The Unpaved Road of Non-Coding RNA Structure–Function Relationships: Current Knowledge, Available Methodologies, and Future Trends
Abstract
1. Prevalence of Non-Coding Genes in the Genomes of Complex Eukaryotes
2. General Principles and Rules Governing RNA Structure
3. Functional Relevance of Structural Elements in Non-Coding RNAs
3.1. Hairpin Loops
3.2. Bulges and Internal Loops
3.3. Pseudoknots
3.4. Kissing Hairpins
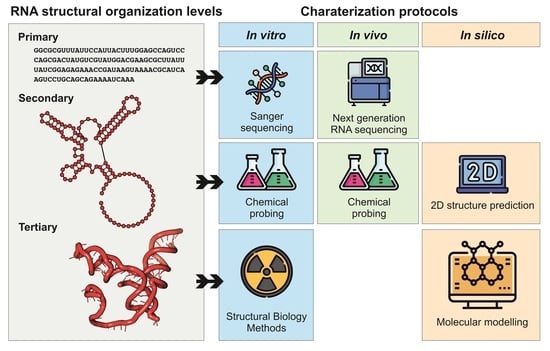
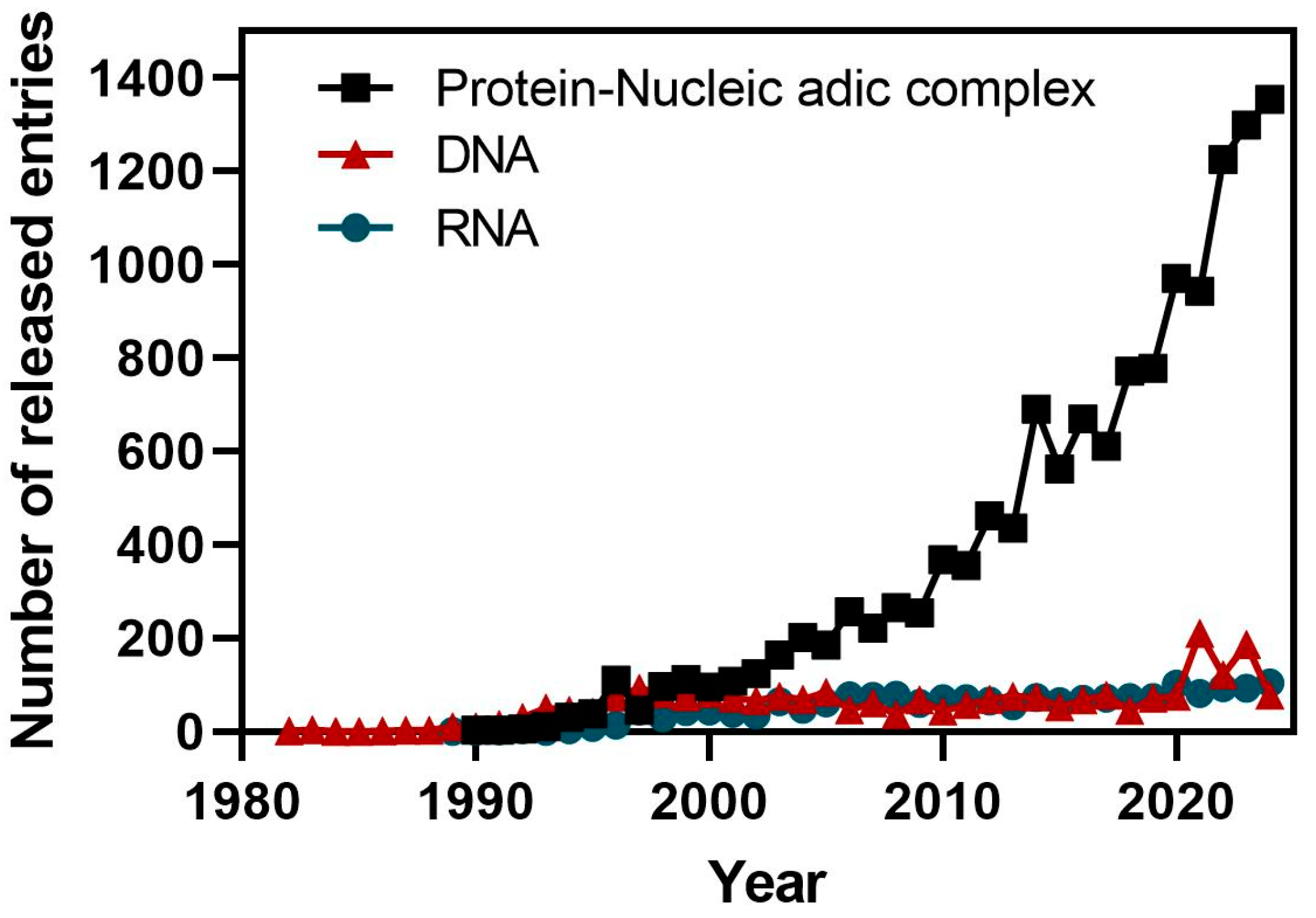
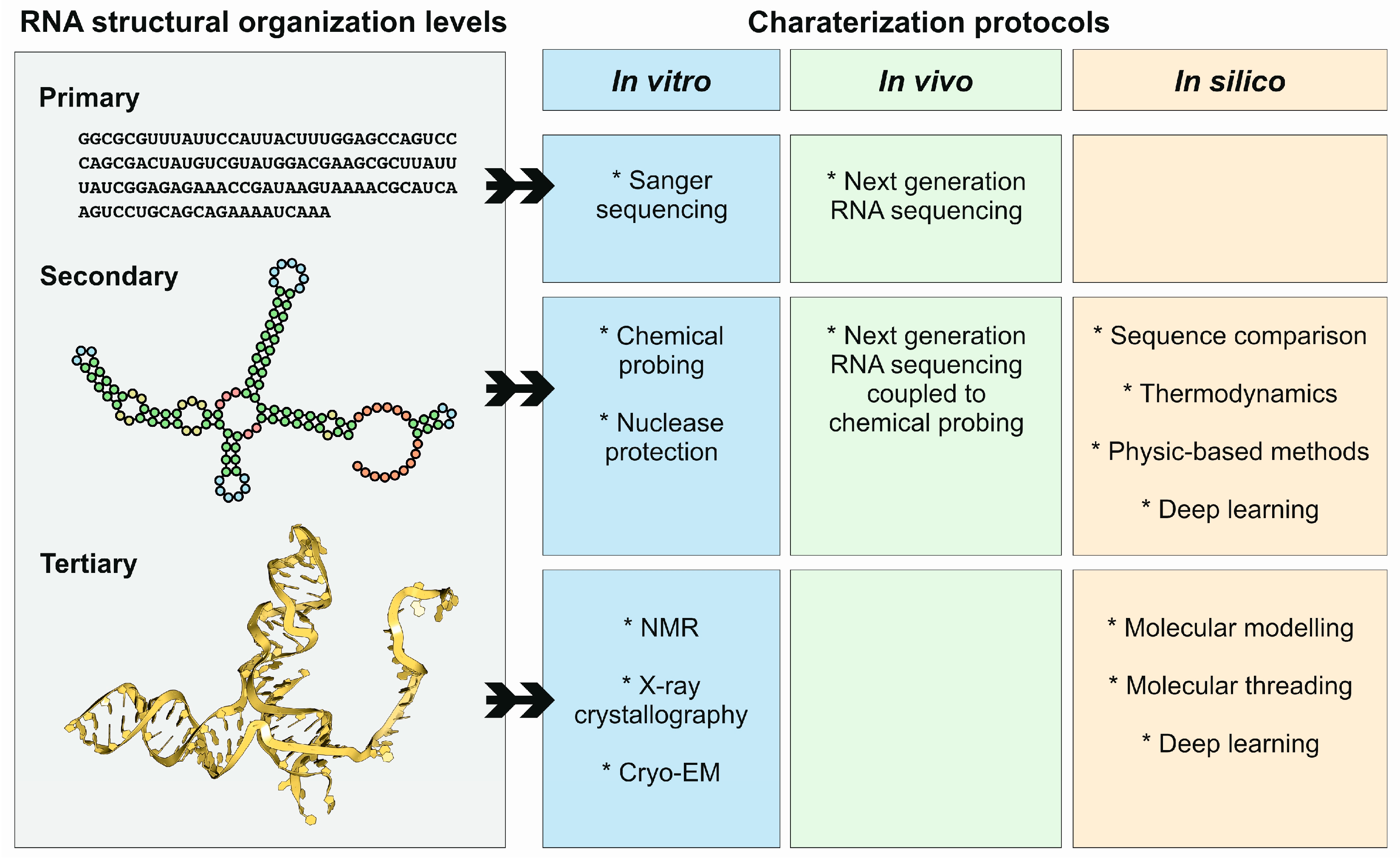
4. Methods and Protocols to Study ncRNA Structures
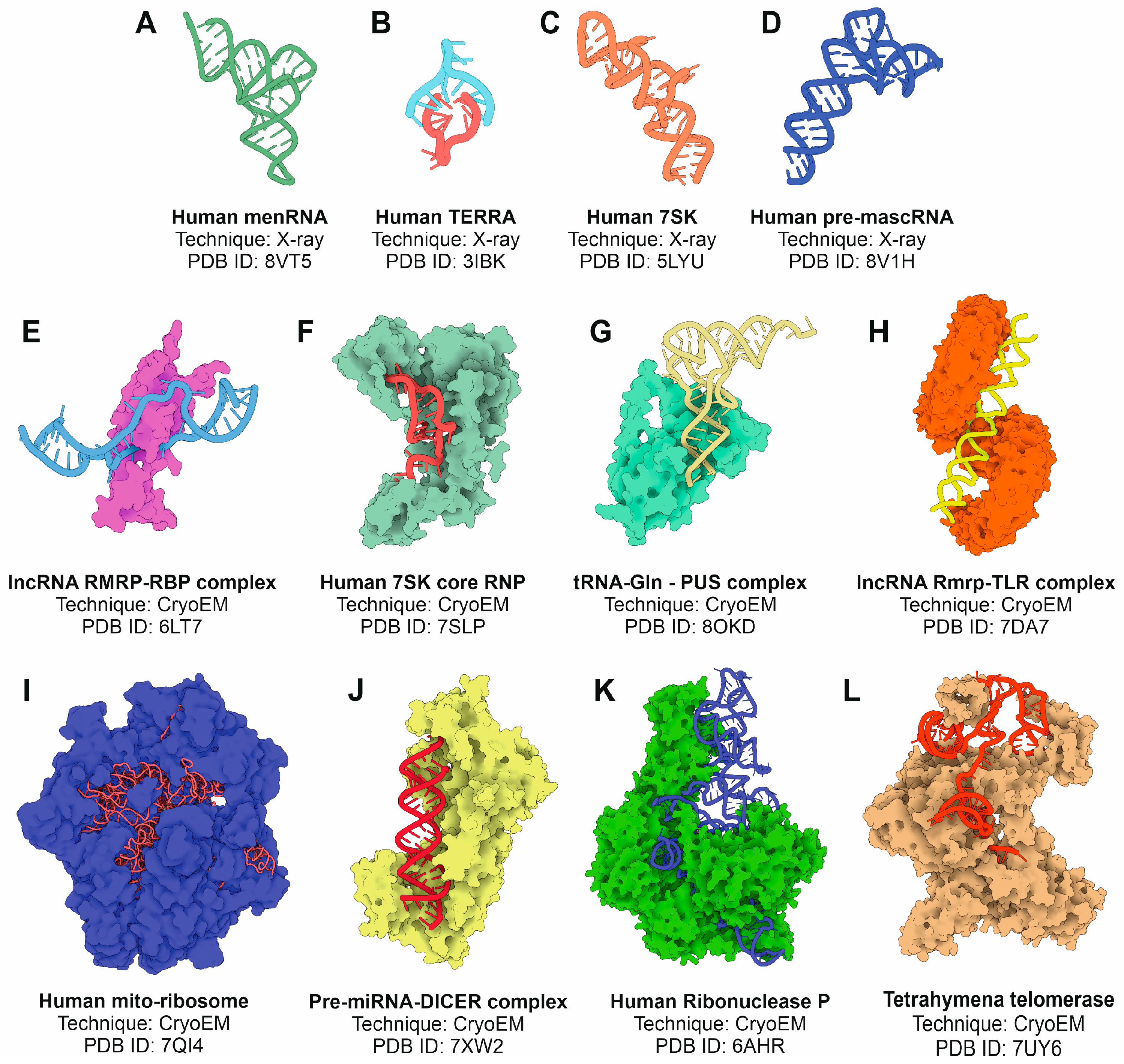
4.1. X-Ray Crystallography
4.2. Cryo-Electron Microscopy
4.3. In Vivo Methods
4.4. In Silico Methods
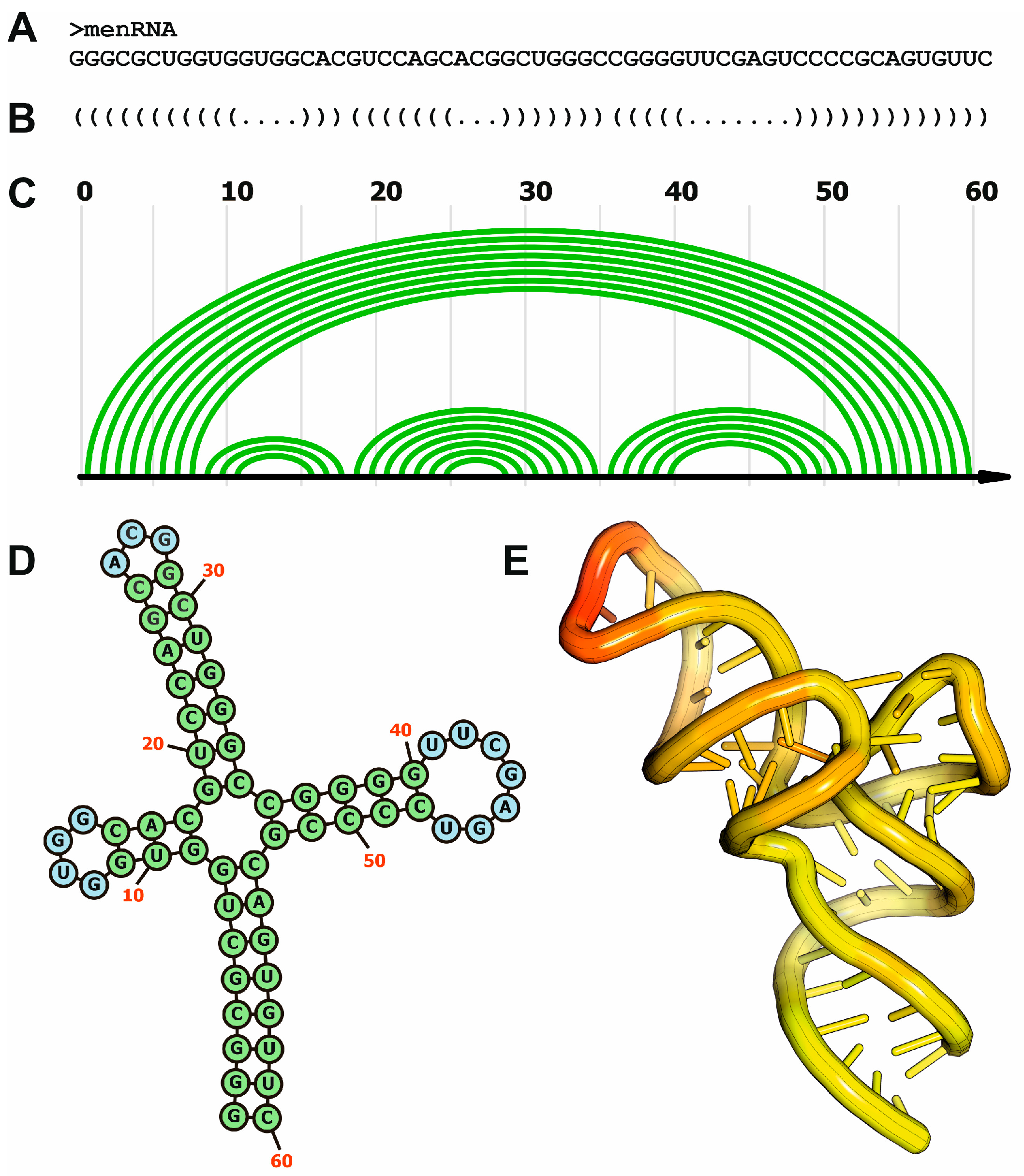
4.4.1. Methods for RNA Secondary Structure Prediction
4.4.2. Methods for RNA Tertiary Structure Prediction
5. Perspectives and Further Developments
- Integration of multiscale data: combining atomic-level structural information with systemic data on ncRNA localization, interaction networks, and dynamics will enable a holistic understanding of ncRNA function. Hybrid approaches integrating cryo-EM and chemical probing with single-cell RNA sequencing and spatial transcriptomics are particularly promising.
- High-throughput structural characterization: automation in cryo-EM and microfluidics-based methods for RNA crystallography could facilitate the high-throughput determination of ncRNA structures, accelerating the discovery of novel functional motifs.
- Dynamic and contextual studies: capturing ncRNA structures in their native cellular environment remains a significant challenge. Emerging techniques, such as cryo-electron tomography (cryo-ET) and in situ structural studies, aim to bridge this gap by visualizing ncRNAs within intact cells.
- Functional modulation and rational drug design: structural insights into ncRNAs have profound implications for drug discovery. Small molecules targeting ncRNA structures or their interactions with proteins could provide new therapeutic avenues for diseases associated with dysregulated ncRNAs, such as cancer and neurodegenerative disorders.
- Evolutionary perspectives: structural comparisons across species can reveal conserved motifs and inform functional hypotheses. Integrating structural biology with evolutionary genomics will help identify universally important ncRNA structures and their roles in diverse organisms.
- Artificial intelligence: the use of deep-learning approaches to infer ncRNA structure and function will increase our understanding of their functions, increasing the knowledge about the molecular players related to cell physiology and human disease.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, C.A.; Hitz, B.C.; Sloan, C.A.; Chan, E.T.; Davidson, J.M.; Gabdank, I.; Hilton, J.A.; Jain, K.; Baymuradov, U.K.; Narayanan, A.K.; et al. The Encyclopedia of DNA elements (ENCODE): Data portal update. Nucleic Acids Res. 2018, 46, D794–D801. [Google Scholar] [CrossRef]
- Mattick, J.S.; Gagen, M.J. The evolution of controlled multitasked gene networks: The role of introns and other noncoding RNAs in the development of complex organisms. Mol. Biol. Evol. 2001, 18, 1611–1630. [Google Scholar] [CrossRef] [PubMed]
- Villa, T.; Porrua, O. Pervasive transcription: A controlled risk. FEBS J. 2023, 290, 3723–3736. [Google Scholar] [CrossRef]
- Carroll, S.B. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell 2008, 134, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Tjian, R. Transcription regulation and animal diversity. Nature 2003, 424, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mudge, J.M.; Carbonell-Sala, S.; Diekhans, M.; Martinez, J.G.; Hunt, T.; Jungreis, I.; Loveland, J.E.; Arnan, C.; Barnes, I.; Bennett, R.; et al. GENCODE 2025: Reference gene annotation for human and mouse. Nucleic Acids Res. 2024, 53, D966–D975. [Google Scholar] [CrossRef]
- Chen, L.L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Assmann, S.M.; Chou, H.L.; Bevilacqua, P.C. Rock, scissors, paper: How RNA structure informs function. Plant Cell 2023, 35, 1671–1707. [Google Scholar] [CrossRef]
- Tinoco, I., Jr.; Bustamante, C. How RNA folds. J. Mol. Biol. 1999, 293, 271–281. [Google Scholar] [CrossRef]
- Mathews, D.H.; Turner, D.H. Prediction of RNA secondary structure by free energy minimization. Curr. Opin. Struct. Biol. 2006, 16, 270–278. [Google Scholar] [CrossRef]
- Juan, V.; Wilson, C. RNA secondary structure prediction based on free energy and phylogenetic analysis. J. Mol. Biol. 1999, 289, 935–947. [Google Scholar] [CrossRef]
- Lemieux, S.; Major, F. RNA canonical and non-canonical base pairing types: A recognition method and complete repertoire. Nucleic Acids Res. 2002, 30, 4250–4263. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.; Zhou, Y.; Zhong, Z.; Toh, D.F.; Chen, G. RNA triplexes: From structural principles to biological and biotech applications. Wiley Interdiscip. Rev. RNA 2015, 6, 111–128. [Google Scholar] [CrossRef]
- Brion, P.; Westhof, E. Hierarchy and dynamics of RNA folding. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 113–137. [Google Scholar] [CrossRef]
- Westhof, E.; Fritsch, V. RNA folding: Beyond Watson-Crick pairs. Structure 2000, 8, R55–R65. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, I., Jr.; Li, P.T.; Bustamante, C. Determination of thermodynamics and kinetics of RNA reactions by force. Q. Rev. Biophys. 2006, 39, 325–360. [Google Scholar] [CrossRef]
- Liphardt, J.; Onoa, B.; Smith, S.B.; Tinoco, I., Jr.; Bustamante, C. Reversible unfolding of single RNA molecules by mechanical force. Science 2001, 292, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Vieregg, J.; Cheng, W.; Bustamante, C.; Tinoco, I., Jr. Measurement of the effect of monovalent cations on RNA hairpin stability. J. Am. Chem. Soc. 2007, 129, 14966–14973. [Google Scholar] [CrossRef]
- Guckian, K.M.; Schweitzer, B.A.; Ren, R.X.; Sheils, C.J.; Paris, P.L.; Tahmassebi, D.C.; Kool, E.T. Experimental Measurement of Aromatic Stacking Affinities in the Context of Duplex DNA. J. Am. Chem. Soc. 1996, 118, 8182–8183. [Google Scholar] [CrossRef]
- Xia, T.; SantaLucia, J., Jr.; Burkard, M.E.; Kierzek, R.; Schroeder, S.J.; Jiao, X.; Cox, C.; Turner, D.H. Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs. Biochemistry 1998, 37, 14719–14735. [Google Scholar] [CrossRef]
- Serra, M.J.; Turner, D.H. Predicting thermodynamic properties of RNA. Methods Enzymol. 1995, 259, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Broyde, S. Setting the stage for predicting RNA thermodynamic properties and their structural components. Biophys. J. 1996, 70, 1571–1572. [Google Scholar] [CrossRef] [PubMed]
- Lilley, D.M. Structures of helical junctions in nucleic acids. Q. Rev. Biophys. 2000, 33, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Herschlag, D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995, 270, 20871–20874. [Google Scholar] [CrossRef]
- Lilley, D.M. The origins of RNA catalysis in ribozymes. Trends Biochem. Sci. 2003, 28, 495–501. [Google Scholar] [CrossRef]
- Draper, D.E.; Grilley, D.; Soto, A.M. Ions and RNA folding. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 221–243. [Google Scholar] [CrossRef]
- Draper, D.E. RNA folding: Thermodynamic and molecular descriptions of the roles of ions. Biophys. J. 2008, 95, 5489–5495. [Google Scholar] [CrossRef]
- Denesyuk, N.A.; Hori, N.; Thirumalai, D. Molecular Simulations of Ion Effects on the Thermodynamics of RNA Folding. J. Phys. Chem. B 2018, 122, 11860–11867. [Google Scholar] [CrossRef]
- dell’Erba, M.G.; Zemba, G.R. Thermodynamics of a model for RNA folding. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2009, 79, 011913. [Google Scholar] [CrossRef]
- Leontis, N.B.; Lescoute, A.; Westhof, E. The building blocks and motifs of RNA architecture. Curr. Opin. Struct. Biol. 2006, 16, 279–287. [Google Scholar] [CrossRef]
- Gregorian, R.S., Jr.; Crothers, D.M. Determinants of RNA hairpin loop-loop complex stability. J. Mol. Biol. 1995, 248, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Dale, T.; Smith, R.; Serra, M.J. A test of the model to predict unusually stable RNA hairpin loop stability. RNA 2000, 6, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Sobczak, K.; Wilczynska, U.; Drath, M.; Jasinska, A.; Kaczynska, D.; Krzyzosiak, W.J. Structural features of microRNA (miRNA) precursors and their relevance to miRNA biogenesis and small interfering RNA/short hairpin RNA design. J. Biol. Chem. 2004, 279, 42230–42239. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.M.; Shivashankar, V.; Ma, E.J.; Baryza, J.L.; Nutiu, R. Functional Atlas of Primary miRNA Maturation by the Microprocessor. Mol. Cell 2020, 80, 892–902. [Google Scholar] [CrossRef]
- Garg, A.; Shang, R.; Cvetanovic, T.; Lai, E.C.; Joshua-Tor, L. The structural landscape of Microprocessor-mediated processing of pri-let-7 miRNAs. Mol. Cell 2024, 84, 4175–4190. [Google Scholar] [CrossRef]
- Xiong, X.; Kang, X.; Zheng, Y.; Yue, S.; Zhu, S. Identification of loop nucleotide polymorphisms affecting microRNA processing and function. Mol. Cells 2013, 36, 518–526. [Google Scholar] [CrossRef]
- Dang, T.L.; Le, C.T.; Le, M.N.; Nguyen, T.D.; Nguyen, T.L.; Bao, S.; Li, S.; Nguyen, T.A. Select amino acids in DGCR8 are essential for the UGU-pri-miRNA interaction and processing. Commun. Biol. 2020, 3, 344. [Google Scholar] [CrossRef]
- McIntyre, G.J.; Yu, Y.H.; Lomas, M.; Fanning, G.C. The effects of stem length and core placement on shRNA activity. BMC Mol. Biol. 2011, 12, 34. [Google Scholar] [CrossRef]
- Zhang, D.; Jue, D.; Smith, N.; Zhong, C.; Finnegan, E.J.; de Feyter, R.; Wang, M.B.; Greaves, I. Asymmetric bulges within hairpin RNA transgenes influence small RNA size, secondary siRNA production and viral defence. Nucleic Acids Res. 2024, 52, 9904–9916. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Ding, Y.; Lu, X.; Zhang, G.; Yang, J.; Zheng, H.; Wang, H.; Jiang, Y.; Xu, L. LncRNA Structural Characteristics in Epigenetic Regulation. Int. J. Mol. Sci. 2017, 18, 2659. [Google Scholar] [CrossRef]
- Cerase, A.; Tartaglia, G.G. Long non-coding RNA-polycomb intimate rendezvous. Open Biol. 2020, 10, 200126. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Bulkley, D.; Wang, J.; Valenstein, M.L.; Yario, T.A.; Steitz, T.A.; Steitz, J.A. Structural insights into the stabilization of MALAT1 noncoding RNA by a bipartite triple helix. Nat. Struct. Mol. Biol. 2014, 21, 633–640. [Google Scholar] [CrossRef]
- Mondal, M.; Mukherjee, S.; Halder, S.; Bhattacharyya, D. Stacking geometry for non-canonical G:U wobble base pair containing dinucleotide sequences in RNA: Dispersion-corrected DFT-D study. Biopolymers 2015, 103, 328–338. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. RNA bulges as architectural and recognition motifs. Structure 2000, 8, R47–R54. [Google Scholar] [CrossRef]
- Correll, C.C.; Munishkin, A.; Chan, Y.L.; Ren, Z.; Wool, I.G.; Steitz, T.A. Crystal structure of the ribosomal RNA domain essential for binding elongation factors. Proc. Natl. Acad. Sci. USA 1998, 95, 13436–13441. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, K.; Lim, C. Arrangement of 3D structural motifs in ribosomal RNA. Nucleic Acids Res. 2010, 38, 3512–3522. [Google Scholar] [CrossRef]
- Macrae, I.J.; Zhou, K.; Li, F.; Repic, A.; Brooks, A.N.; Cande, W.Z.; Adams, P.D.; Doudna, J.A. Structural basis for double-stranded RNA processing by Dicer. Science 2006, 311, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, B.K.; Erwin, J.A.; Song, J.J.; Lee, J.T. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 2008, 322, 750–756. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Staple, D.W.; Butcher, S.E. Pseudoknots: RNA structures with diverse functions. PLoS Biol. 2005, 3, e213. [Google Scholar] [CrossRef]
- Huang, F.W.; Li, L.Y.; Reidys, C.M. Sequence-structure relations of pseudoknot RNA. BMC Bioinform. 2009, 10 (Suppl. S1), S39. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Kim, C.H.; Bustamante, C.; Tinoco, I., Jr. Characterization of the mechanical unfolding of RNA pseudoknots. J. Mol. Biol. 2008, 375, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.F.; Chang, K.C.; Chen, Y.L.; Hsieh, P.S.; Lee, A.I.; Tu, J.Y.; Chen, Y.T.; Wen, J.D. Formation of frameshift-stimulating RNA pseudoknots is facilitated by remodeling of their folding intermediates. Nucleic Acids Res. 2021, 49, 6941–6957. [Google Scholar] [CrossRef]
- Su, L.; Chen, L.; Egli, M.; Berger, J.M.; Rich, A. Minor groove RNA triplex in the crystal structure of a ribosomal frameshifting viral pseudoknot. Nat. Struct. Biol. 1999, 6, 285–292. [Google Scholar] [CrossRef]
- Theimer, C.A.; Blois, C.A.; Feigon, J. Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function. Mol. Cell 2005, 17, 671–682. [Google Scholar] [CrossRef]
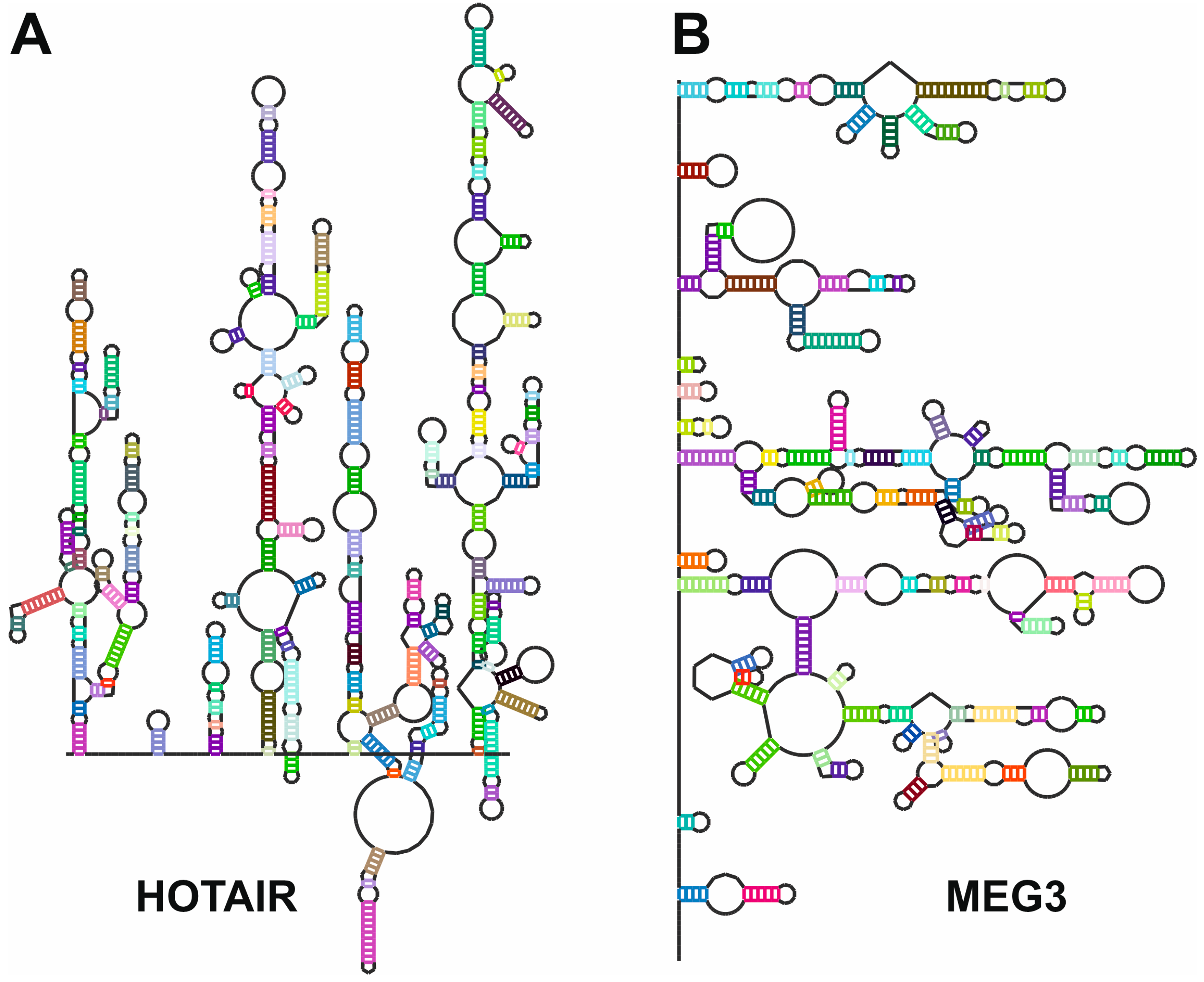
- Uroda, T.; Anastasakou, E.; Rossi, A.; Teulon, J.M.; Pellequer, J.L.; Annibale, P.; Pessey, O.; Inga, A.; Chillon, I.; Marcia, M. Conserved Pseudoknots in lncRNA MEG3 Are Essential for Stimulation of the p53 Pathway. Mol. Cell 2019, 75, 982–995. [Google Scholar] [CrossRef]
- Turner, D.H. Thermodynamics of base pairing. Curr. Opin. Struct. Biol. 1996, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E.; Freier, S.M.; Spector, D.L. 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 2008, 135, 919–932. [Google Scholar] [CrossRef]
- Tsai, M.C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Yang, L.; Froberg, J.E.; Lee, J.T. Long noncoding RNAs: Fresh perspectives into the RNA world. Trends Biochem. Sci. 2014, 39, 35–43. [Google Scholar] [CrossRef]
- Naganuma, T.; Nakagawa, S.; Tanigawa, A.; Sasaki, Y.F.; Goshima, N.; Hirose, T. Alternative 3′-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J. 2012, 31, 4020–4034. [Google Scholar] [CrossRef]
- Rivas, E.; Clements, J.; Eddy, S.R. A statistical test for conserved RNA structure shows lack of evidence for structure in lncRNAs. Nat. Methods 2017, 14, 45–48. [Google Scholar] [CrossRef]
- Sussman, J.L.; Lin, D.; Jiang, J.; Manning, N.O.; Prilusky, J.; Ritter, O.; Abola, E.E. Protein Data Bank (PDB): Database of three-dimensional structural information of biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 1078–1084. [Google Scholar] [CrossRef]
- Lawson, C.L.; Berman, H.M.; Chen, L.; Vallat, B.; Zirbel, C.L. The Nucleic Acid Knowledgebase: A new portal for 3D structural information about nucleic acids. Nucleic Acids Res. 2024, 52, D245–D254. [Google Scholar] [CrossRef]
- Neidle, S. Beyond the double helix: DNA structural diversity and the PDB. J. Biol. Chem. 2021, 296, 100553. [Google Scholar] [CrossRef]
- Lietzke, S.E.; Barnes, C.L.; Kundrot, C.E. Crystallization and structure determination of RNA. Curr. Opin. Struct. Biol. 1995, 5, 645–649. [Google Scholar] [CrossRef]
- Ferre-D’Amare, A.R.; Zhou, K.; Doudna, J.A. A general module for RNA crystallization. J. Mol. Biol. 1998, 279, 621–631. [Google Scholar] [CrossRef]
- Golden, B.L.; Kundrot, C.E. RNA crystallization. J. Struct. Biol. 2003, 142, 98–107. [Google Scholar] [CrossRef]
- Vicens, Q.; Gooding, A.R.; Laederach, A.; Cech, T.R. Local RNA structural changes induced by crystallization are revealed by SHAPE. RNA 2007, 13, 536–548. [Google Scholar] [CrossRef]
- Zhang, J.; Ferre-D’Amare, A.R. Post-crystallization Improvement of RNA Crystal Diffraction Quality. Methods Mol. Biol. 2015, 1316, 13–24. [Google Scholar] [CrossRef]
- Carrasco, N.; Buzin, Y.; Tyson, E.; Halpert, E.; Huang, Z. Selenium derivatization and crystallization of DNA and RNA oligonucleotides for X-ray crystallography using multiple anomalous dispersion. Nucleic Acids Res. 2004, 32, 1638–1646. [Google Scholar] [CrossRef]
- Zhang, J.; Ferre-D’Amare, A.R. Post-crystallization Improvement of RNA Crystals by Synergistic Ion Exchange and Dehydration. Bio Protoc. 2015, 5, e1578. [Google Scholar] [CrossRef]
- Pley, H.W.; Flaherty, K.M.; McKay, D.B. Three-dimensional structure of a hammerhead ribozyme. Nature 1994, 372, 68–74. [Google Scholar] [CrossRef]
- Scott, W.G.; Finch, J.T.; Klug, A. The crystal structure of an all-RNA hammerhead ribozyme: A proposed mechanism for RNA catalytic cleavage. Cell 1995, 81, 991–1002. [Google Scholar] [CrossRef]
- Edwards, A.L.; Garst, A.D.; Batey, R.T. Determining structures of RNA aptamers and riboswitches by X-ray crystallography. Methods Mol. Biol. 2009, 535, 135–163. [Google Scholar] [CrossRef]
- Serganov, A.; Polonskaia, A.; Phan, A.T.; Breaker, R.R.; Patel, D.J. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature 2006, 441, 1167–1171. [Google Scholar] [CrossRef]
- Itoh, Y.; Chiba, S.; Sekine, S.; Yokoyama, S. Crystal structure of human selenocysteine tRNA. Nucleic Acids Res. 2009, 37, 6259–6268. [Google Scholar] [CrossRef]
- Zong, X.; Nakagawa, S.; Freier, S.M.; Fei, J.; Ha, T.; Prasanth, S.G.; Prasanth, K.V. Natural antisense RNA promotes 3′ end processing and maturation of MALAT1 lncRNA. Nucleic Acids Res. 2016, 44, 2898–2908. [Google Scholar] [CrossRef]
- Skeparnias, I.; Zhang, J. Structural basis of NEAT1 lncRNA maturation and menRNA instability. Nat. Struct. Mol. Biol. 2024, 31, 1650–1654. [Google Scholar] [CrossRef]
- Ma, H.; Jia, X.; Zhang, K.; Su, Z. Cryo-EM advances in RNA structure determination. Signal Transduct. Target. Ther. 2022, 7, 58. [Google Scholar] [CrossRef]
- Bonilla, S.L.; Kieft, J.S. The promise of cryo-EM to explore RNA structural dynamics. J. Mol. Biol. 2022, 434, 167802. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Xie, J.; Nowak, J.S.; Luo, B.; Zhang, C.; Jia, G.; Zou, J.; Huang, D.; Glatt, S.; et al. RNA sample optimization for cryo-EM analysis. Nat. Protoc. 2024. [Google Scholar] [CrossRef]
- Bonilla, S.L.; Jang, K. Challenges, advances, and opportunities in RNA structural biology by Cryo-EM. Curr. Opin. Struct. Biol. 2024, 88, 102894. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, S.L.; Vicens, Q.; Kieft, J.S. Cryo-EM reveals an entangled kinetic trap in the folding of a catalytic RNA. Sci. Adv. 2022, 8, eabq4144. [Google Scholar] [CrossRef]
- Yan, C.; Hang, J.; Wan, R.; Huang, M.; Wong, C.C.; Shi, Y. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science 2015, 349, 1182–1191. [Google Scholar] [CrossRef]
- Yan, C.; Wan, R.; Bai, R.; Huang, G.; Shi, Y. Structure of a yeast activated spliceosome at 3.5 A resolution. Science 2016, 353, 904–911. [Google Scholar] [CrossRef]
- Yan, C.; Wan, R.; Bai, R.; Huang, G.; Shi, Y. Structure of a yeast step II catalytically activated spliceosome. Science 2017, 355, 149–155. [Google Scholar] [CrossRef]
- Bertram, K.; Agafonov, D.E.; Dybkov, O.; Haselbach, D.; Leelaram, M.N.; Will, C.L.; Urlaub, H.; Kastner, B.; Luhrmann, R.; Stark, H. Cryo-EM Structure of a Pre-catalytic Human Spliceosome Primed for Activation. Cell 2017, 170, 701–713. [Google Scholar] [CrossRef]
- Bertram, K.; Agafonov, D.E.; Liu, W.T.; Dybkov, O.; Will, C.L.; Hartmuth, K.; Urlaub, H.; Kastner, B.; Stark, H.; Luhrmann, R. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature 2017, 542, 318–323. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, C.; Hang, J.; Finci, L.I.; Lei, J.; Shi, Y. An Atomic Structure of the Human Spliceosome. Cell 2017, 169, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Y.; Susac, L.; Chan, H.; Basu, R.; Zhou, Z.H.; Feigon, J. Structure of Telomerase with Telomeric DNA. Cell 2018, 173, 1179–1190. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Wang, Y.; Song, H.; Zhou, Z.H.; Feigon, J. Structure of active human telomerase with telomere shelterin protein TPP1. Nature 2022, 604, 578–583. [Google Scholar] [CrossRef]
- Palka, C.; Forino, N.M.; Hentschel, J.; Das, R.; Stone, M.D. Folding heterogeneity in the essential human telomerase RNA three-way junction. RNA 2020, 26, 1787–1800. [Google Scholar] [CrossRef]
- Leitao, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Noncoding RNA 2022, 8, 10. [Google Scholar] [CrossRef]
- Partin, A.C.; Zhang, K.; Jeong, B.C.; Herrell, E.; Li, S.; Chiu, W.; Nam, Y. Cryo-EM Structures of Human Drosha and DGCR8 in Complex with Primary MicroRNA. Mol. Cell 2020, 78, 411–422. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Cheng, H.; Ke, X.; Sun, L.; Zhang, Q.C.; Wang, H.W. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-miRNA Substrate. Cell 2018, 173, 1191–1203. [Google Scholar] [CrossRef]
- Lee, H.; Roh, S.H. Cryo-EM structures of human DICER dicing a pre-miRNA substrate. FEBS J. 2024, 291, 3072–3079. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Trinh, T.A.; Bao, S.; Nguyen, T.A. Secondary structure RNA elements control the cleavage activity of DICER. Nat. Commun. 2022, 13, 2138. [Google Scholar] [CrossRef]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science 2009, 326, 1275–1279. [Google Scholar] [CrossRef]
- Tomasello, G.; Armenia, I.; Molla, G. The Protein Imager: A full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 2020, 36, 2909–2911. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.M. Advances in RNA structure analysis by chemical probing. Curr. Opin. Struct. Biol. 2010, 20, 295–304. [Google Scholar] [CrossRef]
- Kubota, M.; Tran, C.; Spitale, R.C. Progress and challenges for chemical probing of RNA structure inside living cells. Nat. Chem. Biol. 2015, 11, 933–941. [Google Scholar] [CrossRef]
- Yu, B.; Li, P.; Zhang, Q.C.; Hou, L. Differential analysis of RNA structure probing experiments at nucleotide resolution: Uncovering regulatory functions of RNA structure. Nat. Commun. 2022, 13, 4227. [Google Scholar] [CrossRef]
- Spitale, R.C.; Flynn, R.A.; Zhang, Q.C.; Crisalli, P.; Lee, B.; Jung, J.W.; Kuchelmeister, H.Y.; Batista, P.J.; Torre, E.A.; Kool, E.T.; et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 2015, 519, 486–490. [Google Scholar] [CrossRef]
- Baek, S.C.; Kim, B.; Jang, H.; Kim, K.; Park, I.S.; Min, D.H.; Kim, V.N. Structural atlas of human primary microRNAs generated by SHAPE-MaP. Mol. Cell 2024, 84, 1158–1172. [Google Scholar] [CrossRef]
- Mustoe, A.M.; Busan, S.; Rice, G.M.; Hajdin, C.E.; Peterson, B.K.; Ruda, V.M.; Kubica, N.; Nutiu, R.; Baryza, J.L.; Weeks, K.M. Pervasive Regulatory Functions of mRNA Structure Revealed by High-Resolution SHAPE Probing. Cell 2018, 173, 181–195. [Google Scholar] [CrossRef]
- Zeller, M.J.; Favorov, O.; Li, K.; Nuthanakanti, A.; Hussein, D.; Michaud, A.; Lafontaine, D.A.; Busan, S.; Serganov, A.; Aube, J.; et al. SHAPE-enabled fragment-based ligand discovery for RNA. Proc. Natl. Acad. Sci. USA 2022, 119, e2122660119. [Google Scholar] [CrossRef]
- Somarowthu, S.; Legiewicz, M.; Chillon, I.; Marcia, M.; Liu, F.; Pyle, A.M. HOTAIR forms an intricate and modular secondary structure. Mol. Cell 2015, 58, 353–361. [Google Scholar] [CrossRef]
- Bugnon, L.A.; Edera, A.A.; Prochetto, S.; Gerard, M.; Raad, J.; Fenoy, E.; Rubiolo, M.; Chorostecki, U.; Gabaldon, T.; Ariel, F.; et al. Secondary structure prediction of long noncoding RNA: Review and experimental comparison of existing approaches. Brief. Bioinform. 2022, 23, bbac205. [Google Scholar] [CrossRef]
- Novikova, I.V.; Hennelly, S.P.; Sanbonmatsu, K.Y. Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res. 2012, 40, 5034–5051. [Google Scholar] [CrossRef]
- Monroy-Eklund, A.; Taylor, C.; Weidmann, C.A.; Burch, C.; Laederach, A. Structural analysis of MALAT1 long noncoding RNA in cells and in evolution. RNA 2023, 29, 691–704. [Google Scholar] [CrossRef]
- Clemson, C.M.; Hutchinson, J.N.; Sara, S.A.; Ensminger, A.W.; Fox, A.H.; Chess, A.; Lawrence, J.B. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 2009, 33, 717–726. [Google Scholar] [CrossRef]
- Lin, Y.; Schmidt, B.F.; Bruchez, M.P.; McManus, C.J. Structural analyses of NEAT1 lncRNAs suggest long-range RNA interactions that may contribute to paraspeckle architecture. Nucleic Acids Res. 2018, 46, 3742–3752. [Google Scholar] [CrossRef]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The lncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Fang, R.; Moss, W.N.; Rutenberg-Schoenberg, M.; Simon, M.D. Probing Xist RNA Structure in Cells Using Targeted Structure-Seq. PLoS Genet. 2015, 11, e1005668. [Google Scholar] [CrossRef]
- Shcherbakova, I.; Brenowitz, M. Perturbation of the hierarchical folding of a large RNA by the destabilization of its Scaffold’s tertiary structure. J. Mol. Biol. 2005, 354, 483–496. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, B.; Ding, X.; Liu, T.; Hu, X.; Yip, K.Y.; Yang, Z.R.; Mathews, D.H.; Lu, Z.J. Improved prediction of RNA secondary structure by integrating the free energy model with restraints derived from experimental probing data. Nucleic Acids Res. 2015, 43, 7247–7259. [Google Scholar] [CrossRef]
- Reeder, J.; Hochsmann, M.; Rehmsmeier, M.; Voss, B.; Giegerich, R. Beyond Mfold: Recent advances in RNA bioinformatics. J. Biotechnol. 2006, 124, 41–55. [Google Scholar] [CrossRef]
- Hofacker, I.L. RNA secondary structure analysis using the Vienna RNA package. Curr. Protoc. 2009, 26, 12.2.1–12.2.16. [Google Scholar] [CrossRef]
- Ali, S.E.; Mittal, A.; Mathews, D.H. RNA Secondary Structure Analysis Using RNAstructure. Curr. Protoc. 2023, 3, e846. [Google Scholar] [CrossRef]
- Ding, Y.; Chan, C.Y.; Lawrence, C.E. Sfold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Res. 2004, 32, W135–W141. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Fernandez, N.; Cordiner, R.A.; Young, R.S.; Hug, N.; Macias, S.; Caceres, J.F. Genetic variation and RNA structure regulate microRNA biogenesis. Nat. Commun. 2017, 8, 15114. [Google Scholar] [CrossRef]
- Long, D.; Lee, R.; Williams, P.; Chan, C.Y.; Ambros, V.; Ding, Y. Potent effect of target structure on microRNA function. Nat. Struct. Mol. Biol. 2007, 14, 287–294. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef]
- Ontiveros-Palacios, N.; Cooke, E.; Nawrocki, E.P.; Triebel, S.; Marz, M.; Rivas, E.; Griffiths-Jones, S.; Petrov, A.I.; Bateman, A.; Sweeney, B. Rfam 15: RNA families database in 2025. Nucleic Acids Res. 2024, 53, D258–D267. [Google Scholar] [CrossRef]
- Sato, K.; Hamada, M. Recent trends in RNA informatics: A review of machine learning and deep learning for RNA secondary structure prediction and RNA drug discovery. Brief. Bioinform. 2023, 24, bbad186. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, Z.; Fan, X.; Yuan, Z.; Mao, Q.; Yao, Y. Review of machine learning methods for RNA secondary structure prediction. PLoS Comput. Biol. 2021, 17, e1009291. [Google Scholar] [CrossRef]
- Singh, J.; Hanson, J.; Paliwal, K.; Zhou, Y. RNA secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning. Nat. Commun. 2019, 10, 5407. [Google Scholar] [CrossRef]
- Townshend, R.J.L.; Eismann, S.; Watkins, A.M.; Rangan, R.; Karelina, M.; Das, R.; Dror, R.O. Geometric deep learning of RNA structure. Science 2021, 373, 1047–1051. [Google Scholar] [CrossRef]
- Miao, Z.; Westhof, E. RNA Structure: Advances and Assessment of 3D Structure Prediction. Annu. Rev. Biophys. 2017, 46, 483–503. [Google Scholar] [CrossRef]
- Thiel, B.C.; Flamm, C.; Hofacker, I.L. RNA structure prediction: From 2D to 3D. Emerg. Top. Life Sci. 2017, 1, 275–285. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.J. RNA 3D Structure Prediction Using Coarse-Grained Models. Front. Mol. Biosci. 2021, 8, 720937. [Google Scholar] [CrossRef]
- Kryshtafovych, A.; Schwede, T.; Topf, M.; Fidelis, K.; Moult, J. Critical assessment of methods of protein structure prediction (CASP)-Round XV. Proteins 2023, 91, 1539–1549. [Google Scholar] [CrossRef]
- Miao, Z.; Adamiak, R.W.; Antczak, M.; Boniecki, M.J.; Bujnicki, J.; Chen, S.J.; Cheng, C.Y.; Cheng, Y.; Chou, F.C.; Das, R.; et al. RNA-Puzzles Round IV: 3D structure predictions of four ribozymes and two aptamers. RNA 2020, 26, 982–995. [Google Scholar] [CrossRef]
- Rother, M.; Milanowska, K.; Puton, T.; Jeleniewicz, J.; Rother, K.; Bujnicki, J.M. ModeRNA server: An online tool for modeling RNA 3D structures. Bioinformatics 2011, 27, 2441–2442. [Google Scholar] [CrossRef]
- Parisien, M.; Major, F. The MC-Fold and MC-Sym pipeline infers RNA structure from sequence data. Nature 2008, 452, 51–55. [Google Scholar] [CrossRef]
- Watkins, A.M.; Das, R. RNA 3D Modeling with FARFAR2, Online. Methods Mol. Biol. 2023, 2586, 233–249. [Google Scholar] [CrossRef]
- Watkins, A.M.; Rangan, R.; Das, R. FARFAR2: Improved De Novo Rosetta Prediction of Complex Global RNA Folds. Structure 2020, 28, 963–976. [Google Scholar] [CrossRef]
- Biesiada, M.; Purzycka, K.J.; Szachniuk, M.; Blazewicz, J.; Adamiak, R.W. Automated RNA 3D Structure Prediction with RNAComposer. Methods Mol. Biol. 2016, 1490, 199–215. [Google Scholar] [CrossRef]
- Biesiada, M.; Pachulska-Wieczorek, K.; Adamiak, R.W.; Purzycka, K.J. RNAComposer and RNA 3D structure prediction for nanotechnology. Methods 2016, 103, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Purzycka, K.J.; Popenda, M.; Szachniuk, M.; Antczak, M.; Lukasiak, P.; Blazewicz, J.; Adamiak, R.W. Automated 3D RNA structure prediction using the RNAComposer method for riboswitches. Methods Enzymol. 2015, 553, 3–34. [Google Scholar] [CrossRef]
- Sarzynska, J.; Popenda, M.; Antczak, M.; Szachniuk, M. RNA tertiary structure prediction using RNAComposer in CASP15. Proteins 2023, 91, 1790–1799. [Google Scholar] [CrossRef]
- Palermo, G.; Casalino, L.; Magistrato, A.; Andrew McCammon, J. Understanding the mechanistic basis of non-coding RNA through molecular dynamics simulations. J. Struct. Biol. 2019, 206, 267–279. [Google Scholar] [CrossRef]
- Kuhrova, P.; Mlynsky, V.; Zgarbova, M.; Krepl, M.; Bussi, G.; Best, R.B.; Otyepka, M.; Sponer, J.; Banas, P. Improving the Performance of the Amber RNA Force Field by Tuning the Hydrogen-Bonding Interactions. J. Chem. Theory Comput. 2019, 15, 3288–3305. [Google Scholar] [CrossRef]
- Tan, D.; Piana, S.; Dirks, R.M.; Shaw, D.E. RNA force field with accuracy comparable to state-of-the-art protein force fields. Proc. Natl. Acad. Sci. USA 2018, 115, E1346–E1355. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kaur, S.; Baronti, L.; Petzold, K.; Chen, A.A. A two-dimensional replica-exchange molecular dynamics method for simulating RNA folding using sparse experimental restraints. Methods 2019, 162–163, 96–107. [Google Scholar] [CrossRef]
- Bell, D.R.; Cheng, S.Y.; Salazar, H.; Ren, P. Capturing RNA Folding Free Energy with Coarse-Grained Molecular Dynamics Simulations. Sci. Rep. 2017, 7, 45812. [Google Scholar] [CrossRef]
- Dawson, W.K.; Maciejczyk, M.; Jankowska, E.J.; Bujnicki, J.M. Coarse-grained modeling of RNA 3D structure. Methods 2016, 103, 138–156. [Google Scholar] [CrossRef]
- Paciello, G.; Acquaviva, A.; Ficarra, E.; Deriu, M.A.; Macii, E. A molecular dynamics study of a miRNA:mRNA interaction. J. Mol. Model. 2011, 17, 2895–2906. [Google Scholar] [CrossRef]
- Xia, Z.; Clark, P.; Huynh, T.; Loher, P.; Zhao, Y.; Chen, H.W.; Ren, P.; Rigoutsos, I.; Zhou, R. Molecular dynamics simulations of Ago silencing complexes reveal a large repertoire of admissible ‘seed-less’ targets. Sci. Rep. 2012, 2, 569. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, A.L.; Enguita, F.J. The Unpaved Road of Non-Coding RNA Structure–Function Relationships: Current Knowledge, Available Methodologies, and Future Trends. Non-Coding RNA 2025, 11, 20. https://doi.org/10.3390/ncrna11020020
Leitão AL, Enguita FJ. The Unpaved Road of Non-Coding RNA Structure–Function Relationships: Current Knowledge, Available Methodologies, and Future Trends. Non-Coding RNA. 2025; 11(2):20. https://doi.org/10.3390/ncrna11020020
Chicago/Turabian StyleLeitão, Ana Lúcia, and Francisco J. Enguita. 2025. "The Unpaved Road of Non-Coding RNA Structure–Function Relationships: Current Knowledge, Available Methodologies, and Future Trends" Non-Coding RNA 11, no. 2: 20. https://doi.org/10.3390/ncrna11020020
APA StyleLeitão, A. L., & Enguita, F. J. (2025). The Unpaved Road of Non-Coding RNA Structure–Function Relationships: Current Knowledge, Available Methodologies, and Future Trends. Non-Coding RNA, 11(2), 20. https://doi.org/10.3390/ncrna11020020