The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology
Abstract
1. Introduction
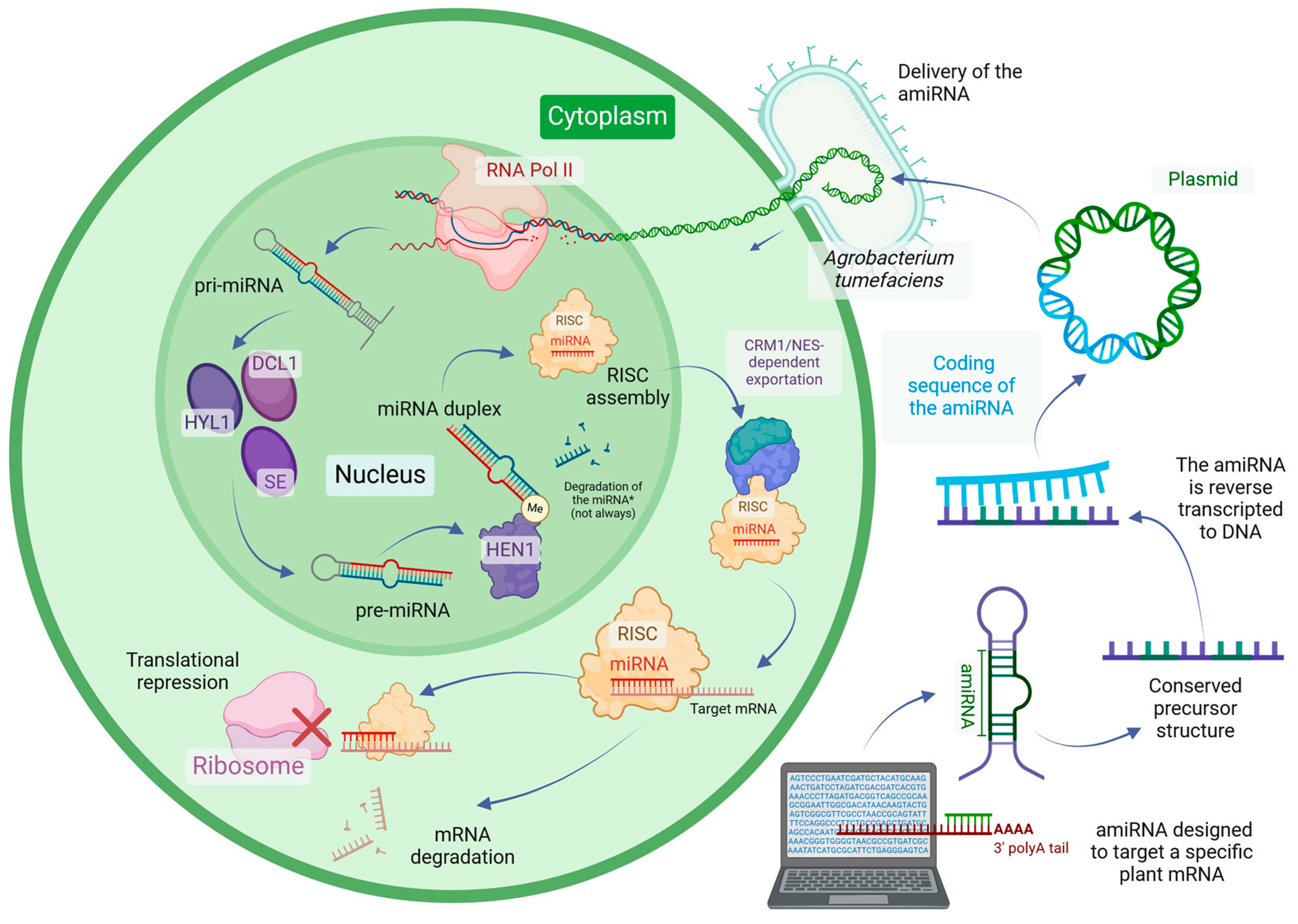
2. The Essentials of amiRNA Design
3. Advances in the Development and Improvement of Plant amiRNAs
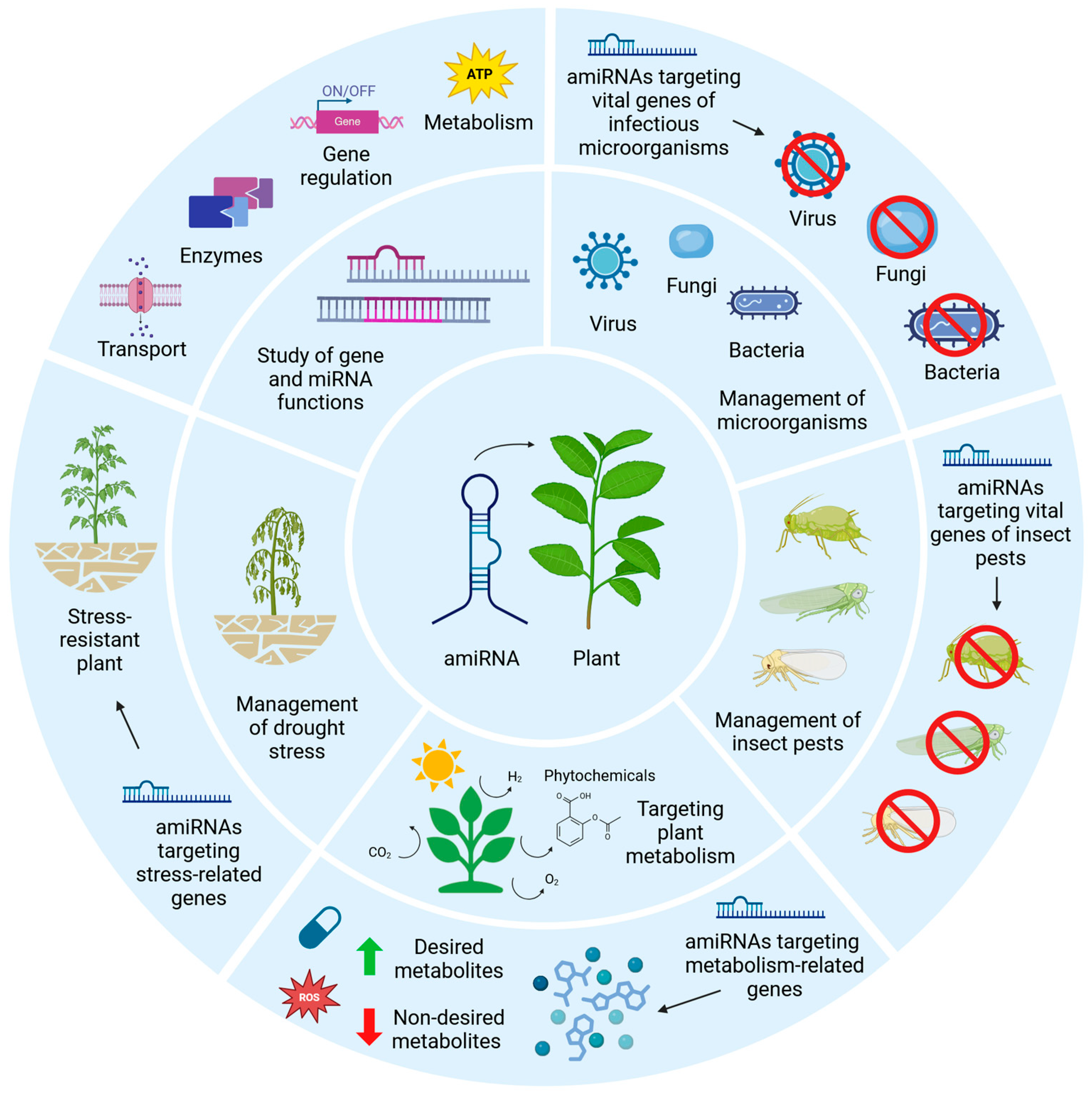
4. Applications of amiRNAs Against Infectious Diseases
5. Applications of amiRNAs Against Insect Pests
6. Applications of amiRNAs in Plant Metabolism
7. Applications of amiRNAs Against Drought Stress
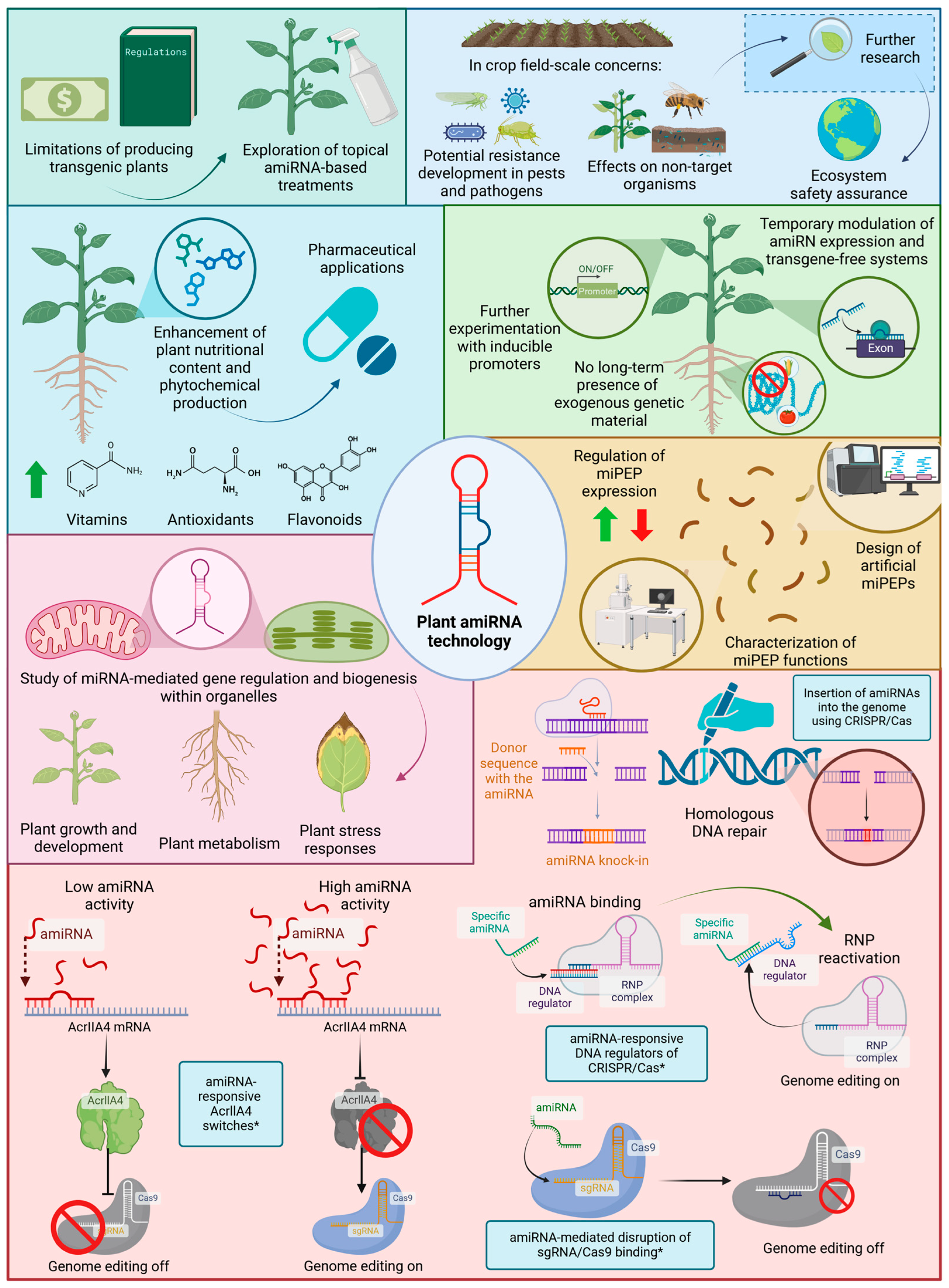
8. Concluding Remarks
9. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern plant biotechnology as a strategy in addressing climate change and attaining food security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Adedibu, P.A.; Shkryl, Y.; Emmanuel, F.O.; Tekutyeva, L.; Balabanova, L. Modern Plant Biotechnology: An Antidote against Global Food Insecurity. Agronomy 2023, 13, 2038. [Google Scholar] [CrossRef]
- Qaim, M. Role of New Plant Breeding Technologies for Food Security and Sustainable Agricultural Development. Appl. Econ. Perspect. Policy 2020, 42, 129–150. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Bacha, S.A.S.; Iqbal, B. Advancing agro-ecological sustainability through emerging genetic approaches in crop improvement for plants. Funct. Integr. Genomics 2023, 23, 145. [Google Scholar] [CrossRef]
- Rajam, M.V. RNA silencing technology: A boon for crop improvement. J. Biosci. 2020, 45, 118. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhury, A.; Chauhan, S.; Ghosh, A.; Tiwari, R.; Rajam, M.V. RNA interference and crop protection against biotic stresses. Physiol. Mol. Biol. Plants 2021, 27, 2357–2377. [Google Scholar] [CrossRef]
- Rajput, M.; Choudhary, K.; Kumar, M.; Vivekanand, V.; Chawade, A.; Ortiz, R.; Pareek, N. RNA Interference and CRISPR/Cas Gene Editing for Crop Improvement: Paradigm Shift towards Sustainable Agriculture. Plants 2021, 10, 1914. [Google Scholar] [CrossRef]
- Jiang, C.H.; Li, Z.J.; Zheng, L.Y.; Yu, Y.Y.; Niu, D.D. Small RNAs: Efficient and miraculous effectors that play key roles in plant–microbe interactions. Mol. Plant Pathol. 2023, 24, 999–1013. [Google Scholar] [CrossRef]
- Li, M.; Yu, B. Recent advances in the regulation of plant miRNA biogenesis. RNA Biol. 2021, 18, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, Y.; Chen, S.; Shi, M.; Jiang, X.; He, Z.; Gao, S. Mechanisms of MicroRNA Biogenesis and Stability Control in Plants. Front. Plant Sci. 2022, 13, 844149. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Axtell, M.J. MicroRNAs in Plants: Key Findings from the Early Years. Plant Cell 2019, 31, 1206–1207. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Agarwala, N.; Singh, K.; Sunkar, R.; Tuteja, N. NcRNAs in plant development and stress responses. Plant Physiol. Biochem. 2024, 214, 108950. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, N.; Laufs, P. Plant miRNA integrated functions in development and reproduction. Front. Plant Physiol. 2023, 1, 1271423. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, S.; Wang, F.; Meng, X.; Ma, Y.; Guan, J.; Zhang, F. The roles of microRNAs in horticultural plant disease resistance. Front. Genet. 2023, 14, 1137471. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Non-Coding RNAs in Response to Drought Stress. Int. J. Mol. Sci. 2021, 22, 12519. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in abiotic stress response and characteristics regulation of plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Angulo-Bejarano, P.I.; Bandyopadhyay, A.; Sharma, A.; Paul, S. Regulatory roles of noncoding RNAs in callus induction and plant cell dedifferentiation. Plant Cell Rep. 2023, 42, 689–705. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Xu, M.; Zhao, J.; Xu, D. Molecular mechanism of miRNA mediated biosynthesis of secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2024, 208, 108524. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, C.; Ahmed, S.S.S.J.; Ramalingam, S.; Selvaraj, D.; Srivastava, A.; Paul, S.; Sharma, A. Identification of microRNAs from Medicinal Plant Murraya koenigii by High-Throughput Sequencing and Their Functional Implications in Secondary Metabolite Biosynthesis. Plants 2022, 11, 46. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, S.; Yu, B. MicroRNA biogenesis, degradation and activity in plants. Cell. Mol. Life Sci. 2015, 72, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Knop, K.; Dolata, J.; Taube, M.; Bajczyk, M.; Barciszewska-Pacak, M.; Pacak, A.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Posttranscriptional coordination of splicing and miRNA biogenesis in plants. Wiley Interdiscip. Rev. RNA 2017, 8, e1403. [Google Scholar] [CrossRef]
- Gao, Z.; Nie, J.; Wang, H. MicroRNA biogenesis in plant. Plant Growth Regul. 2021, 93, 1–12. [Google Scholar] [CrossRef]
- Gangadhar, B.H.; Venkidasamy, B.; Samynathan, R.; Saranya, B.; Chung, I.M.; Thiruvengadam, M. Overview of miRNA biogenesis and applications in plants. Biologia 2021, 76, 2309–2327. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Hu, Y.; Zhao, L.; Xin, S.; Ni, Q.; Zhang, P.; Chen, M. Biogenesis, Functions, Interactions, and Resources of Non-Coding RNAs in Plants. Int. J. Mol. Sci. 2022, 23, 3695. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, B. MicroRNA production in Arabidopsis. Front. Plant Sci. 2023, 14, 1096772. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Sun, Z.; Li, H.; Liu, H. The Biosynthesis Process of Small RNA and Its Pivotal Roles in Plant Development. Int. J. Mol. Sci. 2024, 25, 7680. [Google Scholar] [CrossRef]
- Budak, H.; Akpinar, B.A. Plant miRNAs: Biogenesis, organization and origins. Funct. Integr. Genomics 2015, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Jodder, J. Regulation of pri-MIRNA processing: Mechanistic insights into the miRNA homeostasis in plant. Plant Cell Rep. 2021, 40, 783–798. [Google Scholar] [CrossRef]
- Hajieghrari, B.; Farrokhi, N. Plant RNA-mediated gene regulatory network. Genomics 2022, 114, 409–442. [Google Scholar] [CrossRef]
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696. [Google Scholar] [CrossRef]
- Zhang, B.; You, C.; Zhang, Y.; Zeng, L.; Hu, J.; Zhao, M.; Chen, X. Linking key steps of microRNA biogenesis by TREX-2 and the nuclear pore complex in Arabidopsis. Nat. Plants 2020, 6, 957–969. [Google Scholar] [CrossRef]
- Cambiagno, D.A.; Giudicatti, A.J.; Arce, A.L.; Gagliardi, D.; Li, L.; Yuan, W.; Lundberg, D.S.; Weigel, D.; Manavella, P.A. HASTY modulates miRNA biogenesis by linking pri-miRNA transcription and processing. Mol. Plant 2021, 14, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Lin, J.; Wu, Y.; Guo, H.; Shao, Y.; Wang, F.; Wang, X.; Mo, X.; Zheng, S.; et al. CRD1, an Xpo1 domain protein, regulates miRNA accumulation and crown root development in rice. Plant J. 2019, 100, 328–342. [Google Scholar] [CrossRef]
- Bologna, N.G.; Iselin, R.; Abriata, L.A.; Sarazin, A.; Pumplin, N.; Jay, F.; Grentzinger, T.; Dal Peraro, M.; Voinnet, O. Nucleo-cytosolic Shuttling of ARGONAUTE1 Prompts a Revised Model of the Plant MicroRNA Pathway. Mol. Cell 2018, 69, 709–719.e5. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jia, T.; Chen, X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef]
- Guo, L.; Lu, Z. The Fate of miRNA* Strand through Evolutionary Analysis: Implication for Degradation As Merely Carrier Strand or Potential Regulatory Molecule? PLoS ONE 2010, 5, e11387. [Google Scholar] [CrossRef]
- Trevisan, S.; Nonis, A.; Begheldo, M.; Manoli, A.; Palme, K.; Caporale, G.; Ruperti, B.; Quaggiotti, S. Expression and tissue-specific localization of nitrate-responsive miRNAs in roots of maize seedlings. Plant Cell Environ. 2012, 35, 1137–1155. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Q.; Jin, L.G.; Qiu, L.J. MiR1511 co-regulates with miR1511* to cleave the GmRPL4a gene in soybean. Chinese Sci. Bull. 2012, 57, 3804–3810. [Google Scholar] [CrossRef]
- Hackenberg, M.; Shi, B.J.; Gustafson, P.; Langridge, P. Characterization of phosphorus-regulated miR399 and miR827 and their isomirs in barley under phosphorus-sufficient and phosphorus-deficient conditions. BMC Plant Biol. 2013, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Xia, J.; Jiang, C.; Qi, B.; Ling, X.; Lin, S.; Zhang, W.; Guo, J.; Jin, H.; Zhao, H. Bacillus cereus AR156 primes induced systemic resistance by suppressing miR825/825* and activating defense-related genes in Arabidopsis. J. Integr. Plant Biol. 2016, 58, 426–439. [Google Scholar] [CrossRef]
- Liu, W.W.; Meng, J.; Cui, J.; Luan, Y.S. Characterization and Function of MicroRNA*s in Plants. Front. Plant Sci. 2017, 8, 2200. [Google Scholar] [CrossRef]
- Basso, M.F.; Ferreira, P.C.G.; Kobayashi, A.K.; Harmon, F.G.; Nepomuceno, A.L.; Molinari, H.B.C.; Grossi-de-Sa, M.F. MicroRNAs and new biotechnological tools for its modulation and improving stress tolerance in plants. Plant Biotechnol. J. 2019, 17, 1482–1500. [Google Scholar] [CrossRef]
- Amritha, P.P.; Shah, J.M. Can genetic engineering-based methods for gene function identification be eclipsed by genome editing in plants? A comparison of methodologies. Mol. Genet. Genomics 2021, 296, 485–500. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef]
- Ossowski, S.; Schwab, R.; Weigel, D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008, 53, 674–690. [Google Scholar] [CrossRef]
- Sablok, G.; Pérez-Quintero, Á.L.; Hassan, M.; Tatarinova, T.V.; López, C. Artificial microRNAs (amiRNAs) engineering—On how microRNA-based silencing methods have affected current plant silencing research. Biochem. Biophys. Res. Commun. 2011, 406, 315–319. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Trivedi, P.K. Artificial microRNA mediated gene silencing in plants: Progress and perspectives. Plant Mol. Biol. 2014, 86, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, A.E.; de la Torre-Montaña, A.; Martín-García, T.; Carbonell, A. Artificial Small RNAs for Functional Genomics in Plants. In RNA-Based Technologies for Functional Genomics in Plants. Concepts and Strategies in Plant Sciences; Tang, G., Teotia, S., Tang, X., Singh, D., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–29. [Google Scholar]
- Pandey, P.; Mysore, K.S.; Senthil-Kumar, M. Recent Advances in Plant Gene Silencing Methods. In Plant Gene Silencing. Methods in Molecular Biology; Mysore, K.S., Senthil-Kumar, M., Eds.; Humana Press Inc.: New York, NY, USA, 2022; Volume 2408, pp. 1–22. [Google Scholar]
- Kumar, M.; Panwar, V.; Chaudhary, V.; Kumar, R. Artificial miRNAs: A potential tool for genetic improvement of horticultural crops. Sci. Hortic. 2024, 331, 113160. [Google Scholar] [CrossRef]
- Chauhan, S.; Yogindran, S.; Rajam, M.V. Role of miRNAs in biotic stress reactions in plants. Indian J. Plant Physiol. 2017, 22, 514–529. [Google Scholar] [CrossRef]
- Cisneros, A.E.; Carbonell, A. Artificial Small RNA-Based Silencing Tools for Antiviral Resistance in Plants. Plants 2020, 9, 669. [Google Scholar] [CrossRef]
- Tiwari, R.; Rajam, M.V. RNA- and miRNA-interference to enhance abiotic stress tolerance in plants. J. Plant Biochem. Biotechnol. 2022, 31, 689–704. [Google Scholar] [CrossRef]
- Mann, C.W.G.; Sawyer, A.; Gardiner, D.M.; Mitter, N.; Carroll, B.J.; Eamens, A.L. RNA-Based Control of Fungal Pathogens in Plants. Int. J. Mol. Sci. 2023, 24, 12391. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Yang, Y.; Schmid, M.; Wang, Y. MiRNA Mediated Regulation and Interaction between Plants and Pathogens. Int. J. Mol. Sci. 2021, 22, 2913. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, W.; Taning, C.N.T.; Smagghe, G.; Wang, J.J. Regulatory roles of microRNAs in insect pests: Prospective targets for insect pest control. Curr. Opin. Biotechnol. 2021, 70, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, J.; Li, C. Research Progress on miRNAs and Artificial miRNAs in Insect and Disease Resistance and Breeding in Plants. Genes 2024, 15, 1200. [Google Scholar] [CrossRef]
- Yan, H.; Deng, X.; Cao, Y.; Huang, J.; Ma, L.; Zhao, B. A novel approach for the construction of plant amiRNA expression vectors. J. Biotechnol. 2011, 151, 9–14. [Google Scholar] [CrossRef]
- Charrier, A.; Vergne, E.; Joffrion, C.; Richer, A.; Dousset, N.; Chevreau, E. An artificial miRNA as a new tool to silence and explore gene functions in apple. Transgenic Res. 2019, 28, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.F.; Tan, J.; Zhang, W.; Yang, W.; Zhuang, T.; Lu, W.; Qiu, Y.; Du, X.; Zhuang, X.; Zhou, T.; et al. Transgenic expression of artificial microRNA targeting soybean mosaic virus P1 gene confers virus resistance in plant. Transgenic Res. 2024, 33, 149–157. [Google Scholar] [CrossRef]
- Betti, F.; Ladera-Carmona, M.J.; Weits, D.A.; Ferri, G.; Iacopino, S.; Novi, G.; Svezia, B.; Kunkowska, A.B.; Santaniello, A.; Piaggesi, A.; et al. Exogenous miRNAs induce post-transcriptional gene silencing in plants. Nat. Plants 2021, 7, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; López, C.; Daròs, J.A. Fast-Forward Identification of Highly Effective Artificial Small RNAs Against Different Tomato spotted wilt virus Isolates. Mol. Plant-Microbe Interact. 2019, 32, 142–156. [Google Scholar] [CrossRef]
- Lunardon, A.; Kariuki, S.M.; Axtell, M.J. Expression and processing of polycistronic artificial microRNAs and trans-acting siRNAs from transiently introduced transgenes in Solanum lycopersicum and Nicotiana benthamiana. Plant J. 2021, 106, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Xu, K.; Li, D.; Hu, H.; Zhou, F.; Song, P.; Yu, Y.; Wei, Q.; Liu, Q.; et al. Clay nanosheet-mediated delivery of recombinant plasmids expressing artificial miRNAs via leaf spray to prevent infection by plant DNA viruses. Hortic. Res. 2020, 7, 179. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Meng, Y.; Chen, W. Gold nanoparticles (AuNPs) can rapidly deliver artificial microRNA (AmiRNA)-ATG6 to silence ATG6 expression in Arabidopsis. Plant Cell Rep. 2023, 42, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, A.A.; Suhorukova, A.V.; Kabardaeva, K.V.; Goldenkova-Pavlova, I.V. Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Toppino, L.; Kooiker, M.; Lindner, M.; Dreni, L.; Rotino, G.L.; Kater, M.M. Reversible male sterility in eggplant (Solanum melongena L.) by artificial microRNA-mediated silencing of general transcription factor genes. Plant Biotechnol. J. 2011, 9, 684–692. [Google Scholar] [CrossRef]
- Yan, F.; Lu, Y.; Wu, G.; Peng, J.; Zheng, H.; Lin, L.; Chen, J. A simplified method for constructing artificial microRNAs based on the osa-MIR528 precursor. J. Biotechnol. 2012, 160, 146–150. [Google Scholar] [CrossRef]
- Carbonell, A.; Takeda, A.; Fahlgren, N.; Johnson, S.C.; Cuperus, J.T.; Carrington, J.C. New Generation of Artificial MicroRNA and Synthetic Trans-Acting Small Interfering RNA Vectors for Efficient Gene Silencing in Arabidopsis. Plant Physiol. 2014, 165, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Jover-Gil, S.; Paz-Ares, J.; Micol, J.L.; Ponce, M.R. Multi-gene silencing in Arabidopsis: A collection of artificial microRNAs targeting groups of paralogs encoding transcription factors. Plant J. 2014, 80, 149–160. [Google Scholar] [CrossRef]
- Fahim, M.; Larkin, P.J. Designing Effective AmiRNA and Multimeric AmiRNA Against Plant Viruses. In Methods in Molecular Biology; Taxman, D., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 942, pp. 357–377. [Google Scholar]
- Zhang, N.; Zhang, D.; Chen, S.L.; Gong, B.Q.; Guo, Y.; Xu, L.; Zhang, X.N.; Li, J.F. Engineering Artificial MicroRNAs for Multiplex Gene Silencing and Simplified Transgenic Screen. Plant Physiol. 2018, 178, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Saakre, M.; Jaiswal, S.; Rathinam, M.; Raman, K.V.; Tilgam, J.; Paul, K.; Sreevathsa, R.; Pattanayak, D. Host-Delivered RNA Interference for Durable Pest Resistance in Plants: Advanced Methods, Challenges, and Applications. Mol. Biotechnol. 2024, 66, 1786–1805. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.; Pereira, A.M.; Lopes, A.L.; Coimbra, S. The best CRISPR/Cas9 versus RNA interference approaches for Arabinogalactan proteins’ study. Mol. Biol. Rep. 2020, 47, 2315–2325. [Google Scholar] [CrossRef]
- Zand Karimi, H.; Innes, R.W. Molecular mechanisms underlying host-induced gene silencing. Plant Cell 2022, 34, 3183–3199. [Google Scholar] [CrossRef]
- Liu, S.R.; Zhou, J.J.; Hu, C.G.; Wei, C.L.; Zhang, J.Z. MicroRNA-Mediated Gene Silencing in Plant Defense and Viral Counter-Defense. Front. Microbiol. 2017, 8, 1801. [Google Scholar] [CrossRef]
- Yu, S.; Pilot, G. Testing the efficiency of plant artificial microRNAs by transient expression in Nicotiana benthamiana reveals additional action at the translational level. Front. Plant Sci. 2014, 5, 622. [Google Scholar] [CrossRef]
- Cisneros, A.E.; Martín-García, T.; Primc, A.; Kuziuta, W.; Sánchez-Vicente, J.; Aragonés, V.; Daròs, J.A.; Carbonell, A. Transgene-free, virus-based gene silencing in plants by artificial microRNAs derived from minimal precursors. Nucleic Acids Res. 2023, 51, 10719–10736. [Google Scholar] [CrossRef]
- Kaur, M.; Manchanda, P.; Kalia, A.; Ahmed, F.K.; Nepovimova, E.; Kuca, K.; Abd-Elsalam, K.A. Agroinfiltration Mediated Scalable Transient Gene Expression in Genome Edited Crop Plants. Int. J. Mol. Sci. 2021, 22, 10882. [Google Scholar] [CrossRef]
- Parizotto, E.A.; Dunoyer, P.; Rahm, N.; Himber, C.; Voinnet, O. In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 2004, 18, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, S.; Alves Junior, L.; Merkle, T. Improvement of the design and generation of highly specific plant knockdown lines using primary synthetic microRNAs (pri-smiRNAs). BMC Res. Notes 2010, 3, 59. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Sun, J.; Luo, Y.; Wang, M.B.; Fan, Y.L.; Wang, L. A simple artificial microRNA vector based on ath-miR169d precursor from Arabidopsis. Mol. Biol. Rep. 2010, 37, 903–909. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Li, Y.; Yu, D. A new strategy for construction of artificial miRNA vectors in Arabidopsis. Planta 2012, 235, 1421–1429. [Google Scholar] [CrossRef]
- Schwab, R.; Ossowski, S.; Riester, M.; Warthmann, N.; Weigel, D. Highly Specific Gene Silencing by Artificial MicroRNAs in Arabidopsis. Plant Cell 2006, 18, 1121–1133. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Snyder, J.A.; Park, Y.B.; Li, Y.F.; Sunkar, R.; Axtell, M.J. Sliced microRNA targets and precise loop-first processing of MIR319 hairpins revealed by analysis of the Physcomitrella patens degradome. RNA 2009, 15, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhong, X.; Jiang, S.; Zhai, C.; Ma, L. Improved method for constructing plant amiRNA vectors with blue-white screening and MAGIC. Biotechnol. Lett. 2011, 33, 1683–1688. [Google Scholar] [CrossRef]
- Jiang, F.; Song, Y.; Han, Q.; Zhu, C.; Wen, F. The choice of target site is crucial in artificial miRNA-mediated virus resistance in transgenic Nicotiana tabacum. Physiol. Mol. Plant Pathol. 2011, 76, 2–8. [Google Scholar] [CrossRef]
- Warthmann, N.; Chen, H.; Ossowski, S.; Weigel, D.; Herve, P. Highly Specific Gene Silencing by Artificial miRNAs in Rice. PLoS ONE 2008, 3, e1829. [Google Scholar] [CrossRef]
- Chen, S.; Songkumarn, P.; Liu, J.; Wang, G.L. A Versatile Zero Background T-Vector System for Gene Cloning and Functional Genomics. Plant Physiol. 2009, 150, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, N.; Shen, W.; Li, J.F. Engineered Artificial MicroRNA Precursors Facilitate Cloning and Gene Silencing in Arabidopsis and Rice. Int. J. Mol. Sci. 2019, 20, 5620. [Google Scholar] [CrossRef] [PubMed]
- Teotia, S.; Wang, X.; Zhou, N.; Wang, M.; Liu, H.; Qin, J.; Han, D.; Li, C.; Li, C.E.; Pan, S.; et al. A high-efficiency gene silencing in plants using two-hit asymmetrical artificial MicroRNAs. Plant Biotechnol. J. 2023, 21, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, B.; Chi, M.; Han, D.; Tang, H.; Tang, G.; Xiang, Y. Design, Construction, and Validation of Artificial MicroRNA Vectors Using Agrobacterium-Mediated Transient Expression System. In Methods in Molecular Biology; Fett-Neto, A., Ed.; Humana Press: New York, NY, USA, 2016; Volume 1405, pp. 149–162. [Google Scholar]
- Pegler, J.L.; Grof, C.P.L.; Eamens, A.L. The Plant MicroRNA Pathway: The Production and Action Stages. In Methods in Molecular Biology; de Folter, S., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1932, pp. 15–39. [Google Scholar]
- Eamens, A.L.; Smith, N.A.; Curtin, S.J.; Wang, M.B.; Waterhouse, P.M. The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA 2009, 15, 2219–2235. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Cai, T.; Hu, Y.; Chen, Y.; Hodges, E.; Ni, F.; Wu, L.; Li, S.; Zhou, H.; Long, C.; et al. Sorting of Small RNAs into Arabidopsis Argonaute Complexes Is Directed by the 5′ Terminal Nucleotide. Cell 2008, 133, 116–127. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. Computational analysis of miRNA targets in plants: Current status and challenges. Brief. Bioinform. 2011, 12, 115–121. [Google Scholar] [CrossRef]
- Attri, K.; Zhang, Z.; Singh, A.; Sharrock, R.A.; Xie, Z. Rapid sequence and functional diversification of a miRNA superfamily targeting calcium signaling components in seed plants. New Phytol. 2022, 235, 1082–1095. [Google Scholar] [CrossRef]
- Brousse, C.; Liu, Q.; Beauclair, L.; Deremetz, A.; Axtell, M.J.; Bouché, N. A non-canonical plant microRNA target site. Nucleic Acids Res. 2014, 42, 5270–5279. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Lu, Y.; Zinta, G.; Lang, Z.; Zhu, J.K. UTR-Dependent Control of Gene Expression in Plants. Trends Plant Sci. 2018, 23, 248–259. [Google Scholar] [CrossRef]
- Ren, Y.; Li, M.; Wang, W.; Lan, W.; Schenke, D.; Cai, D.; Miao, Y. MicroRNA840 (MIR840) accelerates leaf senescence by targeting the overlapping 3′UTRs of PPR and WHIRLY3 in Arabidopsis thaliana. Plant J. 2022, 109, 126–143. [Google Scholar] [CrossRef]
- Narjala, A.; Nair, A.; Tirumalai, V.; Vivek Hari Sundar, G.; Shivaprasad, P.V. A conserved sequence signature is essential for robust plant miRNA biogenesis. Nucleic Acids Res. 2020, 48, 3103–3118. [Google Scholar] [CrossRef]
- Bhagwat, B.; Chi, M.; Su, L.; Tang, H.; Tang, G.; Xiang, Y. An in vivo Transient Expression System Can Be Applied for Rapid and Effective Selection of Artificial MicroRNA Constructs for Plant Stable Genetic Transformation. J. Genet. Genomics 2013, 40, 261–270. [Google Scholar] [CrossRef]
- Singh, A.; Das, S. Slicing Messengers by Artificial Designs: Artificial MicroRNA Induced Gene Silencing in Polyploid Plants for Functional Genomics and Trait Modification. In RNA-Based Technologies for Functional Genomics in Plants. Concepts and Strategies in Plant Sciences; Tang, G., Teotia, S., Tang, X., Singh, D., Eds.; Springer: Cham, Switzerland, 2021; pp. 77–129. [Google Scholar]
- Komiya, R. Biogenesis of diverse plant phasiRNAs involves an miRNA-trigger and Dicer-processing. J. Plant Res. 2017, 130, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Muhammad, S.; Cao, M.; Wu, L. Biogenesis and regulatory hierarchy of phased small interfering RNAs in plants. Plant Biotechnol. J. 2018, 16, 965–975. [Google Scholar] [CrossRef]
- Huang, J.; Wang, R.; Dai, X.; Feng, J.; Zhang, H.; Zhao, P.X. A microRNA biogenesis-like pathway for producing phased small interfering RNA from a long non-coding RNA in rice. J. Exp. Bot. 2019, 70, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teng, C.; Xia, R.; Meyers, B.C. PhasiRNAs in Plants: Their Biogenesis, Genic Sources, and Roles in Stress Responses, Development, and Reproduction. Plant Cell 2020, 32, 3059–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Meyers, B.C. Plant Small RNAs: Their Biogenesis, Regulatory Roles, and Functions. Annu. Rev. Plant Biol. 2023, 74, 21–51. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, T.; Nang Do, V. Customization of Artificial MicroRNA Design. In MicroRNA Profiling. Methods in Molecular Biology; Rani, S., Ed.; Humana Press Inc.: New York, NY, USA, 2017; Volume 1509, pp. 235–243. [Google Scholar]
- Guo, Y.; Han, Y.; Ma, J.; Wang, H.; Sang, X.; Li, M. Undesired small RNAs originate from an artificial microRNA precursor in transgenic petunia (Petunia hybrida). PLoS ONE 2014, 9, e98783. [Google Scholar] [CrossRef]
- Hu, J.; Deng, X.; Shao, N.; Wang, G.; Huang, K. Rapid construction and screening of artificial microRNA systems in Chlamydomonas reinhardtii. Plant J. 2014, 79, 1052–1064. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, D.; Sheen, J. Epitope-tagged protein-based artificial miRNA screens for optimized gene silencing in plants. Nat. Protoc. 2014, 9, 939–949. [Google Scholar] [CrossRef]
- Carbonell, A.; Fahlgren, N.; Mitchell, S.; Cox, K.L.; Reilly, K.C.; Mockler, T.C.; Carrington, J.C. Highly specific gene silencing in a monocot species by artificial microRNAs derived from chimeric miRNA precursors. Plant J. 2015, 82, 1061–1075. [Google Scholar] [CrossRef]
- Castro, Á.; Quiroz, D.; Sánchez, E.; Miccono, M.d.l.Á.; Aguirre, C.; Ramírez, A.; Montes, C.; Prieto, H. Synthesis of an artificial Vitis vinifera miRNA 319e using overlapping long primers and its application for gene silencing. J. Biotechnol. 2016, 233, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; Mao, W.; Guan, Y.; Zhang, Z. An effective artificial microRNA vector based on Fv-miR166 precursor from strawberry. Sci. Hortic. 2019, 256, 108643. [Google Scholar] [CrossRef]
- Guo, Z.; Kuang, Z.; Zhao, Y.; Deng, Y.; He, H.; Wan, M.; Tao, Y.; Wang, D.; Wei, J.; Li, L.; et al. PmiREN2.0: From data annotation to functional exploration of plant microRNAs. Nucleic Acids Res. 2022, 50, D1475–D1482. [Google Scholar] [CrossRef]
- Ataei, S.; Ahmadi, J.; Marashi, S.A.; Abolhasani, I. AmiR-P3: An AI-based microRNA prediction pipeline in plants. PLoS ONE 2024, 19, e0308016. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, H.J.; Kwak, K.J.; Lee, K.; Hong, S.W.; Kang, H. MicroRNA400-Guided Cleavage of Pentatricopeptide Repeat Protein mRNAs Renders Arabidopsis thaliana More Susceptible to Pathogenic Bacteria and Fungi. Plant Cell Physiol. 2014, 55, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Tholt, G.; Ivanics, M.; Várallyay, É.; Jenes, B.; Havelda, Z. Polycistronic artificial miRNA-mediated resistance to Wheat dwarf virus in barley is highly efficient at low temperature. Mol. Plant Pathol. 2016, 17, 427–437. [Google Scholar] [CrossRef]
- Luan, Y.; Cui, J.; Wang, W.; Meng, J. MiR1918 enhances tomato sensitivity to Phytophthora infestans infection. Sci. Rep. 2016, 6, 35858. [Google Scholar] [CrossRef]
- Mitter, N.; Zhai, Y.; Bai, A.X.; Chua, K.; Eid, S.; Constantin, M.; Mitchell, R.; Pappu, H.R. Evaluation and identification of candidate genes for artificial microRNA-mediated resistance to tomato spotted wilt virus. Virus Res. 2016, 211, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lin, C.; Du, J.; Song, Y.; Jiang, M.; Liu, H.; Zhou, S.; Wen, F.; Zhu, C. Dimeric artificial microRNAs mediate high resistance to RSV and RBSDV in transgenic rice plants. Plant Cell Tissue Organ Cult. 2016, 126, 127–139. [Google Scholar] [CrossRef]
- Wagaba, H.; Patil, B.L.; Mukasa, S.; Alicai, T.; Fauquet, C.M.; Taylor, N.J. Artificial microRNA-derived resistance to Cassava brown streak disease. J. Virol. Methods 2016, 231, 38–43. [Google Scholar] [CrossRef]
- Carbonell, A.; Daròs, J.A. Artificial microRNAs and synthetic trans-acting small interfering RNAs interfere with viroid infection. Mol. Plant Pathol. 2017, 18, 746–753. [Google Scholar] [CrossRef]
- Petchthai, U.; Le Yee, C.S.; Wong, S.M. Resistance to CymMV and ORSV in artificial microRNA transgenic Nicotiana benthamiana plants. Sci. Rep. 2018, 8, 9958. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; Lisón, P.; Daròs, J.A. Multi-targeting of viral RNAs with synthetic trans-acting small interfering RNAs enhances plant antiviral resistance. Plant J. 2019, 100, 720–737. [Google Scholar] [CrossRef]
- Liang, C.; Hao, J.; Li, J.; Baker, B.; Luo, L. Artificial microRNA-mediated resistance to cucumber green mottle mosaic virus in Nicotiana benthamiana. Planta 2019, 250, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Prasad, M. Silencing AC1 of Tomato leaf curl virus using artificial microRNA confers resistance to leaf curl disease in transgenic tomato. Plant Cell Rep. 2020, 39, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Persson Hodén, K.; Liao, Z.; Åsman, A.; Dixelius, C. Phytophthora infestans Ago1-associated miRNA promotes potato late blight disease. New Phytol. 2022, 233, 443–457. [Google Scholar] [CrossRef]
- Berbati, M.; Kaldis, A.; Voloudakis, A. Efficient artificial microRNA-mediated resistance against zucchini yellow mosaic virus in zucchini via agroinfiltration. J. Virol. Methods 2023, 321, 114805. [Google Scholar] [CrossRef]
- Guo, H.; Song, X.; Wang, G.; Yang, K.; Wang, Y.; Niu, L.; Chen, X.; Fang, R. Plant-Generated Artificial Small RNAs Mediated Aphid Resistance. PLoS ONE 2014, 9, e97410. [Google Scholar] [CrossRef]
- Faisal, M.; Abdel-Salam, E.M.; Alatar, A.A. Artificial microRNA-Based RNA Interference and Specific Gene Silencing for Developing Insect Resistance in Solanum lycopersicum. Agronomy 2021, 11, 136. [Google Scholar] [CrossRef]
- Agrawal, A.; Rajamani, V.; Reddy, V.S.; Mukherjee, S.K.; Bhatnagar, R.K. Transgenic plants over-expressing insect-specific microRNA acquire insecticidal activity against Helicoverpa armigera: An alternative to Bt-toxin technology. Transgenic Res. 2015, 24, 791–801. [Google Scholar] [CrossRef]
- Saini, R.P.; Raman, V.; Dhandapani, G.; Malhotra, E.V.; Sreevathsa, R.; Kumar, P.A.; Sharma, T.R.; Pattanayak, D. Silencing of HaAce1 gene by host-delivered artificial microRNA disrupts growth and development of Helicoverpa armigera. PLoS ONE 2018, 13, e0194150. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Fishilevich, E.; Doran, R.L.; Lee, K.; de Campos, S.B.; German, M.A.; Narva, K.E.; Waterhouse, P.M. Plin-amiR, a pre-microRNA-based technology for controlling herbivorous insect pests. Plant Biotechnol. J. 2020, 18, 1925–1932. [Google Scholar] [CrossRef]
- Yogindran, S.; Rajam, M.V. Host-derived artificial miRNA-mediated silencing of ecdysone receptor gene provides enhanced resistance to Helicoverpa armigera in tomato. Genomics 2021, 113, 736–747. [Google Scholar] [CrossRef]
- Tian, B.; Li, J.; Oakley, T.R.; Todd, T.C.; Trick, H.N. Host-Derived Artificial MicroRNA as an Alternative Method to Improve Soybean Resistance to Soybean Cyst Nematode. Genes 2016, 7, 122. [Google Scholar] [CrossRef]
- Zubair, M.; Khan, M.Z.; Rauf, I.; Raza, A.; Shah, A.H.; Hassan, I.; Amin, I.; Mansoor, S. Artificial micro RNA (amiRNA)-mediated resistance against whitefly (Bemisia tabaci) targeting three genes. Crop Prot. 2020, 137, 105308. [Google Scholar] [CrossRef]
- Chi, M.; Bhagwat, B.; Lane, W.D.; Tang, G.; Su, Y.; Sun, R.; Oomah, B.D.; Wiersma, P.A.; Xiang, Y. Reduced polyphenol oxidase gene expression and enzymatic browning in potato (Solanum tuberosum L.) with artificial microRNAs. BMC Plant Biol. 2014, 14, 62. [Google Scholar] [CrossRef]
- Kaur, S.; Spillane, C. Reduction in Carotenoid Levels in the Marine Diatom Phaeodactylum tricornutum by Artificial MicroRNAs Targeted Against the Endogenous Phytoene Synthase Gene. Mar. Biotechnol. 2015, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, F.; Das, S.S.; Sanan-Mishra, N.; Khan, H. Artificial miRNA-mediated down-regulation of two monolignoid biosynthetic genes (C3H and F5H) cause reduction in lignin content in jute. Plant Mol. Biol. 2015, 89, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.; Shu, L.; Zhuang, X.; Liu, Y.; Chen, J.; Hu, Z. Sustainable photosynthetic H2-production mediated by artificial miRNA silencing of OEE2 gene in green alga Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2015, 40, 5609–5616. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Chen, M.; Zhuang, X.; Wang, C.; Wang, J.; Hu, Z. Improved photobio-H2 production regulated by artificial miRNA targeting psbA in green microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2018, 11, 36. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Hu, C.; Sun, T.; Zeng, Z.; Cai, X.; Li, H.; Hu, Z. Optogenetic regulation of artificial microRNA improves H2 production in green alga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2017, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Li, H.; Wang, J.; Hu, Z. Artificial miRNA inhibition of phosphoenolpyruvate carboxylase increases fatty acid production in a green microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2017, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Ozseyhan, M.E.; Li, P.; Na, G.N.; Li, Z.; Wang, C.; Lu, C. Improved fatty acid profiles in seeds of Camelina sativa by artificial microRNA mediated FATB gene suppression. Biochem. Biophys. Res. Commun. 2018, 503, 621–624. [Google Scholar] [CrossRef]
- Wyrzykowska, A.; Pieczynski, M.; Szweykowska-Kulinska, Z. Construction of Artificial Mirnas to Prevent Drought Stress in Solanum Tuberosum. In Environmental Responses in Plants. Methods in Molecular Biology; Duque, P., Ed.; Humana Press: New York, NY, USA, 2016; Volume 1398, pp. 271–290. [Google Scholar]
- Li, S.; Zhang, N.; Zhu, X.; Ma, R.; Yang, J.; Tang, X.; Si, H. Enhanced drought tolerance with artificial microRNA-mediated StProDH1 gene silencing in potato. Crop Sci. 2020, 60, 1462–1471. [Google Scholar] [CrossRef]
- Azadi, H.; Samiee, A.; Mahmoudi, H.; Jouzi, Z.; Rafiaani Khachak, P.; De Maeyer, P.; Witlox, F. Genetically modified crops and small-scale farmers: Main opportunities and challenges. Crit. Rev. Biotechnol. 2016, 36, 434–446. [Google Scholar] [CrossRef]
- Purnhagen, K.; Wesseler, J. EU Regulation of New Plant Breeding Technologies and Their Possible Economic Implications for the EU and Beyond. Appl. Econ. Perspect. Policy 2021, 43, 1621–1637. [Google Scholar] [CrossRef]
- Azadi, H.; Taheri, F.; Ghazali, S.; Movahhed Moghaddam, S.; Siamian, N.; Goli, I.; Choobchian, S.; Pour, M.; Özgüven, A.I.; Janečková, K.; et al. Genetically modified crops in developing countries: Savior or traitor? J. Clean. Prod. 2022, 371, 133296. [Google Scholar] [CrossRef]
- Entine, J.; Felipe, M.S.S.; Groenewald, J.H.; Kershen, D.L.; Lema, M.; McHughen, A.; Nepomuceno, A.L.; Ohsawa, R.; Ordonio, R.L.; Parrott, W.A.; et al. Regulatory approaches for genome edited agricultural plants in select countries and jurisdictions around the world. Transgenic Res. 2021, 30, 551–584. [Google Scholar] [CrossRef]
- Touzdjian Pinheiro Kohlrausch Távora, F.; de Assis dos Santos Diniz, F.; de Moraes Rêgo-Machado, C.; Chagas Freitas, N.; Barbosa Monteiro Arraes, F.; Chumbinho de Andrade, E.; Furtado, L.L.; Osiro, K.O.; Lima de Sousa, N.; Cardoso, T.B.; et al. CRISPR/Cas- and Topical RNAi-Based Technologies for Crop Management and Improvement: Reviewing the Risk Assessment and Challenges Towards a More Sustainable Agriculture. Front. Bioeng. Biotechnol. 2022, 10, 913728. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Holeva, M.C.; Sklavounos, A.; Rajeswaran, R.; Pooggin, M.M.; Voloudakis, A.E. Topical Application of Double-Stranded RNA Targeting 2b and CP Genes of Cucumber mosaic virus Protects Plants against Local and Systemic Viral Infection. Plants 2021, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, B.; Zheng, W.; Bauer, M.J.; Havecker, E.R.; Mai, J.T.; Hoffer, P.H.; Sanders, R.A.; Eads, B.D.; Caruano-Yzermans, A.; Taylor, D.N.; et al. Topically delivered 22 nt siRNAs enhance RNAi silencing of endogenous genes in two species. Planta 2021, 254, 60. [Google Scholar] [CrossRef] [PubMed]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. DsRNA Uptake in Plant Pests and Pathogens: Insights into RNAi-Based Insect and Fungal Control Technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Christiaens, O.; Sweet, J.; Dzhambazova, T.; Urru, I.; Smagghe, G.; Kostov, K.; Arpaia, S. Implementation of RNAi-based arthropod pest control: Environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 2022, 95, 1–15. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; dos Santos, E.Á.; Smagghe, G.; Zotti, M.J. Management of Pest Insects and Plant Diseases by Non-Transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Beernink, B.M.; Amanat, N.; Li, V.H.; Manchur, C.L.; Whyard, S.; Belmonte, M.F. SIGS vs. HIGS: Opportunities and challenges of RNAi pest and pathogen control strategies. Can. J. Plant Pathol. 2024, 46, 675–689. [Google Scholar] [CrossRef]
- Niu, J.; Shen, G.; Christiaens, O.; Smagghe, G.; He, L.; Wang, J. Beyond insects: Current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag. Sci. 2018, 74, 2680–2687. [Google Scholar] [CrossRef]
- Arpaia, S.; Christiaens, O.; Giddings, K.; Jones, H.; Mezzetti, B.; Moronta-Barrios, F.; Perry, J.N.; Sweet, J.B.; Taning, C.N.T.; Smagghe, G.; et al. Biosafety of GM Crop Plants Expressing dsRNA: Data Requirements and EU Regulatory Considerations. Front. Plant Sci. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Feng, H.; Jander, G. Engineering pest tolerance through plant-mediated RNA interference. Curr. Opin. Plant Biol. 2021, 60, 102029. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-Based Biocontrol Products: Market Status, Regulatory Aspects, and Risk Assessment. Front. Insect Sci. 2021, 1, 818037. [Google Scholar] [CrossRef]
- Un Jan Contreras, S.; Gardner, C.M. Environmental fate and behaviour of antibiotic resistance genes and small interference RNAs released from genetically modified crops. J. Appl. Microbiol. 2022, 133, 2877–2892. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, D.; Jogam, P.; Allini, V.R.; Abbagani, S.; Alok, A. The present and potential future methods for delivering CRISPR/Cas9 components in plants. J. Genet. Eng. Biotechnol. 2020, 18, 25. [Google Scholar] [CrossRef]
- He, Y.; Mudgett, M.; Zhao, Y. Advances in gene editing without residual transgenes in plants. Plant Physiol. 2022, 188, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sandoval, E.; Dierschke, T.; Fisher, T.J.; Bowman, J.L. Efficient and Inducible Use of Artificial MicroRNAs in Marchantia polymorpha. Plant Cell Physiol. 2016, 57, 281–290. [Google Scholar] [CrossRef]
- Rozov, S.M.; Permyakova, N.V.; Sidorchuk, Y.V.; Deineko, E.V. Optimization of Genome Knock-In Method: Search for the Most Efficient Genome Regions for Transgene Expression in Plants. Int. J. Mol. Sci. 2022, 23, 4416. [Google Scholar] [CrossRef] [PubMed]
- Collonnier, C.; Guyon-Debast, A.; Maclot, F.; Mara, K.; Charlot, F.; Nogué, F. Towards mastering CRISPR-induced gene knock-in in plants: Survey of key features and focus on the model Physcomitrella patens. Methods 2017, 121–122, 103–117. [Google Scholar] [CrossRef]
- Awwad, D.A. Beyond classic editing: Innovative CRISPR approaches for functional studies of long non-coding RNA. Biol. Methods Protoc. 2019, 4, bpz017. [Google Scholar] [CrossRef]
- Zadabbas Shahabadi, H.; Akbarzadeh, A.; Ofoghi, H.; Kadkhodaei, S. Site-specific gene knock-in and bacterial phytase gene expression in Chlamydomonas reinhardtii via Cas9 RNP-mediated HDR. Front. Plant Sci. 2023, 14, 1150436. [Google Scholar] [CrossRef]
- Li, Y.; Huang, C.; Liu, Y.; Zeng, J.; Yu, H.; Tong, Z.; Yuan, X.; Sui, X.; Fang, D.; Xiao, B.; et al. CRISPR/Cas9-mediated seamless gene replacement in protoplasts expands the resistance spectrum to TMV-U1 strain in regenerated Nicotiana tabacum. Plant Biotechnol. J. 2023, 21, 2641–2653. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Wu, P.Y.; Chang, C.H.; Huang, L.F. High-yield BMP2 expression in rice cells via CRISPR and endogenous αAmy3 promoter. Appl. Microbiol. Biotechnol. 2024, 108, 206. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.K.; Kim, W.C. The strategy of knock-in with homology-directed genome editing in the model ornamental plant Petunia using CRISPR/Cas9 ribonucleoprotein complex. Sci. Hortic. 2024, 326, 112714. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Méndez-García, A.; Chamu-García, V.; Rodríguez, A.L.; Bandyopadhyay, A.; Paul, S. The applications of CRISPR/Cas-mediated microRNA and lncRNA editing in plant biology: Shaping the future of plant non-coding RNA research. Planta 2024, 259, 32. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Shih, J.; Wheatley, M.S.; Yang, Y. Predictable NHEJ Insertion and Assessment of HDR Editing Strategies in Plants. Front. Genome Ed. 2022, 4, 825236. [Google Scholar] [CrossRef]
- Permyakova, N.V.; Marenkova, T.V.; Belavin, P.A.; Zagorskaya, A.A.; Sidorchuk, Y.V.; Deineko, E.V. CRISPR/Cas9-Mediated Targeted DNA Integration: Rearrangements at the Junction of Plant and Plasmid DNA. Int. J. Mol. Sci. 2022, 23, 8636. [Google Scholar] [CrossRef]
- Hirosawa, M.; Fujita, Y.; Saito, H. Cell-Type-Specific CRISPR Activation with MicroRNA-Responsive AcrllA4 Switch. ACS Synth. Biol. 2019, 8, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.D.; Aschenbrenner, S.; Grosse, S.; Rapti, K.; Domenger, C.; Fakhiri, J.; Mastel, M.; Börner, K.; Eils, R.; Grimm, D.; et al. Cell-specific CRISPR-Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 2019, 47, e75. [Google Scholar] [CrossRef]
- Calvache, C.; Vazquez-Vilar, M.; Selma, S.; Uranga, M.; Fernández-del-Carmen, A.; Daròs, J.A.; Orzáez, D. Strong and tunable anti-CRISPR/Cas activities in plants. Plant Biotechnol. J. 2022, 20, 399–408. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Hyden, B.; Tuskan, G.A.; Abraham, P.E.; Yang, X. Expanding the application of anti-CRISPR proteins in plants for tunable genome editing. Plant Physiol. 2023, 192, 60–64. [Google Scholar] [CrossRef]
- Yun, D.; Jung, C. MiRNA-Responsive CRISPR-Cas System via a DNA Regulator. Biosensors 2023, 13, 975. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Z.; Liu, Y. RNAi-mediated control of CRISPR functions. Theranostics 2020, 10, 6661–6673. [Google Scholar] [CrossRef]
- Chen, Q.J.; Deng, B.H.; Gao, J.; Zhao, Z.Y.; Chen, Z.L.; Song, S.R.; Wang, L.; Zhao, L.P.; Xu, W.P.; Zhang, C.X.; et al. A miRNA-Encoded Small Peptide, vvi-miPEP171d1, Regulates Adventitious Root Formation. Plant Physiol. 2020, 183, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Gautam, H.; Sharma, A.; Trivedi, P.K. Plant microProteins and miPEPs: Small molecules with much bigger roles. Plant Sci. 2023, 326, 111519. [Google Scholar] [CrossRef]
- Ormancey, M.; Thuleau, P.; Combier, J.P.; Plaza, S. The Essentials on microRNA-Encoded Peptides from Plants to Animals. Biomolecules 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Sanyal, I.; Rai, S.P.; Lata, C. An overview on miRNA-encoded peptides in plant biology research. Genomics 2021, 113, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Couzigou, J.M.; Lauressergues, D.; Bécard, G.; Combie, J.P. MiRNA-encoded peptides (miPEPs): A new tool to analyze the roles of miRNAs in plant biology. RNA Biol. 2015, 12, 1178–1180. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Song, S.; Wang, L.; Xu, W.; Zhang, C.; Wang, S.; Liu, H.; Ma, C. Vvi-miPEP172b and vvi-miPEP3635b increase cold tolerance of grapevine by regulating the corresponding MIRNA genes. Plant Sci. 2022, 325, 111450. [Google Scholar] [CrossRef]
- Mandal, K.; Boro, P.; Chattopadhyay, S. Micro-RNA based gene regulation: A potential way for crop improvements. Plant Gene 2021, 27, 100312. [Google Scholar] [CrossRef]
- Pandita, D. MiRNA-and RNAi-Mediated Metabolic Engineering in Plants. In Metabolic Engineering in Plants; Aftab, T., Hakeem, K.R., Eds.; Springer: Singapore, 2022; pp. 171–186. [Google Scholar]
- Li, W.X.; Huang, J.Z.; Zhao, H.J.; Tan, Y.Y.; Cui, H.R.; Poirier, Y.; Shu, Q.Y. Production of low phytic acid rice by hairpin RNA- and artificial microRNA-mediated silencing of OsMIK in seeds. Plant Cell Tissue Organ Cult. 2014, 119, 15–25. [Google Scholar] [CrossRef]
- Sabzehzari, M.; Naghavi, M.R. Phyto-miRNAs-based regulation of metabolites biosynthesis in medicinal plants. Gene 2019, 682, 13–24. [Google Scholar] [CrossRef]
- Jeena, G.S.; Singh, N.; Shikha; Shukla, R.K. An insight into microRNA biogenesis and its regulatory role in plant secondary metabolism. Plant Cell Rep. 2022, 41, 1651–1671. [Google Scholar] [CrossRef]
- Ali, N.A.; Song, W.; Huang, J.; Wu, D.; Zhao, X. Recent advances and biotechnological applications of RNA metabolism in plant chloroplasts and mitochondria. Crit. Rev. Biotechnol. 2024, 44, 1552–1573. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V. Mitochondria in photosynthetic cells: Coordinating redox control and energy balance. Plant Physiol. 2023, 191, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Betti, F.; Ladera-Carmona, M.J.; Perata, P.; Loreti, E. RNAi Mediated Hypoxia Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 9394. [Google Scholar] [CrossRef]
- Anand, A.; Pandi, G. Noncoding RNA: An Insight into Chloroplast and Mitochondrial Gene Expressions. Life 2021, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Wang, P.; Wang, G. Non-coding RNA Regulated Cross-Talk Between Mitochondria and Other Cellular Compartments. Front. Cell Dev. Biol. 2021, 9, 688523. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Srivastava, A.; Bandyopadhyay, A.; Paul, S. The elusive roles of chloroplast microRNAs: An unexplored facet of the plant transcriptome. Plant Mol. Biol. 2022, 109, 667–671. [Google Scholar] [CrossRef]
- Small, I. RNAi for revealing and engineering plant gene functions. Curr. Opin. Biotechnol. 2007, 18, 148–153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Vázquez, L.A.; Castro-Pacheco, A.M.; Pérez-Vargas, R.; Velázquez-Jiménez, J.F.; Paul, S. The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology. Non-Coding RNA 2025, 11, 19. https://doi.org/10.3390/ncrna11020019
Bravo-Vázquez LA, Castro-Pacheco AM, Pérez-Vargas R, Velázquez-Jiménez JF, Paul S. The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology. Non-Coding RNA. 2025; 11(2):19. https://doi.org/10.3390/ncrna11020019
Chicago/Turabian StyleBravo-Vázquez, Luis Alberto, Ana Marta Castro-Pacheco, Rodrigo Pérez-Vargas, Joceline Fernanda Velázquez-Jiménez, and Sujay Paul. 2025. "The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology" Non-Coding RNA 11, no. 2: 19. https://doi.org/10.3390/ncrna11020019
APA StyleBravo-Vázquez, L. A., Castro-Pacheco, A. M., Pérez-Vargas, R., Velázquez-Jiménez, J. F., & Paul, S. (2025). The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology. Non-Coding RNA, 11(2), 19. https://doi.org/10.3390/ncrna11020019






