Abstract
The discovery of the involvement of microRNAs (miRNAs) in cystic fibrosis (CF) has generated increasing interest in the past years, due to their possible employment as a novel class of drugs to be studied in pre-clinical settings of therapeutic protocols for cystic fibrosis. In this narrative review article, consider and comparatively evaluate published laboratory information of possible interest for the development of miRNA-based therapeutic protocols for cystic fibrosis. We consider miRNAs involved in the upregulation of CFTR, miRNAs involved in the inhibition of inflammation and, finally, miRNAs exhibiting antibacterial activity. We suggest that antago-miRNAs and ago-miRNAs (miRNA mimics) can be proposed for possible validation of therapeutic protocols in pre-clinical settings.
1. Introduction
Cystic fibrosis (CF) is an hereditary genetic disease caused by a dysregulation of the cystic fibrosis transmembrane regulator (CFTR) gene by a chronic hyperinflammatory state and by frequent and severe bacterial infection of the lungs [1,2,3]. The discovery of the involvement of microRNAs (miRNAs) in cystic fibrosis (CF) [4,5,6,7] has generated increasing interest in the past years, due to the possible employment as a novel and very promising class of drugs able to mimic or inhibit miRNA functions. These novel drugs are expected to be employed in pre-clinical settings of therapeutic protocols for cystic fibrosis [8,9]. In this narrative review article, we considered and comparatively evaluated published laboratory information of possible interest for the development of miRNA-based therapeutic protocols for cystic fibrosis. We considered the miRNAs involved in the upregulation of CFTR, the miRNAs involved in the inhibition of inflammation and, finally, the miRNAs exhibiting antibacterial activity. We suggest that molecules exhibiting ago-miRNA and anti-miRNA activity can be proposed for possible validation of therapeutic protocols in pre-clinical settings. The motivation for studying miRNA therapeutics for CFTR upregulation in cystic fibrosis is based on the fact that CFTR correctors and modulators are beneficial for about 80% of the patients with cystic fibrosis [10,11]. Therefore, for an important number of CF patients, therapeutic interventions to modulate CFTR should be implemented. In addition to these considerations, anti-inflammatory therapies and approaches against bacterial resistance to antibiotic therapy are still major challenges in the management and treatment of CF patients [12,13]. In this respect, studies on novel therapeutic approaches, including those based on miRNA targeting or mimicking, are highly demanded.
2. MicroRNA Therapeutics: From Laboratory Investigations to Clinical Trials
It is well established that microRNAs are very important non-coding RNAs directing the post-transcriptional regulation of gene expression both in normal and pathological tissues [9,14,15,16]. Several review articles are available describing the biogenesis of miRNAs, their processing, and the mechanisms of translational suppression or degradation of target mRNAs [17,18,19,20]. Micro RNAs are a class of small, single-stranded non-coding RNAs that function as a guide molecule in RNA silencing and hence modulate gene expression [17,18,19,20]. The discovery of the deep involvement of miRNAs in several human pathologies has generated interest in pre-clinical studies, demonstrating the possible application of the so-called “MicroRNA Therapeutics” for the development of clinical protocols [21,22,23]. MicroRNA therapeutics can be divided into at least two major categories: one based on inhibiting miRNA activity, and the second based on mimicking the miRNA biological activity (Figure 1).
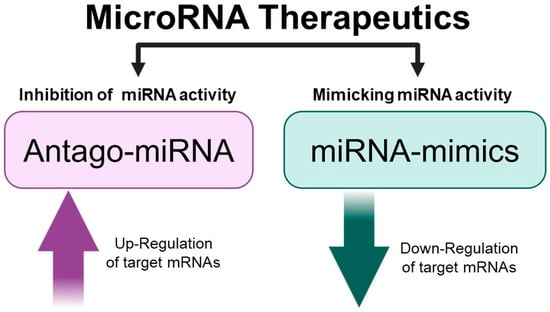
Figure 1.
MicroRNA therapeutics. Picture created using Bio-Render.com (7 November 2024).
2.1. The Anti-miRNA Approach: Counteracting miRNAs Causing Pathological Conditions
The significant progress in understanding the molecular basis of human pathologies has revealed that several miRNAs are up-regulated when pathological tissues are compared to their normal counterparts. This is well established in cancer research, where several onco-miRNAs and metastatic-miRNAs have been demonstrated to be deeply associated with cancer [24,25,26,27]. In this particular field of investigation, a large number of review articles and pioneering research studies are available. This approach is based on several preliminary phases: (a) in general, the “pathological miRNAs” are up-regulated in pathological cells or tissues isolated from the patients [28]; (b) “therapeutic” molecules able to interfere with the activity of the “pathological miRNAs” should be available and validated “in vitro” for the highly efficient hybridization to the target miRNA [29]; (c) efficient delivery systems should be available to allow efficient penetration of the anti-miRNA molecules to target cells [30]. This strategy is described in Figure 2.
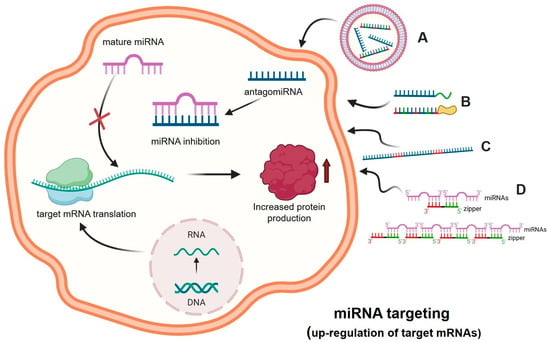
Figure 2.
MicroRNA Therapeutics: the anti-miRNA approach. A forced inhibition of the miRNA activity can be obtained using antago-miRNA (anti-miRNA) oligonucleotides (AMOs) (e.g., DNA, RNA, and nucleic acids analogs such as LNA, PNA, 2′-MOE), delivered with vectors (A) or bioconjugated for an increased cellular uptake (e.g., R-PNAs) and/or a targeted delivery (B). MicroRNA inhibition can also be achieved using anti-miRNA sponge RNA sequences that contain multiple microRNA binding sites (C). The approach based on the use of zipper oligonucleotides is shown in panel (D) [31]. The binding between the miRNA and the anti-miRNA molecules leads to the inactivation of the miRNA, as it can no longer bind to its molecular target, i.e., messenger RNA, thus increasing protein production. Picture created using Bio-Render.com (7 November 2024).
In the scheme outlined in Figure 2, suitably delivered antagomiRNAs interact within the cell with complementary target miRNAs, causing the inhibition of the molecular interactions between these regulatory miRNAs and the binding sites present within the 3′-UTR of the target mRNA. This causes a decrease in the activity of miRNAs in down-regulating mRNAs, which is associated with increased protein production. This experimental approach has been applied to up-regulate the expression of tumor-suppressor genes, whose expression is down-regulated in many cancer types. One of the most intriguing and interesting examples is the interplay between PTEN, miR-221-3p, and antisense molecules against miR-221-3p. PTEN is among the most studied and well-characterized tumor suppressor genes [32]. It encodes PTEN (phosphatase and tensin homologue), a tumor-suppressor protein that antagonizes the phosphoinositide 3-kinase (PI3K)-mTOR) pathway through its lipid phosphatase activity, as reviewed by Song et al. [33]. Loss or down-regulation of PTEN is a hallmark of several cancers [34,35]. Remarkably, the onco-miR miR-221-3p (hyper-expressed in many cancer types) is able to interact with the 3′-UTR of PTEN mRNA, leading to PTEN down-regulation and tumor onset and progression [36,37,38]. Interestingly, anti-miR molecules targeting miR-221-3p have been demonstrated to exert pro-apoptotic and anti-tumor activities both in vitro [39,40] and in vivo [41]. In addition, Xue et al. demonstrated that the treatment of colorectal carcinoma cells with anti-miR221 was able to sensitize the cells to radiation through the activation of PTEN [42]. From a drug-development perspective, the anti-miRNA approach should be considered with caution, as a single miRNA is able to interact with tens or even hundreds of potential target mRNAs [43,44]. Another parameter to consider with great attention is the delivery of anti-miRNA molecules in order to achieve the highest biological activity [44,45].
Another important parameter of the antisense approach is efficiency recognition. In this context, an important alternative/complementary strategy is based on the use of “small RNA zipper” molecules able to connect miRNA molecules end to end, as described by Meng et al. [31]. The interaction between these small RNA zippers and miRNAs generates highly stable RNA-RNA duplexes. In these experimental conditions, miRNA activity is blocked. Using this approach to target the oncomiR miR-221, Meng et al. were able to demonstrate that the miR-221 zipper was able to reverse the oncogenic function of miR-221 in breast cancer cells, restoring the activity of mRNAs that were down-regulated in breast cancer cells by miR-221.
2.2. The “miRNA-Masking” Approach: Inhibiting the Molecular Interactions Between miRNAs and 3′-UTR miR-Binding Sites
One of the alternative strategies for altering miRNA functions is, rather than hybridizing with the microRNAs, to cover the miRNA-binding site present in the 3′UTR of the target mRNAs, therefore preventing miRNA/mRNA recognition. In these experimental conditions, miRNA function is deeply hampered. This strategy, called miRNA-mask or miRNA-masking, is based on the use of a single-stranded 2′-O-methyl-modified oligoribonucleotide (or other chemically modified molecule), that is fully complementary to the miRNA binding site to be “masked”, usually present and functionally active within the 3′-UTR of the mRNA to be modulated [46]. This technology was described and discussed by Pagoni et al. and by Wang et al. [47,48], and a similar concept was the basis of a study reported by Choi et al. [49]. The most important steps of this approach are summarized in Figure 3.
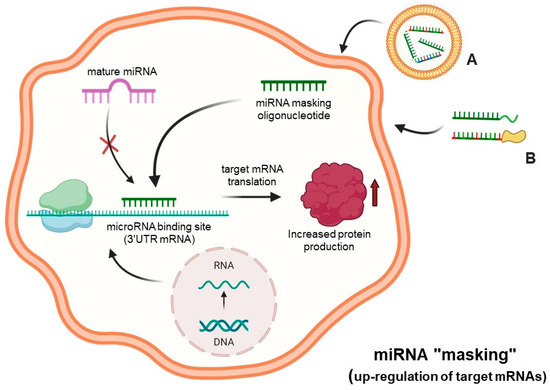
Figure 3.
MicroRNA therapeutics: the “miRNA-masking” approach. The down-regulation of microRNA functions is obtained through miRNA masking oligonucleotides and analogs delivered to cells (A,B), which act by masking the miRNAs binding site of target mRNAs through a direct hybridization of the miRNA “mask” with the 3′UTR region of mRNA. Created using Bio-Render.com (7 November 2024).
In the scheme outlined in this figure, suitably delivered miRNA-masking molecules specifically interact within the cell with complementary miRNA-binding sites present within the 3′-UTR of mRNA, causing inhibition or suppression of the molecular interactions of these regulatory miRNAs to the “masked” binding sites. This causes a decrease in the activity of regulatory miRNAs in down-regulating mRNAs, which is associated with an increased protein production.
2.3. MicroRNA Therapeutics: The miRNA Mimicking Approach
In several diseases, pathological genes are up-regulated, which is associated with a high content of their respective mRNAs. Examples include mRNAs coding oncoproteins [50] or, in the case of COVID-19, pro-inflammatory genes coding proteins involved in the COVID-19 “Cytokine Storm” [51]. In most of these cases, miRNAs regulating these up-regulated genes are down-regulated. The treatment of target cells with pre-miRNAs mimicking the activity of these down-regulated miRNAs might be of great interest. The objective of the miRNA therapeutics based on the miRNA mimicking approach is to replace the biological activity of these down-regulated miRNAs.
The experimental approach outlined in Figure 4 can be employed in order to restore the biological activity of miRNAs that are down-regulated or fully suppressed in human pathologies. This is, for example, the case of tumor-suppressor miRNAs targeting mRNAs coding oncoproteins [52,53]. As schematically presented in Figure 4, in representative studies, pre-miRNAs are delivered to target cells, where they generate mature miRNAs that are able to interact with the 3′UTR of the mRNAs to be modulated, causing a sharp inhibition of protein production. Synthetic RNA duplexes containing chemical modifications complexed in lipid or polymer-based vectors are used for therapeutic purposes to enhance stability and cellular absorption. Another method involves the use of expression vectors containing the sequence of the miRNA whose levels need to be increased, under the control of a strong promoter. As a representative example, Phatak et al. studied miR-214-3p, an important tumor suppressor in several cancers [54,55,56]. In particular, they studied the interplay between miR-214-3p and the oncoprotein RAB14 in esophageal cancer cells. The major result of this study was that the forced expression of miR-214-3p was associated with a marked decrease in cellular migration and invasion [56].
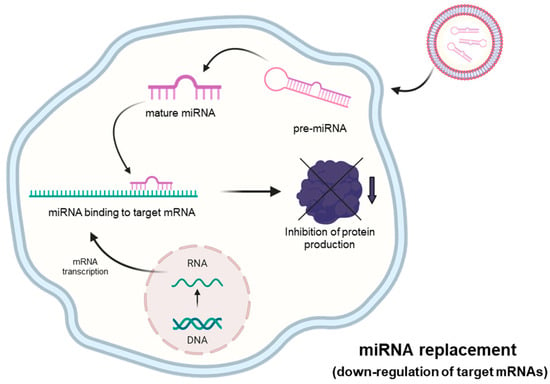
Figure 4.
MicroRNA therapeutics: the “miRNA-replacement” approach. This strategy is based on the use of molecules (mature double-strand microRNA mimics or pre-miRNA oligonucleotides) that can restore physiological levels of miRNA with consequent inhibition of mRNA translation. Created using Bio-Render.com (7 November 2024).
2.4. Pre-Clinical and Clinical Studies Based on miRNA Therapeutics
Several studies (both pre-clinical and clinical) support the concept that miRNA therapeutics deserves great attention for translating laboratory research into clinical practice. In fact, the therapeutic potential of miRNAs for various human pathologies (including cystic fibrosis) is evident, and future studies are expected to validate their possible applicability in clinical settings. The growing interest in these studies is demonstrated by the involvement in miRNA therapeutics of several biopharmaceutical companies, such as Santaris Pharma (Hersholm, Denmark), Roche Pharmaceuticals (Singapore), Regulus Therapeutics (San Diego, CA, USA), Mirna Therapeutics Inc. (Carlsbad, CA, USA), miRagen Therapeutics (Boulder, CO, USA), and EnGeneIC (Sydney, Australia). The involvement of these companies has been reviewed by Zhang et al., 2021 [44]. Examples of biopharmaceutical products for miRNA therapeutics of human diseases are reported in Table 1.

Table 1.
Examples of biopharmaceutical products for miRNA therapeutics targeting human diseases.
2.5. Combining miRNA Therapeutics with Chemotherapy
Several studies have demonstrated that the protocols of miRNA therapeutics can be employed together with chemotherapeutic agents [67,68,69]. For instance, Zurlo et al. combined the treatment of U251 and T98G glioma cells with a peptide nucleic acid targeting miR-221-3p and an anti-tubulin tetrahydrothieno [2,3-c]pyridine derivative and found synergistic activity [67]. A similar approach was followed by Gasparello et al. using sulforaphane and a peptide nucleic acid targeting miR-15b-5p [68]; furthermore, Zurlo et al. found that a combined treatment of glioblastoma U251 cells with an anti-miR-10b-5p exhibits synergistic acidity when combined with an anticancer agent based on 1-(3′,4′,5′-trimethoxyphenyl)-2-aryl-1H-imidazole scaffold [69]. Another example is the demonstration that miRNA therapeutics can overcome cancer resistance to therapy. Studies indicate that combining therapeutic miRNAs with chemotherapy can decrease the required drug doses for cancer treatment [45].
3. Pathophysiology of Cystic Fibrosis: Identification of Target Pathological Networks for the Development of Therapeutic Protocols
In cystic fibrosis, the defects of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) are caused by more than 2000 mutations of the CFTR gene, causing deep alterations in CFTR protein content and functions. These quantitative and/or qualitative alterations deeply affect the homeostasis of chloride, bicarbonate, sodium, and water in the airway surface liquid [1,2,70,71,72]. As reviewed by Cabrini et al., this influences the mucus composition and viscosity and is associated in CF patients with severe conditions of infection and inflammation throughout their whole lives [73]. CF is a multi-organ pathology characterized by the following major pathological conditions: (a) a deep dysregulation of the CFTR [74]; (b) a hyperinflammatory state characterized by an elevated expression of pro-inflammatory genes, such as the interleukin-8 (IL-8) gene; [75] (c) bacterial infection, the most relevant being that caused by Pseudomonas aeruginosa [76]. Micro RNAs are involved in all these pathological conditions, as will be discussed in the following sections.
4. MicroRNAs and Expression of the CFTR Gene
In this field of investigation, we should mention several research papers that have significantly contributed to the notion that CFTR expression and functions are regulated by microRNA. For example, Gillen et al. reported in 2011 evidence suggesting an involvement of microRNAs in regulating CFTR gene expression [77]. In 2012, Ramachandran et al. identified and described a microRNA network that regulates the expression and biosynthesis of wild-type and DeltaF508 mutant CFTR [78]. In Table 2, we report a list of miRNAs that interact with the 3′UTR of CFTR mRNA and regulate CFTR. Summarizing, the miRNAs involved in CFTR regulation are, in addition to miR-145 and miR-494 (reported by Gillen et al.) [77], miR-101 [79], miR-144 [79], miR-223 [80], miR-509 [81], miR-384 [82], miR-200b [83], miR-143-5p [84], miR-335-5p [85], miR-16 [86]. It should be mentioned that these microRNAs can synergize, as demonstrated by Megiorni et al. while studying the biological effect of miR-101-3p and miR-294 [87] and by Papi et al. [88], who demonstrated a synergistic enhancement of the CFTR gene expression following the combined treatment of bronchial epithelial cells with antisense molecules targeting miR-145-5p and miR-101-3p. The experimental approaches to sustain the role of these miRNAs on CFTR gene expression are usually the following: (a) the presence of the miRNA-binding sites within the 3′UTR of the CFTR mRNA; (b) the demonstration that these miRNA-binding sites are conserved throughout molecular evolution (and therefore are functionally important) when the nucleotide CFTR mRNA sequences of different species are compared; (c) the demonstration that pre-miRNAs down-regulated and antago-miRNAs up-regulated luciferase activity in 3′UTR/CFTR)/luc constructs; no or low modulation of luciferase activity should be found in mutated constructs in which the miRNA-binding sites of the 3′UTR/CFTR mRNA are mutated in order to suppress the miRNA/(CFTR) mRNA interaction. The final evidence that supports the role of the studied miRNAs in CFTR gene regulation is that forcing the expression of these microRNAs is associated with deep inhibition of CFTR gene expression, as published by Papi et al. [89]. Conversely, and of great interest in proposing “miRNA Therapeutics” for cystic fibrosis, the treatment of target epithelial cells with inhibitors of miRNAs down-regulating CFTR is associated with the hyperactivation of CFTR gene expression. In this respect, antago-miRNAs based on peptide nucleic acids (PNAs) have been proposed [90] and employed with impactful results by several research groups [91,92,93,94]. One of the most interesting approaches for CFTR upregulation is, in our opinion, targeting miR-145-5p, as generally recognized by concurrent evidences [93,95,96,97,98]. Interestingly, these treatments can be combined to obtain the highest effect on CFTR upregulation, as demonstrated by Papi et al., who reported the synergistic enhancement of the expression of the CFTR gene after the combined treatment of bronchial epithelial Calu-3 cells with PNAs targeting miR-145-5p and miR-101-3p [88]. Table 2 summarizes examples of miRNAs regulating CFTR.

Table 2.
Examples of miRNAs involved in regulation of CFTR, and frequently up-regulated in CF.
5. MicroRNAs and Inflammation in Cystic Fibrosis
CF transmembrane conductance regulator (CFTR)-deficient airway epithelial cells display signaling abnormalities and aberrant intracellular processes, which lead to transcription of inflammatory mediators. Several transcription factors, especially nuclear factor-κB (NF-κB) are activated [99,100]. As mentioned above, the lung environment of CF patients is characterized by high levels of pro-inflammatory cytokines, such as IL-8, IL-6, and TNF-alpha, and decreased levels of anti-inflammatory mediators, such as IL-10, associated with a marked and persistent neutrophil recruitment into the airways. Thus, the possibility of using cytokine modulators to inhibit the exaggerated inflammation in CF represents a potential rational approach (2). After the initial approval of the dual therapy Orkambi® (Lumacaftor/Ivacaftor; Vertex Pharmaceutical, Boston, Mass, USA), interesting data showed that the functional rescue of F508del-CFTR also significantly reduces the mRNA levels of CXCL8, CXCL1, and CXCL2 in response to P. aeruginosa exposure, highlighting the potential anti-inflammatory properties of the corrector/potentiator combination [101,102].
Recently reported evidence suggest that microRNAs are deeply involved in controlling all the different steps of inflammation in cystic fibrosis. Some microRNAs promote inflammation, while most of the studied microRNAs are potent inhibitors of the expression of genes responsible for CF inflammation (examples in Table 3) [103,104,105,106,107,108,109,110,111,112].

Table 3.
Examples of miRNAs involved in the regulation of pro-inflammatory gene expression and frequently down-regulated in CF.
Among pro-inflammatory microRNAs, one example is miR-155, which promotes inflammation in cystic fibrosis by enhancing the expression of IL-8 [103]. In this context, miRNA therapeutics based on the use of anti-miR-155 molecules are expected to inhibit IL-8 expression. On the contrary, many other miRNAs cause a decrease in the production of pro-inflammatory proteins. For instance, Oglesby reported a decrease in IL-8 production associated with the hyperexpression of miR-17 [104]. Ma et al. reported that miR-302b negatively regulates IL-1β production [105], and Fabbri et al. found that miR-93-5p (down-regulated during P. aeruginosa infection) inhibits IL-8 protein synthesis and release [106].
With respect to the mechanism of action of the anti-inflammatory miRNAs, the molecular targets are diverse. Fabbri et al. described a direct interaction between miR-93-5p and IL-8 mRNA [106], while Kalantari et al. reported that miR-718 represses pro-inflammatory cytokine production by targeting PTEN [107]. Interestingly, the NF-kB pathway is a target of the activity of several miRNAs. For instance, Bardin et al. reported that miR-199a-3p reduced IKKβ protein expression, thereby inhibiting NF-κB activation and expression of NF-kB-regulated genes [108]. In addition, Ma et al. [105] and Xu et al. [109] reported the direct targeting of IRAK4 mRNA by miR-302b and miR-93, respectively. A further example of the involvement of miRNAs in the NF-kB pathways is miR-636, which was demonstrated to exhibit a direct interaction with IL1R1 and RANK; these interactions cause a decrease in the levels of IL1R1 and IKKβ proteins, causing inhibition of pro-inflammatory gene expression [110]. A final example is constituted by the effects of miR-93-5p and miR-145-5p on the expression of Toll-like Receptor-4 (TLR-4). In this respect, TLR-4 mRNA was found to be a direct target of miR-93-5p and miR-145-5p by Gao et al. [111] and Wu et al. [112], respectively. Figure 5 shows the expected molecular targets and mechanism of action of miR-93-5p.
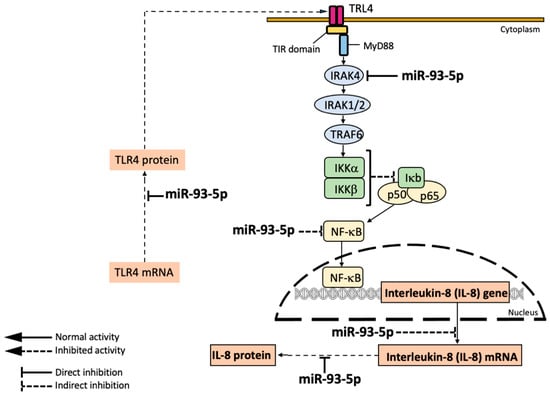
Figure 5.
Mechanism of action and molecular targets of miR-93-5p according to the reports published by Fabbri et al. [106], Xu et al. [109], and Gao et al. [111]. MicroRNA miR-93-5p directly interacts with IL-8 mRNA, thereby inhibiting IL-8 production and release [106]; in addition, miR-93-5p inhibits IRAK1, thereby preventing NF-kB activation and expression of NF-kB-dependent genes, such as IL-8 [109]; in addition, miR-93-5p is able to interact with TLR-4 mRNA [112], thereby down-regulating the NF-kB pathway.
MicroRNA miR-93-5p directly inhibits TLR4 [112] and IRAK4 [109], causing indirect inhibition of NF-kB and of the transcription of the NF-kB regulated IL-8 gene. In addition, miR-93-5p directly interacts with the 3′UTR of IL-8 mRNA, thereby causing the inhibition of IL-8 production and release.
From a therapeutic point of view, a decrease in the expression of pro-inflammatory genes should be obtained with transfection of cellular model systems with pre-miR-93-5p generating, after cell penetration, a bioactive miR-93-5p molecule. The possible use of miR-93-5p for anti-inflammatory therapy of Cystic Fibrosis is supported by the very interesting results published by Gao et al. [111]. In their study, Gao et al. employed an in vivo mouse model of LPS-induced acute lung injury (LPS-ALI). When the levels of miR-93-5p expression were analyzed in the lung tissues and bronchoalveolar fluid, they were found to be significantly down-regulated. Interestingly, when agomiR-93 was injected into LPS-ALI mice, agomiR-93-mediated decrease of lung injury was noted, associated with suppression of the LPS-induced inflammatory response.
With respect to testing miRNA therapeutics products in animal models prior to clinical trials, we would like to underline that, at least in theory, ago-miRNA and anti-miRNA products targeting genes relevant to CF (for instance the human CFTR and IL-8 genes, as discussed in Section 4 and Section 5) can be assayed in animal models, obtaining informative results, since the sequences of both regulatory miRNAs and those of miRNA-binding sites are conserved throughout the molecular evolution [93,106,111].
6. MicroRNAs and the Development of Antibacterial Strategies for Cystic Fibrosis
In cystic fibrosis, the bacterial infection is one of the most leading causes of morbidity and mortality of CF patients. It is well established that Pseudomonas aeruginosa (P. aeruginosa) is the most frequently involved in bacterial infection [113]. In addition, the interplay between inflammation and bacterial infection in CF should be considered [114,115,116]. For instance, the expression of miR-302b is induced by TLR2 and the TLR4/NK-κB pathway during P. aeruginosa infection, and its overexpression activates cytokine release [114]. Moreover, in a paper published in 2016, Li et al. reported that P. aeruginosa infection augments inflammation through the miR-301b repression of c-Myb-mediated immune activation and infiltration [115]. A final example outlining the interplay between P. aeruginosa infection and inflammation is provided by another study, by Li at al., who found that overexpressed miR-539 exacerbates Pseudomonas aeruginosa pneumonia by promoting inflammatory responses [116]. The role of microRNAs in chronic Pseudomonas lung infection in cystic fibrosis has been reviewed by Fesen et al. [117], Ye et al. [118], and Kimura et al. [119]. This issue is of great interest, considering that P. aeruginosa has been considered by the World Health Organization (WHO) as one of the priority bacteria, requiring extensive research and urgent development of new antibiotic treatments [120].
One of the examples of miRNAs involved in P. aeruginosa infection is miRNA-302b, which was demonstrated by Zhou et al. to augment the host defense against bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits [121]. Another example is constituted by the microRNA-302/367 Cluster, which impacts host antimicrobial defense via the regulation of the mitophagic response to P. aeruginosa infection [122]. A product for possible applications as an antibacterial agent is MEG3-4, a miRNA decoy that regulates IL-1β to prevent sepsis during lung infection [123].
An interesting study underlying the interplay between P. aeruginosa infection and microRNAs has been reported by Lozano-Iturbe et al., who found that the binding of P. aeruginosa to CF bronchial epithelial cells deeply alters the composition of the produced exosomes after comparison with healthy control cells [124]. An application of this and similar observations has been reported by Koeppen et al., who demonstrated that let-7b-5p in vesicles secreted by human airway cells is able to reduce biofilm formation and increase antibiotic sensitivity of P. aeruginosa [125]. Taken together, the studies presented in this chapter strongly suggest that the microRNAs involved in P. aeruginosa infection should be considered as molecular targets for the development of antibacterial approaches.
7. Conclusions and Future Perspectives
In this review paper, we have presented and discussed studies supporting the role of microRNAs in the major pathological conditions of cystic fibrosis, i.e., the regulation of CFTR expression, the process of inflammation and bacterial infection, with particular focus on Pseudomonas aeruginosa. These studies support the concept that “microRNA therapeutics (see Figure 1, Figure 2, Figure 3 and Figure 4) might be considered in the future for the possible development of therapeutic protocols for cystic fibrosis. Interestingly, clinical trials based on miRNA therapeutics are ongoing for the treatment of human pathologies (Table 1). It is well established that anti-miRNA molecules down-regulating CFTR (for instance PNAs against miR-101-3p, miR-145-5p and miR-335-5p) are able to restore CFTR activity. In this respect, it should be noted that targeting multiple CFTR-regulating miRNAs might lead to high efficiency in CFTR upregulation. This was reported by Papi et al., who combined treatment with PNAs against miR-101-3p and miR-145-5p to obtain maximal enhancement of CFTR expression [88], and by Megiorni et al., who combined miR-101 and miR-494 synthetic mimics, demonstrating synergism in inhibiting the expression of a reporter construct containing the 3′-UTR of CFTR in luciferase assays [87]. Some miRNAs are involved in different processes, such as miR-145-5p, which is involved in CFTR regulation [77,81,93,95,96,97] and in inhibiting the expression of pro-inflammatory genes [112].
Important issues that should be considered in future experimental studies should clarify the impact of miRNA therapeutics in comparison with other strategies for modifying gene expression using DNA/RNA-based biomolecules, such as siRNAs and ASO. This comparison is necessary, considering that (a) inhibiting one single miRNA might activate the expression of a large cohort of genes, all negatively controlled by the same miRNA, and (b) that mimicking the activity of a single miRNA might inhibit the expression of many different mRNAs containing the binding site of the miRNA itself. In this respect, transcriptomic and proteomic studies should be highly warranted to verify this possibility. Examples of limitations and challenges of the miRNA therapeutic approach for CF are presented (together with possible solutions) in Table 4.

Table 4.
Challenges of the “MicroRNA Therapeutics” approach.
Other issues of miRNA therapeutics were extensively presented and discussed in two review papers by Zhang et al. [44] and by Seyhan [45]. Among future perspectives, we would like to discuss the issues below.
7.1. Combined Treatments
A very interesting field of investigation for the future is the combination of miRNA therapeutics with current chemotherapy. For instance, De Santi was able to demonstrate that CFTR-specific target site blockers (TSBs) masking the miR-145-5p and miR-223-3p binding sites increased CFTR expression and enhanced the effects of ivacaftor/lumacaftor or ivacaftor/tezacaftor [84]. In our opinion, this very important issue deserves to be investigated in great detail, considering that CFTR correctors and modulators are beneficial for about 80% of the patients with cystic fibrosis, but not for the entire population of CF patients [10,11]. Therefore, for an important number of patients, therapeutic interventions to modulate CFTR should be implemented, and combined treatments exploring synergisms between CFTR corrector/potentiators and “miRNA Therapeutics” products might be considered in pre-clinical approaches.
7.2. Innovative Diagnostic Tools for a Personalized miRNA Therapeutics of Cystic Fibrosis
The analysis of the pattern of extracellular circulating microRNAs (EC-miRNA) has been extensively studied in projects focusing on the liquid biopsy of cancer [128,129]. These studies were followed by a number of investigations demonstrating the usefulness of EC-miRNAs as non-invasive biomarkers in a wide range of diseases [130,131]. Although few reports are available on EC-miRNAs in CF, this issue should be deeply investigated, in our opinion, considering the potential importance of miRNA signatures for programming therapeutic interventions, including those based on miRNA therapeutics. It is widely accepted that circulating EC-miRNAs might be important for monitoring the response of patients to therapy. In this respect, Cook et al. demonstrated for the first time that changes in circulating miRNA levels in CF might be predictive of CF-associated complications (hepatic fibrosis), suggesting that serum-based miRNA analysis might be have prognostic value [132]. More recently, Ideozu et al. employed microarray technology to identify aberrantly expressed plasma ECmiRNAs in CF and were able to demonstrate significant differences in the ECmiRNAs pattern when CF patients were compared with non-CF subjects [133]. The results of this study suggest that ECmiRNAs may be clinically relevant in CF. Further studies in this field of investigation are warranted, as also suggested in the review article by De Palma et al., who highlighted recent findings on the potential utility of measuring circulating miRNAs in CF [7]. In particular, it would be very important to find a positive correlation between the analysis of EC-miRNAs and the miRNAs found in vivo in the bronchial epithelium of CF patients, where the increased expression of miRNAs (for instance miR-145, miR-223, and miR-494) correlates with decreased CFTR expression [80]. Although further studies are required to define the importance of EC-miRNAs as diagnostic, prognostic, and predictive biomarkers, the possible interplay between the expression of EC-miRNAs in CF patients and the response to therapy (including miRNA therapeutics) should be considered of top interest.
7.3. Delivery
A further major issue (not covered by the present review) is the delivery of miRNA therapeutics molecules. Several studies are ongoing in this specific issue. PNAs can be efficiently delivered to target cells by the attachment of a cell-penetrating 8-arginine peptide [40]. TSBs have been delivered after encapsulation in poly-lactic-co-glycolic acid (PLGA) nanoparticles [84], PNAs and miRNA mimics can be delivered using argininocalix [4] arenes [134,135]. The research on these very important issues is expected to reinforce the possible application of miRNA therapeutics (also in combination with conventional interventions) for the experimental treatment of cystic fibrosis.
Author Contributions
Conceptualization, formal analysis, resources, writing—original draft preparation, writing—review and editing, project administration, A.F. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fondazione Fibrosi Cistica (FFC), project “Revealing the microRNAs-transcription factors network in cystic fibrosis: from microRNA therapeutics to precision medicine (CF-miRNA-THER)”, Project no. FFC#7/2018. AF and RG are funded by MUR-FISR COVID-miRNAPNA (Project no. FISR2020IP_04128) and by the Interuniversity Consortium for Biotechnology (CIB) (Projects no. CIB-Unife-2020 and CIB-Unife-2022). AF was funded by the University Fund for Scientific Research FAR, (Projects no. FAR-AF-Unife-2023, FAR-AF-Unife-2024) and by the University Fund FIRD 2024 (Projects n° FIRD-Finotti 2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are contained within the article; additional information will be shared upon request to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mall, M.A.; Burgel, P.R.; Castellani, C.; Davies, J.C.; Salathe, M.; Taylor-Cousar, J.L. Cystic fibrosis. Nat. Rev. Dis. Primers. 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.; Ramsey, B.W. Cystic Fibrosis: A Review. JAMA 2023, 329, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.M.; Bonk, M.P.; Hadjiliadis, D. Cystic Fibrosis: Emerging Understanding and Therapies. Annu. Rev. Med. 2019, 70, 197–210. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, P.J.; Greene, C.M. MicroRNA Dysregulation in Cystic Fibrosis. Mediat. Inflamm. 2015, 2015, 529642. [Google Scholar] [CrossRef]
- Sonneville, F.; Ruffin, M.; Guillot, L.; Rousselet, N.; Le Rouzic, P.; Corvol, H.; Tabary, O. New insights about miRNAs in cystic fibrosis. Am. J. Pathol. 2015, 185, 897–908. [Google Scholar] [CrossRef]
- Glasgow, A.M.A.; De Santi, C.; Greene, C.M. Non-coding RNA in cystic fibrosis. Biochem. Soc. Trans. 2018, 46, 619–630. [Google Scholar] [CrossRef]
- De Palma, F.D.E.; Raia, V.; Kroemer, G.; Maiuri, M.C. The Multifaceted Roles of MicroRNAs in Cystic Fibrosis. Diagnostics 2020, 10, 1102. [Google Scholar] [CrossRef]
- Bardin, P.; Sonneville, F.; Corvol, H.; Tabary, O. Emerging microRNA Therapeutic Approaches for Cystic Fibrosis. Front. Pharmacol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- De Santi, C.; Greene, C.M. Challenges facing microRNA therapeutics for cystic fibrosis lung disease. Epigenomics 2020, 12, 179–181. [Google Scholar] [CrossRef]
- Fajac, I.; Sermet, I. Therapeutic Approaches for Patients with Cystic Fibrosis Not Eligible for Current CFTR Modulators. Cells 2021, 10, 2793. [Google Scholar] [CrossRef]
- Bardin, E.; Pastor, A.; Semeraro, M.; Golec, A.; Hayes, K.; Chevalier, B.; Berhal, F.; Prestat, G.; Hinzpeter, A.; Gravier-Pelletier, C.; et al. Modulators of CFTR. Updates on Clinical Development and Future Directions. Eur. J. Med. Chem. 2021, 213, 113195. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.; Simmonds, N.J. Why don’t anti-inflammatories work in cystic fibrosis? Expert Rev. Respir. Med. 2024, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- López-Causapé, C.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. The problems of antibiotic resistance in cystic fibrosis and solutions. Expert Rev. Respir. Med. 2015, 9, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I. Roles of MicroRNAs in Disease Biology. JMA J. 2023, 6, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef]
- Chen, H. microRNA-Based Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 25, 230. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Gazi SHossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097. [Google Scholar] [CrossRef]
- Shademan, B.; Karamad, V.; Nourazarian, A.; Masjedi, S.; Isazadeh, A.; Sogutlu, F.; Avcı, C.B. MicroRNAs as Targets for Cancer Diagnosis: Interests and Limitations. Adv. Pharm. Bull. 2023, 13, 435–445. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—MicroRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Azari, H.; Nazari, E.; Mohit, R.; Asadnia, A.; Maftooh, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Shahidsales, S.; Khazaei, M.; et al. Machine learning algorithms reveal potential miRNAs biomarkers in gastric cancer. Sci. Rep. 2023, 13, 6147. [Google Scholar] [CrossRef]
- Lukiw, W.J. Variability in micro RNA (miRNA) abundance, speciation and complexity amongst different human populations and potential relevance to Alzheimer’s disease (AD). Front. Cell Neurosci. 2013, 7, 133. [Google Scholar] [CrossRef]
- Lennox, K.A.; Owczarzy, R.; Thomas, D.M.; Walder, J.A.; Behlke, M.A. Improved Performance of Anti-miRNA Oligonucleotides Using a Novel Non-Nucleotide Modifier. Mol. Ther. Nucleic Acids 2013, 2, e117. [Google Scholar] [CrossRef]
- Saenz-Pipaon, G.; Dichek, D.A. Targeting and delivery of microRNA-targeting antisense oligonucleotides in cardiovascular diseases. Atherosclerosis 2023, 374, 44–54. [Google Scholar] [CrossRef]
- Meng, L.; Liu, C.; Lü, J.; Zhao, Q.; Deng, S.; Wang, G.; Qiao, J.; Zhang, C.; Zhen, L.; Lu, Y.; et al. Small RNA zippers lock miRNA molecules and block miRNA function in mammalian cells. Nat. Commun. 2017, 8, 13964. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; Dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, G.; Xu, M.; Ying, X.; Li, B.; Cao, F.; Cheng, S.; Xiao, B.; Cheng, M.; Liang, L.; et al. Comprehensive analysis of PTEN-related ceRNA network revealing the key pathways WDFY3-AS2-miR-21-5p/miR-221-3p/miR-222-3p-TIMP3 as potential biomarker in tumorigenesis and prognosis of kidney renal clear cell carcinoma. Mol. Carcinog. 2022, 61, 508–523. [Google Scholar] [CrossRef]
- Zhang, L.; Bu, Z.; Shen, J.; Shang, L.; Chen, Y.; Zhang, P.; Wang, Y. MicroRNA-221 regulates cell activity and apoptosis in acute lymphoblastic leukemia via regulating PTEN. Exp. Ther. Med. 2021, 22, 1133. [Google Scholar] [CrossRef]
- Hou, L.; Tong, X.; Lin, S.; Yu, M.; Ye, W.C.; Xie, M. MiR-221/222 Ameliorates Deoxynivalenol-Induced Apoptosis and Proliferation Inhibition in Intestinal Epithelial Cells by Targeting PTEN. Front. Cell Dev. Biol. 2021, 19, 652939. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Lü, J.; Zhao, Q.; Li, D.; Shen, L.; Wang, Z.; Liu, J.; Xie, D.; Cho, W.C.; et al. Targeted Inhibition of miR-221/222 Promotes Cell Sensitivity to Cisplatin in Triple-Negative Breast Cancer MDA-MB-231 Cells. Front. Genet. 2020, 10, 1278. [Google Scholar] [CrossRef]
- Brognara, E.; Fabbri, E.; Bazzoli, E.; Montagner, G.; Ghimenton, C.; Eccher, A.; Cantù, C.; Manicardi, A.; Bianchi, N.; Finotti, A.; et al. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J. Neurooncol. 2014, 118, 19–28. [Google Scholar] [CrossRef]
- Ali, A.; Grillone, K.; Ascrizzi, S.; Caridà, G.; Fiorillo, L.; Ciliberto, D.; Staropoli, N.; Tagliaferri, P.; Tassone, P.; Di Martino, M.T. LNA-i-miR221 activity in colorectal cancer: A reverse translational investigation. Mol. Ther. Nucleic. Acids 2024, 35, 102221. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Sun, K.; Deng, H.J.; Lei, S.T.; Dong, J.Q.; Li, G.X. Anti-miRNA-221 sensitizes human colorectal carcinoma cells to radiation by upregulating PTEN. World J. Gastroenterol. 2013, 19, 9307–9317. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, D.; Pontis, F.; Moro, M.; Centonze, G.; Bertolini, G.; Milione, M.; Mensah, M.; Segale, M.; Petraroia, I.; Borzi, C.; et al. Cotargeting of miR-126-3p and miR-221-3p inhibits PIK3R2 and PTEN, reducing lung cancer growth and metastasis by blocking AKT and CXCR4 signalling. Mol. Oncol. 2021, 15, 2969–2988. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des. Dev. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef]
- Wang, Z. The principles of MiRNA-masking antisense oligonucleotides technology. Methods Mol. Biol. 2011, 676, 43–49. [Google Scholar] [CrossRef]
- Pagoni, M.; Cava, C.; Sideris, D.C.; Avgeris, M.; Zoumpourlis, V.; Michalopoulos, I.; Drakoulis, N. miRNA-Based Technologies in Cancer Therapy. J. Pers. Med. 2023, 13, 1586. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Lu, Y.; Yang, B. miRNAs at the heart of the matter. J. Mol. Med. 2008, 86, 771–783. [Google Scholar] [CrossRef]
- Choi, W.Y.; Giraldez, A.J.; Schier, A.F. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science 2007, 318, 271–274. [Google Scholar] [CrossRef]
- Castel, P.; Rauen, K.A.; McCormick, F. The duality of human oncoproteins: Drivers of cancer and congenital disorders. Nat. Rev. Cancer 2020, 20, 383–397. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Lewalle, P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021, 11, 708765. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.; Smith, N.; Adekoya, T.O.; Li, Y.; Kim, S.; Rios-Colon, L.; Deep, G.; Niture, S.; Albanese, C.; Suy, S.; et al. Knockdown of microRNA-214-3p Promotes Tumor Growth and Epithelial-Mesenchymal Transition in Prostate Cancer. Cancers 2021, 13, 5875. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.; Lu, L.; Wang, Y. miR-214-3p Regulates Multi-Drug Resistance and Apoptosis in Retinoblastoma Cells by Targeting ABCB1 and XIAP. Onco Targets Ther. 2020, 13, 803–811. [Google Scholar] [CrossRef]
- Phatak, P.; Burrows, W.M.; Creed, T.M.; Youssef, M.; Lee, G.; Donahue, J.M. MiR-214-3p targets Ras-related protein 14 (RAB14) to inhibit cellular migration and invasion in esophageal Cancer cells. BMC Cancer 2022, 22, 1265. [Google Scholar] [CrossRef]
- Huang, P.S.; Liao, C.J.; Huang, Y.H.; Yeh, C.T.; Chen, C.Y.; Tang, H.C.; Chang, C.C.; Lin, K.H. Functional and Clinical Significance of Dysregulated microRNAs in Liver Cancer. Cancers 2021, 13, 5361. [Google Scholar] [CrossRef]
- Chavez, E.; Rodriguez, J.; Drexler, Y.; Fornoni, A. Novel Therapies for Alport Syndrome. Front. Med. 2022, 9, 848389. [Google Scholar] [CrossRef]
- van der Ree, M.H.; de Vree, J.M.; Stelma, F.; Willemse, S.; van der Valk, M.; Rietdijk, S.; Molenkamp, R.; Schinkel, J.; van Nuenen, A.C.; Beuers, U.; et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: A phase 1B, double-blind, randomised controlled trial. Lancet 2017, 389, 709–717. [Google Scholar] [CrossRef]
- Chioccioli, M.; Roy, S.; Newell, R.; Pestano, L.; Dickinson, B.; Rigby, K.; Herazo-Maya, J.; Jenkins, G.; Ian, S.; Saini, G.; et al. A lung targeted miR-29 mimic as a therapy for pulmonary fibrosis. EBioMedicine 2022, 85, 104304. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zain, J.; Rosen, S.T.; Querfeld, C. Emerging drugs for the treatment of cutaneous T-cell lymphoma. Expert. Opin. Emerg. Drugs 2022, 27, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Abplanalp, W.T.; Fischer, A.; John, D.; Zeiher, A.M.; Gosgnach, W.; Darville, H.; Montgomery, R.; Pestano, L.; Allée, G.; Paty, I.; et al. Efficiency and Target Derepression of Anti-miR-92a: Results of a First in Human Study. Nucleic Acid Ther. 2020, 30, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Reid, G.; Kao, S.C.; Pavlakis, N.; Brahmbhatt, H.; MacDiarmid, J.; Clarke, S.; Boyer, M.; van Zandwijk, N. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics 2016, 8, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Valencia, T.; Allerson, C.; Schairer, A.; Flaten, A.; Yheskel, M.; Kersjes, K.; Li, J.; Gatto, S.; Takhar, M.; et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 2019, 10, 4148. [Google Scholar] [CrossRef]
- Zurlo, M.; Romagnoli, R.; Oliva, P.; Gasparello, J.; Finotti, A.; Gambari, R. Synergistic effects of the combined treatment of U251 and T98G glioma cells with an anti-tubulin tetrahydrothieno [2,3-c]pyridine derivative and a peptide nucleic acid targeting miR-221-3p. Int. J. Oncol. 2021, 59, 61. [Google Scholar] [CrossRef]
- Zurlo, M.; Romagnoli, R.; Oliva, P.; Gasparello, J.; Finotti, A.; Gambari, R. Synergistic Effects of A Combined Treatment of Glioblastoma U251 Cells with An Anti-miR-10b-5p Molecule and An AntiCancer Agent Based on 1-(3’,4’,5’-Trimethoxyphenyl)-2-Aryl-1H-Imidazole Scaffold. Int. J. Mol. Sci. 2022, 23, 5991. [Google Scholar] [CrossRef]
- Gasparello, J.; Papi, C.; Zurlo, M.; Gambari, L.; Rozzi, A.; Manicardi, A.; Corradini, R.; Gambari, R.; Finotti, A. Treatment of Human Glioblastoma U251 Cells with Sulforaphane and a Peptide Nucleic Acid (PNA) Targeting miR-15b-5p: Synergistic Effects on Induction of Apoptosis. Molecules 2022, 27, 1299. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Schrijver, I. Cystic Fibrosis: A Review of Associated Phenotypes, Use of Molecular Diagnostic Approaches, Genetic Characteristics, Progress, and Dilemmas. J. Mol. Diagn. 2016, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cabrini, G.; Rimessi, A.; Borgatti, M.; Pinton, P.; Gambari, R. Overview of CF lung pathophysiology. Curr. Opin. Pharmacol. 2022, 64, 102214. [Google Scholar] [CrossRef] [PubMed]
- Fanen, P.; Wohlhuter-Haddad, A.; Hinzpeter, A. Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int. J. Biochem. Cell Biol. 2014, 52, 94–102. [Google Scholar] [CrossRef]
- Roesch, E.A.; Nichols, D.P.; Chmiel, J.F. Inflammation in cystic fibrosis: An update. Pediatr. Pulmonol. 2018, 53, S30–S50. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef]
- Gillen, A.E.; Gosalia, N.; Leir, S.H.; Harris, A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 2011, 438, 25–32. [Google Scholar] [CrossRef]
- Ramachandran, S.; Karp, P.H.; Jiang, P.; Ostedgaard, L.S.; Walz, A.E.; Fisher, J.T.; Keshavjee, S.; Lennox, K.A.; Jacobi, A.M.; Rose, S.D.; et al. A microRNA network regulates expression and biosynthesis of wild-type and DeltaF508 mutant cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA 2012, 109, 13362–13367. [Google Scholar] [CrossRef]
- Hassan, F.; Nuovo, G.J.; Crawford, M.; Boyaka, P.N.; Kirkby, S.; Nana-Sinkam, S.P.; Cormet-Boyaka, E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS ONE 2012, 7, e50837. [Google Scholar] [CrossRef]
- Oglesby, I.K.; Chotirmall, S.H.; McElvaney, N.G.; Greene, C.M. Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in deltaF508 cystic fibrosis airway epithelium. J. Immunol. 2013, 190, 3354–3362. [Google Scholar] [CrossRef]
- Ramachandran, S.; Karp, P.H.; Osterhaus, S.R.; Jiang, P.; Wohlford-Lenane, C.; Lennox, K.A.; Jacobi, A.M.; Praekh, K.; Rose, S.D.; Behlke, M.A.; et al. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am. J. Respir. Cell Mol. Biol. 2013, 49, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Viart, V.; Bergougnoux, A.; Bonini, J.; Varilh, J.; Chiron, R.; Tabary, O.; Molinari, N.; Claustres, M.; Taulan-Cadars, M. Transcription factors and miRNAs that regulate fetal to adult CFTR expression change are new targets for cystic fibrosis. Eur. Respir. J. 2015, 45, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, S.; Kamysz, W.; Jakiela, B.; Sanak, M.; Króliczewski, J.; Bebok, Z.; Bartoszewski, R.; Collawn, J.F. miR-200b downregulates CFTR during hypoxia in human lung epithelial cells. Cell Mol. Biol. Lett. 2017, 22, 23. [Google Scholar] [CrossRef]
- De Santi, C.; Gadi, S.; Swiatecka-Urban, A.; Greene, C.M. Identification of a novel functional miR-143-5p recognition element in the Cystic Fibrosis Transmembrane Conductance Regulator 3′UTR. AIMS Genet. 2018, 5, 53–62. [Google Scholar] [CrossRef]
- Tamanini, A.; Fabbri, E.; Jakova, T.; Gasparello, J.; Manicardi, A.; Corradini, R.; Finotti, A.; Borgatti, M.; Lampronti, I.; Munari, S.; et al. A Peptide-Nucleic Acid Targeting miR-335-5p Enhances Expression of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene with the Possible Involvement of the CFTR Scaffolding Protein NHERF1. Biomedicines 2021, 9, 117. [Google Scholar] [CrossRef]
- Kumar, P.; Bhattacharyya, S.; Peters, K.W.; Glover, M.L.; Sen, A.; Cox, R.T.; Kundu, S.; Caohuy, H.; Frizzell, R.A.; Pollard, H.B.; et al. miR-16 rescues F508del-CFTR function in native cystic fibrosis epithelial cells. Gene Ther. 2015, 22, 908–916. [Google Scholar] [CrossRef]
- Megiorni, F.; Cialfi, S.; Dominici, C.; Quattrucci, S.; Pizzuti, A. Synergistic post-transcriptional regulation of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) by miR-101 and miR-494 specific binding. PLoS ONE 2011, 6, e26601. [Google Scholar] [CrossRef]
- Papi, C.; Gasparello, J.; Zurlo, M.; Manicardi, A.; Corradini, R.; Cabrini, G.; Gambari, R.; Finotti, A. Combined Treatment of Bronchial Epithelial Calu-3 Cells with Peptide Nucleic Acids Targeting miR-145-5p and miR-101-3p: Synergistic Enhancement of the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene. Int. J. Mol. Sci. 2022, 23, 9348. [Google Scholar] [CrossRef]
- Papi, C.; Gasparello, J.; Zurlo, M.; Cosenza, L.C.; Gambari, R.; Finotti, A. The Cystic Fibrosis Transmembrane Conductance Regulator Gene (CFTR) Is under Post-Transcriptional Control of microRNAs: Analysis of the Effects of agomiRNAs Mimicking miR-145-5p, miR-101-3p, and miR-335-5p. Noncoding RNA 2023, 9, 29. [Google Scholar] [CrossRef]
- Gambari, R.; Gasparello, J.; Fabbri, E.; Borgatti, M.; Tamanini, A.; Finotti, A. Peptide Nucleic Acids for MicroRNA Targeting. Methods Mol. Biol. 2020, 2105, 199–215. [Google Scholar] [CrossRef]
- Amato, F.; Tomaiuolo, R.; Nici, F.; Borbone, N.; Elce, A.; Catalanotti, B.; D’Errico, S.; Morgillo, C.M.; De Rosa, G.; Mayol, L.; et al. Exploitation of a very small peptide nucleic acid as a new inhibitor of miR-509-3p involved in the regulation of cystic fibrosis disease-gene expression. Biomed. Res. Int. 2014, 2014, 610718. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, F.; Amato, F.; Morgillo, C.M.; Pinto, B.; Santarpia, G.; Borbone, N.; D’errico, S.; Catalanotti, B.; Piccialli, G.; Castaldo, G.; et al. Peptide Nucleic Acids as miRNA Target Protectors for the Treatment of Cystic Fibrosis. Molecules 2017, 22, 1144. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Tamanini, A.; Jakova, T.; Gasparello, J.; Manicardi, A.; Corradini, R.; Sabbioni, G.; Finotti, A.; Borgatti, M.; Lampronti, I.; et al. A Peptide Nucleic Acid against MicroRNA miR-145-5p Enhances the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Calu-3 Cells. Molecules 2017, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Tamanini, A.; Jakova, T.; Gasparello, J.; Manicardi, A.; Corradini, R.; Finotti, A.; Borgatti, M.; Lampronti, I.; Munari, S.; et al. Treatment of human airway epithelial Calu-3 cells with a peptide-nucleic acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the cystic fibrosis Transmembrane Conductance Regulator gene. Eur. J. Med. Chem. 2021, 209, 112876. [Google Scholar] [CrossRef]
- Finotti, A.; Gasparello, J.; Fabbri, E.; Tamanini, A.; Corradini, R.; Dechecchi, M.C.; Cabrini, G.; Gambari, R. Enhancing the Expression of CFTR Using Antisense Molecules against MicroRNA miR-145-5p. Am. J. Respir. Crit. Care Med. 2019, 199, 1443–1444. [Google Scholar] [CrossRef]
- Lutful Kabir, F.; Ambalavanan, N.; Liu, G.; Li, P.; Solomon, G.M.; Lal, C.V.; Mazur, M.; Halloran, B.; Szul, T.; Gerthoffer, W.T.; et al. MicroRNA-145 Antagonism Reverses TGF-beta Inhibition of F508del CFTR Correction in Airway Epithelia. Am. J. Respir. Crit. Care Med. 2018, 197, 632–643. [Google Scholar] [CrossRef]
- Sultan, S.; Rozzi, A.; Gasparello, J.; Manicardi, A.; Corradini, R.; Papi, C.; Finotti, A.; Lampronti, I.; Reali, E.; Cabrini, G.; et al. A Peptide Nucleic Acid (PNA) Masking the miR-145-5p Binding Site of the 3′UTR of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) mRNA Enhances CFTR Expression in Calu-3 Cells. Molecules 2020, 25, 1677. [Google Scholar] [CrossRef]
- De Santi, C.; Fernández, E.F.; Gaul, R.; Vencken, S.; Glasgow, A.; Oglesby, I.K.; Hurley, K.; Hawkins, F.; Mitash, N.; Mu, F.; et al. Precise Targeting of miRNA Sites Restores CFTR Activity in CF Bronchial Epithelial Cells. Mol. Ther. 2020, 28, 1190–1199. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Kerem, E.; Ferkol, T.; Elizur, A. Airway inflammation in cystic fibrosis: Molecular mechanisms and clinical implications. Thorax 2013, 68, 1157–1162. [Google Scholar] [CrossRef]
- Ghigo, A.; Prono, G.; Riccardi, E.; De Rose, V. Dysfunctional Inflammation in Cystic Fibrosis Airways: From Mechanisms to Novel Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 1952. [Google Scholar] [CrossRef]
- Harwood, K.H.; McQuade, R.M.; Jarnicki, A.; Schneider-Futschik, E.K. Anti-Inflammatory Influences of Cystic Fibrosis Transmembrane Conductance Regulator Drugs on Lung Inflammation in Cystic Fibrosis. Int. J. Mol. Sci. 2021, 22, 7606. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, M.; Roussel, L.; Maillé, E.; Rousseau, S.; Brochiero, E. Vx-809/Vx-770 treatment reduces inflammatory response to Pseudomonas aeruginosa in primary differentiated cystic fibrosis bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L635–L641. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Balakathiresan, N.S.; Dalgard, C.; Gutti, U.; Armistead, D.; Jozwik, C.; Srivastava, M.; Pollard, H.B.; Biswas, R. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J. Biol. Chem. 2011, 286, 11604–11615. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, I.K.; Vencken, S.F.; Agrawal, R.; Gaughan, K.; Molloy, K.; Higgins, G.; McNally, P.; McElvaney, N.G.; Mall, M.A.; Greene, C.M. miR-17 overexpression in cystic fibrosis airway epithelial cells decreases interleukin-8 production. Eur. Respir. J. 2015, 46, 1350–1360. [Google Scholar] [CrossRef]
- Ma, T.; Liu, X.; Cen, Z.; Xin, C.; Guo, M.; Zou, C.; Song, W.; Xie, R.; Wang, K.; Zhou, H.; et al. MicroRNA-302b negatively regulates IL-1β production in response to MSU crystals by targeting IRAK4 and EphA2. Arthritis Res. Ther. 2018, 20, 34. [Google Scholar] [CrossRef]
- Fabbri, E.; Borgatti, M.; Montagner, G.; Bianchi, N.; Finotti, A.; Lampronti, I.; Bezzerri, V.; Dechecchi, M.C.; Cabrini, G.; Gambari, R. Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa-mediated induction of proinflammatory responses. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1144–1155. [Google Scholar] [CrossRef]
- Kalantari, P.; Harandi, O.F.; Agarwal, S.; Rus, F.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Caffrey, D.R.; Golenbock, D.T. miR-718 represses proinflammatory cytokine production through targeting phosphatase and tensin homolog (PTEN). J. Biol. Chem. 2017, 292, 5634–5644. [Google Scholar] [CrossRef]
- Bardin, P.; Marchal-Duval, E.; Sonneville, F.; Blouquit-Laye, S.; Rousselet, N.; Le Rouzic, P.; Corvol, H.; Tabary, O. Small RNA and transcriptome sequencing reveal the role of miR-199a-3p in inflammatory processes in cystic fibrosis airways. J. Pathol. 2018, 245, 410–420. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, H.; Yang, X.; Wang, L.; Su, L.; Liu, K.; Gu, Q.; Xu, X. MicroRNA-93 inhibits inflammatory cytokine production in LPS-stimulated murine macrophages by targeting IRAK4. FEBS Lett. 2014, 588, 1692–1698. [Google Scholar] [CrossRef]
- Bardin, P.; Foussignière, T.; Rousselet, N.; Rebeyrol, C.; Porter, J.C.; Corvol, H.; Tabary, O. miR-636: A Newly-Identified Actor for the Regulation of Pulmonary Inflammation in Cystic Fibrosis. Front. Immunol. 2019, 10, 2643. [Google Scholar] [CrossRef]
- Gao H, Xiao D, Gao L, Li MicroRNA-93 contributes to the suppression of lung inflammatory responses in LPS-induced acute lung injury in mice via the TLR4/MyD88/NF-kappaB signaling pathway. Int. J. Mol. Med. 2020, 46, 561–570. [CrossRef] [PubMed]
- Wu, M.; Liu, F.; Yan, L.; Huang, R.; Hu, R.; Zhu, J.; Li, S.; Long, C. MiR-145-5p restrains chondrogenic differentiation of synovium-derived mesenchymal stem cells by suppressing TLR4. Nucleosides Nucleotides Nucleic Acids 2022, 41, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C. Pseudomonas aeruginosa in cystic fibrosis: Pathogenesis and persistence. Paediatr. Respir. Rev. 2002, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Mourenza, Á.; Lorente-Torres, B.; Durante, E.; Llano-Verdeja, J.; Aparicio, J.F.; Fernández-López, A.; Gil, J.A.; Mateos, L.M.; Letek, M. Understanding microRNAs in the Context of Infection to Find New Treatments against Human Bacterial Pathogens. Antibiotics 2022, 11, 356. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Li, R.; Zhou, X.; Zhang, S.; Yu, M.; Ye, Y.; Wang, Y.; Huang, C.; Wu, M. Pseudomonas aeruginosa infection augments inflammation through miR-301b repression of c-Myb-mediated immune activation and infiltration. Nat. Microbiol. 2016, 1, 16132. [Google Scholar] [CrossRef]
- Li, J.; Yang, Q.; Gao, X.; Chen, F.; Gu, X.; Zhou, X.; Chen, L.; Liu, J.; Wu, M. Overexpressed miR-539 exacerbates Pseudomonas aeruginosa puenmonia by promoting inflammatory responses. Precis. Clin. Med. 2023, 6, pbad012. [Google Scholar] [CrossRef]
- Fesen, K.; Silveyra, P.; Fuentes, N.; Nicoleau, M.; Rivera, L.; Kitch, D.; Graff, G.R.; Siddaiah, R. The role of microRNAs in chronic pseudomonas lung infection in Cystic fibrosis. Respir. Med. 2019, 151, 133–138. [Google Scholar] [CrossRef]
- Ye, Y.; Richard Sun, Y.H.; Fitzpatrick, F.; Greene, C.M. microRNAs: A new class of endogenous antimicrobials for the treatment of infections in cystic fibrosis and beyond. Future Microbiol. 2024, 19, 1041–1043. [Google Scholar] [CrossRef]
- Kimura, M.; Kothari, S.; Gohir, W.; Camargo, J.F.; Husain, S. MicroRNAs in infectious diseases: Potential diagnostic biomarkers and therapeutic targets. Clin. Microbiol. Rev. 2023, 36, e0001523. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Ye, Y.; Zhao, K.; Zhuang, Y.; Li, Y.; Wei, Y.; Wu, M. MicroRNA-302b augments host defense to bacteria by regulating inflammatory responses via feedback to TLR/IRAK4 circuits. Nat. Commun. 2014, 5, 3619. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Pu, Q.; Zhou, C.; Lin, P.; Gao, P.; Zhang, X.; Chu, Y.; Yue, B.; Wu, M. MicroRNA-302/367 Cluster Impacts Host Antimicrobial Defense via Regulation of Mitophagic Response Against Pseudomonas aeruginosa Infection. Front. Immunol. 2020, 11, 569173. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, L.; Pu, Q.; Bu, H.; Zhu, P.; Chen, Z.; Yu, M.; Li, X.; Weiland, T.; Bansal, A.; et al. MEG3-4 is a miRNA decoy that regulates IL-1β abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci. Signal 2018, 11, eaao2387. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Iturbe, V.; Blanco-Agudín, N.; Vázquez-Espinosa, E.; Fernández-Vega, I.; Merayo-Lloves, J.; Vazquez, F.; Girón, R.M.; Quirós, L.M. The Binding of Pseudomonas aeruginosa to Cystic Fibrosis Bronchial Epithelial Model Cells Alters the Composition of the Exosomes They Produce Compared to Healthy Control Cells. Int. J. Mol. Sci. 2024, 25, 895. [Google Scholar] [CrossRef]
- Koeppen, K.; Nymon, A.; Barnaby, R.; Bashor, L.; Li, Z.; Hampton, T.H.; Liefeld, A.E.; Kolling, F.W.; LaCroix, I.S.; Gerber, S.A.; et al. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2105370118. [Google Scholar] [CrossRef]
- Sasaki, S.; Sun, R.; Bui, H.H.; Crosby, J.R.; Monia, B.P.; Guo, S. Steric Inhibition of 5′ UTR Regulatory Elements Results in Upregulation of Human CFTR. Mol. Ther. 2019, 27, 1749–1757. [Google Scholar] [CrossRef]
- Mewa, F.; Greene, C. Knockdown of interleukin-8 in airway epithelial cells. RCSIsmj 2012, 5, 18–23. [Google Scholar]
- Papoutsoglou, P.; Morillon, A. Extracellular Vesicle lncRNAs as Key Biomolecules for Cell-to-Cell Communication and Circulating Cancer Biomarkers. Noncoding RNA 2024, 10, 54. [Google Scholar] [CrossRef]
- Gasparello, J.; Papi, C.; Zurlo, M.; Gambari, L.; Manicardi, A.; Rozzi, A.; Ferrarini, M.; Corradini, R.; Gambari, R.; Finotti, A. MicroRNAs miR-584-5p and miR-425-3p Are Up-Regulated in Plasma of Colorectal Cancer (CRC) Patients: Targeting with Inhibitor Peptide Nucleic Acids Is Associated with Induction of Apoptosis in Colon Cancer Cell Lines. Cancers 2022, 15, 128. [Google Scholar] [CrossRef]
- Colaianni, F.; Zelli, V.; Compagnoni, C.; Miscione, M.S.; Rossi, M.; Vecchiotti, D.; Di Padova, M.; Alesse, E.; Zazzeroni, F.; Tessitore, A. Role of Circulating microRNAs in Liver Disease and HCC: Focus on miR-122. Genes 2024, 15, 1313. [Google Scholar] [CrossRef]
- Pozniak, T.; Shcharbin, D.; Bryszewska, M. Circulating microRNAs in Medicine. Int. J. Mol. Sci. 2022, 23, 3996. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.L.; Pereira, T.N.; Lewindon, P.J.; Shepherd, R.W.; Ramm, G.A. Circulating microRNAs as noninvasive diagnostic biomarkers of liver disease in children with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ideozu, J.E.; Zhang, X.; Rangaraj, V.; McColley, S.; Levy, H. Microarray profiling identifies extracellular circulating miRNAs dysregulated in cystic fibrosis. Sci. Rep. 2019, 9, 15483. [Google Scholar] [CrossRef]
- Gasparello, J.; Manicardi, A.; Casnati, A.; Corradini, R.; Gambari, R.; Finotti, A.; Sansone, F. Efficient cell penetration and delivery of peptide nucleic acids by an argininocalixarene. Sci. Rep. 2019, 9, 3036. [Google Scholar] [CrossRef]
- Gasparello, J.; Lomazzi, M.; Papi, C.; D’Aversa, E.; Sansone, F.; Casnati, A.; Donofrio, G.; Gambari, R.; Finotti, A. Efficient Delivery of MicroRNA and AntimiRNA Molecules Using an Argininocalixarene Macrocycle. Mol. Ther. Nucleic Acids 2019, 18, 748–763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).