Abstract
Oral cancer (OC) ranks among the most prevalent head and neck cancers, becoming the eleventh most common cancer worldwide with ~350,000 new cases and 177,000 fatalities annually. The rising trend in the occurrence of OC among young individuals and women who do not have tobacco habits is escalating rapidly. Surgical procedures, radiation therapy, and chemotherapy are among the most prevalent treatment options for oral cancer. To achieve better therapy and an early detection of the cancer, it is essential to understand the disease’s etiology at the molecular level. Saliva, the most prevalent body fluid obtained non-invasively, holds a collection of distinct non-coding RNA pools (ncRNAomes) that can be assessed as biomarkers for identifying oral cancer. Non-coding signatures, which are transcripts lacking a protein-coding function, have been identified as significant in the progression of various cancers, including oral cancer. This review aims to examine the role of various salivary ncRNAs (microRNA, circular RNA, and lncRNA) associated with disease progression and to explore their functions as potential biomarkers for early disease identification to ensure better survival outcomes for oral cancer patients.
1. Introduction
Oral squamous cell carcinoma (OSCC), commonly referred to as oral cancer (OC), is the most common form of head and neck cancer, originating in the tongue, lips, and floor of the mouth. It ranks among the top ten causes of cancer-related death rates globally [1,2,3]. In India, OSCC or OC are the most observed, accounting for almost one-third of the overall cases globally [3,4]. According to recently released statistics in GLOBOCAN, there were around 3,77,713 new cases of OC worldwide in 2020, while there were over 177,757 fatalities [1]. The prevalent risk factors for OSCC encompass betel quid chewing with areca nut, excessive tobacco use or smoking, alcohol intake, inadequate oral hygiene, and nutritional deficiencies. Additional factors include environmental effects, persistent viral infections such as human papillomavirus (HPV), and microbial infections [2,3,5]. OSCC originates from the mucosal epithelial cells found in the oral cavity [6,7]. The genetic features of OSCC exhibit complexity and significant heterogeneity [6,8]. Even with progress in understanding and scientific discoveries over the last two decades, the 5-year survival rate for oral cancer patients continues to fall short of 50% [3,9]. The disease’s aggressiveness and heterogeneity, coupled with late diagnosis, the absence of early detection markers, ineffective chemotherapeutic drugs, therapy resistance, and side effects, frequently hinder its management [2,10]. Despite some encouraging outcomes, EGFR-targeted treatment and PD-1/PD-L1 immune therapy, which have been approved by the US Food and Drug Administration (FDA), have had minimal success [2,11,12].
Recent advancements in high throughput genome sequencing have shown that over 98% of the human genome consists of non-coding transcripts that do not translate into any protein [2]. Long non-coding RNA (lncRNA) has garnered considerable interest among non-coding RNAs for its role as either a tumor suppressor or an oncogene. lncRNAs are considered biomarkers in oral pre-malignant lesions due to their over or under-expression and have been linked to tumor initiation, progression, and metastasis, along with drug resistance [3]. Similarly, circular RNA (circRNA) has been studied, potentially serving as a biomarker due to its ability to sponge miRNA thereby regulating gene expression. Various microRNAs (miRNAs) are examined for their multifaceted roles in the hallmarks of cancer and are considered potential biomarkers for cancer detection and prognosis [3]. RNAseq studies have identified over 200 ncRNAs (including miRNA, lncRNA, circRNA, snoRNA, and piRNA) linked to OC progression [2].
In OSCC, various investigators have observed abnormalities in different lncRNAs, including MALAT1, HOTTAIR, HOTTIP, and TUG1 [6,13,14,15,16,17,18]. With its role as a tumor suppressor and tumor-promoting oncogenic driver, lncRNA might be the origin of the functional dysregulation linked to all pathophysiological abnormalities in OSCC [6]. miRNA-145, recognized as a significant tumor suppressor miRNA, is crucial in the regulation of apoptosis [19,20] and is often found to be downregulated in various cancers [20,21,22,23]. The downregulation of miRNA-145 has been shown in an animal model of OSCC [24]. miRNA-184 has the potential to target numerous genes and plays a role in inhibiting neuroblastoma cell survival by targeting AKT2, the serine/threonine kinase [25]. The Wong lab demonstrated that miRNA-184 was crucial in the anti-apoptotic and proliferative events associated with OSCC [20,26].
Saliva, an isotonic fluid produced by the parotid, submandibular, and submaxillary salivary glands, bathes the oral cavity [27,28]. Saliva is a distinctive biological fluid characterized by a diverse range of proteins, polypeptides, nucleic acids, electrolytes, and hormones. The salivary glands produce a hypotonic exocrine secretion, exhibiting a pH range of 7.2 to 7.4 [29]. Saliva serves as the initial biological medium that interacts with external substances, protecting the mucosa of the upper digestive tract, particularly in the oral cavity and pharynx. Human saliva exhibits a total antioxidant capacity that surpasses that of blood plasma [30]. Saliva comprises polypeptides, immunoglobulin, and enzymes such as lactoferrin, lysozyme, and histamine. These polypeptides are essential in defense mechanisms against free radicals, preventing oral cancer [31,32]. Multiple agents induce carcinogenic effects by altering the biochemical composition of human saliva. Staterin is an acidic salivary protein that inhibits the accumulation of calcium phosphate in the salivary glands’ excretory ducts and modulates tooth enamel’s solubility [33,34,35]. The concentration of statherin in the saliva of patients with oral cancer is diminished, resulting in reduced function within the oral cavity [35,36].
Cystatins are proteins characterized by their chemical structure and function as inhibitors of the enzyme cysteine proteases. Cystatin SA-I, with a molecular weight of 14 kDa, has been identified in the saliva of patients diagnosed with oral squamous cell carcinoma. This protein exhibits greater levels in the saliva of patients prior to treatment than in the saliva of treated patients, suggesting its potential as a biomarker for oral squamous cell carcinoma [35,37]. Epidermal growth factor (EGF) is a protein crucial in regulating the balance of the oral mucosa and the mucosa of the upper gastrointestinal tract. It additionally promotes the healing of wounds in the oral environment.
The saliva of patients with oral cancer shows a reduced concentration of EGF, leading to a diminished potential for the renewal of the epithelium of the oral mucosa in these individuals [35,38,39]. Epithelial markers (CA125, CA19-9, tissue polypeptide antigen, carcinoembryonic antigen, CYFRA 21-1) are found in higher concentrations in the saliva of patients diagnosed with OSCC [35]. Matrix metalloproteinases (MMPs) are enzymes that play a role in developing oral cancer progression.
The uncontrolled activity of MMPs in tumor tissues primarily contributes to the degradation of proteins such as collagen, elastin, and fibronectin. The saliva of patients with OSCC has shown elevated MMP-2 and MMP-9 activity [35,40,41]. The composition and qualities of saliva change in response to the interactions between the environment, microbiome, and host response. Reports indicate that P. gingivalis infection significantly enhances the invasive potential of OSCC cells through the upregulation of IL-8 and MMPs [42]. F. nucleatum promotes the production of cytokines, including TNF, IL-6, 8, 10, and 12, as well as ROS and kinase, which enhance the progression of oral cancer [43,44]. Reactive oxygen species (ROS), including hydrogen peroxide and oxygen radicals, along with reactive nitrogen species (RNS) such as nitric oxides, reactive lipids, and metabolites like malondialdehyde and hydroxy-2-nonenal, together with MMPs produced by immune cells, can lead to DNA damage in epithelial cells. This occurs via tumor cell toll-like receptors, which trigger the nuclear translocation of the transcription factor nuclear factor-kappa β (NF-kβ) and subsequent cytokine production [44].
Saliva also contains a unique repertoire of ncRNA signatures that can play a crucial role in diagnosing OSCC. Salivary lncRNA analysis and site-specific expression profiles aid in the early identification of OSCC and the tracking of post-surgery recurrence [3,45]. It is important to note that relatively fewer studies have been conducted on ncRNAome, such as lncRNA, circRNA, and miRNA, particularly regarding their clinical utility as non-invasive diagnostic and prognostic markers in saliva samples. Biopsies are a definitive procedure for diagnosing oral malignancy; however, the urgency to identify the disease in its early stages and the requirement to explore its molecular background for effective treatment planning have prompted the development of alternative diagnostic methods [28]. The collection of salivary ncRNAomes has potential as (1) it is less invasive and has no such risks that are associated with the collection of the blood [46], and (2) the collection process of saliva is not only inexpensive and more efficient but can also provide information that is not readily available from serum testing [47]. However, saliva’s individual biomarkers lack the sensitivity and specificity to fulfill rigorous diagnostic standards. The issue of excessively high saliva viscosity arises from mucopolysaccharides and mucoproteins, which can disrupt the analytical procedure. Despite this, saliva is valuable for the early detection, diagnosis, and monitoring of applied therapy for oral cancer [35]. This review, therefore, will primarily examine the salivary ncRNA composition linked to disease progression and investigate their roles as potential biomarkers for early disease detection, aiming to improve survival outcomes among individuals with oral cancer.
2. Saliva in Diagnostic Application and the Emergence of Saliva Omics
The significance of saliva in diagnosis is increasingly recognized, and its application as a diagnostic tool for systemic disorders is growing [48]. A trending approach to studying saliva and its components and functions is saliva omics. Saliva omics encompasses biomarkers categorized into genomes, transcripts, proteins, epigenomes, metabolites, and microbiomes [28]. Saliva omics offer a comprehensive method for evaluating diseases, tracking progress, and enabling personalized, non-invasive diagnostic solutions. The study of genomics in saliva provides valuable genetic information from host cells and microorganisms, enabling the analysis of mutations, polymorphisms, and epigenetic changes associated with diseases like oral cancer, thus aiding in risk assessment and personalized medicine [49,50]. Transcriptomics uncovers RNA molecules, including mRNA, miRNA, and lncRNA, that indicate alterations in gene expression due to disease. Specific salivary miRNAs and lncRNAs are associated with conditions such as cardiovascular and autoimmune diseases, facilitating disease monitoring and evaluating treatment responses [51]. Proteomics examines the diverse proteins in saliva, such as enzymes, antibodies, and cytokines. These can reveal oral and systemic health issues, including diabetes and cancer, by identifying inflammatory markers or malignancies for early diagnosis and ongoing disease monitoring. Finally, metabolomics examines small molecules and metabolic byproducts, revealing changes associated with cancer, stress, and metabolic disorders, thus providing insights into alterations in cellular stress and energy metabolism [52].
Multiple research groups have concluded that ncRNAome signatures in saliva represent an attractive, non-invasive, cost-effective strategy for diagnosing and prognosis OSCC [53,54,55,56]. ncRNAs may serve as promising candidates in cancer diagnosis and therapy. This occurs as ncRNAs simultaneously target various druggable and non-druggable targets and signaling events. Additionally, they exhibit tissue specificity, unique RNA characteristics for quick detection, enhanced tissue-related activity, and a significantly more stable structure [57]. In a particular investigation, miR136, miR-147, miR1250, miR-148a, miR632, miR646, miR668, miR877, miR503, miR220a, miR323-5p, miR-24, and miR-27b have been determined for diagnostic purposes in patients with OSCC [58,59]. Duz lab found that miR139-5p levels were reduced in the saliva of patients with tongue cancer [59,60].
3. Crosstalk Between ncRNAome and Oral Microbiota in OSCC
There is increasing evidence that members of the human microbiome are closely linked to a diverse range of cancer types. The microorganisms in the oral cavity can exist as commensals, symbionts, and pathogens [61,62,63,64]. These microbes and their host keep a balanced condition where they are advantageous to one another. During disease states like periodontitis, there may be an increase in the growth of specific commensal microbiota, accompanied by a decline in other oral microbiota within the oral cavity [9,64,65]. The oral microbiome is suggested as a potential diagnostic marker for oral cancer [66]. Various organisms have been identified to increase in OSCC samples, including Capnocytophaga gingivalis, Prevotella melaninogenica, and S. mitis [67]; F. nucleatum [65]; Pseudomonas aeruginosa [68]; Campylobacter concisus, Prevotella salivae, Prevotella loeschii, and Fusobacterium oral taxon 204 [69]; the genera Fusobacterium, Dialister, Peptostreptococcus, Filifactor, Peptococcus, Catonella, and Parvimonas [70]; as well as Prevotella oris, Neisseria flava, Neisseria flavescens/subflava, F. nucleatum ss polymorphum, Aggregatibacter segnis, and Fusobacterium periodonticum [71].
Yang’s lab investigated the changes in the microbiome throughout the progression of cancer, from early to late stages, and observed a notable increase in Fusobacteria. They discovered that F. periodonticum, Parvimonas micra, Streptococcus constellation, Haemophilus influenza, and Filifactor alocis were linked to OSCC at the species level, and their abundance gradually rose from stage 1 to stage 4 [72].
Viruses have historically been linked to the potential development of OSCC [66]. Numerous meta-analyses have shown that infection with human papillomavirus (HPV) increases the possibility of OSCC by as much as three times [73,74]. The average incidence of HPV among OSCC patients is approximately 25% [73,75,76]. Additionally, other viruses have been identified in OSCC samples, either as individual infections or in co-infections with HPV. Nevertheless, it is unclear how they contribute to the disease [76,77,78,79]. Numerous research groups have been investigating the link between the herpes simplex virus (HSV), an adenovirus, and oral cancer in both clinical subjects and animal models [80,81]. A recent investigation has indicated that HSV-1 is dominant in the oral cavity and tumor tissue of OSCC subjects, yet it has no significant impact on OSCC proliferation and invasion [82]. The prevalence of Candida is notably high, accounting for 78.8% of all OSCC cases, while Saccharomyces represents 76.8% of the OSCC cases, in contrast to a lower presence in healthy controls [83]. The dysbiosis of oral bacteria, resulting from the consumption of broad-spectrum antibiotics, may significantly facilitate the development of hyperplastic Candidiasis, which could lead to OSCC [84]. Candida infection induces cytokines like IL8 and TNF-a, which stimulate TLRs that can interact with the NF-kB inflammatory process in oral cancer metastasis [85]. The causality of oral oncogenesis arises from the interaction between the environmental microbiome and the host oral mucosa, influencing a wide range of non-coding RNAs and the host’s tumor immune surveillance and cytotoxicity. Growing evidence connects Fusobacterium nucleatum to tumorigenesis [86,87,88]. A research study showed that F. nucleatum infection could trigger epithelial-mesenchymal transition (EMT) in oral epithelial cells and outlined a possible signaling pathway that governs the induction of EMT [89]. Further exploration demonstrated the alterations of lncRNA and potential hub genes in oral epithelial cells in response to F. nucleatum infection, providing novel insights into the transition from normal to malignant transformation initiated by oral bacterial infection [89]. The analysis results indicate that LINC00460, LINC00511, LINC01160, LINC00702, and MNX1-AS1 may serve as key regulators of the hub genes associated with malignant transformation caused by Fusobacterium nucleatum infection [89]. LINC00460 was identified to exhibit a co-expression pattern with VEGFA, which has been demonstrated to be overexpressed in aggressive OSCC [90]. MNX1-AS1 serves as a natural antisense transcript of MNX1 and has been identified as a potential oncogenic driver in multiple cancer types [91,92,93]. MNX1-AS1 overexpression may induce an epithelial–mesenchymal transition (EMT) and stimulate breast cancer’s Akt/mTOR pathway [93]. Further investigation is required to elucidate the role of MX1-AS1 and LINC00460 in oral cancer progression. A recent report indicates that the infection of oral epithelial cells with F. nucleatum results in elevated expression levels of MIR4435-2HG, which can then specifically bind to another non-coding RNA, microRNA-296-5p, leading to a downregulation of its expression. A reduction follows this in mi-296-5p’s ability to block the expression of its target gene Akt2, which subsequently activates the expression of the transcription factor SNAI1 and contributes to the transition into the mesenchymal phenotype of infected oral epithelial cells [94].
Infection with P. gingivalis has been positively correlated with advanced clinical staging, poor differentiation, and lymph node metastasis among individuals with OSCC [88,95]. A recent research investigation reveals that the P. gingivalis outer membrane vesicles (OMVs) enhance the invasion and proliferation of OSCC cells in vitro [59]. Additional research indicated that sRNA23392 was prevalent in P. gingivalis OMVs and facilitated the invasion and migration of OSCC cells by targeting desmocollin-2 (DSC2) [96]. sRNAs of miRNA size, typically ranging from 15 to 25 nt in length, primarily interact with target mRNAs via incomplete base pairing, influencing the translation and/or stability of the mRNAs [97]. DSC2, a member of the desmosomal cadherin family, has been identified as playing a role in tumor development. sRNA23392 inhibitors reduced the migration and invasion of OSCC cells induced by P. gingivalis OMV [96]. A recent report indicates that P. gingivalis may enhance OSCC development by modulating cyclin D1 expression through the miR-21/PDCD4/AP-1 negative feedback signaling pathway [95]. The levels of P. gingivalis DNA showed a positive correlation with the expression of miR-21 and c-Jun while exhibiting a negative correlation with PDCD4 expression in clinical OSCC samples. The expression of miR-21 increased as the expression of programmed cell death 4 (PDCD4) decreased [95]. The expression of cyclin miR-21, PDCD4, and AP-1 influenced D1. Blocking c-Jun, blocking miR-21 expression, or overexpressing PDCD4 disrupted the pathway, decreasing cyclin D1 expression and hindering cell proliferation [95,98].
4. Targeting Salivary ncRNAome for Oral Cancer Detection
ncRNAs present a promising avenue for OC detection owing to their distinct advantages: (a) Non-Invasive Collection: Saliva collection is convenient and suited for repeated sampling, facilitating early detection and ongoing monitoring [99]. (b) Cancer-Specific Markers: ncRNAomes play a crucial role in regulating gene expression and serve as indicators of tumor growth, metastasis, and treatment resistance, making them a dependable marker for cancer [100]. (c) Local Cellular Reflection: ncRNAs from oral tumor cells enter saliva, directly capturing oral-specific cancer changes. (d) Early Detection: Specific lncRNAs, such as HOTAIR, MALAT1, and H19, exhibit changed levels during the early stages of cancer, facilitating timely intervention [100]. (e) Cost-Effective: Saliva-based lncRNA testing is affordable, particularly with RT-PCR or sequencing, facilitating more accessible routine screening [101].
This review will highlight several seminal works that illustrate the interplay between the expression of specific ncRNA signatures and the progression of OSCC.
5. Role of Salivary ncRNAome as Potential Diagnostic Biomarkers
Saliva and other less invasive sampling methods, including oral swabs, brush biopsies, and scrapers, have emerged as practical tools for detecting biomarkers of OSCC. Researchers have discovered several non-coding RNAs (ncRNAs) in saliva that hold clinical significance for OSCC (Table 1, Figure 1). Among these biomarkers, lncRNAs have attracted considerable interest due to their diagnostic and prognostic capabilities.

Table 1.
Representative examples of salivary ncRNAs associated with OSCC.
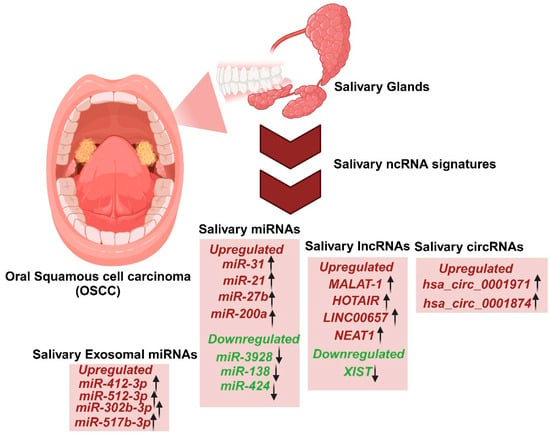
Figure 1.
Salivary ncRNAome signatures as diagnostic markers for the detection of oral squamous cell carcinoma (OSCC).
For instance, it was demonstrated that lncRNAs HOTAIR and MALAT1 are significantly elevated in the saliva of OSCC patients, with higher HOTAIR expression correlating with nodal metastasis, making it a promising non-invasive biomarker for OSCC management [112,113,114]. However, such studies often face limitations, including small sample sizes. These findings underscore the potential of lncRNAs as diagnostic tools but highlight the need for further validation. Salivary miRNAs such as miR-21, miR-31, miR-27b have also shown promise as OSCC biomarkers due to their role in cancer progression [114]. Additionally, Zhao et al. identified dysregulated circRNAs in the saliva of OSCC patients, such as overexpressed hsa_circ_0001874 and hsa_circ_0001971, further expanding the pool of potential diagnostic markers [56].
Despite these advances, further research is needed to validate the diagnostic and prognostic roles of circulatory and salivary lncRNAs and other biomarkers in OSCC. Such studies could pave the way for more effective, non-invasive diagnostic tools and improve the management of OSCC. In the following sections, we will try to explore a few unique ncRNA signatures for a potential biomarker.
5.1. Role of Salivary miRNAs as a Biomarker for OSCC
Several pieces of evidence suggest that salivary miRNAs are an up-and-coming method for diagnosing and prognosing OSCC. A careful investigation of these may be crucial in formulating innovative approaches to OSCC treatment.
miR-31 is significantly upregulated in saliva compared to plasma, indicating its local contribution to tumorigenesis and its sensitivity in detecting tumors, regardless of size or stage. Notably, levels of miR-31 diminish after tumor excision, creating a clear connection between its expression and the presence of tumors [115]. Nonetheless, it fails to distinguish OSCC from dysplastic potentially malignant disorders (PMDs), indicating its potential as an early biomarker [116]. miR-31 has been identified as upregulated in OSCC. It functions as an oncogenic miRNA by targeting SIRT3 thereby disrupting mitochondrial activity (Figure 2) [102]. miR-31 targets SIRT3 to promote OSCC invasion. OSCC tumors show an increase in miR-31 levels and a decrease in SIRT3 expression (Figure 2). The expression of SIRT3 reduced tumor cell migration and invasion, which was enhanced by miR-31 [103]. miR-31-SIRT3 hindered the mitochondrial membrane potential and structural integrity. The dysregulation of this axis also played a role in the development of oxidative stress—furthermore, miR-31 altered tumor cells from aerobic metabolism to glycolytic metabolism [103]. Mitochondrial dysfunction and the adaptation of aerobic glycolysis for energy production are common occurrences in malignancies [102,117]. Conversely, miR-27b is upregulated in OSCC saliva, positioning it as a promising diagnostic marker and oncogenic miRNA [118].
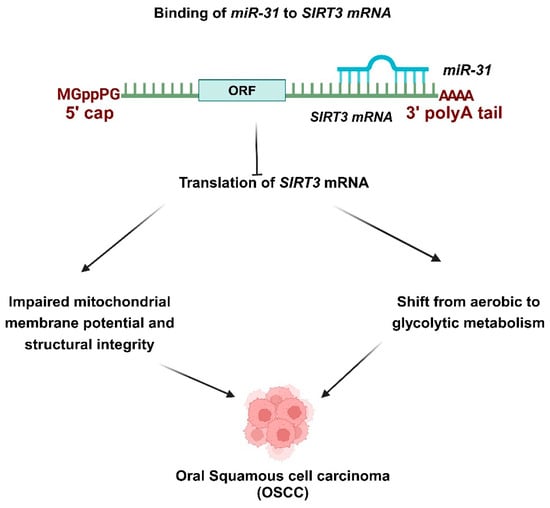
Figure 2.
Role of miR-31 in OSCC progression. miR-31 targets SIRT3, facilitating the invasion of OSCC. OSCC tumors exhibit elevated levels of miR-31 and reduced expression of SIRT3. miR-31-SIRT3 impaired the mitochondrial membrane potential and structural integrity. This axis’s dysregulation also contributed to the emergence of oxidative stress. Additionally, miR-31 transformed tumor cells from aerobic metabolism to glycolytic metabolism. miR-31 has been recognized as upregulated in OSCC and acts as an oncogenic miRNA by targeting SIRT3, which disrupts mitochondrial activity. miR-31 targets SIRT3, facilitating the invasion of OSCC. OSCC tumors exhibit elevated levels of miR-31 and reduced expressions of SIRT3. The expression of SIRT3 diminished the tumor cell migration and invasion amplified by miR-31. miR-31-SIRT3 impaired the mitochondrial membrane potential and structural integrity. This axis’s dysregulation also contributed to the development of oxidative stress. Additionally, miR-31 transformed tumor cells from aerobic metabolism to glycolytic metabolism.
miR-3928 serves as a key regulator in the process of carcinogenesis [119,120]. A study indicates that salivary miR-3928 functions as a tumor suppressor in OSCC. The expression of miR-3928 decreased substantially in the OSCC group (67-fold) and the oral potentially malignant disorder (OLP) group (sixfold) as compared to healthy controls. The results indicate that miR-3928 holds promise as a significant biomarker and may serve as a tumor inhibitor in therapeutic strategies [104]. In a particular study, liquid biopsy assays from saliva revealed that miR-138 and miR-424 exhibited decreased expression levels in saliva samples from OSCC and OPMD patients compared to healthy controls. These emerging early diagnostic biomarkers effectively distinguish between OSCC patients, OPMD patients, and healthy subjects [121].
The Arun lab indicated that in OSCC, the dysregulation of the miR-200 family and EMT-related genes may contribute to tumor metastasis and therapeutic resistance [104,122,123]. Several studies have shown increased salivary miR-200a in patients diagnosed with OSCC [123,124,125,126]. Another investigation reveals that the expression levels of two biomarkers, miR-200 and miR-34, are lower in patients than in healthy individuals. In contrast, the expression level of miR-24 is higher in patients than in healthy individuals [127]. A separate study demonstrated the upregulation of miR-412-3p, miR-512-3p, miR-302b-3p, and miR-517b-3p in the extracellular vesicle salivary samples of OSCC patients when compared to healthy controls, highlighting their involvement in OSCC [128,129]. These findings underscore the diagnostic, prognostic, and therapeutic potential of salivary miRNAs in OSCC while highlighting the need for further research to validate their clinical utility.
5.2. Role of Salivary lncRNAs as a Biomarker for OSCC
Salivary lncRNAs have emerged as critical biomarkers in OSCC due to their diverse roles in tumor progression, metastasis, and prognosis. Among them, HOTAIR stands out for its significant overexpression in the saliva of OSCC patients with lymph node metastasis, compared to other lncRNAs such as HULC, MALAT-1, MEG-3, NEAT-1, and UCA1 [55]. Metastasis is recognized as the primary factor contributing to mortality in OSCC [130]. Consequently, HOTAIR could serve as a potential predictor of patient survival [55]. miR-326 expression is downregulated in OSCC, and results suggest that HOTAIR functions as a competitive endogenous RNA by sponging miR-326, which regulates the derepression of metastasis-associated gene 2 (MTA2) [131] (Figure 3A). MTA2 is a component of the Twist complex that inhibits E-cadherin expression [132]. MTA2 can enhance the epithelial-mesenchymal transition (EMT) and the progression of multiple cancers, negatively impacting their prognosis [133,134]. Overexpression of MTA2 was noted in OSCC cell lines [131].
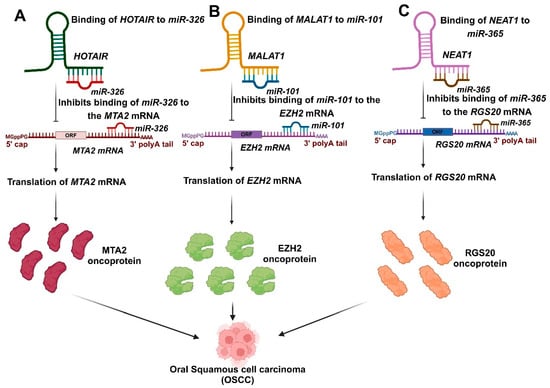
Figure 3.
The involvement of salivary lncRNAs in the progression of OSCC. (A). The expression of miR-326 is reduced in OSCC, and the findings indicate that HOTAIR acts as a competitive endogenous RNA by sponging miR-326, which regulates the derepression of metastasis-associated gene 2 (MTA2). (B). MALAT1 influences the progression of OSCC by negatively regulating miR-101, which promotes the expression of EZH2. (C). NEAT1 has been identified to enhance proliferation, migration, and invasion by sponging miR-365, thereby facilitating the expression of RGS20 in OSCC.
A research investigation also identified MALAT1 in saliva and observed no significant difference in the salivary levels of MALAT1 among metastatic and non-metastatic cases, challenging the established link between MALAT1 and the metastatic potential of OSCC [55]. The overexpression of MALAT1 plays a crucial role in governing gene expression, enhancing cell motility, and promoting tumor development as well as epithelial-mesenchymal invasion (EMT). MALAT1 has been associated with the nuclear accumulation of oncogenes NF-κB, P65, β-catenin, and P-β-catenin, leading to these effects. This resulted in the deregulation of Wnt/β-catenin signaling and an elevated production of MMP-2, MMP-7, and MMP-9 [106,135,136]. Nonetheless, a recent study revealed that OSCC patients exhibited elevated MALAT1 levels and reduced salivary miRNA-124 levels compared to normal controls [106]. A recent study indicated that MALAT1 was upregulated, whereas miR-101 was downregulated in OSCC. MALAT1 impacts the progression of OSCC through the negative regulation of miR-101. Additionally, bioinformatics analysis identified EZH2 as the target of miR-101. MALAT1 promotes EZH2 expression by modulating miR-101, presenting a potential novel target for OSCC treatment [15] (Figure 3B).
Salivary LINC00657 and miR-106a may be effective diagnostic markers for oral squamous cell carcinoma. Salivary LINC00657 demonstrates an elevated diagnostic accuracy (83.3%) in distinguishing between OSCC grade II and III [54]. In the same way, the Xu lab showed that the overexpression of LINC00657 was associated with a higher pathological stage, suggesting a generally lower survival rate [108]. The results indicated a reduced expression of salivary miR-106a in the OSCC group compared to the OLP and control groups, suggesting that the overexpression of LINC00657 may be downregulating miR-106a expression. Additionally, salivary miR106a may be included among the tumor suppressor miRNAs associated with OSCC [54]. Recent findings by Shieh et al. linked the absence of lncRNA XIST in saliva to a higher risk of OSCC [137].
In OSCC, NEAT1 exhibited a significantly higher expression in the saliva of OSCC patients than healthy individuals’ oral mucosa [53,55]. NEAT1 has been identified to enhance proliferation, migration, and invasion by sponging miR-365 in OSCC [110]. RGS20 was recognized as a direct target of miR-365, and its overexpression hindered the miR-365-induced suppression of OSCC cell proliferation and invasion. RGS20 enhanced cell viability, motility, and the protein expression of cyclin D1 and N-cadherin, while reducing the protein level of E-cadherin, indicating the oncogenic role of RGS20 in OSCC. The regulation of RGS20 protein levels by NEAT1/miR-365 indicates that NEAT1 functions as a ceRNA for miR-365, thereby promoting the expression of RGS20 [110] (Figure 3C). NEAT1 also has the potential to enhance proliferation and epithelial-mesenchymal transition (EMT) while inhibiting apoptosis by activating the VEGF-A and Notch signaling pathways in vitro, indicating its role as a regulatory factor in OSCC [109].
The salivary lncRNAs collectively provide essential insights into the progression of OSCC. Their varied mechanisms, including transcriptional regulation and ceRNA activity, highlight the complexity of OSCC pathogenesis and the necessity for additional research to translate these findings into clinical applications.
5.3. Role of Salivary circRNAs as a Biomarker for OSCC
There is only limited evidence of salivary circRNAs as diagnostic markers for OSCC. When considered alongside the roles of salivary lncRNAs, circRNAs add another layer of complexity to the molecular landscape of OSCC. Both classes of RNA share mechanisms such as sponging miRNAs and interacting with key signaling pathways, emphasizing their interconnected contributions to tumor progression, metastasis, and treatment resistance. The Bahn lab reported on the prevalence of circRNAs in saliva and their involvement in intracellular signaling cascades in addition to the inflammatory response [128,129,138]. A specific study identified over 400 circRNAs extracted from cell-free saliva in healthy controls [138]. The half-life (t1/2) of circRNA is 48 h, roughly four times longer than that of mRNAs, suggesting enhanced stability [139,140]. The stability, abundance, and favorable half-life of circRNAs indicate their potential as biomarkers [139,141].
A specific study reported that there were 12 upregulated and 20 downregulated circRNAs in the saliva of OSCC patients compared to healthy controls [56]. In the analysis of differentially expressed circRNAs, hsa_circ_0001874, hsa_circ_0001971, and hsa_circ_0008068 showed significant upregulation in the OSCC group compared to the healthy group. Clinical data revealed that salivary hsa_circ_0001874 was correlated with TNM stage (p = 0.006) and tumor grade (p = 0.023), while hsa_circ_0001971 showed a correlation with TNM stage (p = 0.019) [56]. Furthermore, the analysis revealed that the expression levels of salivary hsa_circ_0001874 and hsa_circ_0001971 were significantly lower in the postoperative samples than in the preoperative samples (p < 0.001). Therefore, salivary hsa_circ_0001874 and hsa_circ_0001971 may be used as biomarkers to diagnose OSCC [56]. hsa_circ_0001971 and hsa_circ_0001874 may exert oncogenic effects, at least in part, via their subsequent signaling networks such as hsa_circ_0001971/miR-186/SHP2 and hsa_circ_0001874/miR-296-5p/PLK1. MiR-186 has been identified as a tumor suppressor in OSCC, with its downregulation linked to increased expression of the oncogenic factor protein tyrosine phosphatase SHP2 and the activation of growth-promoting signaling pathways [142]. Similarly, miR-296 functions as a tumor suppressor, and its reduced expression is associated with increased levels of the PLK1 oncogenic factor in non-small cell lung cancer cells [143]. circ_0001847 and circ_0001971 inhibited the expression of miR-296-5p and miR-186 through their binding to these miRNAs [111]. Additionally, it was shown that miR-296-5p and miR-186 overexpression could bind to circ_0001847 and circ_0001971, respectively, and significantly lower their levels, suggesting that these circRNAs and miRNAs sponged one another [111]. The two signaling pathways of circ_0001971/miR-186/SHP2 and circ_0001874/miR-296-5p/PLK1 govern the oncogenic impacts of hsa_circ_0001971 and hsa_circ_0001874 in the progression of OSCC (Figure 4). SHP2 serves as a crucial activator for the oncogenic effect of PLK1 [144]. The impaired regulation of hsa_circ_0001971 may influence SHP2 and PLK1, whereas hsa_circ_0001874 impacts only PLK1 [111].
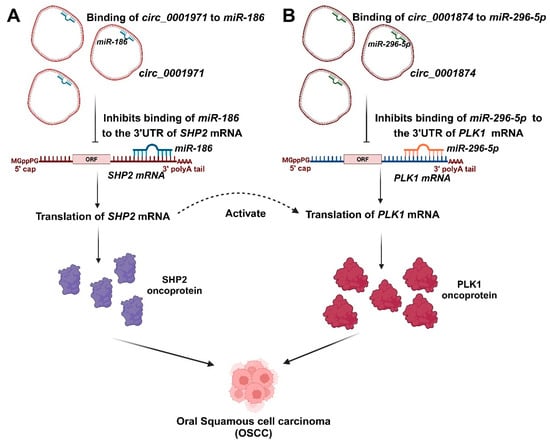
Figure 4.
The involvement of salivary circRNAs in the progression of OSCC. (A). Circ_0001971 inhibited the expression of miR-186, resulting in the increased expression of the SHP2 oncoprotein. (B). Circ_0001874 inhibited the expression of miR-296-5p, resulting in the increased expression of the PLK1 oncoprotein. The dysregulation of hsa_circ_0001971 may affect SHP2 and PLK1, while hsa_circ_0001874 influences only PLK1. SHP2 is an essential activator for the oncogenic activity of PLK1.
Collectively, these non-coding RNAs hold promise for advancing our understanding of OSCC pathogenesis and developing novel diagnostic tools and targeted therapies.
6. Conclusions
In this review, we specifically focused on the role of salivary ncRNA signatures (including miRNA, lncRNA, and circRNA) in advancing OC (Figure 1). The ncRNAs influence numerous oncogenic and tumor-suppressive pathways, thus influencing the progression of OC. Exploring the role of salivary ncRNAs as potential biomarkers for oral cancer has revealed an intriguing potential for OC detection. Numerous findings have shown that the elevated mortality and morbidity in OC are primarily linked to the challenges of achieving a swift diagnosis and effective management. Consequently, obtaining a timely diagnosis is essential for effectively managing the spread and metastasis of OC, ultimately enhancing the overall life expectancies of patients [145]. Oral dysbiosis, characterized by an imbalance in the oral microbial community, has garnered considerable interest because of its intricate connection to oral cancer. A growing body of evidence connects oral oncogenesis causality to the environmental microbiome and its interaction with the host oral mucosa, which drives the abundance of ncRNA-promoting oral cancer. The comprehensive ncRNA signatures derived from saliva reveal their potential as non-invasive diagnostic and prognostic biomarkers for OC. In our current review, we have observed several molecular signaling mechanisms by which these ncRNA signatures influenced OSCC progression. Further investigation is essential to grasp the mechanisms that underlie OSCC comprehensively. Numerous clinical trials have been carried out involving ncRNAs, particularly focusing on miRNAs as diagnostic and therapeutic biomarkers [146]. A clinical study has commenced to assess both the specificity and sensitivity of miRNA-412 and miR-512 in extracellular vesicles derived from saliva regarding the malignant advancement of OC (ClinicalTrials.gov Identifier: NCT04913545) [2]. The significance of lncRNA MALAT1 and its target miR-124 in diagnostics and therapy has been explored in saliva samples from OC patients (ClinicalTrials.gov Identifier: NCT05708209) [2]. A patent (US 20230227914A1) describes a method for detecting head and neck cancer in the oral cavity or throat, including oral squamous cell carcinoma. This involves identifying the amount of expression of two or more miRNAs in a biological sample from a subject. The selected miRNAs include hsa-let-7a, hsa-miR-16, hsa-miR-21, hsa-miR-451, hsa-miR-486-5p, and hsa-miR-92a-3p. In comparison with cancer-free reference samples, the levels of expression of these miRNAs in the biological sample indicate the presence of head and neck cancer of the oral cavity of the throat [98]. Another patent (US20230203493A1) revealed that lncRNA biomarkers are associated with oral squamous cell carcinoma and can be utilized in diagnosing and treating this condition. The biomarkers include lncRNA RP11-875O11.3, LINC01679, AP000695.4, RP11-339B21.10, RP11-426C22.4, RP11-426C22.5, and/or AP000695.6. The biomarkers are utilized to develop products aimed at diagnosing oral squamous cell carcinoma. The biomarkers are also utilized in formulating a pharmaceutical composition aimed at managing oral squamous cell carcinoma and another pharmaceutical composition for the same condition [147]. Despite the significant efforts to uncover the basis for detecting mRNA and proteins as biomarkers for diseases, there remains a limited understanding of ncRNAome as the emerging category of biomarkers found in body fluids [148]. However, further extensive pre-clinical research is necessary to effectively incorporate salivary ncRNAome into clinical practice for OC diagnosis. There is an urgent need for an efficient biomarker for OC detection. This is an open question, and further investigations into the salivary ncRNAome in relation to oral cancer will further deepen our understanding of the condition.
Author Contributions
Conceptualization: S.D.; data curation: S.B. and S.S.; writing—original draft: All authors; review and editing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors appreciate the reviewers for dedicating their time and effort to evaluate the manuscript. We are grateful for all the insightful comments and suggestions that have contributed to enhancing the quality of the manuscript. All the figures were created using Biorender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Biswas, B.; Manoj Appadan, A.; Shah, J.; Pal, J.K.; Basu, S.; Sur, S. Non-Coding RNAs in Oral Cancer: Emerging Roles and Clinical Applications. Cancers 2023, 15, 3752. [Google Scholar] [CrossRef] [PubMed]
- Kalmatte, A.; Rekha, P.D.; Ratnacaram, C.K. Emerging Cell Cycle Related Non-Coding RNA Biomarkers from Saliva and Blood for Oral Squamous Cell Carcinoma. Mol. Biol. Rep. 2023, 50, 9479–9496. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Satyanarayana, L.; Asthana, S.; Shivalingesh, K.K.; Goutham, B.S.; Ramachandra, S. Oral Cancer Statistics in India on the Basis of First Report of 29 Population-Based Cancer Registries. J. Oral Maxillofac. Pathol. 2018, 22, 18–26. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral Cancer: Etiology and Risk Factors: A Review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Dey Ghosh, R.; Guha Majumder, S. Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma. Cancers 2022, 14, 5590. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The Molecular Biology of Head and Neck Cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef]
- Rischin, D.; Ferris, R.L.; Le, Q.-T. Overview of Advances in Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3225–3226. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota during Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Sujir, N.; Ahmed, J.; Pai, K.; Denny, C.; Shenoy, N. Challenges in Early Diagnosis of Oral Cancer: Cases Series. Acta Stomatol. Croat. 2019, 53, 174–180. [Google Scholar] [CrossRef]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Devel. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef] [PubMed]
- Gharat, S.A.; Momin, M.; Bhavsar, C. Oral Squamous Cell Carcinoma: Current Treatment Strategies and Nanotechnology-Based Approaches for Prevention and Therapy. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 363–400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, S.; Cai, G.; Kong, L.; Zhang, T.; Ren, Y.; Wu, Y.; Mei, M.; Zhang, L.; Wang, X. Long Non Coding RNA MALAT1 Promotes Tumor Growth and Metastasis by Inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. Sci. Rep. 2015, 5, 15972. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, X.; Yang, M.; Lu, L.; Zhou, Q. Long Non-Coding RNA TUG1 Promotes Progression of Oral Squamous Cell Carcinoma through Upregulating FMNL2 by Sponging MiR-219. Am. J. Cancer Res. 2017, 7, 1899–1912. [Google Scholar]
- Xiao, L.; Wang, W.; Zhao, J.; Xu, H.; Li, S.; Yang, X. LncRNA MALAT1 Promotes Cell Proliferation and Invasion by Regulating the MiR-101/EZH2 Axis in Oral Squamous Cell Carcinoma. Oncol. Lett. 2020, 20, 164. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Liu, T.; Wang, X.; et al. Long Non-Coding RNA HOTAIR Promotes Tumor Cell Invasion and Metastasis by Recruiting EZH2 and Repressing E-Cadherin in Oral Squamous Cell Carcinoma. Int. J. Oncol. 2015, 46, 2586–2594. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H. Expression of Long Noncoding RNA-HOX Transcript Antisense Intergenic RNA in Oral Squamous Cell Carcinoma and Effect on Cell Growth. Tumour Biol. 2015, 36, 8573–8578. [Google Scholar] [CrossRef]
- Liang, J.; Liang, L.; Ouyang, K.; Li, Z.; Yi, X. MALAT1 Induces Tongue Cancer Cells’ EMT and Inhibits Apoptosis through Wnt/β-Catenin Signaling Pathway. J. Oral Pathol. Med. 2017, 46, 98–105. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Bramsen, J.B.; Lamy, P.; Villadsen, S.B.; Fristrup, N.; Sørensen, K.D.; Ulhøi, B.; Borre, M.; Kjems, J.; Dyrskjøt, L.; et al. MiR-145 Induces Caspase-Dependent and -Independent Cell Death in Urothelial Cancer Cell Lines with Targeting of an Expression Signature Present in Ta Bladder Tumors. Oncogene 2010, 29, 1073–1084. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary MicroRNAs in Oral Cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Naoe, T. MicroRNA-143 and -145 in Colon Cancer. DNA Cell Biol. 2007, 26, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread Deregulation of MicroRNA Expression in Human Prostate Cancer. Oncogene 2008, 27, 1788–1793. [Google Scholar] [CrossRef]
- Ichimi, T.; Enokida, H.; Okuno, Y.; Kunimoto, R.; Chiyomaru, T.; Kawamoto, K.; Kawahara, K.; Toki, K.; Kawakami, K.; Nishiyama, K.; et al. Identification of Novel MicroRNA Targets Based on MicroRNA Signatures in Bladder Cancer. Int. J. Cancer 2009, 125, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, X.-Y.; Gong, R.-G.; Li, A.; Yang, S.; Cao, Y.-T.; Wen, Y.-M.; Wang, C.-M.; Yi, X.-Z. The Expression Profile of MicroRNAs in a Model of 7,12-Dimethyl-Benz[a]Anthrance-Induced Oral Carcinogenesis in Syrian Hamster. J. Exp. Clin. Cancer Res. 2009, 28, 64. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, S.; Ma, H.; Zhao, M.; Myers, J.N.; Weber, R.S.; Sturgis, E.M.; Wei, Q. A Functional Variant at the MiR-184 Binding Site in TNFAIP2 and Risk of Squamous Cell Carcinoma of the Head and Neck. Carcinogenesis 2011, 32, 1668–1674. [Google Scholar] [CrossRef]
- Wong, T.-S.; Liu, X.-B.; Wong, B.Y.-H.; Ng, R.W.-M.; Yuen, A.P.-W.; Wei, W.I. Mature MiR-184 as Potential Oncogenic MicroRNA of Squamous Cell Carcinoma of Tongue. Clin. Cancer Res. 2008, 14, 2588–2592. [Google Scholar] [CrossRef]
- Punj, A. Secretions of Human Salivary Gland. In Salivary Glands—New Approaches in Diagnostics and Treatment; IntechOpen: London, UK, 2019; ISBN 9781789849882. [Google Scholar]
- Rajendran, P.; Sekar, R.; Zahra, H.A.; Jayaraman, S.; Rajagopal, P.; Abdallah, B.M.; Ali, E.M.; Abdelsalam, S.A.; Veeraraghavan, V. Salivaomics to Decode Non-Coding RNAs in Oral Cancer. A Narrative Review. Noncoding RNA Res. 2023, 8, 376–384. [Google Scholar] [CrossRef]
- Chicharro, J.L.; Lucía, A.; Pérez, M.; Vaquero, A.F.; Ureña, R. Saliva Composition and Exercise. Sports Med. 1998, 26, 17–27. [Google Scholar] [CrossRef]
- Ziobro, A.; Bartosz, G. A Comparison of the Total Antioxidant Capacity of Some Human Body Fluids. Cell. Mol. Biol. Lett. 2003, 8, 415–419. [Google Scholar]
- Nagler, R.M.; Klein, I.; Zarzhevsky, N.; Drigues, N.; Reznick, A.Z. Characterization of the Differentiated Antioxidant Profile of Human Saliva. Free Radic. Biol. Med. 2002, 32, 268–277. [Google Scholar] [CrossRef]
- Burke, J.C.; Evans, C.A.; Crosby, T.R.; Mednieks, M.I. Expression of Secretory Proteins in Oral Fluid after Orthodontic Tooth Movement. Am. J. Orthod. Dentofacial Orthop. 2002, 121, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Dozic, I.; Todorovic, T. Antimicrobial Peptides of Human Saliva. Stomatol. Glas. Srb. 2005, 52, 208–216. [Google Scholar] [CrossRef]
- Todorović, T.; Dozić, I.; Mandić, B.; Marjanović, M. Antioxidant role of saliva in maintaining oral health. Vojnosanit. Pregl. 2005, 62, 575–579. [Google Scholar] [CrossRef]
- Andjelski-Radicevic, B.; Dozic, I. Biochemical Markers in Saliva in Patients with Oral Cancer. Stomatol. Glas. Srb. 2020, 67, 201–207. [Google Scholar] [CrossRef]
- Contucci, A.M.; Inzitari, R.; Agostino, S.; Vitali, A.; Fiorita, A.; Cabras, T.; Scarano, E.; Messana, I. Statherin Levels in Saliva of Patients with Precancerous and Cancerous Lesions of the Oral Cavity: A Preliminary Report. Oral Dis. 2005, 11, 95–99. [Google Scholar] [CrossRef]
- Shintani, S.; Hamakawa, H.; Ueyama, Y.; Hatori, M.; Toyoshima, T. Identification of a Truncated Cystatin SA-I as a Saliva Biomarker for Oral Squamous Cell Carcinoma Using the SELDI ProteinChip Platform. Int. J. Oral Maxillofac. Surg. 2010, 39, 68–74. [Google Scholar] [CrossRef]
- Bernardes, V.F.; Gleber-Netto, F.O.; Sousa, S.F.; Silva, T.A.; Abreu, M.H.N.G.; Aguiar, M.C.F. EGF in Saliva and Tumor Samples of Oral Squamous Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 528–533. [Google Scholar] [CrossRef]
- Balicki, R.; Grabowska, S.Z.; Citko, A. Salivary Epidermal Growth Factor in Oral Cavity Cancer. Oral Oncol. 2005, 41, 48–55. [Google Scholar] [CrossRef]
- Ghallab, N.A.; Shaker, O.G. Serum and Salivary Levels of Chemerin and MMP-9 in Oral Squamous Cell Carcinoma and Oral Premalignant Lesions. Clin. Oral Investig. 2017, 21, 937–947. [Google Scholar] [CrossRef]
- Shpitzer, T.; Hamzany, Y.; Bahar, G.; Feinmesser, R.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R.M. Salivary Analysis of Oral Cancer Biomarkers. Br. J. Cancer 2009, 101, 1194–1198. [Google Scholar] [CrossRef]
- Ha, N.H.; Park, D.G.; Woo, B.H.; Kim, D.J.; Choi, J.I.; Park, B.S.; Kim, Y.D.; Lee, J.H.; Park, H.R. Porphyromonas Gingivalis Increases the Invasiveness of Oral Cancer Cells by Upregulating IL-8 and MMPs. Cytokine 2016, 86, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, K.R.; Yilmaz, Ö. Prelude to Oral Microbes and Chronic Diseases: Past, Present and Future. Microbes Infect. 2015, 17, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, X.; Cheng, J.; Liu, Z.; Zhou, H.; Liao, J.; Wang, X.; Liu, H. Oral Microbiome and Its Relationship with Oral Cancer. J. Cancer Res. Ther. 2024, 20, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, G.; Deva Magendhra Rao, A.K.; Manikandan, M.; Arun, K.; Vinothkumar, V.; Revathidevi, S.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Expression Profiling of Long Non-Coding RNA Identifies Linc-RoR as a Prognostic Biomarker in Oral Cancer. Tumour Biol. 2017, 39, 1010428317698366. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C.; Pickler, R.; Grap, M.J.; Elswick, R.K., Jr. Comparison of Biomarkers in Blood and Saliva in Healthy Adults. Nurs. Res. Pract. 2012, 2012, 246178. [Google Scholar] [CrossRef]
- Hofman, L.F. Human Saliva as a Diagnostic Specimen. J. Nutr. 2001, 131, 1621S–1625S. [Google Scholar] [CrossRef]
- Sindhu, S.; Jagannathan, N. Saliva: A Cutting Edge in Diagnostic Procedures. J. Oral Dis. 2014, 2014, 168584. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in Diagnostic Applications of Saliva in Human Organ Diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Shah, F.D.; Begum, R.; Vajaria, B.N.; Patel, K.R.; Patel, J.B.; Shukla, S.N.; Patel, P.S. A Review on Salivary Genomics and Proteomics Biomarkers in Oral Cancer. Indian J. Clin. Biochem. 2011, 26, 326–334. [Google Scholar] [CrossRef]
- Patil, S.; Arakeri, G.; Alamir, A.W.H.; Awan, K.H.; Baeshen, H.; Ferrari, M.; Patil, S.; Fonseca, F.P.; Brennan, P.A. Role of Salivary Transcriptomics as Potential Biomarkers in Oral Cancer: A Systematic Review. J. Oral Pathol. Med. 2019, 48, 871–879. [Google Scholar] [CrossRef]
- Esperouz, F.; Ciavarella, D.; Santarelli, A.; Lorusso, M.; Lo Muzio, L.; Laino, L.; Lo Russo, L. Saliva-Based Biomarkers in Oral Squamous Cell Carcinoma Using OMICS Technologies: A Systematic Review. Oral 2024, 4, 293–302. [Google Scholar] [CrossRef]
- Gibb, E.A.; Enfield, K.S.S.; Stewart, G.L.; Lonergan, K.M.; Chari, R.; Ng, R.T.; Zhang, L.; MacAulay, C.E.; Rosin, M.P.; Lam, W.L. Long Non-Coding RNAs Are Expressed in Oral Mucosa and Altered in Oral Premalignant Lesions. Oral Oncol. 2011, 47, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Tarrad, N.A.F.; Hassan, S.; Shaker, O.G.; AbdelKawy, M. Salivary LINC00657 and MiRNA-106a as Diagnostic Biomarkers for Oral Squamous Cell Carcinoma, an Observational Diagnostic Study. BMC Oral Health 2023, 23, 994. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wu, Z.; Zhang, J.; Su, B. Salivary LncRNA as a Potential Marker for Oral Squamous Cell Carcinoma Diagnosis. Mol. Med. Rep. 2013, 7, 761–766. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Wang, J.; Ouyang, S.-B.; Huang, Z.-K.; Liao, L. Salivary Circular RNAs Hsa_circ_0001874 and Hsa_circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2018, 47, 2511–2521. [Google Scholar] [CrossRef]
- Mazumder, S.; Datta, S.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Liquid Biopsy: MiRNA as a Potential Biomarker in Oral Cancer. Cancer Epidemiol. 2019, 58, 137–145. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J. Dent. Res. 2014, 93, 86S–93S. [Google Scholar] [CrossRef]
- Koneru, S.; Tanikonda, R. Salivaomics—A Promising Future in Early Diagnosis of Dental Diseases. Dent. Res. J. 2014, 11, 11–15. [Google Scholar]
- Duz, M.B.; Karatas, O.F.; Guzel, E.; Turgut, N.F.; Yilmaz, M.; Creighton, C.J.; Ozen, M. Identification of MiR-139-5p as a Saliva Biomarker for Tongue Squamous Cell Carcinoma: A Pilot Study. Cell. Oncol. 2016, 39, 187–193. [Google Scholar] [CrossRef]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The Oral Microbiota—A Mechanistic Role for Systemic Diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The Emerging Role of Oral Microbiota in Oral Cancer Initiation, Progression and Stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-C.; Chang, L.-C.; Huang, H.-D.; Peng, C.-Y.; Chuang, C.-Y.; Chen, Y.-T.; Lu, M.-Y.; Chiu, Y.-W.; Chen, P.-Y.; Yang, S.-F. Oral Microbial Dysbiosis and Its Performance in Predicting Oral Cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The Salivary Microbiota as a Diagnostic Indicator of Oral Cancer: A Descriptive, Non-Randomized Study of Cancer-Free and Oral Squamous Cell Carcinoma Subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef]
- Kakabadze, M.Z.; Paresishvili, T.; Karalashvili, L.; Chakhunashvili, D.; Kakabadze, Z. Oral Microbiota and Oral Cancer: Review. Oncol. Rev. 2020, 14, 476. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in Oral Microbiota Associated with Oral Cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Nasher, A.T.; Idris, A.M.; Chen, T. Robust Species Taxonomy Assignment Algorithm for 16S RRNA NGS Reads: Application to Oral Carcinoma Samples. J. Oral Microbiol. 2015, 7, 28934. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, H.A.; Cox, M.R.; Eslick, G.D. Association between Human Papillomavirus (HPV) and Oesophageal Squamous Cell Carcinoma: A Meta-Analysis. Epidemiol. Infect. 2014, 142, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.S.; Rahman, B.; Ridda, I.; Newall, A.T.; Tabrizi, S.N.; Garland, S.M.; Segelov, E.; Seale, H.; Crowe, P.J.; Moa, A.; et al. The Aetiological Role of Human Papillomavirus in Oesophageal Squamous Cell Carcinoma: A Meta-Analysis. PLoS ONE 2013, 8, e69238. [Google Scholar] [CrossRef]
- Petrick, J.L.; Wyss, A.B.; Butler, A.M.; Cummings, C.; Sun, X.; Poole, C.; Smith, J.S.; Olshan, A.F. Prevalence of Human Papillomavirus among Oesophageal Squamous Cell Carcinoma Cases: Systematic Review and Meta-Analysis. Br. J. Cancer 2014, 110, 2369–2377. [Google Scholar] [CrossRef]
- Syrjänen, K.; Syrjänen, S. Detection of Human Papillomavirus in Esophageal Papillomas: Systematic Review and Meta-Analysis. Apmis 2013, 121, 363–374. [Google Scholar] [CrossRef]
- Carpén, T.; Syrjänen, S.; Jouhi, L.; Randen-Brady, R.; Haglund, C.; Mäkitie, A.; Mattila, P.S.; Hagström, J. Epstein-Barr Virus (EBV) and Polyomaviruses Are Detectable in Oropharyngeal Cancer and EBV May Have Prognostic Impact. Cancer Immunol. Immunother. 2020, 69, 1615–1626. [Google Scholar] [CrossRef]
- Drop, B.; Strycharz-Dudziak, M.; Kliszczewska, E.; Polz-Dacewicz, M. Coinfection with Epstein-Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma BK Virus (BKPyV) in Laryngeal, Oropharyngeal and Oral Cavity Cancer. Int. J. Mol. Sci. 2017, 18, 2752. [Google Scholar] [CrossRef]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.-A.; Sand, L. Human Papilloma Virus, Herpes Simplex Virus and Epstein Barr Virus in Oral Squamous Cell Carcinoma from Eight Different Countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar]
- Djuric, M.; Jankovic, L.; Jovanovic, T.; Pavlica, D.; Brkic, S.; Knezevic, A.; Markovic, D.; Milasin, J. Prevalence of Oral Herpes Simplex Virus Reactivation in Cancer Patients: A Comparison of Different Techniques of Viral Detection. J. Oral Pathol. Med. 2009, 38, 167–173. [Google Scholar] [CrossRef]
- Lou, E.; Kellman, R.M.; Shillitoe, E.J. Effect of Herpes Simplex Virus Type-1 on Growth of Oral Cancer in an Immunocompetent, Orthotopic Mouse Model. Oral Oncol. 2002, 38, 349–356. [Google Scholar] [CrossRef]
- Koivikko, T.; Rodrigues, P.C.; Vehviläinen, M.; Hyvönen, P.; Sundquist, E.; Arffman, R.K.; Al-Samadi, A.; Välimaa, H.; Salo, T.; Risteli, M. Detection of Herpes Simplex Virus in Oral Tongue Squamous Cell Carcinoma. Front. Pharmacol. 2023, 14, 1182152. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Litlekalsøy, J.; Ahmed, I.A.; Martinsen, E.M.H.; Furriol, J.; Javier-Lopez, R.; Elsheikh, M.; Gaafar, N.M.; Morgado, L.; Mundra, S.; et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients from Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front. Cell. Infect. Microbiol. 2021, 11, 673465. [Google Scholar] [CrossRef] [PubMed]
- Mohd Bakri, M.; Mohd Hussaini, H.; Rachel Holmes, A.; David Cannon, R.; Mary Rich, A. Revisiting the Association between Candidal Infection and Carcinoma, Particularly Oral Squamous Cell Carcinoma. J. Oral Microbiol. 2010, 2, 5780. [Google Scholar] [CrossRef]
- Salgado, R.; Fonseca, D.; Marques, A.; Napoleao, S.; França, T.; Akashi, K.; Prado, C.; Baiocchi, G.; Plaça, D.; Filgueiras, I.; et al. The Network Interplay of Type 1 Interferon and Toll-like Receptor Signaling Cascades Hallmarks the Immune Response against Candida spp. Infections. Res. Sq. 2021, 11, 10–21203. [Google Scholar]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of Oral Microbiome on Oral Cancers, a Review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of Oral Microbiota in Tumor and Non-Tumor Tissues of Patients with Oral Squamous Cell Carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef]
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The Prevalence Rate of Periodontal Pathogens and Its Association with Oral Squamous Cell Carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhang, Z.; Li, Y.; Li, Q.; Geng, F.; Liu, J.; Pan, Y. Analysis of Differentially Expressed Genes in Oral Epithelial Cells Infected with Fusobacterium Nucleatum for Revealing Genes Associated with Oral Cancer. J. Cell. Mol. Med. 2021, 25, 892–904. [Google Scholar] [CrossRef]
- Sales, C.B.S.; Buim, M.E.C.; de Souza, R.O.; de Faro Valverde, L.; Mathias Machado, M.C.; Reis, M.G.; Soares, F.A.; Ramos, E.A.G.; Gurgel Rocha, C.A. Elevated VEGFA MRNA Levels in Oral Squamous Cell Carcinomas and Tumor Margins: A Preliminary Study. J. Oral Pathol. Med. 2016, 45, 481–485. [Google Scholar] [CrossRef]
- Huang, S.; Sun, Y. Long Noncoding RNA MNX1-AS1 Functions as a Competing Endogenous RNA to Regulate Epithelial-Mesenchymal Transition by Sponging MiR-744-5p in Colorectal Cancer. Biosci. Biotechnol. Biochem. 2021, 85, 568–578. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, Y.; Wang, J.; Yang, X.; Wen, L.; Feng, J. LncRNA MNX1-AS1 Promotes Glioblastoma Progression through Inhibition of MiR-4443. Oncol. Res. 2019, 27, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Pan, Y.; Pan, Y.; Wang, O. MNX1-AS1 Is a Functional Oncogene That Induces EMT and Activates the AKT/MTOR Pathway and MNX1 in Breast Cancer. Cancer Manag. Res. 2019, 11, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium Nucleatum Promotes Epithelial-Mesenchymal Transiton through Regulation of the LncRNA MIR4435-2HG/MiR-296-5p/Akt2/SNAI1 Signaling Pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, H.; Liu, J.; Pan, C.; Zhang, D.; Li, X.; Pan, Y. Porphyromonas Gingivalis Infection Promoted the Proliferation of Oral Squamous Cell Carcinoma Cells through the MiR-21/PDCD4/AP-1 Negative Signaling Pathway. ACS Infect. Dis. 2019, 5, 1336–1347. [Google Scholar] [CrossRef]
- Liu, D.; Liu, S.; Liu, J.; Miao, L.; Zhang, S.; Pan, Y. SRNA23392 Packaged by Porphyromonas Gingivalis Outer Membrane Vesicles Promotes Oral Squamous Cell Carcinomas Migration and Invasion by Targeting Desmocollin-2. Mol. Oral Microbiol. 2021, 36, 182–191. [Google Scholar] [CrossRef]
- Choi, J.-W.; Um, J.-H.; Cho, J.-H.; Lee, H.-J. Tiny RNAs and Their Voyage via Extracellular Vesicles: Secretion of Bacterial Small RNA and Eukaryotic MicroRNA. Exp. Biol. Med. 2017, 242, 1475–1481. [Google Scholar] [CrossRef]
- Tran, N.; Khoury, S. Biomarkers of Oral, Pharyngeal and Laryngeal Cancers. U.S. Patent 17/888, 20 July 2023. [Google Scholar]
- Chu, H.-W.; Chang, K.-P.; Hsu, C.-W.; Chang, I.Y.-F.; Liu, H.-P.; Chen, Y.-T.; Wu, C.-C. Identification of Salivary Biomarkers for Oral Cancer Detection with Untargeted and Targeted Quantitative Proteomics Approaches. Mol. Cell. Proteom. 2019, 18, 1796–1806. [Google Scholar] [CrossRef]
- Markopoulos, A.K.; Michailidou, E.Z.; Tzimagiorgis, G. Salivary Markers for Oral Cancer Detection. Open Dent. J. 2010, 4, 172–178. [Google Scholar] [CrossRef]
- Kolenda, T.; Ryś, M.; Guglas, K.; Teresiak, A.; Bliźniak, R.; Mackiewicz, J.; Lamperska, K. Quantification of Long Non-Coding RNAs Using QRT-PCR: Comparison of Different CDNA Synthesis Methods and RNA Stability. Arch. Med. Sci. 2021, 17, 1006–1015. [Google Scholar] [CrossRef]
- Lee, M.R.; Mantel, C.; Lee, S.A.; Moon, S.-H.; Broxmeyer, H.E. MiR-31/SDHA Axis Regulates Reprogramming Efficiency through Mitochondrial Metabolism. Stem Cell Rep. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Hung, P.-S.; Tu, H.-F.; Kao, S.-Y.; Yang, C.-C.; Liu, C.-J.; Huang, T.-Y.; Chang, K.-W.; Lin, S.-C. MiR-31 Is Upregulated in Oral Premalignant Epithelium and Contributes to the Immortalization of Normal Oral Keratinocytes. Carcinogenesis 2014, 35, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, A.; Mohajertehran, F.; Aghaee-Bakhtiari, S.H.; Ayatollahi, H.; Douzandeh, K.; Pakfetrat, A.; Mohtasham, N. Downregulation of Salivary MiR-3928 as a Potential Biomarker in Patients with Oral Squamous Cell Carcinoma and Oral Lichen Planus. Clin. Exp. Dent. Res. 2024, 10, e877. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qu, Y.; Dang, S.; Yao, B.; Ji, M. MiR-145 Inhibits Oral Squamous Cell Carcinoma (OSCC) Cell Growth by Targeting c-Myc and Cdk6. Cancer Cell Int. 2013, 13, 51. [Google Scholar] [CrossRef]
- Shalaby, R.; Ibrahim, S.; Kotb, A.A.W.; Baz, S.; Hafed, L.; Shaker, O.; Afifi, S. MALAT1 as a Potential Salivary Biomarker in Oral Squamous Cell Carcinoma through Targeting MiRNA-124. Oral Dis. 2024, 30, 2075–2083. [Google Scholar] [CrossRef]
- Zhang, C.-Z. Long Intergenic Non-Coding RNA 668 Regulates VEGFA Signaling through Inhibition of MiR-297 in Oral Squamous Cell Carcinoma. Biochem. Biophys. Res. Commun. 2017, 489, 404–412. [Google Scholar] [CrossRef]
- Xu, F.-Y.; Xu, X.; Hu, X.-D. LINC00657 Promotes Malignant Progression of Oral Squamous Cell Carcinoma via Regulating MicroRNA-150. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2482–2490. [Google Scholar]
- He, K.; Zhu, Z.-B.; Shu, R.; Hong, A. LncRNA NEAT1 Mediates Progression of Oral Squamous Cell Carcinoma via VEGF-A and Notch Signaling Pathway. World J. Surg. Oncol. 2020, 18, 261. [Google Scholar] [CrossRef]
- Huang, G.; He, X.; Wei, X.-L. LncRNA NEAT1 Promotes Cell Proliferation and Invasion by Regulating MiR-365/RGS20 in Oral Squamous Cell Carcinoma. Oncol. Rep. 2018, 39, 1948–1956. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, S.; Zhang, X.; Zhao, S.; Cheng, M.; Fan, X.; Cai, Y.; Liao, L. Deregulation of Hsa_circ_0001971/MiR-186 and Hsa_circ_0001874/MiR-296 Signaling Pathways Promotes the Proliferation of Oral Squamous Carcinoma Cells by Synergistically Activating SHP2/PLK1 Signals. Sci. Rep. 2021, 11, 20561. [Google Scholar]
- Ghafouri-Fard, S.; Shoorei, H.; Anamag, F.T.; Taheri, M. The Role of Non-Coding RNAs in Controlling Cell Cycle Related Proteins in Cancer Cells. Front. Oncol. 2020, 10, 608975. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Sun, Z. Screening and Validation of Plasma Long Non-Coding RNAs as Biomarkers for the Early Diagnosis and Staging of Oral Squamous Cell Carcinoma. Oncol. Lett. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers 2017, 2017, 7243968. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Lin, S.-C.; Yang, C.-C.; Cheng, H.-W.; Chang, K.-W. Exploiting Salivary MiR-31 as a Clinical Biomarker of Oral Squamous Cell Carcinoma. Head Neck 2012, 34, 219–224. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, A.N.; Sharma, R.; Mateen, S.; Shukla, B.; Singh, A.; Chandel, S. Circulating MicroRNA-21 Expression as a Novel Serum Biomarker for Oral Sub-Mucous Fibrosis and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1053–1057. [Google Scholar]
- Zhu, B.; Cao, X.; Zhang, W.; Pan, G.; Yi, Q.; Zhong, W.; Yan, D. MicroRNA-31-5p Enhances the Warburg Effect via Targeting FIH. FASEB J. 2019, 33, 545–556. [Google Scholar] [CrossRef]
- Huang, F.; Xin, C.; Lei, K.; Bai, H.; Li, J.; Chen, Q. Noncoding RNAs in Oral Premalignant Disorders and Oral Squamous Cell Carcinoma. Cell. Oncol. 2020, 43, 763–777. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Zhao, J. Down-Regulation of MiR-3928 Promoted Osteosarcoma Growth. Cell. Physiol. Biochem. 2014, 33, 1547–1556. [Google Scholar] [CrossRef]
- Mulcahy, E.Q.X.; Zhang, Y.; Colόn, R.R.; Cain, S.R.; Gibert, M.K., Jr.; Dube, C.J.; Hafner, M.; Abounader, R. MicroRNA 3928 Suppresses Glioblastoma through Downregulation of Several Oncogenes and Upregulation of P53. Int. J. Mol. Sci. 2022, 23, 3930. [Google Scholar] [CrossRef]
- Rocchetti, F.; Tenore, G.; Macali, F.; Vicidomini, T.; Podda, G.M.; Fantozzi, P.J.; Silvestri, V.; Porzio, V.; Valentini, V.; Ottini, L.; et al. Expression Analysis of Circulating MicroRNAs in Saliva and Plasma for the Identification of Clinically Relevant Biomarkers for Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Cancers 2024, 16, 2990. [Google Scholar] [CrossRef]
- Arunkumar, G.; Deva Magendhra Rao, A.; Manikandan, M.; Prasanna Srinivasa Rao, H.; Subbiah, S.; Ilangovan, R.; Murugan, A.; Munirajan, A. Dysregulation of MiR-200 Family MicroRNAs and Epithelial-Mesenchymal Transition Markers in Oral Squamous Cell Carcinoma. Oncol. Lett. 2017, 15, 649–657. [Google Scholar] [CrossRef]
- Koopaie, M.; Akhbari, P.; Fatahzadeh, M.; Kolahdooz, S. Identification of Common Salivary MiRNA in Oral Lichen Planus and Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. BMC Oral Health 2024, 24, 1177. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA Alterations and Associated Aberrant DNA Methylation Patterns across Multiple Sample Types in Oral Squamous Cell Carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary MicroRNA: Discovery, Characterization, and Clinical Utility for Oral Cancer Detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Shahidi, M.; Manifar, S.; Jafari, S.; Mashhadi Abbas, F.; Barati, M.; Mortazavi, H.; Shirkhoda, M.; Farzanegan, A.; Elmi Rankohi, Z. Diagnostic and Prognostic Relevance of Salivary MicroRNA-21, -125a, -31 and -200a Levels in Patients with Oral Lichen Planus—A Short Report. Cell. Oncol. 2018, 41, 329–334. [Google Scholar] [CrossRef]
- Bahrami, N.; Pirrafiee, M.; Azadi, F.; Azimnejad, R.; Fotook Kiaei, S.Z.; Abbasi, A.J.; Kazempour Dizaji, M.; Lookzadeh, S.; Nejatollahi, S.M.R.; Daustani, M.; et al. Biomarkers for Oral Squamous Cell Carcinoma (MiR-24, MiR-200, and MiR-34): Screening and Detection MicroRNA. Asian Pac. J. Cancer Prev. 2024, 25, 2265–2269. [Google Scholar] [CrossRef]
- Saikishore, R.; Velmurugan, P.; Ranjithkumar, D.; Latha, R.; Sathiamoorthi, T.; Arun, A.; Ravi, A.V.; Sivakumar, S. The Circular RNA-MiRNA Axis: A Special RNA Signature Regulatory Transcriptome as a Potential Biomarker for OSCC. Mol. Ther. Nucleic Acids 2020, 22, 352–361. [Google Scholar] [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary Extracellular Vesicle-Associated MiRNAs as Potential Biomarkers in Oral Squamous Cell Carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef]
- Scapoli, L.; Palmieri, A.; Lo Muzio, L.; Pezzetti, F.; Rubini, C.; Girardi, A.; Farinella, F.; Mazzotta, M.; Carinci, F. MicroRNA Expression Profiling of Oral Carcinoma Identifies New Markers of Tumor Progression. Int. J. Immunopathol. Pharmacol. 2010, 23, 1229–1234. [Google Scholar] [CrossRef]
- Tao, D.; Zhang, Z.; Liu, X.; Zhang, Z.; Fu, Y.; Zhang, P.; Yuan, H.; Liu, L.; Cheng, J.; Jiang, H. LncRNA HOTAIR Promotes the Invasion and Metastasis of Oral Squamous Cell Carcinoma through Metastasis-Associated Gene 2. Mol. Carcinog. 2020, 59, 353–364. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Shen, G. Metastasis-Associated Protein 2 (MTA2) Promotes the Metastasis of Non-Small-Cell Lung Cancer through the Inhibition of the Cell Adhesion Molecule Ep-CAM and E-Cadherin. Jpn. J. Clin. Oncol. 2015, 45, 755–766. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Chou, Y.-E.; Ko, C.-P.; Yang, S.-F.; Hsieh, S.-C.; Lin, C.-L.; Hsieh, Y.-H.; Chen, K.-C. Metastasis Tumor-Associated Protein-2 Knockdown Suppresses the Proliferation and Invasion of Human Glioma Cells in Vitro and in Vivo. J. Neurooncol. 2014, 120, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ji, J.; Cai, Q.; Shi, M.; Chen, X.; Yu, Y.; Liu, B.; Zhu, Z.; Zhang, J. MTA2 Promotes Gastric Cancer Cells Invasion and Is Transcriptionally Regulated by Sp1. Mol. Cancer 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, X.-J.; Lin, Y.; Chen, Q.-Y.; Sun, W.-J.; Tang, L.; Liang, Q.-X. LncRNA MALAT1 Expression Inhibition Suppresses Tongue Squamous Cell Carcinoma Proliferation, Migration and Invasion by Inactivating PI3K/Akt Pathway and Downregulating MMP-9 Expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 198–206. [Google Scholar] [PubMed]
- Iwai, S.; Yonekawa, A.; Harada, C.; Hamada, M.; Katagiri, W.; Nakazawa, M.; Yura, Y. Involvement of the Wnt-β-Catenin Pathway in Invasion and Migration of Oral Squamous Carcinoma Cells. Int. J. Oncol. 2010, 37, 1095–1103. [Google Scholar] [CrossRef]
- Shieh, T.-M.; Liu, C.-J.; Hsia, S.-M.; Ningrum, V.; Liao, C.-C.; Lan, W.-C.; Shih, Y.-H. Lack of Salivary Long Non-Coding RNA XIST Expression Is Associated with Increased Risk of Oral Squamous Cell Carcinoma: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 4622. [Google Scholar] [CrossRef]
- Bahn, J.H.; Zhang, Q.; Li, F.; Chan, T.-M.; Lin, X.; Kim, Y.; Wong, D.T.W.; Xiao, X. The Landscape of MicroRNA, Piwi-Interacting RNA, and Circular RNA in Human Saliva. Clin. Chem. 2015, 61, 221–230. [Google Scholar] [CrossRef]
- Panta, P.; Wong, D.T.W. Salivary Biomarkers in Oral Cancer. In Oral Cancer Detection; Springer International Publishing: Cham, Switzerland, 2019; pp. 265–295. ISBN 9783319612546. [Google Scholar]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and Properties of a Novel Potential Biomarker for Cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Wang, F.; Nazarali, A.J.; Ji, S. Circular RNAs as Potential Biomarkers for Cancer Diagnosis and Therapy. Am. J. Cancer Res. 2016, 6, 1167–1176. [Google Scholar]
- Cai, Z.; Hao, X.-Y.; Liu, F.-X. MicroRNA-186 Serves as a Tumor Suppressor in Oral Squamous Cell Carcinoma by Negatively Regulating the Protein Tyrosine Phosphatase SHP2 Expression. Arch. Oral Biol. 2018, 89, 20–25. [Google Scholar] [CrossRef]
- Xu, C.; Li, S.; Chen, T.; Hu, H.; Ding, C.; Xu, Z.; Chen, J.; Liu, Z.; Lei, Z.; Zhang, H.-T.; et al. MiR-296-5p Suppresses Cell Viability by Directly Targeting PLK1 in Non-Small Cell Lung Cancer. Oncol. Rep. 2016, 35, 497–503. [Google Scholar] [CrossRef]
- Vittal, K.; Pandian, S.S.; Joseph, L.D.; Raj, S.G. Immunohistochemical Expression of Polo-like Kinase 1 in Oral Squamous Cell Carcinoma and Oral Submucous Fibrosis. Indian J. Dent. Res. 2018, 29, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, S.; Menon, M.B. Non-Coding Transcript Variants of Protein-Coding Genes—What Are They Good For? RNA Biol. 2018, 15, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, F. Biomarkers Related to Oral Squamous Cell Carcinoma and Methods of Diagnosis and Treatment Thereof. U.S. Patent 18/000,197, 29 June 2023. [Google Scholar]
- Wong, D.T.W. Salivary Extracellular Noncoding RNA: Emerging Biomarkers for Molecular Diagnostics. Clin. Ther. 2015, 37, 540–551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).