giallourakis, C.C.; et al. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2876–2881. [Google Scholar] [CrossRef]
- Yang, F. Promoter antisense RNAs: Beyond transcription by-products of active promoters. RNA Biol. 2022, 19, 533–540. [Google Scholar] [CrossRef]
- Lu, X.; Sachs, F.; Ramsay, L.; Jacques, P.E.; Goke, J.; Bourque, G.; Ng, H.H. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat. Struct. Mol. Biol. 2014, 21, 423–425. [Google Scholar] [CrossRef]
- Huo, X.; Ji, L.; Zhang, Y.; Lv, P.; Cao, X.; Wang, Q.; Yan, Z.; Dong, S.; Du, D.; Zhang, F.; et al. The Nuclear Matrix Protein SAFB Cooperates with Major Satellite RNAs to Stabilize Heterochromatin Architecture Partially through Phase Separation. Mol. Cell 2020, 77, 368–383.e7. [Google Scholar] [CrossRef] [PubMed]
- Velazquez Camacho, O.; Galan, C.; Swist-Rosowska, K.; Ching, R.; Gamalinda, M.; Karabiber, F.; De La Rosa-Velazquez, I.; Engist, B.; Koschorz, B.; Shukeir, N.; et al. Major satellite repeat RNA stabilize heterochromatin retention of Suv39h enzymes by RNA-nucleosome association and RNA:DNA hybrid formation. Elife 2017, 6, e25293. [Google Scholar] [CrossRef]
- Lu, J.Y.; Chang, L.; Li, T.; Wang, T.; Yin, Y.; Zhan, G.; Han, X.; Zhang, K.; Tao, Y.; Percharde, M.; et al. Homotypic clustering of L1 and B1/Alu repeats compartmentalizes the 3D genome. Cell Res. 2021, 31, 613–630. [Google Scholar] [CrossRef]
- Hall, L.L.; Carone, D.M.; Gomez, A.V.; Kolpa, H.J.; Byron, M.; Mehta, N.; Fackelmayer, F.O.; Lawrence, J.B. Stable C0T-1 Repeat RNA Is Abundant and Is Associated with Euchromatic Interphase Chromosomes. Cell 2014, 156, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, R.S.; Boteva, L.; Soares, D.C.; Naughton, C.; Dun, A.R.; Buckle, A.; Ramsahoye, B.; Bruton, P.C.; Saleeb, R.S.; Arnedo, M.; et al. SAF-A Regulates Interphase Chromosome Structure through Oligomerization with Chromatin-Associated RNAs. Cell 2017, 169, 1214–1227.e18. [Google Scholar] [CrossRef]
- Feretzaki, M.; Pospisilova, M.; Valador Fernandes, R.; Lunardi, T.; Krejci, L.; Lingner, J. RAD51-dependent recruitment of TERRA lncRNA to telomeres through R-loops. Nature 2020, 587, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, E.; Lingner, J. TERRA long noncoding RNA: At the interphase of telomere damage, rescue and signaling. Curr. Opin. Cell Biol. 2024, 91, 102437. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Yamazaki, T.; Hirose, T. Satellite RNAs: Emerging players in subnuclear architecture and gene regulation. EMBO J. 2023, 42, e114331. [Google Scholar] [CrossRef]
- Miyata, K.; Takahashi, A. Pericentromeric repetitive ncRNA regulates chromatin interaction and inflammatory gene expression. Nucleus 2022, 13, 74–78. [Google Scholar] [CrossRef]
- Lopes, M.; Louzada, S.; Gama-Carvalho, M.; Chaves, R. Pericentromeric satellite RNAs as flexible protein partners in the regulation of nuclear structure. Wiley Interdiscip. Rev. RNA 2024, 15, e1868. [Google Scholar] [CrossRef]
- Brönner, C.; Salvi, L.; Zocco, M.; Ugolini, I.; Halic, M. Accumulation of RNA on chromatin disrupts heterochromatic silencing. Genome Res. 2017, 27, 1174–1183. [Google Scholar] [CrossRef]
- Burton, A.; Brochard, V.; Galan, C.; Ruiz-Morales, E.R.; Rovira, Q.; Rodriguez-Terrones, D.; Kruse, K.; Le Gras, S.; Udayakumar, V.S.; Chin, H.G.; et al. Heterochromatin establishment during early mammalian development is regulated by pericentromeric RNA and characterized by non-repressive H3K9me3. Nat. Cell Biol. 2020, 22, 767–778. [Google Scholar] [CrossRef]
- Chen, F.X.; Xie, P.; Collings, C.K.; Cao, K.; Aoi, Y.; Marshall, S.A.; Rendleman, E.J.; Ugarenko, M.; Ozark, P.A.; Zhang, A.; et al. PAF1 regulation of promoter-proximal pause release via enhancer activation. Science 2017, 357, 1294–1298. [Google Scholar] [CrossRef]
- Flynn, R.A.; Do, B.T.; Rubin, A.J.; Calo, E.; Lee, B.; Kuchelmeister, H.; Rale, M.; Chu, C.; Kool, E.T.; Wysocka, J.; et al. 7SK-BAF axis controls pervasive transcription at enhancers. Nat. Struct. Mol. Biol. 2016, 23, 231–238. [Google Scholar] [CrossRef]
- Berg, M.G.; Singh, L.N.; Younis, I.; Liu, Q.; Pinto, A.M.; Kaida, D.; Zhang, Z.; Cho, S.; Sherrill-Mix, S.; Wan, L.; et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell 2012, 150, 53–64. [Google Scholar] [CrossRef]
- Kaida, D.; Berg, M.G.; Younis, I.; Kasim, M.; Singh, L.N.; Wan, L.; Dreyfuss, G. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 2010, 468, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Will, C.L.; Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a003707. [Google Scholar] [CrossRef] [PubMed]
- Tilgner, H.; Knowles, D.G.; Johnson, R.; Davis, C.A.; Chakrabortty, S.; Djebali, S.; Curado, J.; Snyder, M.; Gingeras, T.R.; Guigo, R. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 2012, 22, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.G.; Terns, R.M.; Terns, M.P. Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, L.Y.; Luo, X.Q.; Pan, Q.; Sun, Y.M.; Zeng, Z.C.; Chen, T.Q.; Huang, W.; Fang, K.; Wang, W.T.; et al. Chromatin-associated orphan snoRNA regulates DNA damage-mediated differentiation via a non-canonical complex. Cell Rep. 2022, 38, 110421. [Google Scholar] [CrossRef]
- Sridhar, B.; Rivas-Astroza, M.; Nguyen, T.C.; Chen, W.; Yan, Z.; Cao, X.; Hebert, L.; Zhong, S. Systematic Mapping of RNA-Chromatin Interactions In Vivo. Curr. Biol. 2017, 27, 602–609. [Google Scholar] [CrossRef]
- Schubert, T.; Pusch, M.C.; Diermeier, S.; Benes, V.; Kremmer, E.; Imhof, A.; Langst, G. Df31 protein and snoRNAs maintain accessible higher-order structures of chromatin. Mol. Cell 2012, 48, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, Y.; Shi, M.; Ye, B.; Yin, M.; Li, P.; Shi, S.; Jin, Y.; Zhang, Z.; Zhang, M.Q.; et al. Profiling and characterization of constitutive chromatin-enriched RNAs. iScience 2022, 25, 105349. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, C.; Xu, Z.; Li, H.; Ye, B.; Li, X.; Czajkowsky, D.M.; Shao, Z. Quantitative catalogue of mammalian mitotic chromosome-associated RNAs. Sci. Data 2024, 11, 43. [Google Scholar] [CrossRef]
- Meng, Y.; Yi, X.; Li, X.; Hu, C.; Wang, J.; Bai, L.; Czajkowsky, D.M.; Shao, Z. The non-coding RNA composition of the mitotic chromosome by 5′-tag sequencing. Nucleic Acids Res. 2016, 44, 4934–4946. [Google Scholar] [CrossRef]
- Ding, D.-Q.; Okamasa, K.; Katou, Y.; Oya, E.; Nakayama, J.-i.; Chikashige, Y.; Shirahige, K.; Haraguchi, T.; Hiraoka, Y. Chromosome-associated RNA–protein complexes promote pairing of homologous chromosomes during meiosis in Schizosaccharomyces pombe. Nat. Commun. 2019, 10, 5598. [Google Scholar] [CrossRef]
- Nakajima, R.; Sato, T.; Ogawa, T.; Okano, H.; Noce, T. A noncoding RNA containing a SINE-B1 motif associates with meiotic metaphase chromatin and has an indispensable function during spermatogenesis. PLoS ONE 2017, 12, e0179585. [Google Scholar] [CrossRef]
- Ding, D.-Q.; Okamasa, K.; Yamane, M.; Tsutsumi, C.; Haraguchi, T.; Yamamoto, M.; Hiraoka, Y. Meiosis-Specific Noncoding RNA Mediates Robust Pairing of Homologous Chromosomes in Meiosis. Science 2012, 336, 732–736. [Google Scholar] [CrossRef]
- Shichino, Y.; Yamashita, A.; Yamamoto, M. Meiotic long non-coding meiRNA accumulates as a dot at its genetic locus facilitated by Mmi1 and plays as a decoy to lure Mmi1. Open Biol. 2014, 4, 140022. [Google Scholar] [CrossRef]
- Jonkers, I.; Monkhorst, K.; Rentmeester, E.; Grootegoed, J.A.; Grosveld, F.; Gribnau, J. Xist RNA is confined to the nuclear territory of the silenced X chromosome throughout the cell cycle. Mol. Cell Biol. 2008, 28, 5583–5594. [Google Scholar] [CrossRef][Green Version]
- Rošić, S.; Köhler, F.; Erhardt, S. Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J. Cell Biol. 2014, 207, 335–349. [Google Scholar] [CrossRef]
- Ren, B.; Zhong, Y.; Yang, Y.; Chang, S.; Li, Y.; You, M.; Shan, G.; Wang, X.; Chen, E. Chromatin-associated α-satellite RNA maintains chromosome stability by reestablishing SAF-A in the mitotic cell cycle. Nucleic Acids Res. 2025, 53, gkaf294. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Luo, M.; Xie, G.; Wang, X.; Li, Q.; Gao, L.; Yu, H.; Yu, X. Ribosomal RNA regulates chromosome clustering during mitosis. Cell Discov. 2022, 8, 51. [Google Scholar] [CrossRef]
- Chu, C.; Quinn, J.; Chang, H.Y. Chromatin isolation by RNA purification (ChIRP). J. Vis. Exp. 2012, 25, 3912. [Google Scholar] [CrossRef]
- Mumbach, M.R.; Granja, J.M.; Flynn, R.A.; Roake, C.M.; Satpathy, A.T.; Rubin, A.J.; Qi, Y.; Jiang, Z.; Shams, S.; Louie, B.H.; et al. HiChIRP reveals RNA-associated chromosome conformation. Nat. Methods 2019, 16, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.D.; Wang, C.I.; Kharchenko, P.V.; West, J.A.; Chapman, B.A.; Alekseyenko, A.A.; Borowsky, M.L.; Kuroda, M.I.; Kingston, R.E. The genomic binding sites of a noncoding RNA. Proc. Natl. Acad. Sci. USA 2011, 108, 20497–20502. [Google Scholar] [CrossRef]
- Tenorio, M.; Cruz-Ruiz, S.; Encarnación-Guevara, S.; Hernández, M.; Corona-Gomez, J.A.; Sheccid-Santiago, F.; Serwatowska, J.; López-Perdomo, S.; Flores-Aguirre, C.D.; Arenas-Moreno, D.M.; et al. MAYEX is an old long noncoding RNA recruited for X chromosome dosage compensation in a reptile. Science 2024, 385, 1347–1354. [Google Scholar] [CrossRef]
- Bujisic, B.; Lee, H.G.; Xu, L.; Weissbein, U.; Rivera, C.; Topisirovic, I.; Lee, J.T. 7SL RNA and signal recognition particle orchestrate a global cellular response to acute thermal stress. Nat. Commun. 2025, 16, 1630. [Google Scholar] [CrossRef]
- Simon, M.D.; Pinter, S.F.; Fang, R.; Sarma, K.; Rutenberg-Schoenberg, M.; Bowman, S.K.; Kesner, B.A.; Maier, V.K.; Kingston, R.E.; Lee, J.T. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature 2013, 504, 465–469. [Google Scholar] [CrossRef]
- Ito, S.; Ueno, A.; Ueda, T.; Ogura, R.; Sako, S.; Gabata, Y.; Murashita, J.; Takahashi, H.; Ukimura, O. A testis-specific lncRNA functions as a post-transcriptional regulator of MDM2 and stimulates apoptosis of testicular germ cell tumor cells. Cell Death Discov. 2024, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Engreitz, J.M.; Pandya-Jones, A.; McDonel, P.; Shishkin, A.; Sirokman, K.; Surka, C.; Kadri, S.; Xing, J.; Goren, A.; Lander, E.S.; et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 2013, 341, 1237973. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, N.; Wu, W.; Chen, W.; Jiang, Y.; Chen, J.; Huang, X.; Wen, X.; Xu, J.; Jin, Q.; et al. Genome-wide colocalization of RNA-DNA interactions and fusion RNA pairs. Proc. Natl. Acad. Sci. USA 2019, 116, 3328–3337. [Google Scholar] [CrossRef]
- Li, X.; Zhou, B.; Chen, L.; Gou, L.T.; Li, H.; Fu, X.D. GRID-seq reveals the global RNA-chromatin interactome. Nat. Biotechnol. 2017, 35, 940–950. [Google Scholar] [CrossRef]
- Kato, M.; Carninci, P. Genome-Wide Technologies to Study RNA-Chromatin Interactions. Noncoding RNA 2020, 6, 20. [Google Scholar] [CrossRef]
- Bell, J.C.; Jukam, D.; Teran, N.A.; Risca, V.I.; Smith, O.K.; Johnson, W.L.; Skotheim, J.M.; Greenleaf, W.J.; Straight, A.F. Chromatin-associated RNA sequencing (ChAR-seq) maps genome-wide RNA-to-DNA contacts. Elife 2018, 7, e27024. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Agostini, F.; Suzuki, A.M.; Hashimoto, K.; Pascarella, G.; Gimenez, J.; Roos, L.; Nash, A.J.; Ghilotti, M.; Cameron, C.J.F.; et al. RADICL-seq identifies general and cell type-specific principles of genome-wide RNA-chromatin interactions. Nat. Commun. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, A.A.; Zharikova, A.A.; Galitsyna, A.A.; Luzhin, A.V.; Rubanova, N.M.; Golov, A.K.; Petrova, N.V.; Logacheva, M.D.; Kantidze, O.L.; Ulianov, S.V.; et al. Studying RNA-DNA interactome by Red-C identifies noncoding RNAs associated with various chromatin types and reveals transcription dynamics. Nucleic Acids Res. 2020, 48, 6699–6714. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Chen, Y.; Li, M.; Zhou, F.; Li, K.; Cao, H.; Ni, M.; Liu, Y.; Gu, Z.; et al. In Situ Capture of Chromatin Interactions by Biotinylated dCas9. Cell 2017, 170, 1028–1043.e19. [Google Scholar] [CrossRef]
- Ding, C.; Chen, G.; Luan, S.; Gao, R.; Fan, Y.; Zhang, Y.; Wang, X.; Li, G.; Foda, M.F.; Yan, J.; et al. Simultaneous profiling of chromatin-associated RNA at targeted DNA loci and RNA-RNA Interactions through TaDRIM-seq. Nat. Commun. 2025, 16, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kidder, B.L. CARIP-Seq and ChIP-Seq: Methods to Identify Chromatin-Associated RNAs and Protein-DNA Interactions in Embryonic Stem Cells. J. Vis. Exp. 2018, 135, e57481. [Google Scholar] [CrossRef]
- Kurup, J.T.; Kidder, B.L. Identification of H4K20me3- and H3K4me3-associated RNAs using CARIP-Seq expands the transcriptional and epigenetic networks of embryonic stem cells. J. Biol. Chem. 2018, 293, 15120–15135. [Google Scholar] [CrossRef]
- Fang, J.; Ma, Q.; Chu, C.; Huang, B.; Li, L.; Cai, P.; Batista, P.J.; Tolentino, K.E.M.; Xu, J.; Li, R.; et al. PIRCh-seq: Functional classification of non-coding RNAs associated with distinct histone modifications. Genome Biol. 2019, 20, 292. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, X.; Zhang, Y.; Xu, W.; Yang, Y.; Zhang, Q.; Hu, Z.; Xing, F.; Sun, Q.; Li, G.; et al. The landscape of promoter-centred RNA-DNA interactions in rice. Nat. Plants 2022, 8, 157–170. [Google Scholar] [CrossRef]
- Gavrilov, A.A.; Sultanov, R.I.; Magnitov, M.D.; Galitsyna, A.A.; Dashinimaev, E.B.; Lieberman Aiden, E.; Razin, S.V. RedChIP identifies noncoding RNAs associated with genomic sites occupied by Polycomb and CTCF proteins. Proc. Natl. Acad. Sci. USA 2022, 119, e2116222119. [Google Scholar] [CrossRef] [PubMed]
- Khyzha, N.; Henikoff, S.; Ahmad, K. Profiling RNA at chromatin targets in situ by antibody-targeted tagmentation. Nat. Methods 2022, 19, 1383–1392. [Google Scholar] [CrossRef]
- Shu, X.; Kato, M.; Takizawa, S.; Suzuki, Y.; Carninci, P. RADIP technology comprehensively identifies H3K27me3-associated RNA-chromatin interactions. Nucleic Acids Res. 2024, 52, e104. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sun, W.; Lyu, Y.; Ju, F.; Sun, W.; Chen, J.; Ma, H.; Yang, S.; Zhou, X.; Wu, N.; et al. Chrom-seq identifies RNAs at chromatin marks. Sci. Adv. 2024, 10, eadn1397. [Google Scholar] [CrossRef]
- Dumas, L.; Herviou, P.; Dassi, E.; Cammas, A.; Millevoi, S. G-Quadruplexes in RNA Biology: Recent Advances and Future Directions. Trends Biochem. Sci. 2021, 46, 270–283. [Google Scholar] [CrossRef]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459–474. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; Balasubramanian, S. An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2. J. Am. Chem. Soc. 2012, 134, 11974–11976. [Google Scholar] [CrossRef]
- Takahama, K.; Takada, A.; Tada, S.; Shimizu, M.; Sayama, K.; Kurokawa, R.; Oyoshi, T. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem. Biol. 2013, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Takahama, K.; Kino, K.; Arai, S.; Kurokawa, R.; Oyoshi, T. Identification of Ewing’s sarcoma protein as a G-quadruplex DNA- and RNA-binding protein. FEBS J. 2011, 278, 988–998. [Google Scholar] [CrossRef]
- Conlon, E.G.; Lu, L.; Sharma, A.; Yamazaki, T.; Tang, T.; Shneider, N.A.; Manley, J.L. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife 2016, 5, 17820. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Harvey, S.E.; Hu, X.; Cheng, C. RNA G-quadruplex secondary structure promotes alternative splicing via the RNA-binding protein hnRNPF. Genes Dev. 2017, 31, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Marcel, V.; Tran, P.L.; Sagne, C.; Martel-Planche, G.; Vaslin, L.; Teulade-Fichou, M.P.; Hall, J.; Mergny, J.L.; Hainaut, P.; Van Dyck, E. G-quadruplex structures in TP53 intron 3: Role in alternative splicing and in production of p53 mRNA isoforms. Carcinogenesis 2011, 32, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Teixeira, G.S.; Martins, L.; Marques, M.R.; de Souza, A.P.; Line, S.R. G-quadruplex formation enhances splicing efficiency of PAX9 intron 1. Hum. Genet. 2015, 134, 37–44. [Google Scholar] [CrossRef]
- Fisette, J.F.; Montagna, D.R.; Mihailescu, M.R.; Wolfe, M.S. A G-rich element forms a G-quadruplex and regulates BACE1 mRNA alternative splicing. J. Neurochem. 2012, 121, 763–773. [Google Scholar] [CrossRef]
- Wang, X.; Goodrich, K.J.; Gooding, A.R.; Naeem, H.; Archer, S.; Paucek, R.D.; Youmans, D.T.; Cech, T.R.; Davidovich, C. Targeting of Polycomb Repressive Complex 2 to RNA by Short Repeats of Consecutive Guanines. Mol. Cell 2017, 65, 1056–1067.e5. [Google Scholar] [CrossRef] [PubMed]
- Beltran, M.; Tavares, M.; Justin, N.; Khandelwal, G.; Ambrose, J.; Foster, B.M.; Worlock, K.B.; Tvardovskiy, A.; Kunzelmann, S.; Herrero, J.; et al. G-tract RNA removes Polycomb repressive complex 2 from genes. Nat. Struct. Mol. Biol. 2019, 26, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.K.; Sahakyan, A.B.; Balasubramanian, S. Structural Analysis using SHALiPE to Reveal RNA G-Quadruplex Formation in Human Precursor MicroRNA. Angew. Chem. Int. Ed. Engl. 2016, 55, 8958–8961. [Google Scholar] [CrossRef]
- Mirihana Arachchilage, G.; Dassanayake, A.C.; Basu, S. A potassium ion-dependent RNA structural switch regulates human pre-miRNA 92b maturation. Chem. Biol. 2015, 22, 262–272. [Google Scholar] [CrossRef]
- Sahakyan, A.B.; Murat, P.; Mayer, C.; Balasubramanian, S. G-quadruplex structures within the 3′ UTR of LINE-1 elements stimulate retrotransposition. Nat. Struct. Mol. Biol. 2017, 24, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Karaulanov, E.; Musheev, M.; Trnka, P.; Schäfer, A.; Grummt, I.; Niehrs, C. GADD45A binds R-loops and recruits TET1 to CpG island promoters. Nat. Genet. 2019, 51, 217–223. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Bondarenko, V.; Wongsurawat, T.; Yenamandra, S.P.; Jenjaroenpun, P. Toward predictive R-loop computational biology: Genome-scale prediction of R-loops reveals their association with complex promoter structures, G-quadruplexes and transcriptionally active enhancers. Nucleic Acids Res. 2018, 46, 7566–7585. [Google Scholar] [CrossRef]
- Bayona-Feliu, A.; Barroso, S.; Muñoz, S.; Aguilera, A. The SWI/SNF chromatin remodeling complex helps resolve R-loop-mediated transcription-replication conflicts. Nat. Genet. 2021, 53, 1050–1063. [Google Scholar] [CrossRef]
- Lim, G.; Hohng, S. Single-molecule fluorescence studies on cotranscriptional G-quadruplex formation coupled with R-loop formation. Nucleic Acids Res. 2020, 48, 9195–9203. [Google Scholar] [CrossRef]
- Mondal, T.; Subhash, S.; Vaid, R.; Enroth, S.; Uday, S.; Reinius, B.; Mitra, S.; Mohammed, A.; James, A.R.; Hoberg, E.; et al. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015, 6, 7743. [Google Scholar] [CrossRef]
- Hansen, A.S.; Hsieh, T.S.; Cattoglio, C.; Pustova, I.; Saldana-Meyer, R.; Reinberg, D.; Darzacq, X.; Tjian, R. Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol. Cell 2019, 76, 395–411.e13. [Google Scholar] [CrossRef]
- Saldana-Meyer, R.; Rodriguez-Hernaez, J.; Escobar, T.; Nishana, M.; Jacome-Lopez, K.; Nora, E.P.; Bruneau, B.G.; Tsirigos, A.; Furlan-Magaril, M.; Skok, J.; et al. RNA Interactions Are Essential for CTCF-Mediated Genome Organization. Mol. Cell 2019, 76, 412–422.e5. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Tanasie, N.-L.; Zernia, S.; Stigler, J. Single-molecule imaging reveals a direct role of CTCF’s zinc fingers in SA interaction and cluster-dependent RNA recruitment. Nucleic Acids Res. 2024, 52, 6490–6506. [Google Scholar] [CrossRef]
- Beltran, M.; Yates, C.M.; Skalska, L.; Dawson, M.; Reis, F.P.; Viiri, K.; Fisher, C.L.; Sibley, C.R.; Foster, B.M.; Bartke, T.; et al. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 2016, 26, 896–907. [Google Scholar] [CrossRef]
- Davidovich, C.; Wang, X.; Cifuentes-Rojas, C.; Goodrich, K.J.; Gooding, A.R.; Lee, J.T.; Cech, T.R. Toward a consensus on the binding specificity and promiscuity of PRC2 for RNA. Mol. Cell 2015, 57, 552–558. [Google Scholar] [CrossRef]
- Davidovich, C.; Zheng, L.; Goodrich, K.J.; Cech, T.R. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 2013, 20, 1250–1257. [Google Scholar] [CrossRef]
- Hemphill, W.O.; Fenske, R.; Gooding, A.R.; Cech, T.R. PRC2 direct transfer from G-quadruplex RNA to dsDNA has implications for RNA-binding chromatin modifiers. Proc. Natl. Acad. Sci. USA 2023, 120, e2220528120. [Google Scholar] [CrossRef]
- Song, J.; Gooding, A.R.; Hemphill, W.O.; Love, B.D.; Robertson, A.; Yao, L.; Zon, L.I.; North, T.E.; Kasinath, V.; Cech, T.R. Structural basis for inactivation of PRC2 by G-quadruplex RNA. Science 2023, 381, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.K.; Blanco, M.R.; Walkup, W.G.t.; Bonesteele, G.; Urbinati, C.R.; Banerjee, A.K.; Chow, A.; Ettlin, O.; Strehle, M.; Peyda, P.; et al. Denaturing purifications demonstrate that PRC2 and other widely reported chromatin proteins do not appear to bind directly to RNA in vivo. Mol. Cell 2024, 84, 1271–1289.e12. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Blum, R.; Rosenberg, M.; Lee, J.T. Re-analysis of CLAP data affirms PRC2 as an RNA binding protein. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kung, J.T.; Kesner, B.; An, J.Y.; Ahn, J.Y.; Cifuentes-Rojas, C.; Colognori, D.; Jeon, Y.; Szanto, A.; del Rosario, B.C.; Pinter, S.F.; et al. Locus-specific targeting to the X chromosome revealed by the RNA interactome of CTCF. Mol. Cell 2015, 57, 361–375. [Google Scholar] [CrossRef]
- Wulfridge, P.; Yan, Q.; Rell, N.; Doherty, J.; Jacobson, S.; Offley, S.; Deliard, S.; Feng, K.; Phillips-Cremins, J.E.; Gardini, A.; et al. G-quadruplexes associated with R-loops promote CTCF binding. Mol. Cell 2023, 83, 3064–3079.e5. [Google Scholar] [CrossRef]
- Zhao, X.; Li, D.; Pu, J.; Mei, H.; Yang, D.; Xiang, X.; Qu, H.; Huang, K.; Zheng, L.; Tong, Q. CTCF cooperates with noncoding RNA MYCNOS to promote neuroblastoma progression through facilitating MYCN expression. Oncogene 2016, 35, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Saravanan, B.; Walavalkar, K.; Farooq, U.; Singh, A.K.; Radhakrishnan, S.; Thakur, J.; Pandit, A.; Henikoff, S.; Notani, D. Active enhancers strengthen insulation by RNA-mediated CTCF binding at chromatin domain boundaries. Genome Res. 2023, 33, 1–17. [Google Scholar] [CrossRef]
- Xiang, J.-F.; Yin, Q.-F.; Chen, T.; Zhang, Y.; Zhang, X.-O.; Wu, Z.; Zhang, S.; Wang, H.-B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014, 24, 513–531. [Google Scholar] [CrossRef]
- Saldana-Meyer, R.; Gonzalez-Buendia, E.; Guerrero, G.; Narendra, V.; Bonasio, R.; Recillas-Targa, F.; Reinberg, D. CTCF regulates the human p53 gene through direct interaction with its natural antisense transcript, Wrap53. Genes Dev. 2014, 28, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Skalska, L.; Begley, V.; Beltran, M.; Lukauskas, S.; Khandelwal, G.; Faull, P.; Bhamra, A.; Tavares, M.; Wellman, R.; Tvardovskiy, A.; et al. Nascent RNA antagonizes the interaction of a set of regulatory proteins with chromatin. Mol. Cell 2021, 81, 2944–2959.e10. [Google Scholar] [CrossRef]
- Hyder, U.; D’Orso, I. Nascent RNA: Friend or foe of the chromatin bound? Mol. Cell 2021, 81, 2871–2872. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Aguilar, R.; Kesner, B.; Lee, H.G.; Kriz, A.J.; Chu, H.P.; Lee, J.T. Jpx RNA regulates CTCF anchor site selection and formation of chromosome loops. Cell 2021, 184, 6157–6173.e24. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Wang, C.; Du, Z.; Zhang, S.; Wen, X.; Zhou, L.; Li, H.; Chen, H.; Li, D.; et al. Oplr16 serves as a novel chromatin factor to control stem cell fate by modulating pluripotency-specific chromosomal looping and TET2-mediated DNA demethylation. Nucleic Acids Res. 2020, 48, 3935–3948. [Google Scholar] [CrossRef]
- Skalska, L.; Beltran-Nebot, M.; Ule, J.; Jenner, R.G. Regulatory feedback from nascent RNA to chromatin and transcription. Nat. Rev. Mol. Cell Biol. 2017, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.S.R.; Pavitra, E.; Bandaru, S.S.; Varaprasad, G.L.; Nagaraju, G.P.; Malla, R.R.; Huh, Y.S.; Han, Y.K. HOTAIR: A potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol. Cancer 2023, 22, 65. [Google Scholar] [CrossRef]
- Kuo, F.-C.; Neville, M.J.; Sabaratnam, R.; Wesolowska-Andersen, A.; Phillips, D.; Wittemans, L.B.L.; van Dam, A.D.; Loh, N.Y.; Todorčević, M.; Denton, N.; et al. HOTAIR interacts with PRC2 complex regulating the regional preadipocyte transcriptome and human fat distribution. Cell Rep. 2022, 40, 111136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; Dean, A. Chromatin-Associated RNAs Regulate Gene Expression and Chromatin Structure. Non-Coding RNA 2025, 11, 68. https://doi.org/10.3390/ncrna11050068
Xie B, Dean A. Chromatin-Associated RNAs Regulate Gene Expression and Chromatin Structure. Non-Coding RNA. 2025; 11(5):68. https://doi.org/10.3390/ncrna11050068
Chicago/Turabian StyleXie, Bingning, and Ann Dean. 2025. "Chromatin-Associated RNAs Regulate Gene Expression and Chromatin Structure" Non-Coding RNA 11, no. 5: 68. https://doi.org/10.3390/ncrna11050068
APA StyleXie, B., & Dean, A. (2025). Chromatin-Associated RNAs Regulate Gene Expression and Chromatin Structure. Non-Coding RNA, 11(5), 68. https://doi.org/10.3390/ncrna11050068






