MicroRNAs: A Novel Approach for Monitoring Treatment Response in Major Depressive Disorder?
Abstract
1. Introduction
2. MicroRNAs in Major Depressive Disorder
3. MicroRNAs in Monitoring Treatment Response
3.1. Tricyclic Antidepressants
3.2. Serotonin Selective Reuptake Inhibitor Antidepressants
3.2.1. Fluoxetine
3.2.2. Fluvoxamine
3.2.3. Sertraline
3.2.4. Paroxetine
3.2.5. Citalopram
3.2.6. Escitalopram
3.3. Serotonin and Noradrenaline Reuptake Inhibitor Antidepressants
3.3.1. Venlafaxine
3.3.2. Duloxetine
3.4. Mirtazapine
3.5. Electroconvulsive Therapy
4. Adequate Sampling for miRNAs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Zhang, L.; Yang, C.; Li, X.; Li, Z. Global, Regional, and National Epidemiology of Depression in Working-Age Individuals, 1990–2019. Depress. Anxiety 2024, 2024, 4747449. [Google Scholar] [CrossRef]
- Yang, C.-M.; Lee, S.-Y. Effect of Untreated Depression in Adolescence on the Suicide Risk and Attempt in Male Young Adults. Korean J. Psychosom. Med. 2020, 28, 29–35. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant Depression: Definition, Prevalence, Detection, Management, and Investigational Interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Kaurani, L. Clinical Insights into MicroRNAs in Depression: Bridging Molecular Discoveries and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 2866. [Google Scholar] [CrossRef] [PubMed]
- Luoni, A.; Riva, M.A. MicroRNAs and Psychiatric Disorders: From Aetiology to Treatment. Pharmacol. Ther. 2016, 167, 13–27. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Dinan, T.G.; Cryan, J.F. Little Things on Which Happiness Depends: MicroRNAs as Novel Therapeutic Targets for the Treatment of Anxiety and Depression. Mol. Psychiatry 2012, 17, 359–376. [Google Scholar] [CrossRef]
- Hansen, K.F.; Obrietan, K. MicroRNA as Therapeutic Targets for Treatment of Depression. Neuropsychiatr. Dis. Treat. 2013, 9, 1011–1021. [Google Scholar] [CrossRef]
- Dwivedi, Y. Emerging Role of MicroRNAs in Major Depressive Disorder: Diagnosis and Therapeutic Implications. Dialogues Clin. Neurosci. 2014, 16, 43–61. [Google Scholar] [CrossRef]
- Cai, L.; Xu, J.; Liu, J.; Luo, H.; Yang, R.; Gui, X.; Wei, L. MiRNAs in Treatment-Resistant Depression: A Systematic Review. Mol. Biol. Rep. 2024, 51, 638. [Google Scholar] [CrossRef]
- Kamran Ameer, M.; Ikram, M.; Imran Khan, M.; Wahab, F.; Imran Naseer, M.; Ullah, N. Emerging Biomarkers in Major Depressive Disorder: Diagnostic, Prognostic, and Therapeutic Implications. INNOSC Theranostics Pharmacol. Sci. 2025, 4404. [Google Scholar] [CrossRef]
- Cătană, C.-S.; Crișan, C.-A.; Opre, D.; Berindan-Neagoe, I. Diagnostic and Prognostic Value of MicroRNAs for Alzheimer’s Disease: A Comprehensive Meta-Analysis. Med. Pharm. Rep. 2020, 93, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.B.; Liu, J.J.; Villaescusa, J.C.; Åberg, E.; Brené, S.; Wegener, G.; Mathé, A.A.; Lavebratt, C. Elevation of Il6 Is Associated with Disturbed Let-7 Biogenesis in a Genetic Model of Depression. Transl. Psychiatry 2016, 6, e869. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.A.; Lima-Bastos, S.; Gobira, P.H.; Lisboa, S.F. Endocannabinoid Signaling and Epigenetics Modifications in the Neurobiology of Stress-Related Disorders. Neuronal Signal. 2023, 7, NS20220034. [Google Scholar] [CrossRef]
- Bahi, A.; Dreyer, J.-L. Lentiviral-Mediated Let-7d MicroRNA Overexpression Induced Anxiolytic- and Anti-Depressant-like Behaviors and Impaired Dopamine D3 Receptor Expression. Eur. Neuropsychopharmacol. 2018, 28, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Magri, C.; Barbon, A.; Bonvicini, C.; Segala, M.; Congiu, C.; Bignotti, S.; Milanesi, E.; Trabucchi, L.; Cattane, N.; et al. Proteasome System Dysregulation and Treatment Resistance Mechanisms in Major Depressive Disorder. Transl. Psychiatry 2015, 5, e687. [Google Scholar] [CrossRef]
- Cattaneo, A.; Ferrari, C.; Turner, L.; Mariani, N.; Enache, D.; Hastings, C.; Kose, M.; Lombardo, G.; McLaughlin, A.P.; Nettis, M.A.; et al. Whole-Blood Expression of Inflammasome- and Glucocorticoid-Related MRNAs Correctly Separates Treatment-Resistant Depressed Patients from Drug-Free and Responsive Patients in the BIODEP Study. Transl. Psychiatry 2020, 10, 232. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Franco Guerreiro-Costa, L.N.; Lins-Silva, D.; Faria Guimaraes, D.; Souza, L.S.; Leal, G.C.; Caliman-Fontes, A.T.; Beanes, G.; Costa, R.D.S.; Quarantini, L.C. MicroRNAs as Diagnostic Biomarkers and Predictors of Antidepressant Response in Major Depressive Disorder: A Systematic Review. Cureus 2024, 16, e56910. [Google Scholar] [CrossRef]
- Ding, R.; Su, D.; Zhao, Q.; Wang, Y.; Wang, J.-Y.; Lv, S.; Ji, X. The Role of MicroRNAs in Depression. Front. Pharmacol. 2023, 14, 1129186. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Wang, L.; Lan, T.; Gao, R.; Wang, W.; Yu, S.Y. MicroRNA-26a-3p Rescues Depression-like Behaviors in Male Rats via Preventing Hippocampal Neuronal Anomalies. J. Clin. Investig. 2021, 131, e148853. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.; Ding, Y.M.; Gui, X.W.; Wu, L.X.; Tian, S.; Wu, W. MicroRNA-26a-2 Maintains Stress Resiliency and Antidepressant Efficacy by Targeting the Serotonergic Autoreceptor HTR1A. Biochem. Biophys. Res. Commun. 2019, 511, 440–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Chu, Z.; Dang, Y.; Zhu, J.; Su, X. MicroRNA-101 in the Ventrolateral Orbital Cortex (VLO) Modulates Depressive-like Behaviors in Rats and Targets Dual-Specificity Phosphatase 1 (DUSP1). Brain Res. 2017, 1669, 55–62. [Google Scholar] [CrossRef]
- Issler, O.; Haramati, S.; Paul, E.D.; Maeno, H.; Navon, I.; Zwang, R.; Gil, S.; Mayberg, H.S.; Dunlop, B.W.; Menke, A.; et al. MicroRNA 135 Is Essential for Chronic Stress Resiliency, Antidepressant Efficacy, and Intact Serotonergic Activity. Neuron 2014, 83, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Chang, J. The Important Roles of MicroRNAs in Depression: New Research Progress and Future Prospects. J. Mol. Med. 2021, 99, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wei, S.; Luo, M.; Tang, Z.; Lin, Q.; Wang, X.; Luo, M.; He, Y.; Wang, C.; Wei, D.; et al. MiR-139-5p Has an Antidepressant-like Effect by Targeting Phosphodiesterase 4D to Activate the CAMP/PKA/CREB Signaling Pathway. Ann. Transl. Med. 2021, 9, 1594. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Pan, J.; Wang, X.; Zhao, Y.; Guo, Z. Hippocampal MiRNA-144 Modulates Depressive-Like Behaviors in Rats by Targeting PTP1B. Neuropsychiatr. Dis. Treat. 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Wang, P.; Li, X.; Song, Z.; Wei, C.; Zhang, Q.; Luo, B.; Liu, Z.; Yang, Y.; et al. Clinical and Preclinical Evaluation of MiR-144-5p as a Key Target for Major Depressive Disorder. CNS Neurosci. Ther. 2023, 29, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yuan, P.; Wang, Y.; Hunsberger, J.G.; Elkahloun, A.; Wei, Y.; Damschroder-Williams, P.; Du, J.; Chen, G.; Manji, H.K. Evidence for Selective MicroRNAs and Their Effectors as Common Long-Term Targets for the Actions of Mood Stabilizers. Neuropsychopharmacology 2009, 34, 1395–1405. [Google Scholar] [CrossRef]
- Lou, D.; Wang, J.; Wang, X. MiR-124 Ameliorates Depressive-like Behavior by Targeting STAT3 to Regulate Microglial Activation. Mol. Cell. Probes 2019, 48, 101470. [Google Scholar] [CrossRef]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased Microglial Exosomal MiR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after RmTBI. Mol. Ther. 2020, 28, 503–522. [Google Scholar] [CrossRef]
- Anand, S.; Foot, N.; Ang, C.; Gembus, K.M.; Keerthikumar, S.; Adda, C.G.; Mathivanan, S.; Kumar, S. Arrestin-Domain Containing Protein 1 (Arrdc1) Regulates the Protein Cargo and Release of Extracellular Vesicles. Proteomics 2018, 18, e1800266. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–Syntenin–ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting during Melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Lin, C.-C.; Tsai, M.-C.; Lee, C.-T.; Sun, M.-H.; Huang, T.-L. Antidepressant Treatment Increased Serum MiR-183 and MiR-212 Levels in Patients with Major Depressive Disorder. Psychiatry Res. 2018, 270, 232–237. [Google Scholar] [CrossRef]
- Hung, Y.Y.; Chou, C.K.; Yang, Y.C.; Fu, H.C.; Loh, E.W.; Kang, H.Y. Exosomal Let-7e, Mir-21-5p, Mir-145, Mir-146a and Mir-155 in Predicting Antidepressants Response in Patients with Major Depressive Disorder. Biomedicines 2021, 9, 1428. [Google Scholar] [CrossRef]
- Goossens, J.; Morrens, M.; Coppens, V. The Potential Use of Peripheral Blood Mononuclear Cells as Biomarkers for Treatment Response and Outcome Prediction in Psychiatry: A Systematic Review. Mol. Diagn. Ther. 2021, 25, 283–299. [Google Scholar] [CrossRef]
- Grosse, L.; Carvalho, L.A.; Birkenhager, T.K.; Hoogendijk, W.J.; Kushner, S.A.; Drexhage, H.A.; Bergink, V. Circulating Cytotoxic T Cells and Natural Killer Cells as Potential Predictors for Antidepressant Response in Melancholic Depression. Restoration of T Regulatory Cell Populations after Antidepressant Therapy. Psychopharmacology 2016, 233, 1679–1688. [Google Scholar] [CrossRef]
- Weizman, R.; Laor, N.; Podliszewski, E.; Notti, I.; Djaldetti, M.; Bessler, H. Cytokine Production in Major Depressed Patients before and after Clomipramine Treatment. Biol. Psychiatry 1994, 35, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 Inflammasome Is Activated in Mononuclear Blood Cells from Patients with Major Depressive Disorder. Brain. Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.M.; Kellermann, O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef]
- Lo Iacono, L.; Ielpo, D.; Parisi, C.; Napoli, G.; Accoto, A.; Di Segni, M.; Babicola, L.; D’Addario, S.L.; Guzzo, S.M.; Pascucci, T.; et al. MicroRNA-34a Regulates 5-HT2C Expression in Dorsal Raphe and Contributes to the Anti-Depressant-like Effect of Fluoxetine. Neuropharmacology 2021, 190, 108559. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Gao, K.; Shen, B.; Zhang, K.; Zhang, Z.; Wang, C. MicroRNA-135b-5p Downregulation Causes Antidepressant Effects by Regulating SIRT1 Expression. Biochem. Genet. 2021, 59, 1582–1598. [Google Scholar] [CrossRef]
- Silver, H.; Susser, E.; Danovich, L.; Bilker, W.; Youdim, M.; Goldin, V.; Weinreb, O. SSRI Augmentation of Antipsychotic Alters Expression of GABA A Receptor and Related Genes in PMC of Schizophrenia Patients. Int. J. Neuropsychopharmacol. 2011, 14, 573–584. [Google Scholar] [CrossRef]
- Ahmadimanesh, M.; Etemad, L.; Rad, D.M.; Ghahremani, M.H.; Mohammadpour, A.H.; Esfehani, R.J.; Jowsey, P.; Behdani, F.; Moallem, S.A.; Abbaszadegan, M.R. Effect of Citalopram and Sertraline on the Expression of MiRNA- 124, 132, and 16 and Their Protein Targets in Patients with Depression. Iran. J. Basic Med. Sci. 2023, 26, 820–829. [Google Scholar] [CrossRef]
- Kuang, W.H.; Dong, Z.Q.; Tian, L.T.; Li, J. MicroRNA-451a, MicroRNA-34a-5p, and MicroRNA-221-3p as Predictors of Response to Antidepressant Treatment. Braz. J. Med. Biol. Res. 2018, 51, 1–9. [Google Scholar] [CrossRef]
- Lopez, J.P.; Pereira, F.; Richard-Devantoy, S.; Berlim, M.; Chachamovich, E.; Fiori, L.M.; Niola, P.; Turecki, G.; Jollant, F. Co-Variation of Peripheral Levels of MiR-1202 and Brain Activity and Connectivity during Antidepressant Treatment. Neuropsychopharmacology 2017, 42, 2043–2051. [Google Scholar] [CrossRef]
- Fang, Y.; Qiu, Q.; Zhang, S.; Sun, L.; Li, G.; Xiao, S.; Li, X. Changes in MiRNA-132 and MiR-124 Levels in Non-Treated and Citalopram-Treated Patients with Depression. J. Affect. Disord. 2018, 227, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Zhao, J.; Liu, C.; Qu, X.; Li, Y. MiR-155 Is Involved in Major Depression Disorder and Antidepressant Treatment via Targeting SIRT1. Biosci. Rep. 2018, 38, BSR20181139. [Google Scholar] [CrossRef]
- Bocchio-Chiavetto, L.; Maffioletti, E.; Bettinsoli, P.; Giovannini, C.; Bignotti, S.; Tardito, D.; Corrada, D.; Milanesi, L.; Gennarelli, M. Blood MicroRNA Changes in Depressed Patients during Antidepressant Treatment. Eur. Neuropsychopharmacol. 2013, 23, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Bocchio-Chiavetto, L.; Zanardini, R.; Milanesi, E.; Placentino, A.; Gennarelli, M. Reduced Peripheral Brain-Derived Neurotrophic Factor MRNA Levels Are Normalized by Antidepressant Treatment. Int. J. Neuropsychopharmacol. 2010, 13, 103–108. [Google Scholar] [CrossRef]
- Enatescu, V.R.; Papava, I.; Enatescu, I.; Antonescu, M.; Anghel, A.; Seclaman, E.; Sirbu, I.O.; Marian, C. Circulating Plasma Micro RNAs in Patients with Major Depressive Disorder Treated with Antidepressants: A Pilot Study. Psychiatry Investig. 2016, 13, 549–557. [Google Scholar] [CrossRef]
- Marshe, V.S.; Islam, F.; Maciukiewicz, M.; Fiori, L.M.; Yerko, V.; Yang, J.; Turecki, G.; Foster, J.A.; Kennedy, S.H.; Blumberger, D.M.; et al. Validation Study of MicroRNAs Previously Associated with Antidepressant Response in Older Adults Treated for Late-Life Depression with Venlafaxine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109867. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Wu, X.-Y.; Jin, X.; Sheng, X.-M.; Fan, Y. MiR-204-5p Plays a Critical Role in the Pathogenesis of Depression and Anti-Depression Action of Venlafaxine in the Hippocampus of Mice. Curr. Med. Chem. 2024, 31, 3412–3425. [Google Scholar] [CrossRef]
- Pan, B.; Liu, Y. Effects of Duloxetine on MicroRNA Expression Profile in Frontal Lobe and Hippocampus in a Mouse Model of Depression. Int. J. Clin. Exp. Pathol. 2015, 8, 15454–15461. [Google Scholar] [PubMed]
- Lopez, J.P.; Fiori, L.M.; Cruceanu, C.; Lin, R.; Labonte, B.; Cates, H.M.; Heller, E.A.; Vialou, V.; Ku, S.M.; Gerald, C.; et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p Are Markers of Antidepressant Response and Regulate MAPK/Wnt-System Genes. Nat. Commun. 2017, 8, 15497. [Google Scholar] [CrossRef]
- Fiori, L.M.; Lopez, J.P.; Richard-Devantoy, S.; Berlim, M.; Chachamovich, E.; Jollant, F.; Foster, J.; Rotzinger, S.; Kennedy, S.H.; Turecki, G. Investigation of MiR-1202, MiR-135a, and MiR-16 in Major Depressive Disorder and Antidepressant Response. Int. J. Neuropsychopharmacol. 2017, 20, 619–623. [Google Scholar] [CrossRef]
- Kato, M.; Ogata, H.; Tahara, H.; Shimamoto, A.; Takekita, Y.; Koshikawa, Y.; Nishida, K.; Nonen, S.; Higasa, K.; Kinoshita, T. Multiple Pre-Treatment MiRNAs Levels in Untreated Major Depressive Disorder Patients Predict Early Response to Antidepressants and Interact with Key Pathways. Int. J. Mol. Sci. 2022, 23, 3873. [Google Scholar] [CrossRef]
- McGrory, C.L.; Ryan, K.M.; Kolshus, E.; McLoughlin, D.M. Peripheral Blood E2F1 MRNA in Depression and Following Electroconvulsive Therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Israel-Elgali, I.; Hertzberg, L.; Shapira, G.; Segev, A.; Krieger, I.; Nitzan, U.; Bloch, Y.; Pillar, N.; Mayer, O.; Weizman, A.; et al. Blood Transcriptional Response to Treatment-Resistant Depression during Electroconvulsive Therapy. J. Psychiatr. Res. 2021, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Y.; Xu, G.; Geng, B.; Cui, Q. Transcriptome Analysis Reveals Non-Identical MicroRNA Profiles between Arterial and Venous Plasma. Oncotarget 2017, 8, 28471–28480. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Zhu, W.; Xu, J.; Guo, R.; Cheng, W.; Wang, F.; Qi, L.-W.; Chen, Y.; et al. A Six-MicroRNA Panel in Plasma Was Identified as a Potential Biomarker for Lung Adenocarcinoma Diagnosis. Oncotarget 2017, 8, 6513–6525. [Google Scholar] [CrossRef]
- Monzo, M.; Santasusagna, S.; Moreno, I.; Martinez, F.; Hernández, R.; Muñoz, C.; Castellano, J.J.; Moreno, J.; Navarro, A. Exosomal MicroRNAs Isolated from Plasma of Mesenteric Veins Linked to Liver Metastases in Resected Patients with Colon Cancer. Oncotarget 2017, 8, 30859–30869. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.-H.; Kwak, T.; Kim, H.; Jeon, S.; Bae, S.; Yang, Y.; Son, S.; Cha, W.; Yang, M.; Lee, E.; et al. A New Quantitative Real-Time PCR Method to Measure Human MiRNAs Using the PROMER Technology. Biochem. Biophys. Res. Commun. 2024, 741, 151069. [Google Scholar] [CrossRef]
- Metcalf, G.A.D. MicroRNAs: Circulating Biomarkers for the Early Detection of Imperceptible Cancers via Biosensor and Machine-Learning Advances. Oncogene 2024, 43, 2135–2142. [Google Scholar] [CrossRef]
- Hu, P.; Cao, Q.; Feng, H.; Liu, Y.; Chen, Y.; Xu, J.; Feng, W.; Sun, H.; Ding, H.; Wang, C.; et al. MicroRNA-451a Is a Candidate Biomarker and Therapeutic Target for Major Depressive Disorder. Gen. Psychiatry 2024, 37, e101291. [Google Scholar] [CrossRef]
- He, J.; Xie, J.; Zhou, G.; Jia, C.; Han, D.; Li, D.; Wei, J.; Li, Y.; Huang, R.; Li, C.; et al. Active Fraction of Polyrhachis Vicina Roger (AFPR) Ameliorate Depression Induced Inflammation Response by FTO/MiR-221-3p/SOCS1 Axis. J. Inflamm. Res. 2023, 16, 6329–6348. [Google Scholar] [CrossRef]
- Webb, L.M.; Phillips, K.E.; Ho, M.C.; Veldic, M.; Blacker, C.J. The Relationship between DNA Methylation and Antidepressant Medications: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 826. [Google Scholar] [CrossRef]
- Inserra, A.; Campanale, A.; Rezai, T.; Romualdi, P.; Rubino, T. Epigenetic Mechanisms of Rapid-Acting Antidepressants. Transl. Psychiatry 2024, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Duclot, F.; Kabbaj, M. Epigenetic Mechanisms Underlying the Role of Brain-Derived Neurotrophic Factor in Depression and Response to Antidepressants. J. Exp. Biol. 2015, 218, 21–31. [Google Scholar] [CrossRef]
- Serafini, G.; Trabucco, A.; Corsini, G.; Escelsior, A.; Amerio, A.; Aguglia, A.; Nasrallah, H.; Amore, M. The Potential of MicroRNAs as Putative Biomarkers in Major Depressive Disorder and Suicidal Behavior. Biomark. Neuropsychiatry 2021, 5, 100035. [Google Scholar] [CrossRef]
- Wu, M.-S.; Li, X.-J.; Liu, C.-Y.; Xu, Q.; Huang, J.-Q.; Gu, S.; Chen, J.-X. Effects of Histone Modification in Major Depressive Disorder. Curr. Neuropharmacol. 2021, 20, 1261–1277. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Alkaraki, A.K.; Gammoh, O.; Tambuwala, M.M.; Mishra, V.; Mishra, Y.; Hassan, S.S.; El-Tanani, M. Deciphering Depression: Epigenetic Mechanisms and Treatment Strategies. Biology 2024, 13, 638. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Li, Y.; Fan, C.; Wang, L.; Wang, W.; Chen, S.; Yu, S.Y. MicroRNA-204-5p Reduction in Rat Hippocampus Contributes to Stress-Induced Pathology via Targeting RGS12 Signaling Pathway. J. Neuroinflamm. 2021, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, Y.; Zhang, K.; Wu, Y.; Li, Y.; Lan, T.; Zhuang, X.; Chen, S.; Yu, S. MicroRNA-204-5p Deficiency within the VmPFC Region Contributes to Neuroinflammation and Behavioral Disorders via the JAK2/STAT3 Signaling Pathway in Rats. Adv. Sci. 2025, 2403080. [Google Scholar] [CrossRef]
- Buch, A.M.; Liston, C. Dissecting Diagnostic Heterogeneity in Depression by Integrating Neuroimaging and Genetics. Neuropsychopharmacology 2021, 46, 156–175. [Google Scholar] [CrossRef]
- Kuehner, C. Why Is Depression More Common among Women than among Men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef] [PubMed]
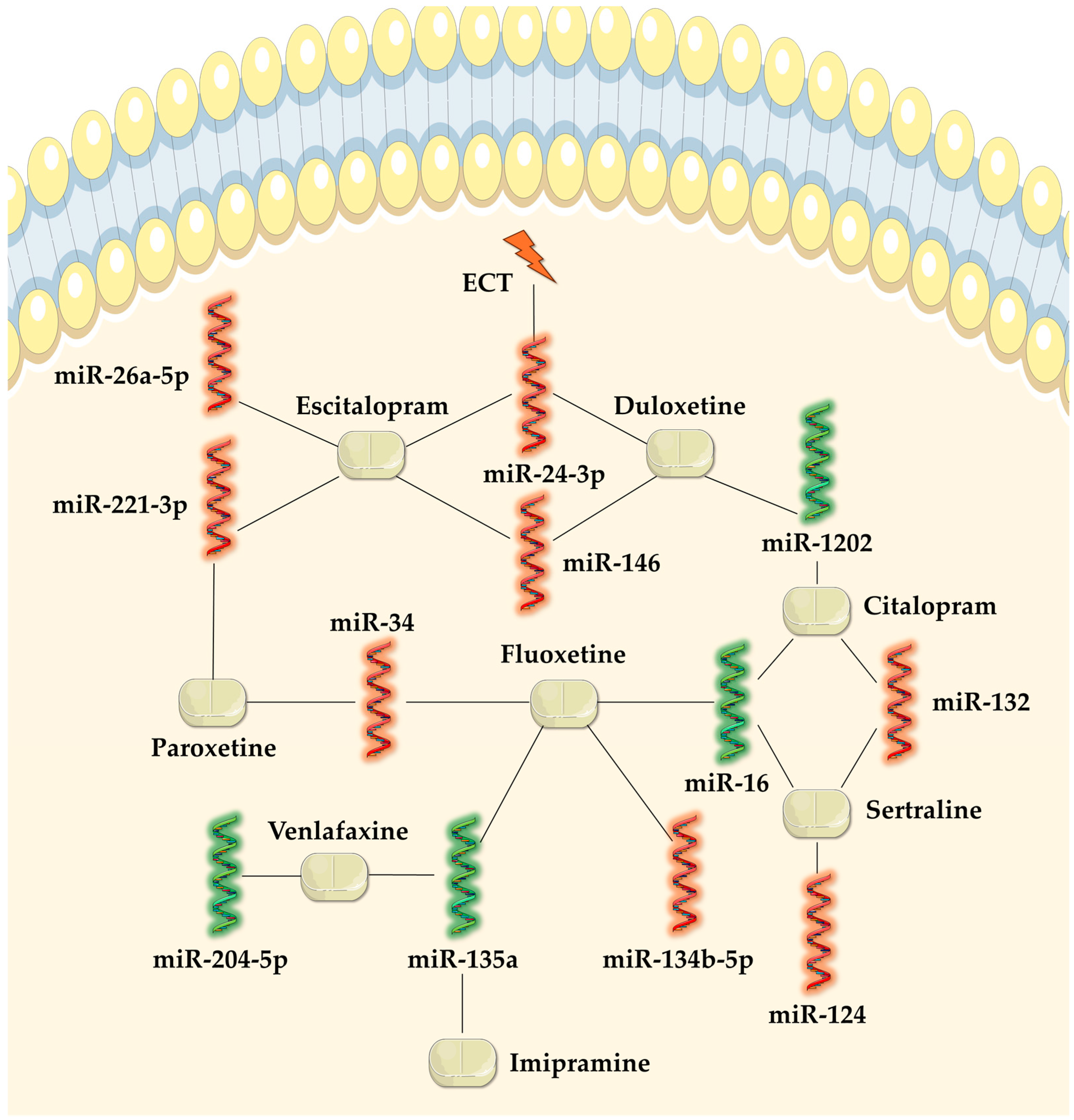
| Antidepressant Class | Relation to miRNAs | Mechanisms of Modulated miRNAs in MDD Without Antidepressant Treatment | References | |
|---|---|---|---|---|
| TCA | ↑ miR-135a | Downregulated in MDD, resulting in a decreased expression of the SERT and 5HT-1A receptor in the raphe nucleus and contributing to reduced serotonergic transmission. Overexpression also maintained the depressive-like phenotype. | [22,41] | |
| SSRI | ||||
| ↓ miR-34a | Upregulated in MDD, resulting in a decreased expression of the 5HT2C receptor in the dorsal raphe nucleus. | [45] | ||
| ↓ miR-135b-5p | Upregulated in MDD, leading to a dysfunctional expression of the SIRT1 gene, which maintains a depressive-like behavior. | [46] | ||
| ↑ miR-16 | Downregulated in MDD, leading to a decreased SERT expression and impaired serotonergic transmission. | [44,48] | ||
| ↑ miR-451a | Downregulated expression in a CRS mouse model. Its reduced expression may affect dendritic spine plasticity through poor inhibition of the CRS-induced corticotropin-releasing factor receptor 1 expression. | [49,70] | ||
| ↓ miR-155 | Upregulated in MDD, leading to a dysfunctional expression of the SIRT1 gene, which maintains a depressive-like behavior. | [52] | ||
| ↓ miR-124 | Upregulated in MDD, reducing the expression of the BDNF gene. | [48] | ||
| ↓ miR-132 | [48,51] | |||
| ↓ miR-221-3p | Upregulated expression in MDD was correlated with a decreased expression of SOCS1 (cytokine inhibitory signaling protein), which led to increased cellular inflammation. | [49,55,71] | ||
| ↓ miR-146 | Upregulated in MDD and involved in several signaling pathways (Wnt, cancer, endocytosis, axon guidance, and MAPK). | [55,59] | ||
| ↓ miR-24-3p | [55,59,63] | |||
| ↓ miR-26-5p | [55] | |||
| SNRI | ↑ miR-1202 | Affected the expression of metabotropic receptor GRM4, thus regulating the glutamatergic system. Decreased peripheral levels reduced the antidepressant response and maintained depressive symptoms. | [50,60] | |
| ↓ miR-204-5p | Upregulated expression was associated with reduced BDNF gene expression. | [57] | ||
| ↓ miR-425-3p | Upregulated in MDD and involved in the MAPK and Wnt signaling pathways. | [59] | ||
| ↑ miR-135a | Downregulated in MDD, resulting in a decreased expression of the SERT and 5HT-1A receptor in the raphe nucleus and contributing to reduced serotonergic transmission. Overexpression also maintained the depressive-like phenotype. | [22,41] | ||
| ↓ miR-146 | Upregulated in MDD and involved in several signaling pathways (Wnt, cancer, endocytosis, axon guidance, and MAPK). | [55,59] | ||
| ↓ miR-24-3p | [55,59,63] | |||
| ECT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cătană, C.-S.; Marta, M.M.; Ungureanu, D.; Crișan, C.-A. MicroRNAs: A Novel Approach for Monitoring Treatment Response in Major Depressive Disorder? Non-Coding RNA 2025, 11, 21. https://doi.org/10.3390/ncrna11020021
Cătană C-S, Marta MM, Ungureanu D, Crișan C-A. MicroRNAs: A Novel Approach for Monitoring Treatment Response in Major Depressive Disorder? Non-Coding RNA. 2025; 11(2):21. https://doi.org/10.3390/ncrna11020021
Chicago/Turabian StyleCătană, Cristina-Sorina, Monica Mihaela Marta, Daniel Ungureanu, and Cătălina-Angela Crișan. 2025. "MicroRNAs: A Novel Approach for Monitoring Treatment Response in Major Depressive Disorder?" Non-Coding RNA 11, no. 2: 21. https://doi.org/10.3390/ncrna11020021
APA StyleCătană, C.-S., Marta, M. M., Ungureanu, D., & Crișan, C.-A. (2025). MicroRNAs: A Novel Approach for Monitoring Treatment Response in Major Depressive Disorder? Non-Coding RNA, 11(2), 21. https://doi.org/10.3390/ncrna11020021







