Abstract
Background/Objectives: Exposure of high-fat diet (HFD)-fed mice to polychlorinated biphenyls (PCBs) results in metabolic dysfunction-associated steatotic liver disease (MASLD) and progression to metabolic dysfunction-associated steatohepatitis (MASH). The mechanisms by which HFD diet and PCBs increase MASLD are unclear. Previously, we identified differences in HFD-fed mouse liver tRNA modifications with single oral exposures to the dioxin-like PCB126, the non-dioxin-like PCB mixture Aroclor 1260 (Ar1260), or the combination of Ar1260 + PCB126. Methods: Here, we used small RNA sequencing and the tRNA analysis of expression (tRAX) pipeline to examine if PCB exposures alter the tRNA transcriptome, including tRNA-derived fragments (tRFs), in the livers of the PCB-exposed mice. Results: Each PCB exposure produced distinct hepatic tRNA transcriptomes with more tRNAs decreased than increased. Only tRNA-Glu-TTC-1 was reduced with all three PCB exposures. More changes in tRFs were identified with Ar1260 alone or in combination with PCB126 than with PCB126 alone. Four tRF-3s were upregulated in both PCB126 and Ar1260 + PCB126 co-exposed mice, suggesting PCB126 as responsible for this increase. We previously reported that PCB126 exposure increased hepatic Angiogenin (ANG) protein which generates tRF-3s. Four previously reported tRNA modifications corresponded to positions of PCB-associated tRNA modifications identified by tRAX: m1A, m6A, ms2t6A, and Ψ. Conclusions: Overall, the differences in hepatic tRNAs and tRFs with three different PCB exposures suggest that PCB exposures play an unexplored role in regulating translation in mouse liver.
1. Introduction
Exposure to environmental pollutants contributes to human diseases including cancer and cardiovascular, respiratory, and neurodegenerative diseases (reviewed in [1]) and to metabolic dysfunction-associated steatotic liver disease (MASLD) [2,3,4,5]. The “exposome” that contributes to these diseases includes not only exposure to chemicals in the environment, but also lifestyle factors including diet and social/behavioral effectors. The global prevalence of MASLD, previously known as nonalcoholic fatty liver disease (NAFLD), has increased to ~25% of the population, driven largely by the rise in obesity and insulin resistance (metabolic syndrome) [6]. However, environmental exposures to pollutants acting as metabolism- and endocrine-disrupting chemicals (MDCs and EDCs), including polychlorinated biphenyls (PCBs) are associated with MASLD [5]. Self-reported workplace exposure of MASLD patients to toxicants, including arsenic, halogenated refrigerants, metals, and pesticides, was associated with a more aggressive MASLD phenotype and hepatocellular carcinoma (HCC) [4]. Recent studies have demonstrated that environmental pollutants alter chemical modifications to RNAs, referred to as the “epitranscriptome” that result in transcriptional and phenotypic changes in the livers of model organisms (reviewed in [7,8]).
tRNAs are highly abundant non-coding RNAs that are extensively modified and have rigid secondary structures allowing them to be aminoacylated (charged) with an amino acid (aa) at the 3′ end (aminoacyl-tRNA, aa-tRNA) [9]. The mouse genome contains 400 “high confidence” tRNAs decoding the standard 20 aa plus one selenocysteine tRNA: tRNA-SeC-TCA-1 [10]. The mouse genome has 47 tRNA isoacceptors: tRNA isotypes loaded with the same aa, but having a different anticodon sequence [11]. tRNA isoacceptors are expressed in a tissue-specific manner [12]. tRNAs that use synonymous codons for the same aa but different sequences in the rest of the molecule are called isodecoders [13,14].
tRNAs have the most chemical modifications of all cellular RNAs with an average of 13 modifications per eukaryotic tRNA and ~100 distinct modifications (reviewed in [9]). These modifications regulate tRNA stability, codon recognition, and the biogenesis of tRNA-derived small RNAs (tDRs). Pseudouridine (Ψ) is the most common eukaryotic tRNA modification in eukaryotes [15]. Additional common tRNA modifications include 5-methylcytosine (m5C), 7-methylguanosine (m7G), and 3-methylcytosine (m3C) additional common tRNA modifications [15]. The anticodon loop (ACL) is the site of hyper-modifications at position 34, the wobble position of the aa-charged tRNA that recognizes cognate codons in mRNA within ribosomes for translation and enhancing reading frame maintenance (reviewed in [16]). We reported that the three PCB exposures, Aroclor1260 (a mixture of non-dioxin-like (NDL) PCB congeners), PCB126 (a dioxin-like (DL) PCB congener), or the combination of Aroclor1260 + PCB126, differentially affected the liver epitranscriptome of high-fat diet (HFD)-fed male C57Bl/6J mice with changes in six post-transcriptional chemical modifications that are located in tRNAs: Am (2-O-methyladenosine), m1A (1-methyladenosine), m2,2G (N2,N2-dimethylguanosine), m5U (5-methylurdine), m6A (N6-methyladenosine), and Ψ [17].
The expression of the various specific tRNA-modifying enzymes, including methyltransferases, e.g., METTL1/2/6/8, TRMT6, and TRMT61A, are dysregulated in HCC (reviewed in [18,19]). We reported that the transcript and protein abundance of specific tRNA modifiers were altered with individual PCB exposures, e.g., TRMG61A, an m1A writer, was increased with PCB126 exposure, although m1A was reduced [20], but we did not examine the tRNA expression. The role of tRNA modifications in MASLD is currently unknown.
tDRs, also called shorter tRNA-derived small RNAs (tsRNAs) or tRNA-derived fragments (tRFs), are generated by cleavage of pre- or mature tRNAs at specific sites [21]. Hereafter, we will use the term tRFs. tRFs perform diverse biological functions and have cell- and tissue-specific expression and their expression patterns are associated with human disease (reviewed in [22,23,24,25,26,27]). Only a fraction of mature tRNAs are processed; thus, tRFs accumulate without a decline in the full-length tRNA [28]. To date, there is no uniform consensus nomenclature for tRFs. Hence, multiple names for the same or similar tRFs are found in the literature. There are six classes of tRFs: 5′-tRF, tRF-3, 3′-tRNA halves, tRF-1, internal tRFs, and “other tRFs” (reviewed in [28]). tRFs are formed by stress-induced ribonucleases including RNase I, DICER, Angiogenin (ANG), and ELAC (Elac ribonuclease Z 2) [28]. tRF-1 (tDR-1) is derived from the 3′ end of the precursor tRNA within the nucleus by ELAC cleavage [29]. tRF-5 and tRF-3 are generated by DICER and RNAse cleavage. ANG generates various tRFs, including tRF-3s, and stress-induced tRNA halves (5′-tiR and 3′-tiR) [30]. Some tRFs act like miRNAs and repress translation, although the findings are controversial [31]. A recent study identified 18 tRFs, including four alanine, two lysine, and one each of serine, arginine, histidine, and glycine tRFs, in the livers of HFD-fed (11 weeks) diet-induced obesity (DIO) C57Bl/6J mice with hepatic lipid droplet accumulation versus control mice [32]. To our knowledge, no one has examined the impact of HFD and PCB exposures on the mouse liver tRNA and/or tRF transcriptome.
In this study, we leveraged our previously generated miRNA-seq data for livers from HFD-fed C57Bl/6J male mice with vehicle control (corn oil) or a single oral exposure to Aroclor1260 or PCB126 individually or in combination [20] to identify how these PCB exposures affected the tRNA transcriptome. The livers from these PCB exposures showed lipid accumulation and fibrosis [33]. Serum markers commensurate with MASH were reported, with specific proteomic [33], miRNA, and mRNA transcriptome profiles [20], RNA modifications [17], and altered molecular pathways for each individual PCB exposure [17,20,33]. Here, we tested the hypothesis that HFD in combination with PCB exposures alters hepatic tRNA and tDR expression. We identified PCB exposure-specific unique and overlapping changes in tRNA and tDR abundance.
2. Results
2.1. PCB Exposures in HFD-Fed Mice Affect Hepatic tRNA Abundance
PCB exposures are implicated in the development of human MASLD [34]. PCBs undergo enterohepatic circulation [35]. Human exposures to potent DL PCBs, including PCB126, are only 0.02% of the total PCB exposure reported in the National Health and Nutrition Examination Survey (NHANES) database [36]. While these exposures are low, they are important due to the high potency of DL PCBs for aryl hydrocarbon receptor (AHR) activation. Because the NDL PCB mixture Ar1260 (20 mg/kg) did not activate AHR [37], PCB126 (20 µg/kg) was added to Ar1260 in our recent mouse studies [17,20,33,38,39]. The co-exposure to PCB126 + Ar1260 increases the human relevance of the administered PCB mixture. These data are the rationale for our MASLD/MASH mouse model in which male C57Bl6/J mice were fed an HFD (12 weeks) and received a single oral gavage of vehicle control (corn oil), Ar1260, PCB126, or the combination of Ar1260 + PCB126 after one week on the HFD (Figure S1). These mice had serum and hepatic changes modeling human MASH [33]. We reported large differences (99.58%) in the liver proteomes of the HFD-fed male mice exposed to Ar1260, PCB126, or the combination of Ar1260 + PCB126 [33]. In addition, liquid chromatography–mass spectrometry (LC-MS) identified seven chemical modification changes in total RNA samples from these same PCB-exposed HFD-fed mouse livers compared to HFD control [17]. These modifications are known to be in tRNAs [40].
Here, we used the tRAX pipeline [41] to identify mature tRNAs and tRFs in the HFD-fed mouse livers with the three PCB exposures using our previously reported miRNA-seq data [20] (Figure S2). This analysis was performed on five separate livers (from five mice) for each of the four exposure groups: control (vehicle control), Ar1260, PCB126, and Ar1260 + PCB126. The read distribution of tRNA isotypes suggests that Ar1260 exposure increased the tRNA isotype distribution in the liver, but that PCB126 and the combination of Ar1260 + PCB126 reduced tRNA isotypes (Figure 1A).

Figure 1.
Read distribution of tRNA isotypes in the livers of the HFD-fed mice exposed to PCBs (A) and the impact of PCB exposures on differential tRNA abundance (B). All mice were fed a HFD. In A, HFDcontrol are data from the livers of the HFD-mice treated with vehicle control(corn oil); HFDAro indicates data from the HFD + Ar1260-exposed livers, HFDPCB indicates data from HFD + PCB126-exposed livers, and HFDAroPCB indicates data from the HFD + Ar1260 + PCB126 co-exposed livers samples. (B) The colors red and green indicate increased or decreased tRNA expression respectively in the samples as indicated. The Venn diagram was finalized using BioRender.com (accessed on 27 March 2025).
Differential expression analysis of tRNA isodecoders was performed on the four groups of liver samples using DESeq2. There were 557 tRNAs identified, including many antisense (AS) tRNAs. Table 1, Table 2 and Table 3 show the differentially expressed isodecoder tRNAs for each PCB exposure compared to the HFD control at an FDR < 0.05. Tables S1–S3 list the antisense tRNAs (AS-tRNAs) identified for each of the three PCB exposures vs. HFD-fed mouse liver control. Table 4, Table 5 and Table 6 list the tRFs identified as differentially regulated in each of the three PCB exposures versus the HFD-fed control mouse livers. We searched the published literature for the roles of the identified tRNAs and tRFs and added this information to Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 and S1–S3.

Table 1.
Differentially expressed mature, nuclear-encoded tRNAs in HFD-fed mouse liver with Ar1260 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRFs”, “undefined” indicates that tRAX did not define the type of tRF.

Table 2.
Differentially expressed mature, nuclear-encoded tRNAs in HFD-fed mouse liver with PCB126 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRFs”, “undefined” indicates that tRAX did not define the type of tRF.

Table 3.
Differentially expressed mature, nuclear-encoded tRNAsin mouse liver with HFD + Ar1260 + PCB126 combination exposure vs. HFD control (FDR < 0.05). In the column “Correspondence with tRFs”, “undefined” indicates that tRAX did not define the type of tRF.
Each of the three PCB exposures produced mostly distinct hepatic tRNA transcriptomes with little overlap (Figure 1B, Table 1, Table 2 and Table 3). Only one tRNA change was found to be in common with all three PCB exposure groups: tRNA-Glu-TTC-1 was downregulated (Figure 1B). Additional common tRNA decreases are shown. For example, tRNA-Thr-CGT-2-1 was decreased with either Ar1260 or PCB126 exposure, but not with Ar1260 + PCB126 co-exposure in vivo. Three tRNA-Glu-CTC-1 variants were decreased with both PCB126 and Ar1260 + PCB126 co-exposure. tRNA-Lys-CTT-9 was increased with PCB126 and the combined Ar1260 + PCB126 co-exposures. Table 1, Table 2 and Table 3 detail the properties of the associated amino acid (aa), indicate whether the expression of the tRNA aligns with its AS-tRNA or tRFs, and summarize additional reports pertinent to that tRNA. Four of the nine tRNAs altered with Ar1260 exposure were increased. Two of the nine tRNAs altered with PCB126 exposure were increased. Three of the eight changes in tRNAs were increased with Ar1260 + PCB126 co-exposure. Thus, the abundance of differentially expressed tRNAs was reduced overall by all three PCB exposures. There was no apparent pattern of PCB exposure, tRNA changes, and properties of the carried aa.
Glu-carrying tRNAs were reduced with all three PCB exposures. Two Thr-carrying tRNAs (tRNA-Thr-AGT-1-2 and tRNA-Thr-CGT-2-1, Table 1), tRNA-Gly-GCC-2-8, and tRNA-Ser-GCT—3-1 were decreased with Ar1260 exposure (Table 1). tRNA-Leu- CAG-2-2 and tRNA-Lys-CTT-9 were higher in PCB126-exposed livers (Table 2). With the combined Ar1260 + PCB126 exposures, tRNA-Gly-CCC-2 was decreased (Table 3) as was tRNA-Gly-GCC-2-8 with Ar1260 exposure (Table 1). tRNA-Lys-CTT-9 was increased with PCB126 exposure and in Ar1260 + PCB126 co-exposed livers (Table 2 and Table 3). tRNA-iMet-CAT-2 was increased with Ar1260 + PCB126 co-exposures. Conversely, tRNA-iMet-CAT-2_antisense was decreased with Ar1260 + PCB126 co-exposure and with Ar1260 exposure (Table 1 and Table 3).
Figure 2 shows a Venn diagram of the tRAX-identified liver AS-tRNAs altered by the three PCB exposures vs. the HFD control (Tables S1–S3). Two AS-tRNAS, AS-tRNA-Tyr-GTA-3 and AS-tRNA-His-GTG-1, were decreased by all three PCB exposures (Figure 2). There was no association between the abundance of tRNAs and AS-tRNA in livers with Ar1260 exposure (Table 1). Of the nine tRNAs whose abundance was altered by PCB126 exposure, only one inverse association with an AS-tRNA was detected (Table 2). The abundance of tRNA-Glu-TCC-1 was reduced, but its AS-tRNA was increased by PCB126 exposure (Table 2). For the eight tRNAs altered by Ar1260 + PCB126 co-exposure, tRNA-iMET-CAT-2 and tRNA-Glu-TTC-1 showed an inverse association with their AS-tRNA, decreased and increased, respectively.
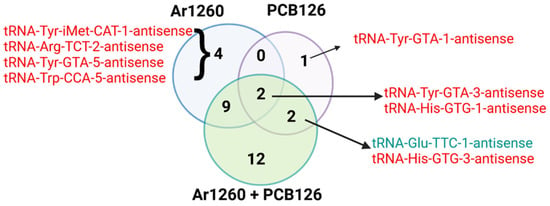
Figure 2.
The impact of PCB exposures on tRNA-antisense abundance. The colors red and green indicate increased or decreased tRNA-antisense expression in the liver samples as indicated. The data are in the Supplementary Tables S1–S3. The Venn diagram was finalized using BioRender.com (accessed on 27 March 2025).
2.2. PCB Exposures in HFD-Fed Mice Affect Hepatic tRF Abundance
We identified 1455 tRFs in the four liver sample groups. Table 4, Table 5 and Table 6 show the differentially expressed tRFs for each PCB exposure compared to the HFD control. Eliminating the AS-tRNAs and the types of tRFs not fully annotated in tRAX [41], but leaving “other”, “partial precount”, and “wholecount” tRFs about which published tRF information [41] was available allowed us to focus our analysis (Table 4, Table 5 and Table 6). More changes in tRFs were detected with Ar1260 (Table 4) than PCB126 (Table 5). Co-exposures to Ar1260 + PCB126 resulted in the most changes in tRFs (Table 6).
Figure 3 shows a Venn diagram of the tRAX-identified liver tRFs altered by the three PCB exposures versus the HFD control. All three PCB exposures altered three undefined tRFs. “tRNA-SeC-TCA-1_other” was increased and “tRNA-Glu-TTC-1_wholecountes”, and “tRNA-Glu-TTC-2_wholecountes” were decreased. Ar1260 and PCB126 exposures showed one common undefined tRF, “tRNA-Leu-CAG-2-2_partialprecounts”, but opposite regulation (Figure 3). Ar1260 and Ar1260 +PCB126 exposures showed two common tRFs with similar regulation: increased “tRNA-Glu-TTC-3-wholecounts” and decreased “tRNA-Ile-AAT-2_other” (Figure 3). Five tRFs, including three tRF-3s (tRNA-iMet-CAT-1, 2, and 3), showed similar regulation with PCB126 or Ar1260 + PCB126 exposures, implicating PCB126 as responsible for these changes relative to HFD alone (Figure 3). ANG is responsible for generating tRF-3s [29]. This increase in tRF-3s is in agreement with the increase in ANG protein in HFD-fed, PCB-126-exposed mouse livers [33].

Figure 3.
The impact of PCB exposures on differential tRF abundance in HFD-fed mouse liver. The colors red and green indicate increased or decreased tRF abundance, respectively, in the liver samples as indicated. The data are in the Supplementary Tables S1–S3. The Venn diagram was finalized using BioRender.com (accessed on 27 March 2025).

Table 4.
The differentially expressed tRFs in HFD-fed mouse liver with Ar1260 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
Table 4.
The differentially expressed tRFs in HFD-fed mouse liver with Ar1260 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
| tRF | Log2FC | Adj p Value | tRF Type | Correspondence with tRNAs (Table 1) or AS-tRNAs (Table S1) | Other Reports |
|---|---|---|---|---|---|
| tRNA-Gln-TTG-4_other | 0.56 | 0.011 | undefined | none | |
| tRNA-Leu-CAA-2-1_trailercounts | 0.52 | 0.021 | tRF-1 | none | |
| tRNA-Val-AAC-4_fiveprime | 0.51 | 0.011 | tRF-5 | none | |
| tRNA-Gly-TCC-1_others | 0.47 | 0.019 | undefined | tRNA increased; no change in AS-tRNA | tRNA-Gly-TCC-1-tRF-3 was increased in the livers of diet-induced obese (DIO) C57Bl/6 mice with lipid droplet accumulation [32]. |
| tRNA-Pro-TGG-3_other | 0.44 | 0.047 | undefined | none | |
| tRNA-Leu-CAG-2-2_partialprecounts | 0.41 | 0.039 | undefined | none | |
| tRNA-Cys-GCA-12_other | 0.40 | 0.040 | undefined | none | |
| tRNA-Thr-AGT-6-1_trailercounts | 0.39 | 0.037 | tRF-1 | none | |
| tRNA-Ile-AAT-2_other | 0.37 | 0.032 | undefined | none | |
| tRNA-Val-CAC-3_fiveprime | 0.34 | 0.030 | tRF-5 | none | tRF-Val-CAC-005 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46]. |
| tRNA-Val-AAC-2_fiveprime | 0.32 | 0.031 | tRF-5 | none | |
| tRNA-Val-AAC-3_fiveprime | 0.32 | 0.032 | tRF-5 | none | |
| tRNA-Val-CAC-4_fiveprime | 0.30 | 0.024 | tRF-5 | none | tRF-Val-CAC-005 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46] |
| tRNA-SeC-TCA-1_other | 0.30 | 0.042 | undefined | none | |
| tRNA-Val-CAC-1_fiveprime | 0.30 | 0.017 | tRF-5 | none | tRF-Val-CAC-005 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46]. |
| tRNA-Val-AAC-5_fiveprime | 0.28 | 0.042 | tRF-5 | tRNA-Val-AAC-5 increased; no AS-tRNA change | tRNA-Val-AAC-5-tRF-5 was increased in the livers of DIO C57Bl/6 mice [32]. |
| tRNA-Val-AAC-5_trailercounts | 0.26 | 0.043 | tRF-1 | tRNA-Val-AAC-5 increased; no AS-tRNA change | |
| tRNA-Gly-TCC-1_other | −0.19 | 0.048 | undefined | none | tRNA-Gly-TCC-1-tRF-3 was increased in the livers of DIO C57Bl/6 mice with lipid droplet accumulation [32] |
| tRNA-iMet-CAT-1_other | −0.32 | 0.018 | undefined | No change in tRNA; AS decreased | |
| tRNA-Thr-AGT-1-2_trailercounts | −0.34 | 0.033 | tRF-1 | tRNA-Thr-AGT-1-2 decreased; no AS-tRNA change | |
| tRNA-Leu-CAA-2_other | −0.36 | 0.034 | undefined | none | tRNA-Leu-CAA was down regulated with increased ER stress [47]. |
| tRNA-Leu-CAG-2-2_partialprecounts | −0.36 | 0.028 | undefined | none | |
| tRNA-Leu-CAA-1_other | −0.37 | 0.042 | undefined | No for tRNA, AS-tRNA was decreased | tRNA-Leu-CAA was down regulated with increased ER stress [47]. |
| tRNA-Leu-CAG-3_other | −0.38 | 0.048 | undefined | No for tRNA, AS-tRNA was decreased | |
| tRNA-Glu-TTC-3_wholecounts | −0.39 | 0.048 | undefined | none | |
| tRNA-Leu-CAA-4_other | −0.39 | 0.031 | undefined | none | tRNA-Leu-CAA was down regulated with increased ER stress [47]. |
| tRNA-Leu-CAA-3_other | −0.39 | 0.021 | undefined | none | tRNA-Leu-CAA is down regulated with increased ER stress [47]. |
| tRNA-Glu-TTC-2_wholecounts | −0.41 | 0.025 | undefined | none | |
| tRNA-Gly-GCC-2-8_trailercounts | −0.44 | 0.035 | tRF-1 | tRNA-Gly-GCC-2-7 increased; no AS-tRNA change | tRNA-Gly-GCC fragments are generated in response to activation of the ER stress response in KGN cells overexpressing IRE1α (ERN1) or in KGN, HeLa, and other cell lines treated with thapsigargin or tunicamycin [48]. |
| tRNA-Ser-GCT-3-1_trailercounts | −0.46 | 0.020 | tRF-1 | tRNA-Ser-GCT-3-1 decreased; no AS-tRNA change | |
| tRNA-Glu-TTC-1_wholecounts | −0.50 | 0.010 | undefined | tRNA-Glu-TTC-1 decreased; no AS-tRNA change | |
| tRNA-Ala-CGC-7_other | −0.62 | 0.001 | undefined | none | tRF-Ala-CGC-006 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46] |

Table 5.
The differentially expressed tDRs in HFD-fed mouse liver with PCB126 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
Table 5.
The differentially expressed tDRs in HFD-fed mouse liver with PCB126 exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
| tRF | Log2FC | Adj p Value | Type of tRF | Correspondence with tRNAs (Table 2) or AS-tRNAs (Table S2) | Other Reports |
|---|---|---|---|---|---|
| tRNA-Leu-CAG-2-2_partialprecounts | 0.59 | 0.003 | undefined | tRNA-Leu-CAG-2-2 was increased; no AS-tRNA change | |
| tRNA-Met-CAT-1_fiveprime | 0.44 | 0.045 | tRF-5 | none | |
| tRNA-SeC-TCA-1_other | 0.374 | 0.012 | undefined | none | |
| tRNA-SeC-TCA-1_threeprime | 0.37 | 0.014 | tRF-3 | none | |
| tRNA-Lys-CTT-9_other | 0.36 | 0.019 | undefined | tRNA-Lys-CTT-9 was increased; no AS-tRNA change | |
| tRNA-Ser-AGA-3_fiveprime | 0.35 | 0.040 | tRF-5 | none | tRNA-Ser-AGA-1-tRF-5 was reduced in the livers of DIO C57Bl/6 mice [32] |
| tRNA-iMet-CAT-1_threeprime | 0.28 | 0.032 | tRF-3 | none | |
| tRNA-iMet-CAT-3_threeprime | 0.28 | 0.033 | tRF-3 | none | |
| tRNA-iMet-CAT-2_threeprime | 0.27 | 0.034 | tRF-3 | none | |
| tRNA-Leu-AAG-3_other | 0.23 | 0.017 | undefined | none | |
| tRNA-Lys-TTT-1_threeprime | 0.21 | 0.046 | tRF-3 | none | tRNA-Lys-TTT-3-tRF-5 was increased in the livers of DIO C57Bl/6 mice [32]. |
| tRNA-Leu-AAG-1_other | 0.20 | 0.040 | undefined | none | |
| tRNA-Leu-AAG-2_other | 0.20 | 0.049 | undefined | none | |
| tRNA-Glu-TTC-2_wholecounts | −0.36 | 0.049 | undefined | none | |
| tRNA-Asp-GTC-1_wholecounts | −0.39 | 0.046 | undefined | none | |
| tRNA-Glu-TTC-1_wholecounts | −0.43 | 0.027 | undefined | tRNA-Glu-TTC-1 was decreased; AS-tRNA was increased | |
| tRNA-Glu-CTC-1-1_partialprecounts | −0.45 | 0.010 | undefined | tRNA-Glu-CTC-1-1 decreased; AS-tRNA change | |
| tRNA-Glu-CTC-1-2_partialprecounts | −0.45 | 0.040 | undefined | tRNA-Glu-CTC-1-2 decreased; AS-tRNA change | |
| tRNA-Glu-CTC-1-3_partialprecounts | −0.45 | 0.040 | undefined | none | |
| tRNA-Thr-CGT-2-1_trailercounts | −0.46 | 0.005 | tRF-1 | tRNA-Thr-CGT-2-1 decreased; AS-tRNA |

Table 6.
The differentially expressed tRFs in HFD-fed mouse liver with Ar1260 + PCB12 co-exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
Table 6.
The differentially expressed tRFs in HFD-fed mouse liver with Ar1260 + PCB12 co-exposure vs. control (FDR < 0.05). In the column “Correspondence with tRNAs or AS-tRNAs”, “none” indicates that tRAX did not identify either a corresponding tRNA or AS-tRNA to the tRF.
| tRF | Log2FC | Adj p Value | Type of tRF | Correspondence with tRNAs (Table 3) or AS-tRNAs (Table S3) | Other Reports |
|---|---|---|---|---|---|
| tRNA-SeC-TCA-1_other | 0.66 | 6.76 × 10−6 | undefined | none | |
| tRNA-iMet-CAT-3_threeprime | 0.51 | 0.00011 | tRF-3 | AS decreased | |
| tRNA-iMet-CAT-1_threeprime | 0.49 | 0.00018 | tRF-3 | none | |
| tRNA-iMet-CAT-2_threeprime | 0.49 | 0.00013 | tRF-3 | tRNA increased; AS decreased | |
| tRNA-Ser-CGA-2_threeprime | 0.44 | 0.003 | tRF-3 | none | |
| tRNA-Lys-CTT-9_other | 0.44 | 0.004 | undefined | tRNA increased; No change AS-tRNA | |
| tRNA-Glu-CTC-5_threeprime | 0.40 | 0.029 | tRF-3 | none | tRNA-Glu-CTC-tRF-5 (fiveprime) was increased in exosomes purified from the blood of patients with metastatic pancreatic cancer to the liver and promoted liver metastasis by increasing the stability of WDR1 (WD Repeat Domain 1) mRNA and increasing WDR1 protein in hepatic stellate cells [49] |
| tRNA-Cys-GCA-5_fiveprime | 0.38 | 0.029 | tRF-5 | none | |
| tRNA-SeC-TCA-1_threeprime | 0.37 | 0.013 | tRF-3 | none | |
| tRNA-Ile-AAT-2_other | 0.34 | 0.046 | undefined | none | |
| tRNA-iMet-CAT-2_fiveprime | 0.33 | 0.049 | tRF-5 | AS-tRNA decreased | |
| tRNA-Ser-GCT-4_threeprime | 0.33 | 0.006 | tRF-3 | none | |
| tRNA-Ser-GCT-3_threeprime | 0.32 | 0.007 | tRF-3 | none | |
| tRNA-Ser-GCT-2_threeprime | 0.32 | 0.007 | tRF-3 | none | |
| tRNA-Arg-ACG-3_threeprime | 0.30 | 0.0005 | tRF-3 | AS-tRNA decreased | |
| tRNA-Phe-GAA-1_other | 0.30 | 0.0120 | undefined | none | |
| tRNA-Ser-AGA-2_other | 0.29 | 0.035 | undefined | none | |
| tRNA-Arg-ACG-1_threeprime | 0.28 | 0.001 | tRF-3 | AS-tRNA decreased | |
| tRNA-Lys-CTT-7_other | 0.28 | 0.038 | undefined | none | |
| tRNA-Ala-CGC-3_threeprime | 0.28 | 0.038 | tRF-3 | none | tRF-ALA-CGC-3 was increased in DIO mouse liver [32]. tRF-Ala-CGC-006 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46] |
| tRNA-Ala-CGC-4_threeprime | 0.29 | 0.038 | tRF-3 | none | tRF-Ala-CGC-006 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46] |
| tRNA-Ala-CGC-7_threeprime | 0.28 | 0.039 | tRF-3 | none | tRF-Ala-CGC-006 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46] |
| tRNA-Ala-TGC-5_threeprime | 0.27 | 0.039 | tRF-3 | none | |
| tRNA-Ala-TGC-7_threeprime | 0.27 | 0.047 | tRF-3 | none | |
| tRNA-Ala-TGC-8_threeprime | 0.27 | 0.046 | tRF-3 | none | |
| tRNA-Ala-TGC-6_threeprime | 0.27 | 0.047 | tRF-3 | none | |
| tRNA-Asn-GTT-2_threeprime | 0.25 | 0.030 | tRF-3 | AS-tRNA decreased | |
| tRNA-Phe-GAA-2_other | 0.23 | 0.041 | undefined | none | |
| tRNA-Asn-GTT-3_threeprime | 0.21 | 0.042 | tRF-3 | none | |
| tRNA-Arg-CCG-3_threeprime | 0.12 | 0.035 | tRF-3 | none | |
| tRNA-Lys-CTT-2_fiveprime | −0.29 | 0.033 | tRF-5 | none | |
| tRNA-Lys-CTT-3_fiveprime | −0.35 | 0.016 | tRF-5 | none | |
| tRNA-Glu-TTC-2_wholecounts | -0.37 | 0.045 | undefined | none | |
| tRNA-Ala-CGC-7_other | −0.44 | 0.024 | undefined | none | tRF-Ala-CGC-006 was elevated in MASLD patient plasma and in the plasma of BALBc mice after 2–6 weeks on a high cholesterol diet [46]. |
| tRNA-Glu-TTC-1_wholecounts | −0.53 | 0.006 | undefined | none | |
| tRNA-Gly-CCC-2_fiveprime | −0.54 | 0.013 | tRF-5 | AS-tRNA increased | tRF-5 tRNA-Gly-CCC-2 was upregulated and positively correlated with the inflammation level and ANG expression in mouse tibialis anterior (TA) muscle after injury from injection of 50 μL of 10 μΜ cardiotoxin [50]. |
| tRNA-Glu-CTC-1-1_partialprecounts | −0.57 | 0.008 | undefined | none | |
| tRNA-Glu-CTC-1-2_partialprecounts | −0.57 | 0.008 | undefined | none | |
| tRNA-Glu-CTC-1-3_partialprecounts | −0.57 | 0.008 | undefined | none | |
| tRNA-Glu-TTC-3_wholecounts | −0.63 | 0.001 | undefined | none | |
| tRNA-Glu-CTC-1_wholecounts | −0.64 | 0.0006 | undefined | none |
Since tRNAs and their tRFs are expected to correlate in terms of direction of expression [51], we examined the data in Venn diagrams (Figure 4). In the Ar1260-exposed livers, two tRNAs that were increased relative to the HFD control showed a corresponding increase in tRFs: tRNA-Thr-AGT-6-1 and tRNA-Val-AAC-5 (Table 1 and Table 4). Similarly, four downregulated tRNAs also showed decreased tRF abundance (Table 1 and Table 4). These concordant results suggest a correlation between these tRNAs and their tRFs in the livers after Ar1260 exposure. With PCB126 exposure, two tRNAs, tRNA-Leu-CAG-2-2 and tRNA-Lys-CTT-9, showed increased abundance and a corresponding increase in tRFs (Table 2 and Table 5). Five tRNAs were decreased with PCB126 exposure and corresponding tRFs were also decreased (Table 2 and Table 5). However, tRNA-Glu-TTC-1 abundance was decreased with PCB126 exposure, whereas a corresponding tRF and the AS-tRNA were increased (Table 2). For the co-exposure to Ar1260 + PCB126, all eight tRNA changes showed concordant changes in tRF abundance (Table 3, Figure 4). Both upregulated tRNAs and all six tRNAs downregulated with Ar1260 + PCB126 co-exposure also showed increased and reduced tRF abundance, respectively (Table 3, Figure 4). Again, the corresponding increases or decreases in tRNAs and their tRFs suggests concordant tRNA processing.
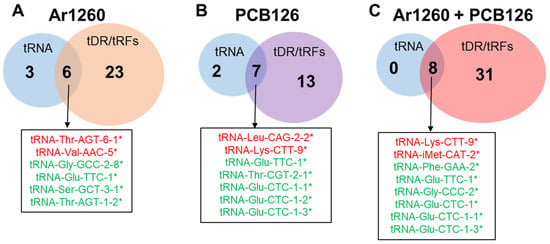
Figure 4.
Association of changes in the abundance of tRNAs and tRFs in HFD-fed mouse liver with PCB exposures. These are tRNAs and tDRs that were differentially expressed in HFD-control versus the indicated PCB exposures. (A) Ar1260 exposure; (B) PCB126 exposure; (C) Ar1260 + PCB126 coexposures. The colors red and green indicate increased or decreased tRNA expression in the samples as indicated. * indicates the same directional change(increased or decreased) of the corresponding tRF(Table 4, Table 5 and Table 6).
However, not all of the detected changes in tRFs were associated with changes in the abundance of parental tRNAs. For example, tRAX detected an increase in tRNA-SeC-TCA-1 fragments with Ar1260, PCB126, and the Ar1260 + PCB126 exposures. However, no change in intact tRNA-Sec-TCA-1 was detected in these samples. Likewise, we observed an increase in tRNA-Met-CAT-1-tRF-5 and tRNA-Met-CAT-1,2,3-tRF-3 with PCB126 and Ar1260 + PCB126 co-exposures, but no increase in mature tRNA-Met-CAT-1 was detected.
2.3. PCB Exposures in HFD-Fed Mice Affect Hepatic tRNA Modifications Detected by RT Misincorporation Terminations by tRAX
tRAX generated a plot of the per-base mismatch frequencies in each tRNA isodecoder, as a dot plot comparison across samples (Figure S5). tRNA positions 6, 9, 24, 25, 26, 32, 34, 37, 56, 58, and 59 showed the widest misincorporations among the tRNA isotypes, implying chemical modifications at those base positions. We previously identified seven PCB exposure-associated modification changes known to be located in tRNAs [52] in total RNA samples isolated from HFD-fed mouse livers [17]. We compared that list of PCB-induced tRNA modifications with the tRNA positions known to contain those modifications and the potential impact on tRF formation. One caveat is that because of the current lack of high throughput-verified detection, “only a small fraction of tRNA modifications have been studied: m5C, m1A, and Ψ” [28]. Four tRNA modifications that correspond to positions of PCB-associated misincorporations were identified: m1A, m6A, ms2t6A, and Ψ. m1A has been identified at positions 9, 14, 22, 57, and 58 in tRNAs [53]. N6-threonylcarbamoyladenosine (t6A) is the most common modification of position 37 in tRNA [40]; however, we did not detect any differences in t6A in Ar1260-, PCB126-, or Ar1260 + PCB126-exposed samples versus control mouse liver samples [17].
3. Discussion
The prevalence of MASLD is increasing [54]. Chronic nutrient overload is a starting point for insulin resistance with impaired glucose and lipid metabolism in the liver, resulting in steatosis initiating the spectrum of MASLD [55]. In addition to obesity, other contributors to MASLD include genetic variants (SNPs) [56], epigenetics [57,58], gut microbiome dysbiosis [59,60,61], and exposure to metabolism-disrupting environmental chemicals (MDCs), e.g., PCBs [34].
Here, we used the tRAX pipeline [41] to identify changes in the tRNA and tRF transcriptome in the livers of HFD-fed male mice exposed to Ar1260, an NDL PCB mixture, PCB126, a DL PCB, and the combination of Ar1260 + PCB126 [33]. These mimic PCB exposures in human populations that are associated with liver abnormalities and metabolic disease [36,62,63]. Our studies have demonstrated mostly unique results with few overlapping changes in proteins [33], mRNA [20], miRNA [20], and RNA modifications [17] in response to these three PCB exposures. We identified for the first time common and unique changes in tRNA and tRF abundance with each of the three PCB exposures. The biological significance of these PCB-exposure-induced changes in the hepatic tRNA transcriptome lies in their potential to disrupt translation processes, ultimately leading to alterations in protein expression.
3.1. tRNAs
Glu-carrying tRNAs were reduced with Ar1260, PCB126, and Ar1260 + PCB126 exposures. Glu is a regulator of hepatic aa metabolism that inhibits inflammatory responses in the liver and has antioxidant activity [64]. We reported that these PCB exposures contribute to MASH development in HFD-fed male mice [33]. Exposures to PCB126, Ar1260, or both Ar1260 + PCB126 did not exacerbate the HFD-induced liver lipid accumulation in the mice [33]. While we did measure Glu (or any other aa) in the liver samples, decreased hepatic Glu increases fat accumulation [64]. Two leu-carrying tRNAs were increased in PCB126-exposed livers. PCB126-exposed mice had lower plasma ALT levels compared to Ar1260-exposed mice and co-exposure Ar1260 + PCB126 attenuated ALT levels compared to Ar1260 alone, suggesting that PCB126 may protect against liver injury [33]. Leu is an essential branched-chain aa (BCAA) metabolized in the liver that decreases hepatic steatosis [64]. Two val-carrying tRNAs were increased with Ar1260 exposure. Val is another essential BCAA that is decreased in liver cirrhosis [65]. We examined the protein abundance of hepatic Glu-metabolizing EPRS1 protein (gene Eprs1, bifunctional glutamate/proline--tRNA ligase), but no change was detected with the PCB exposures [33]. We did not detect protein changes in BCAA-metabolizing enzymes BCKD (branched-chain alpha-ketoacid dehydrogenase kinase), BCAT1 (branched-chain-amino-acid aminotransferase, cytosolic), or BCAT2 (branched-chain-amino-acid aminotransferase, mitochondrial) in the livers of the HFD-fed mice with any of these three PCB exposures [33].
PCB126 and Ar1260 + PCB126-exposed mouse livers showed a decrease in tRNA-Glu-TTC-1, suggesting a PCB126-mediated reduction. Knockdown of human XPOT (exportin for tRNA) increased nuclear tRNA-Glu-TTC-1 in human triple-negative breast cancer (TNBC) cell lines [42]. Exportin hepatic protein abundance was decreased with Ar1260 + PCB126 co-exposure, but no change was detected with PCB126 exposure [33]. Theoretically, decreased exportin protein would be expected to increase nuclear retention of tRNA-Glu-TTC-1. Because small RNAs were extracted from the mouse livers in this study using the miRNAeasy kit which includes both nuclear and cytosolic small RNAs [20], no association appears related to a decrease in both exportin and tRNA-Glu-TTC-1 with Ar1260 + PCB126 co-exposure. Future studies could examine the subcellular distribution of tRNA-glu-TTC-1 in PCB126 or Ar1260 + PCB126-exposed HFD-fed mouse livers.
Ar1260 + PCB126 co-exposure increased hepatic tRNA-iMet-CAT-2. tRNA-iMet initiates protein synthesis, a finding corresponding to the higher number of hepatic proteins detected with Ar1260 + PCB126 co-exposure compared to either Ar1260 or PCB126 exposure alone [33]. Concordantly, the gene ontology (GO) process “regulation of protein metabolic processes” was increased by Ar1260 + PCB126 co-exposure [33], suggesting the need for increased tRNA-iMet-CAT for initiation of translation.
Ar1260 exposure decreased two Thr-carrying tRNAs. One of these tRNAs, tRNA-Thr-CGT-2-1, was also decreased with PCB126 exposure. No Thr-carrying tRNAs were altered in the Ar1260 + PCB126 co-exposure livers. Thr is an essential aa catabolized in the liver [64]. Neither of the hepatic Thr-metabolizing enzymes (TDG, G/T mismatch-specific thymine DNA glycosylase or TDH, L-Threonine dehydrogenase) were altered by any of the three PCB exposures in our HFD-fed mouse model of MASLD/MASH [64].
Ar1260 + PCB126 co-exposure decreased one hepatic gly-carrying tRNA and a different gly-carrying tRNA was decreased with Ar1260 exposure. Gly is a non-essential aa that regulates bile synthesis [66]. Gly was increased in mouse livers on an HFD [67]. Oral gly prevents liver fibrosis in rats [68]. We did not detect any change in the protein levels of SHMT1 (serine hydroxymethyltransferase, cytosolic, which converts serine and tetrahydrofolate to gly and 5,10-methylene tetrahydrofolate) in the livers of any of the three groups of PCB-exposed mice [33]. Likewise, no change in AGT1 (alanine-glyoxylate aminotransferase, Agxt (gene) which converts glycoxylate to gly) protein was detected with these PCB exposures [33].
PCB126 and Ar1260 + PCB126 exposures increased one hepatic lys-carrying tRNA, suggesting regulation by PCB126. Lys is an essential aa that is catabolized in the liver [69]. We did not detect any protein change in LKR/SDH (L-lysine-2-ketoglutarate reductase, Aass (gene), which converts lys to saccharopine, or ALDH7A1 (alpha-aminoadipic semialdehyde dehydrogenase), which converts saccharopine to glutamate [69], in the livers of the HFD-fed mice with these PCB exposures [33].
Taken together, these data suggest that PCB-exposure-induced changes in liver aa-carrying tRNAs are not reflected in alterations in the protein abundance of enzymes involved in their respective hepatic aa metabolism. We did not detect any protein changes in the 29 aa transporters expressed in the liver [70] in response to any of the three PCB exposures in the HFD-fed mouse livers [33]. Uncharged tRNAs accumulate in mammalian cells when aa are limited, trigging the integrated stress response (ISR) [71]. GO process analysis of the proteins altered by these PCB exposures did not indicate IRS [33]. A future goal is to perform metabolic flux analysis using 13C- labeled aa and mouse livers ex vivo [72] to examine how HFD and the three PCB exposures in vivo impact aa metabolism.
3.2. AS-tRNAs
For the majority of tRNAs, there was no correspondence with change in its AS-tRNA. We note that the role of AS-tRNA in mouse liver is unclear. We only found one paper in PubMed [73] reporting AS-tRNAs. In that study, the authors identified sense and antisense mt-tRNAs with “mirrored” expression of some mite T. urticae mt-tRNAs, i.e., tRNA-Arg, tRNA-Glu, tRNA-Leu1, tRNA-Phe, tRNA-Pro, tRNA-Ser2, and tRNA-Tyr, other tRNAs had high-antisense and low-sense tRNA expression, whereas some tRNAs had high-sense and low-antisense reads [73]. We suggest that the application of targeted small RNA sequencing using Illumina technology enabled the detection of AS-tRNA. Further studies are needed on the role of AS-tRNAs in mammals and in MASLD.
3.3. tRFs
The rapid advancement in RNA-seq technologies has identified novel tRNA transcriptome species including tRFs that have roles in cellular biology [74]. Here, we found that PCB exposures increased the abundance of more tRFs than tRNAs. For tRNAs altered by PCB exposures with a corresponding tRF, the tRNA and tRF showed parallel regulation—both increased or decreased. This result supports the expected concordant regulation of the “parental” tRNA and its cleavage to tRFs. However, not all tRNAs showed changes in tRFs. For example, a tRF for tRNA-Leu-CAA-2 was decreased with Ar1260 exposure. However, no change in parental tRNA-Leu-CCA-2 was detected. The opposite patterns of tRNA and tRF and AS-tRNA abundance may relate to PCB-induced alterations in RNA-binding proteins, elongation factors, or ribosomal proteins that may protect some tRNA species from degradation [75,76,77]. tRNA-Leu-CAA is downregulated with endoplasmic reticulum (ER) stress [47]. However, proteome analysis did identify changes in protein abundance in the ER stress pathway [33].
Some of the changes in hepatic tRFs detected here in response to PCB exposures (Table 4, Table 5 and Table 6) were also reported in the steatotic livers of DIO-induced C57BL/6J mice [32], in human MASLD patient plasma [46], and in mouse plasma after 2–6 weeks of high-cholesterol diet-induced MASLD in BALBc mice [46]. For example, tRFs from tRNA-Gly-TCC-1 and tRNA-Val-CAC were increased with Ar1260 exposure. This observation agrees with the increase in tRNA-Gly-TCC-1-tRF-3 and tRNA-Val-CAC in the livers of DIO C57Bl/6 mice [32] and the plasma of MASLD patients [46], respectively. We detected increased tRF-5 fragments from tRNA-Val-AAC with Ar1260 exposure, but not with PCB126 exposure, which also apparently blocked tRNA-Val-AAC cleavage when combined with Ar1260 exposure. tRNA-Val-AAC-5-tRF-5 was increased in the livers of DIO C57Bl/6J mice [32]. Increased tRNA-Val-AAC fragments were detected in mouse liver samples with time after liver isolation and in human HepG2 cells subjected to stressors including starvation, heat shock, hypothermia, hypoxia, and radiation [78]. 5′-tRFs from tRNA-Val inhibit eiF4F complex formation [28]. Interestingly, this corresponds to our observation that Ar1260 exposure produced the fewest changes in the hepatic proteome [33].
However, not all tRF changes agree with these reports. The tRF “tRNA-Gly-TCC-1_other” was lower with Ar1260 exposure but tRNA-Gly-TCC-1-tRF-3 was increased in the steatotic livers of DIO C57Bl/6 mice [32]. This difference may result from the co-exposures to PCBs with HFD to induce MASLD and progression to MASH in our mouse model.
The tRNA endonuclease ANG is highly expressed in liver and generates tRF-3 and 5′tiRFs [79]. We reported that ANG protein was increased in HFD-fed, PCB126-exposed mouse livers [33]. However, we did not detect altered mouse liver protein abundance of DICER or ELAC2 with any of the three PCB exposures [33]. DICER and ELAC2 are responsible for generating tRF-5s and tRF-1 [80,81]. Likewise, no change in IRE1α (ERN1, serine/threonine-protein kinase/endoribonuclease IRE1α) protein that generates tRNA-Gly-GCG-5′tRFs in response to ER stress in cells and in mouse ovary [48] was detected in the HFD-fed, PCB-126 exposed mouse livers [33].
tRFs regulate translation, interact with DNA in regulating transcription, and are mediators in RNA interference, like miRNAs [28]. Here, we observed that PCB126 exposure increased tRFs from tRNA-Lys. tRFs from tRNA-Lys associate with Argonaute 2 (AGO2) [28]. We observed the fewest hepatic miRNA changes with PCB126 exposure in HFD-fed mouse liver [20]. Future studies will be necessary to examine the identities of liver AGO2-associated tRFs and miRNAs and the impact of HFD and PCB exposures on these interactions.
tRNA modifications generally suppress tRF biogenesis [28]. m5C and Ψ were associated with tRF biogenesis [28]. ALKBH3-mediated demethylation of m1A in tRNA makes tRNAs more sensitive to ANG cleavage and tRF generation [80]. Since ANG protein was increased in the PCB126-exposed mouse livers [33], it is possible that the decrease in m1A in PCB126-exposed liver samples [17] is associated with the increase in the five tRF-3s in those samples. However, overall, it is difficult to draw conclusions associating positions of modifications in tRNAs with tRFs identified in our PCB-exposed liver samples. Ongoing efforts in direct-read RNA technologies and bioinformatic analysis will be needed to understand how tRNA modifications affect the tRNA transcriptome.
4. Conclusions
Using tRAX [41], we identified changes in tRNAs and tRFs (tDRs) with PCB exposures in HFD-fed male mouse livers. Our study provides the first evidence of differences in the abundance of individual tRNAs in the livers of HFD-fed male C57Bl/6J mice with three exposures (Ar1260, PCB126, or Ar1260 + PCB126). These findings suggest that PCB exposures play an unexplored role in regulating translation. Further, we report for the first time HFD- and PCB-exposure-induced changes in the abundance of tRFs which may regulate transcript abundance and translation as well as additional processes including extracellular vesicles for communication with other tissues [82]. We speculate that HFD and PCB exposures act as hepatic stressors to generate differences in tRFs. Changes in the hepatic pool of tRNAs is likely to cause differences in translation and thus protein abundance. Additionally, changes in tRNAs can lead to errors in tRNA loading in ribosomes, which decreases the accuracy and efficiency of protein synthesis, leading to mutated proteins with altered function [83]. Future studies will examine if tRNAs associate with mistranslated (altered) proteins in this HFD-fed PCB-exposure model of MASLD progression to MASH. It will be of interest to examine if tRFs are altered in the blood and extracellular vesicles of humans with PCB and other environmental exposures associated with metabolic diseases including MASLD.
Limitations
tRNA structure and modifications affect cDNA synthesis in the RT step prior to RNA-seq, thus reducing the efficiency and accuracy of tRNA-seq analysis [84]. We did not treat the isolated miRNA samples with ALKB or T4 polynucleotide kinase that increase cDNA reads [41]. The presence of tRFs can interfere with estimating the mature tRNA pool [51]. Lastly, we did not explore the mechanism of functional implications of our results.
5. Materials and Methods
5.1. Animal Studies
The experimental design is modeled in Figure S1. The mouse protocol for PCB exposure and HFD was ratified by the University of Louisville Institutional Animal Care and Use Committee (Approval code 18022 on 30 March 2018) [33] and details of this study have been previously reported [33]. In brief, male C57BL/6 mice (8 weeks old) from Jackson Laboratory were randomized (n = 10) into four equal groups. All mice were fed ad libitum a high-fat diet (HFD, 15.2, 42.7, and 42.0% of total calories from protein, carbohydrate, and fat, respectively; TekLad TD88137) throughout the study. At ten weeks of age, each mouse was given a one-time, single oral gavage of either corn oil (vehicle control), Ar1260 (20 mg/kg), PCB126 (20 μg/kg), or a mixture of Ar1260 (20 mg/kg) plus PCB126 (20 μg/kg). These concentrations were selected based on our previous reports that Ar1260 and PCB126 act as “second hits” in HFD-fed mice to induce steatohepatitis [38]. Since humans are exposed to dioxin-like (DL) and non-dioxin-like (NDL) PCB mixtures, PCB126 (a DL-PCB) was added to Ar1260 at doses used in our previous studies [37,38]. At the end of week 12, the mice were fasted for ~ 6 h prior to euthanasia and liver samples were harvested as described [33].
5.2. Short RNA Sequencing
Short RNAs were isolated from five mouse livers/experimental exposure using Qiagen miRNA kits and sequenced as previously described [85]. Briefly, libraries were prepared from 1 µg of mouse liver RNA. The QIAseq miRNA Library Kit (Qiagen) and quality and quantity were validated using an Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The RNA Integrity Number (RIN) for the samples is shown in Table S4. Figure S6 shows a linear regression analysis of RIN vs. read count from the Illumina miRNA-seq data, which shows that higher RIN corresponds with lower read count. Lower RIN scores were not associated with loss of miRNA detection [86]. Sequencing library quantitation was carried out by performing a MiSeq Nano (Illumina, San Diego, CA, USA) test run. Two sequencing runs/sample were performed on the Illumina NextSeq 500 using the NextSeq 500/550 High Output Kit v2.5 (75 cycles). Data as fastq files were downloaded from Illumina’s BaseSpace onto the KY INBRE server for analysis. The raw data of the miRNA-seq are available at the Gene Expression Omnibus (GEO) database: GSE195829.
5.3. Bioinformatics Analysis
Twenty fastq files (four exposures groups: control, Ar1260, PCB126, Ar1260 + PCB126, with five mouse livers per exposure group) were trimmed to remove the Qiagen 3′ Adapter sequence. Quality control (QC) of the raw sequence data was performed using FastQC (version 0.10.1). Minimum quality values for all samples were well above Q30 (1 in 1000 error rate); therefore, no quality trimming was necessary. The number of sequenced reads ranged from 4.4 to 6.9 million across the samples with a median of 5.8 million. Raw fastq files were input to tRAX (tRNA analysis of expression), a tool for identifying tRNAs and tDRs/tRFs and performing differential expression between comparison groups [35,40]. Supplementary Figure S2 shows the tRAX pipeline in which trimmed fastq files are aligned with the Bowtie2 aligner [36,40] prior to abundance estimation and differential expression. For details regarding tRAX’s alignment options, which are specifically designed to take into account the characteristics of tRNAs, see [40]. Sequenced reads were aligned to the Mus musculus (mm10) genome assembly. The alignment rate was high, ranging from 91.7 to 96.2 percent across the samples with a median of 95.7 percent. The pipeline relied on the creation of a mouse tRNA database that included a tRNA scan file generated by tRNAscan-SE [37,40] and downloaded from GtRNAdb [11,38,40] as well as a name map file that converted the tRNAscan-SE IDs to GtRNAdb gene symbols. The tRNA identification and differential expression analysis were performed using a tRAX Python (version 2.7.5) script called processsamples.py. The script was run with the --no frag option to identify mature tRNAs and without the option to identify tRFs. tRAX uses DESeq2 for differential expression analysis which utilizes a negative binomial distribution appropriate for count data. Raw counts are input to DESeq2 and normalized with the relative log expression (RLE) method prior to performing differential expression. A false discovery rate (FDR) of 5% was used to determine significance and reduce the number of false positives in the results. tRAX performed a principal component analysis (PCA, see Figure S7) as part of its pipeline. The samples show wide within-group variability.
tRAX provided a report containing the percentage of reads retained after adapter removal, read mapping rate, proportion of reads mapped to tRNAs versus rRNAs and unannotated regions, percentage of expected read lengths, and range of read count normalization size factors across samples. In the analysis of mature tRNAs, all quality filters were passed with the exception of tRNA read share greater than 50% and at least 70% of reads between 40 and 75 bases in length. In the analysis of tRFs, all filters were passed with the exception of at least 70% of reads between 15 and 50 bases. The read counts, mapping rate, and percentages for these latter filters are shown in Table S5. Reads that aligned to mouse tRNA gene loci with flanking sequences are considered as pre-tRNA reads and were not included in the analysis of mature tRNAs. Supplementary Figure S3 displays the distribution of reads for tRNAs and other small RNA types across samples. Reads referred to as “other” are not mapped to any annotated features.
5.4. Venn Diagrams
The Venn diagrams were drawn using http://bioinformatics.psb.ugent.be/webtools/Venn/. (accessed on 3 April 2025)
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ncrna11030041/s1, Figure S1: Short-term (12 weeks) PCB-exposure in a HFD-fed mouse model of MASH. Figure S2: The tRAX pipeline used to analyze the miRNA-seq liver samples from HFD-fed control, Ar1260, PCB126, and Ar1260 + PCB126 exposed mice. Figure S3. Percentage of uniquely mapped (single), multimapped (mult) and unmapped (unmap) reads for each sample (A) and percentage of uniquely mapped tRNAs, unique_anticodons (equal matches to multiple different tRNAs with the same anticodon), and unique_aminos (equal matches to tRNAs decoding the same amino acid) (B). Figure S4: PCB exposures differentially regulate mouse liver antisense-tRNA. Figure S5. Overall maximum per-base mismatch frequency across all samples. Figure S6: Linear regression analysis of mouse liver miRNA RIN and Read Count from Illumina miRNA-seq analysis. Figure S7: Principal component analysis (PCA) of the tRNA transcriptome for the mouse liver samples. Each point represents the data from one individual mouse liver sample. All mice were fed a HFD and exposed to vehicle control (HFDcontrol), Ar1260 (HFDAro), PCB126 (HFDPCB), or Ar1260 + PCB126 co-exposures (HFDAroPCB). Table S1: Differentially expressed antisense (AS)-tRNAs in HFD-fed mouse liver with Ar1260 exposure vs. HFD-fed control (FDR < 0.05). Table S2: Differentially expressed antisense (AS)-tRNAs in HFD-fed mouse liver with PCB126 exposure vs. HFD-fed control (FDR < 0.05). Table S3: Differentially expressed antisense (AS)-tRNAs in HFD-fed mouse liver with Ar1260 + PCB126 exposure vs. HFD-fed control (FDR < 0.05). Table S4: The RNA Integrity Number (RIN) for the isolated miRNA samples from mouse livers in the four treatment groups indicated. Table S5: Read counts, mapping rate, and tRAX quality measure not meeting expected criteria for some or all samples. References [20,32,46,47,87,88] are cited in the supplementary materials.
Author Contributions
Conceptualization, C.M.K. and J.H.C.; formal analysis, C.M.K., J.H.C. and B.J.P.; investigation, K.M.P.; resources, C.M.K. and M.C.C.; writing—original draft preparation, C.M.K.; writing—review and editing, J.H.C., B.J.P. and E.C.R.; funding acquisition, C.M.K., M.C.C. and E.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institutes of Health grants: NIH R21ES031510, ES031510-01S1, P30ES030283, R35ES028373, R01ES032189, P42ES023716, P20GM113226; P20GM103436, and the Kentucky Council on Postsecondary Education (PON2 415 1900002934).
Institutional Review Board Statement
The mouse protocol for PCB exposure and HFD was ratified by the University of Louisville Institutional Animal Care and Use Committee (Approval code 180222 on 30 March 2018).
Data Availability Statement
The raw data of the miRNA-seq is available at Gene Expression Omnibus (GEO) database: GSE195829.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PCB | polychlorinated biphenyls |
| HFD | high-fat diet |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MASH | metabolic dysfunction-associated steatohepatitis |
| tDRs | tRNA-derived small RNAs |
| tRF | tRNA-derived fragment |
| tsRNAs | shorter tRNA-derived small RNAs |
| AS-tRNA | antisense tRNA |
| NDL | non-dioxin like |
| DL | dioxin-like |
| AHR | arylhydrocarbon receptor |
| ANG | angiogenin |
| MDC | metabolism-disrupting chemicals |
| EDC | endocrine-disrupting chemicals |
| HCC | hepatocellular carcinoma |
| NAFLD | nonalcoholic fatty liver disease |
| ACL | anticodon loop |
| Am | 2-O-methyladenosine |
| m1A | 1-methyladenosine |
| m2,2G | N2,N2-dimethylguanosine) |
| m3C | 3-methylcytosine |
| m5C | 5-methylcytosine |
| m5U | 5-methylurdine |
| m6A | N6-methyladenosin |
| Ψ | pseudouridine |
| ELAC | Elac ribonuclease Z 2 |
| DIO | diet-induced obesity |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
References
- Wu, H.; Eckhardt, C.M.; Baccarelli, A.A. Molecular mechanisms of environmental exposures and human disease. Nat. Rev. Genet. 2023, 24, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Deierlein, A.L.; Rock, S.; Park, S. Persistent Endocrine-Disrupting Chemicals and Fatty Liver Disease. Curr. Environ. Health Rep. 2017, 4, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Samala, N.; Kulkarni, M.; Lele, R.S.; Gripshover, T.C.; Lynn Wise, J.; Rai, S.N.; Cave, M.C. Associations between per- and polyfluoroalkyl substance exposures and metabolic dysfunction associated steatotic liver disease (MASLD) in adult National Health and Nutrition Examination Survey 2017 to 2018. Toxicol. Sci. 2024, 202, 142–151. [Google Scholar] [CrossRef]
- Tovoli, F.; Stefanini, B.; Mandrioli, D.; Mattioli, S.; Vornoli, A.; Sgargi, D.; Manservisi, F.; Piscaglia, F.; Curti, S.; Bolondi, L. Exploring occupational toxicant exposures in patients with metabolic dysfunction-associated steatotic liver disease: A prospective pilot study. Dig. Liver Dis. 2024, 56, 571–578. [Google Scholar] [CrossRef]
- Wahlang, B.; Jin, J.; Beier, J.I.; Hardesty, J.E.; Daly, E.F.; Schnegelberger, R.D.; Falkner, K.C.; Prough, R.A.; Kirpich, I.A.; Cave, M.C. Mechanisms of Environmental Contributions to Fatty Liver Disease. Curr. Environ. Health Rep. 2019, 6, 80–94. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Cayir, A.; Byun, H.-M.; Barrow, T.M. Environmental epitranscriptomics. Environ. Res. 2020, 189, 109885. [Google Scholar] [CrossRef]
- Petri, B.J.; Cave, M.C.; Klinge, C.M. Changes in m6A in Steatotic Liver Disease. Genes 2023, 14, 1653. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.L.; Decker, J.C.; Bolano, A.; Krahn, N. Tuning tRNAs for improved translation. Front. Genet. 2024, 15, 1436860. [Google Scholar] [CrossRef]
- Chan, C.M.; Lykkesfeldt, A.E.; Parker, M.G.; Dowsett, M. Expression of nuclear receptor interacting proteins TIF-1, SUG-1, receptor interacting protein 140, and corepressor SMRT in tamoxifen-resistant breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 3460–3467. [Google Scholar]
- Coughlin, D.J.; Babak, T.; Nihranz, C.; Hughes, T.R.; Engelke, D.R. Prediction and verification of mouse tRNA gene families. RNA Biol. 2009, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.A.; Goodenbour, J.M.; Pan, T. Tissue-specific differences in human transfer RNA expression. PLoS Genet 2006, 2, e221. [Google Scholar] [CrossRef]
- Geslain, R.; Pan, T. Functional analysis of human tRNA isodecoders. J. Mol. Biol. 2010, 396, 821–831. [Google Scholar] [CrossRef]
- Hughes, L.A.; Rudler, D.L.; Siira, S.J.; McCubbin, T.; Raven, S.A.; Browne, J.M.; Ermer, J.A.; Rientjes, J.; Rodger, J.; Marcellin, E.; et al. Copy number variation in tRNA isodecoder genes impairs mammalian development and balanced translation. Nat. Commun. 2023, 14, 2210. [Google Scholar] [CrossRef]
- Zhang, W.; Foo, M.; Eren, A.M.; Pan, T. tRNA modification dynamics from individual organisms to metaepitranscriptomics of microbiomes. Mol. Cell 2022, 82, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Phizicky, E.M. A rationale for tRNA modification circuits in the anticodon loop. RNA 2018, 24, 1277–1284. [Google Scholar] [CrossRef]
- Klinge, C.M.; Piell, K.M.; Petri, B.J.; He, L.; Zhang, X.; Pan, J.; Rai, S.N.; Andreeva, K.; Rouchka, E.C.; Wahlang, B.; et al. Combined exposure to polychlorinated biphenyls and high-fat diet modifies the global epitranscriptomic landscape in mouse liver. Environ. Epigenetics 2021, 7, dvab008. [Google Scholar]
- Cui, W.; Zhao, D.; Jiang, J.; Tang, F.; Zhang, C.; Duan, C. tRNA Modifications and Modifying Enzymes in Disease, the Potential Therapeutic Targets. Int. J. Biol. Sci. 2023, 19, 1146–1162. [Google Scholar] [CrossRef]
- Lin, S.; Kuang, M. RNA modification-mediated mRNA translation regulation in liver cancer: Mechanisms and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 267–281. [Google Scholar] [CrossRef]
- Petri, B.J.; Piell, K.M.; Wahlang, B.; Head, K.Z.; Andreeva, K.; Rouchka, E.C.; Pan, J.; Rai, S.N.; Cave, M.C.; Klinge, C.M. Multiomics analysis of the impact of polychlorinated biphenyls on environmental liver disease in a mouse model. Environ. Toxicol. Pharmacol. 2022, 94, 103928. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Tang, Q.; Tang, F. Oncogenic-tsRNA: A novel diagnostic and therapeutic molecule for cancer clinic. J. Cancer 2024, 15, 5403–5414. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wilson, B.; Kumar, P.; Dutta, A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu. Rev. Genet. 2020, 54, 47–69. [Google Scholar] [CrossRef]
- Jacovetti, C.; Bayazit, M.B.; Regazzi, R. Emerging Classes of Small Non-Coding RNAs With Potential Implications in Diabetes and Associated Metabolic Disorders. Front. Endocrinol. 2021, 12, 670719. [Google Scholar] [CrossRef]
- Murillo-Recio, M.; Martínez de Lejarza Samper, I.M.; Tuñí i Domínguez, C.; Ribas de Pouplana, L.; Torres, A.G. tRNAstudio: Facilitating the study of human mature tRNAs from deep sequencing datasets. Bioinformatics 2022, 38, 2934–2936. [Google Scholar] [CrossRef] [PubMed]
- Pekarsky, Y.; Balatti, V.; Croce, C.M. tRNA-derived fragments (tRFs) in cancer. J. Cell Commun. Signal. 2022, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Grigoriev, A. tatDB: A database of Ago1-mediated targets of transfer RNA fragments. Nucleic Acids Res. 2023, 51, D297–D305. [Google Scholar] [CrossRef]
- Suresh, P.S.; Thankachan, S.; Venkatesh, T. Landscape of Clinically Relevant Exosomal tRNA-Derived Non-coding RNAs. Mol. Biotechnol. 2023, 65, 300–310. [Google Scholar] [CrossRef]
- Muthukumar, S.; Li, C.-T.; Liu, R.-J.; Bellodi, C. Roles and regulation of tRNA-derived small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2024, 25, 359–378. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Hou, Y. Classification, function, and advances in tsRNA in non-neoplastic diseases. Cell Death Dis. 2023, 14, 748. [Google Scholar] [CrossRef]
- Hawwash, N.; Sperrin, M.; Martin, G.P.; Joshu, C.E.; Florido, R.; Platz, E.A.; Renehan, A.G. Waist circumference-years and cancer risk: A prospective study of the association and comparison of predictive performance with waist circumference and body mass index. Br. J. Cancer 2024, 131, 1623–1634. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Zhu, L.; Li, T.; Yan, Z.; Guo, J. Transfer RNA-derived fragments and tRNA halves: Biogenesis, biological functions and their roles in diseases. J. Mol. Med. 2018, 96, 1167–1176. [Google Scholar] [CrossRef]
- Tzur, Y.; Winek, K.; Madrer, N.; Dubnov, S.; Bennett, E.R.; Greenberg, D.S.; Hanin, G.; Gammal, A.; Tam, J.; Arkin, I.T.; et al. Lysine tRNA fragments and miR-194-5p co-regulate hepatic steatosis via β-Klotho and perilipin 2. Mol. Metab. 2024, 79, 101856. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wahlang, B.; Shi, H.; Hardesty, J.E.; Falkner, K.C.; Head, K.Z.; Srivastava, S.; Merchant, M.L.; Rai, S.N.; Cave, M.C.; et al. Dioxin-like and non-dioxin-like PCBs differentially regulate the hepatic proteome and modify diet-induced nonalcoholic fatty liver disease severity. Med. Chem. Res. 2020, 29, 1247–1263. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Jandacek, R.J.; Tso, P. Enterohepatic circulation of organochlorine compounds: A site for nutritional intervention. J. Nutr. Biochem. 2007, 18, 163–167. [Google Scholar] [CrossRef]
- Cave, M.; Appana, S.; Patel, M.; Falkner, K.C.; McClain, C.J.; Brock, G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ. Health Perspect. 2010, 118, 1735–1742. [Google Scholar] [CrossRef]
- Wahlang, B.; Song, M.; Beier, J.I.; Cameron Falkner, K.; Al-Eryani, L.; Clair, H.B.; Prough, R.A.; Osborne, T.S.; Malarkey, D.E.; Christopher States, J.; et al. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 2014, 279, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jan, J.; Hardesty, J.E.; Falkner, K.C.; Prough, R.A.; Balamurugan, A.N.; Mokshagundam, S.P.; Chari, S.T.; Cave, M.C. Polychlorinated biphenyl exposures differentially regulate hepatic metabolism and pancreatic function: Implications for nonalcoholic steatohepatitis and diabetes. Toxicol. Appl. Pharmacol. 2019, 363, 22–33. [Google Scholar] [CrossRef]
- Wahlang, B.; Jin, J.; Hardesty, J.E.; Head, K.Z.; Shi, H.; Falkner, K.C.; Prough, R.A.; Klinge, C.M.; Cave, M.C. Identifying sex differences arising from polychlorinated biphenyl exposures in toxicant-associated liver disease. Food Chem. Toxicol. 2019, 129, 64–76. [Google Scholar] [CrossRef]
- Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 2021, 22, 375–392. [Google Scholar] [CrossRef]
- Holmes, A.D.; Howard, J.M.; Chan, P.P.; Lowe, T.M. tRNA Analysis of eXpression (tRAX): A tool for integrating analysis of tRNAs, tRNA-derived small RNAs, and tRNA modifications. bioRxiv 2022. bioRxiv:2022.2007.2002.498565. [Google Scholar]
- Dai, H.; Yang, X.; Sheng, X.; Wang, Y.; Zhang, S.; Zhang, X.; Hu, L.; Zhang, Z.; Dong, X.; Yin, W.; et al. XPOT Disruption Suppresses TNBC Growth through Inhibition of Specific tRNA Nuclear Exportation and TTC19 Expression to Induce Cytokinesis Failure. Int. J. Biol. Sci. 2023, 19, 5319–5336. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Ito, K.; Chu, C.-S.; Roeder, R.G. Gene-Specific Control of tRNA Expression by RNA Polymerase II. Mol. Cell 2020, 78, 765–778.e767. [Google Scholar] [CrossRef]
- Vilardo, E.; Amman, F.; Toth, U.; Kotter, A.; Helm, M.; Rossmanith, W. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucleic Acids Res. 2020, 48, 6157–6169. [Google Scholar] [CrossRef]
- Pinzaru, A.M.; Tavazoie, S.F. Transfer RNAs as dynamic and critical regulators of cancer progression. Nat. Rev. Cancer 2023, 23, 746–761. [Google Scholar] [CrossRef]
- Huang, P.; Tu, B.; Liao, H.-j.; Huang, F.-z.; Li, Z.-z.; Zhu, K.-y.; Dai, F.; Liu, H.-z.; Zhang, T.-y.; Sun, C.-z. Elevation of plasma tRNA fragments as a promising biomarker for liver fibrosis in nonalcoholic fatty liver disease. Sci. Rep. 2021, 11, 5886. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Coquelet, H.; Leman, G.; Toutain, B.; Petit, C.; Henry, C.; Boissard, A.; Guette, C.; Coqueret, O. tRNA biogenesis and specific aminoacyl-tRNA synthetases regulate senescence stability under the control of mTOR. PLoS Genet. 2021, 17, e1009953. [Google Scholar] [CrossRef]
- Jin, H.; Yeom, J.-H.; Shin, E.; Ha, Y.; Liu, H.; Kim, D.; Joo, M.; Kim, Y.-H.; Kim, H.K.; Ryu, M.; et al. 5′-tRNAGly(GCC) halves generated by IRE1α are linked to the ER stress response. Nat. Commun. 2024, 15, 9273. [Google Scholar] [CrossRef]
- Chen, W.; Peng, W.; Wang, R.; Bai, S.; Cao, M.; Xiong, S.; Li, Y.; Yang, Y.; Liang, J.; Liu, L.; et al. Exosome-derived tRNA fragments tRF-GluCTC-0005 promotes pancreatic cancer liver metastasis by activating hepatic stellate cells. Cell Death Dis. 2024, 15, 102. [Google Scholar] [CrossRef]
- Shen, L.; Liao, T.; Chen, Q.; Lei, Y.; Wang, L.; Gu, H.; Qiu, Y.; Zheng, T.; Yang, Y.; Wei, C.; et al. tRNA-derived small RNA, 5′tiRNA-Gly-CCC, promotes skeletal muscle regeneration through the inflammatory response. J. Cachexia Sarcopenia Muscle 2023, 14, 1033–1045. [Google Scholar] [CrossRef]
- Torres, A.G.; Reina, O.; Stephan-Otto Attolini, C.; Ribas de Pouplana, L. Differential expression of human tRNA genes drives the abundance of tRNA-derived fragments. Proc. Natl. Acad. Sci. USA 2019, 116, 8451–8456. [Google Scholar] [CrossRef] [PubMed]
- Cappannini, A.; Ray, A.; Purta, E.; Mukherjee, S.; Boccaletto, P.; Moafinejad, S.N.; Lechner, A.; Barchet, C.; Klaholz, B.P.; Stefaniak, F.; et al. MODOMICS: A database of RNA modifications and related information. 2023 update. Nucleic Acids Res. 2024, 52, D239–D244. [Google Scholar] [CrossRef] [PubMed]
- Oerum, S.; Dégut, C.; Barraud, P.; Tisné, C. m1A Post-Transcriptional Modification in tRNAs. Biomolecules 2017, 7, 20. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction–Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open 2025, 8, e2454707. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Razavi, H.; Sherman, M.; Allen, A.M.; Anstee, Q.M.; Cusi, K.; Friedman, S.L.; Lawitz, E.; Lazarus, J.V.; Schuppan, D.; et al. Addressing the High and Rising Global Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and Metabolic Dysfunction-Associated Steatohepatitis (MASH): From the Growing Prevalence to Payors’ Perspective. Aliment. Pharmacol. Ther. 2025, 61, 1467–1478. [Google Scholar] [CrossRef]
- Carlsson, B.; Lindén, D.; Brolén, G.; Liljeblad, M.; Bjursell, M.; Romeo, S.; Loomba, R. Review article: The emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2020, 51, 1305–1320. [Google Scholar] [CrossRef]
- Jonas, W.; Schürmann, A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef]
- Maude, H.; Sanchez-Cabanillas, C.; Cebola, I. Epigenetics of Hepatic Insulin Resistance. Front. Endocrinol. 2021, 12, 681356. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. NAFLD 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Wahlang, B.; Alexander, N.C.; Li, X.; Rouchka, E.C.; Kirpich, I.A.; Cave, M.C. Polychlorinated biphenyls altered gut microbiome in CAR and PXR knockout mice exhibiting toxicant-associated steatohepatitis. Toxicol. Rep. 2021, 8, 536–547. [Google Scholar] [CrossRef]
- Cave, M.C.; Pinkston, C.M.; Rai, S.N.; Wahlang, B.; Pavuk, M.; Head, K.Z.; Carswell, G.K.; Nelson, G.M.; Klinge, C.M.; Bell, D.A.; et al. Circulating MicroRNAs, Polychlorinated Biphenyls, and Environmental Liver Disease in the Anniston Community Health Survey. Environ. Health Perspect 2022, 130, 017003. [Google Scholar] [CrossRef] [PubMed]
- Pavuk, M.; Rosenbaum, P.F.; Lewin, M.D.; Serio, T.C.; Rago, P.; Cave, M.C.; Birnbaum, L.S. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, pesticides, and diabetes in the Anniston Community Health Survey follow-up (ACHS II): Single exposure and mixture analysis approaches. Sci. Total Environ. 2023, 877, 162920. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.H. Therapeutic Effects of Amino Acids in Liver Diseases: Current Studies and Future Perspectives. J. Cancer Prev. 2019, 24, 72–78. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, L.A.; Duarte, J.A.G.; Schmieder, R.; Broekaert, D.; Veys, K.; Planque, M.; Vriens, K.; Karasawa, Y.; Napolitano, F.; Fujita, S.; et al. Fat Induces Glucose Metabolism in Nontransformed Liver Cells and Promotes Liver Tumorigenesis. Cancer Res 2021, 81, 1988–2001. [Google Scholar] [CrossRef]
- Rentsch, M.; Puellmann, K.; Sirek, S.; Iesalnieks, I.; Kienle, K.; Mueller, T.; Bolder, U.; Geissler, E.; Jauch, K.-W.; Beham, A. Benefit of Kupffer cell modulation with glycine versus Kupffer cell depletion after liver transplantation in the rat: Effects on postischemic reperfusion injury, apoptotic cell death graft regeneration and survival. Transpl. Int. 2005, 18, 1079–1089. [Google Scholar] [CrossRef]
- Matthews, D.E. Review of Lysine Metabolism with a Focus on Humans. J. Nutr. 2020, 150, 2548S–2555S. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef]
- Labbé, K.; LeBon, L.; King, B.; Vu, N.; Stoops, E.H.; Ly, N.; Lefebvre, A.; Seitzer, P.; Krishnan, S.; Heo, J.M.; et al. Specific activation of the integrated stress response uncovers regulation of central carbon metabolism and lipid droplet biogenesis. Nat. Commun. 2024, 15, 8301. [Google Scholar] [CrossRef] [PubMed]
- Grankvist, N.; Jönsson, C.; Hedin, K.; Sundqvist, N.; Sandström, P.; Björnsson, B.; Begzati, A.; Mickols, E.; Artursson, P.; Jain, M.; et al. Global 13C tracing and metabolic flux analysis of intact human liver tissue ex vivo. Nat. Metab. 2024, 6, 1963–1975. [Google Scholar] [CrossRef]
- Warren, J.M.; Sloan, D.B. Hopeful monsters: Unintended sequencing of famously malformed mite mitochondrial tRNAs reveals widespread expression and processing of sense–antisense pairs. NAR Genom. Bioinform. 2021, 3, lqaa111. [Google Scholar] [CrossRef] [PubMed]
- Magee, R.; Rigoutsos, I. On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res. 2020, 48, 9433–9448. [Google Scholar] [CrossRef]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A emerging complexity of the tRNA world. Nat. Rev. Mol. Cell Biol. 2018, 19, 327. [Google Scholar] [CrossRef]
- Schimmel, P. The emerging complexity of the tRNA world: Mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 2018, 19, 45–58. [Google Scholar] [CrossRef]
- Nawrot, B.; Sochacka, E.; Düchler, M. tRNA structural and functional changes induced by oxidative stress. Cell. Mol. Life Sci. 2011, 68, 4023–4032. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Feng, J.; Liu, Q.; Sun, F.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009, 583, 437–442. [Google Scholar] [CrossRef]
- Oberbauer, V.; Schaefer, M.R. tRNA-Derived Small RNAs: Biogenesis, Modification, Function and Potential Impact on Human Disease Development. Genes 2018, 9, 607. [Google Scholar] [CrossRef]
- Liu, B.; Cao, J.; Wang, X.; Guo, C.; Liu, Y.; Wang, T. Deciphering the tRNA-derived small RNAs: Origin, development, and future. Cell Death Dis. 2021, 13, 24. [Google Scholar] [CrossRef]
- Wilson, B.; Dutta, A. Function and Therapeutic Implications of tRNA Derived Small RNAs. Front. Mol. Biosci. 2022, 9, 888424. [Google Scholar] [CrossRef] [PubMed]
- Tosar, J.P.; Cayota, A. Extracellular tRNAs and tRNA-derived fragments. RNA Biol. 2020, 17, 1149–1167. [Google Scholar] [CrossRef]
- Grewal, S.S. Why should cancer biologists care about tRNAs? tRNA synthesis, mRNA translation and the control of growth. Biochim. Biophys. Acta 2015, 1849, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, W.; Zhou, F.; Pang, Z.; Wang, L.; Pan, T.; Wang, X. Tissue-specific reprogramming of host tRNA transcriptome by the microbiome. Genome Res. 2021, 31, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Muluhngwi, P.; Alizadeh-Rad, N.; Vittitow, S.L.; Kalbfleisch, T.S.; Klinge, C.M. The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells. Sci. Rep. 2017, 7, 5205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhao, M.; Su, Y.; Cong, B.; Wang, Z. RNA quality score evaluation: A preliminary study of RNA integrity number (RIN) and RNA integrity and quality number (RNA IQ). Forensic Sci. Int. 2024, 357, 111976. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Wahlang, B.; Falkner, K.C.; Shi, H.; Jin, J.; Zhou, Y.; Wilkey, D.W.; Merchant, M.L.; Watson, C.T.; Feng, W.; et al. Proteomic Analysis Reveals Novel Mechanisms by Which Polychlorinated Biphenyls Compromise the Liver Promoting Diet-Induced Steatohepatitis. J. Proteome Res. 2019, 18, 1582–1594. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2014. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 3 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).