Abstract
Chromate and dichromate solutions used for the activation and passivation of cadmium and zinc galvanic coatings of metal products are widely used due to their ability to form corrosion-protective films. Therefore, in this article, we examined the kinetic features of the cathodic deposition of Cd and Zn during membrane electrolysis. As a result of comprehensive experimental and theoretical studies, the features of Cd and Zn cathodic depositions were analyzed under different hydrodynamic conditions in a submembrane zone of an anolyte. Experimental physicochemical methods such as the experimental analysis of solutions, analytical modeling, and a statistical analysis were used during the research. A regression dependence for evaluating a reaction rate constant was assessed based on the least-square approximation of the proposed model. As a result, the peculiarities of the cathodic formations for Cd and Zn during the membrane electrolysis process were analyzed. The effect of mechanical mixing with different values of the Reynolds number on the deposition of Cd and Zn on a cathode was evaluated. A change in Cd2+ and Zn2+ ion concentrations was also considered during the research. Overall, the obtained results increased the Cd deposition rate by 2.2 times using an active hydrodynamic environment with the anolyte.
1. Introduction
To reduce the negative impact on the environment of chromates, dichromates, and heavy metal ions, which are present in the galvanic works of machine-building-industry technological processes, experimental studies have been conducted to study the patterns of their secondary use [1].
It is known that chromate and dichromate solutions are used for the activation and passivation of cadmium and zinc galvanic coatings of metal products. Due to the formation of protective conversion chromate films, these coatings receive additional protection against corrosion. It is well-known that chromates and dichromates are toxic compounds and harm the environment. Cd2+ and Zn2+ ions are also harmful; these are formed in the process of the technological processing of parts with Zn and Cd coatings [2].
The formed ions of these metals gradually contaminate the technological baths of galvanic lines and disable them. It leads to the discharge of contaminated process solutions into the sewage of the galvanic site, along with subsequent expensive neutralization and sludge formation.
The membrane electrolysis process is one of the most promising methods for the regeneration and removal of the contaminating heavy metal ions of chromium-containing technological baths from their composition [3]. Thus, thanks to the use of a two-chamber electrolyzer in which the anode chamber is a technological chromium-containing solution with a submerged lead anode and a separate cathode chamber, it is possible to regenerate the composition of this bath. The cathode chamber is filled with a sulfuric acid solution and contains a titanium cathode. One of the walls of the cathode chamber contains a cation-exchange membrane [4].
As shown by cathodic and anodic reactions, Cd2+ and Zn2+ ions pass through the cation exchange and are deposited on the cathode of the cathode chamber in the form of simple substances; i.e., metals. That is, technological baths purify the contaminating ions with a secondary use possibility for the formed metals. The process of chromate regeneration takes place directly in the technological bath.
Many research works worldwide highlight the significance of studies of the membrane electrolysis process. Fischer et al. [5] proposed ways to eliminate heavy metals from leachates using the membrane electrolysis process. As a result, rational material choices for cathodes and anodes were made for the case of Zn, Mn, and Ni. Younesi et al. [6] also described a kinetic mechanism of Cd2+ and Zn2+ ion deposition from their solutions. As a result, the perspectives of applications of the rechargeable sources in electrochemical energy were analyzed [7].
Kubáň and Boček [8] studied the effect of electrolysis on electromembrane extraction. As a result, they demonstrated that electrolysis significantly impacts the performance of the electromembrane extraction process. Fu et al. [9] also proposed an approach to increase the energy efficiency of a CO2 reduction based on a hierarchical SnO2 microsphere catalyst coated on gas-diffusion electrodes.
Danaee et al. [10] investigated the electrocatalytic oxidation of liquids on modified carbon electrodes. As a result, the anodic peak currents proved a linear dependence on the fractional power of the scan rate. Plevová et al. [11] proposed the rational design of a membrane electrode assembly for an alkaline water electrolyzer based on a catalyst-coated membrane.
Chen et al. [12] analyzed in detail the FeN4 active sites to promote an oxygen reduction reaction. As a result, recommendations for designing high-performance electrocatalysts for different electrochemical processes were proposed. Halim et al. [13] proposed interfacial charge storage mechanisms of composite electrodes based on poly(ortho-phenylenediamine)/carbon nanotubes via advanced electrogravimetry.
Finally, Luin and Valant [14] increased the energy efficiency of the electrolysis process for highly concentrated solutions using power-to-solid-energy storage technology.
However, the scientific gaps in the membrane electrolysis process were not eliminated. In particular, the effect of mechanical mixing on metal deposition should be evaluated for different values of the Reynolds number, and the deposition rate for Cd and Zn should be increased.
Therefore, in this article, we aimed to study the kinetic features of the cathodic deposition of Cd and Zn during membrane electrolysis. To achieve this goal, the following objectives were formulated. First, experimental studies were performed under different hydrodynamic conditions in the submembrane zone of an anolyte. Second, a regression dependence for the evaluation of the reaction rate constant was proposed based on the approximation of the proposed mathematical model. Finally, the kinetics of Cd and Zn cathodic formations in the membrane electrolysis process were analyzed.
2. Materials and Methods
2.1. Experimental Studies
The effect of the ion concentration in a chromatizing solution and different hydrodynamic conditions in an anolyte near the membrane zone created with a two-chamber membrane cell were studied. For this purpose, synthetically created aqueous solutions containing sodium dichromate with a content of 50 g/dm3 and a sulfuric acid quantity of 10 g/dm3 were used as the anode chamber. Additional contaminants were Cd2+ and Zn2+ ions in their sulfate form with initial concentrations of 0.013, 0.018, 0.022, 0.027, and 0.089 mol/L. The total volume of the anode chamber was 4.5 dm3. This chamber contained a lead anode with working dimensions of 0.5 × 0.5 × 0.02 dm and a working anode area of 0.5 × 0.5 + 0.5 × (0.5 × 0.5) = 0.375 dm2.
The anode chamber simulated the composition of industrial passivation baths of cadmium and zinc galvanic coatings. A cathode chamber with a 1% aqueous sulfuric acid solution with a volume of 0.75 dm3 was immersed in this chamber. A window was cut in one of the walls of the cathode chamber, into which the cation-exchange membrane (RALEX® CM-PES 11-66; JSC “MEGA Ukraine”, Kyiv, Ukraine) was inserted and hermetically fixed. The part of the membrane that participated in the electrolysis had dimensions of 0.5 × 0.5 dm and an area of 0.25 dm2. The cathode was contained in the cathode chamber in the form of a titanium plate (UNS R50250; JSC “NASOSENERGOMASH Sumy”, Sumy, Ukraine) with a size of 0.6 × 0.2 × 0.01 dm and a working cathode area of 0.18 dm2. The ratio of the cathode area to the anode area was 1:2.08. Due to this ratio between the areas of the electrodes, the electric field between them was unevenly distributed; this contributed to the formation of vortices between the membrane and the edges of the anode. It also contributed to a reduction in the chromate deposits on the outer surface of the membrane and a reduction in the influence of concentration polarization. The force of the direct electric current was maintained at a constant 1.5 A. The current density of the membrane was 6 A/dm2. The lead anode was located near the membrane at 0.05 dm. The temperature of these electrolytic systems was maintained at 18 °C.
Experimental studies were performed under different hydrodynamic conditions in the submembrane zone of the anolyte. The mixing of the anolyte near the membrane zone was mainly due to the action of the direct electric current and diffusion. However, mechanical mixing of the anolyte was also used during the experimental studies with Cd2+ and Zn2+ ions of 0.026 and 0.089 mol/L, respectively. The Reynolds number value was experimentally found. The hydrodynamic diameter of the studies without forced mixing was equal to the diameter of the vortex formed between the membrane and the anode, and the flow rate was calculated according to the movement of the electrolyte in the whirlpool. During the forced mixing of the anolyte, the hydrodynamic diameter corresponded with the diameter of the rectangular pipe and the flow rate was the speed of the liquid passage between the anode and the cation-exchange membrane.
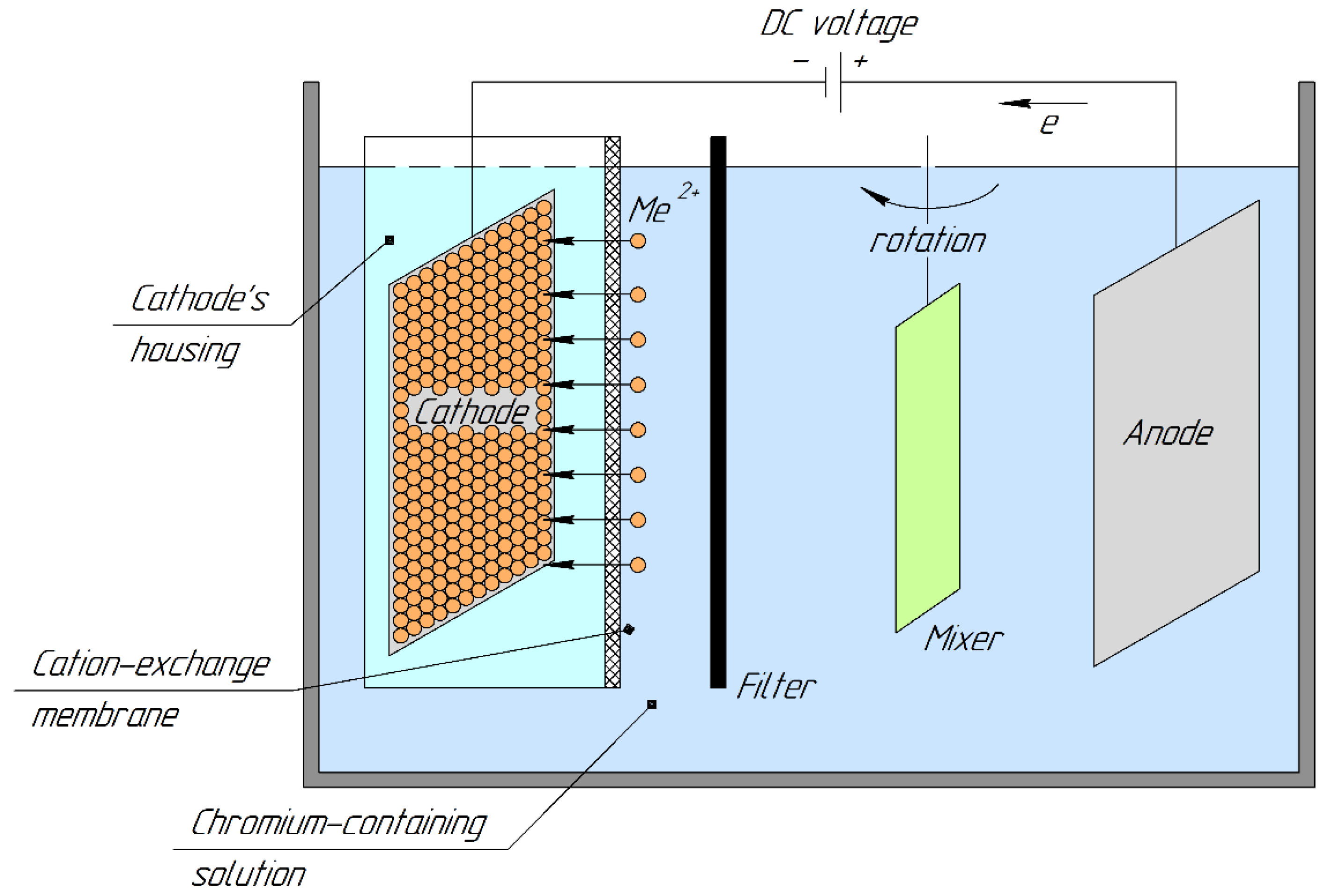
The design scheme of the membrane electrochemical device (electrolyzer) is shown in Figure 1.
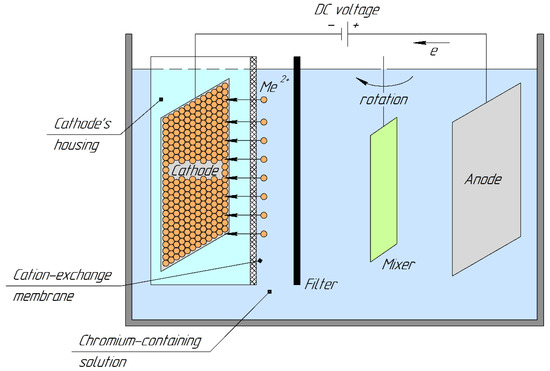
Figure 1.
Design scheme of a membrane electrolyzer.
Its design was as follows. A filter was placed close to the cation-exchange membrane. An external electrode (anode) was placed 0.05 dm from the filter. A flat-bladed mixer was located from the side of the cathode chamber and anode at a distance. It directed the anolyte flow between the cation-exchange membrane and the anode. The cathode masses were determined using the electrogravimetric method.
To increase the efficiency of this electrochemical system and to reduce the effect of concentration polarization, we used mechanical mixing for the anolyte. A mechanical stirrer was placed in the anolyte solution on the side of the membrane and the anode at a distance. The Reynolds numbers were determined as follows:
where dh is the hydrodynamic diameter, m; υ is the flow velocity, m/s; and ν is the kinematic viscosity, m2/s.
Without the use of anolyte mixing (due to the inhomogeneity of the membrane structure and the formation of vortices in the membrane electrolysis process), the hydrodynamic diameter dh equaled the vortex diameter. The flow velocity equaled the speed of the movement of the electrolyte in the vortex.
When using mechanical mixing (for a rectangular pipe, this corresponded with the structure of the module of the membrane window, to which a lead anode of the same size was placed at a distance of about 0.05 dm), the hydrodynamic diameter was equal to:
where H and W are the height and width of the membrane window, respectively.
The flow velocity υ was the liquid passage rate between the anode and the cation-exchange membrane.
Electron microscopy was used to determine the composition of the cathode products, which are described in detail in [15]. Samples of the formed cathode deposits were placed on double-sided carbon adhesive tape. The prepared samples were placed in an electron microscope (REM-106-I; JSC “SELMI”, Sumy, Ukraine) and investigated under a voltage of 20 kV. A secondary electron mode in an electron–optical enhancement range from 600 to 6000 was used.
The composition of the samples was determined by a phase analysis on an X-ray diffractometer (DRON-1-UM; JSC “NASOSENERGOMASH Sumy”, Sumy, Ukraine) in cobalt Kα radiation with a rate of 1 deg/min.
The mass fraction of elements in the local areas of the samples was determined by an X-ray microanalysis. For this purpose, the energy values of the X-ray characteristic peaks were measured for each chemical element.
2.2. Mathematical Modeling
To substantiate and develop a mathematical model, we considered what factors affected the process under study. The mass transfer process of metal cations through the cation-exchange membrane in the membrane electrolysis process is influenced by many factors [16,17]. It is complicated to analytically calculate their impact.
The main negative influencing factor is concentration polarization [18,19]. The concentration polarization result is the accumulation of excess metal ions on the outer surface of the membrane and the possible formation of deposits on the membrane surface as a consequence [20]. In addition to concentration polarization, the resistance to the mass transfer of metal cations across the membrane is influenced by adsorption, the clogging of pores, and a gel-layer formation near the membrane as well as the membrane itself and its structure [21].
The membrane structure is heterogeneous; pores on the walls containing the functional group RSO3 conduct the current and carry ions. The reinforcing fabric of the membrane used in this study was polypropylene and the inert binder was polyethylene. These polymers are not known to conduct electricity, so the membrane itself, thanks to its design, had a relatively high resistance. The membrane manufacturer did not specify the number and pore distribution per unit area, so the resistance of the different parts of the membrane could vary, indicating the different electrical conductivity of its areas.
Therefore, only the experimental data of the membrane could determine the reaction rate. In this regard, the following mathematical model for chemical kinetics was proposed:
where a is the initial concentration of Zn2+ and Cd2+ ions in the anolyte, kg/mol; x is the concentration of ions deposited on the cathode, kg/mol; t is the time, h; k is the reaction rate constant, h−1·(kg/mol)1−n; and n is the dimensionless parameter.
According to this model, the reaction rate was proportional to the product of the concentrations of the reactants; hence, (a − x)n with an unknown coefficient k, which was experimentally evaluated.
After separating the parameters x and t, the initial equation could be rewritten in an integral form:
where τ is the time, h; and ξ is the current concentration of the metal ions deposited on the cathode, kg/mol.
The constant values of the parameters k and n were assumed for a further integration. Under these assumptions, the following general solution of the last equation could be obtained:
Notably, using the L’Hopital rule, this equation could be reduced to a traditional linear model at n → 1:
This function corresponded with the reduced solution for a release process [22]. This additionally proved the reliability of the proposed analytical approach.
Finally, considering the general solution of Equation (5), the following analytical dependence to evaluate the unknown reaction rate constant k of the cathodic deposition of Zn and Cd in the process of membrane electrolysis could be obtained:
This equation could also be proved by its reduction to the linear case n → 1. In this case, the reaction rate constant, h−1, was:
However, to evaluate the parameter k more precisely, this dependence considered the total number N of an experimental dataset of the deposited metal xi at time ti, where I was the number of experimental points (i = 1, 2, …, N). The least-square approximation of Equation (8) was applied:
where R1(k) is the least-square error function.
This error was minimized using the following equation:
.
The regression dependence for evaluating the reaction rate constant k could then be obtained:
Additionally, for a further approximation of the experimentally obtained dependencies for the Cd and Zn masses deposited on the cathode, the following quadratic dependences were considered:
where m is the mass of metal, g; b is the mass rate of the deposition, g/h; and c is the coefficient, g/h2.
Remarkably, the mass rate was an initial slope of an approximating curve for the function of Equation (12).
The unknown parameters b and c depended on the hydrodynamic features of the process (e.g., the Reynolds number). However, they could be evaluated from the available experimental data. For this purpose, the least-square approximation of Equation (12) could be applied:
where R2(b, c) is the least-square error function; and mi is the experimental dataset of the deposited mass, g.
After minimizing this error by using the system of a couple of linear algebraic equations:
or in the matrix form:
the following regression dependence for evaluating the reaction rate constant k could be obtained using the inverse matrix approach:
In the linear case study (relatively small values of c), or vice versa for the quadratic case study (relatively small values of b), the parameters of Formula (12) could be significantly reduced:
As shown below, the first dependence in Equation (17) was applicable for the intense mixing of a solution (an increase in the Reynolds number until transient or turbulent modes were achieved).
Equations (11), (16) and (17) allowed the evaluation of the mass rate parameters b and c as well as the reaction rate k according to the experimental results data, as presented below.
3. Results
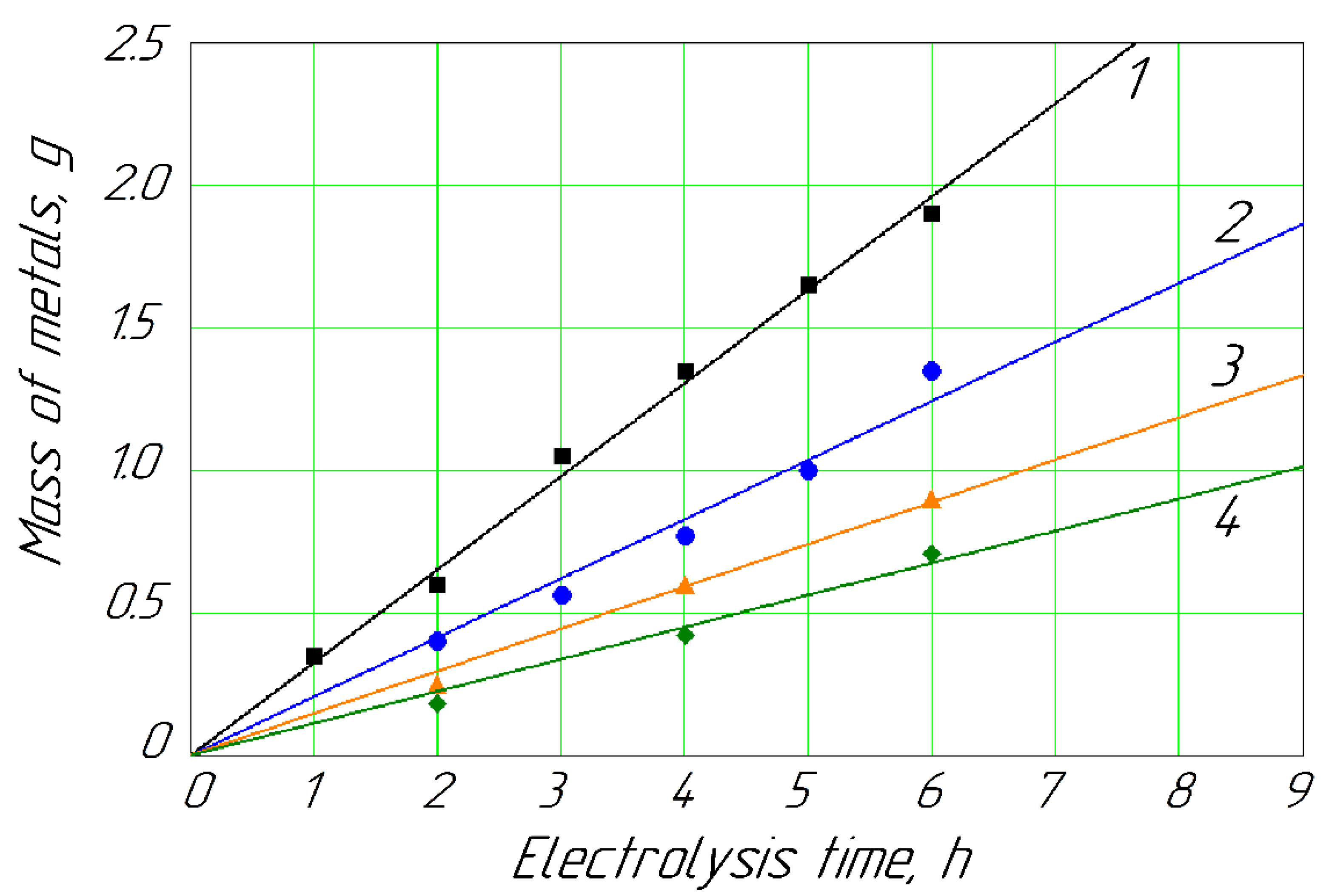
As a result of the conducted experiments, the intensity of the deposition of the metals on the cathode was proportional to their concentrations in the anolyte as a solution of the passivation bath (Figure 2 and Figure 3).
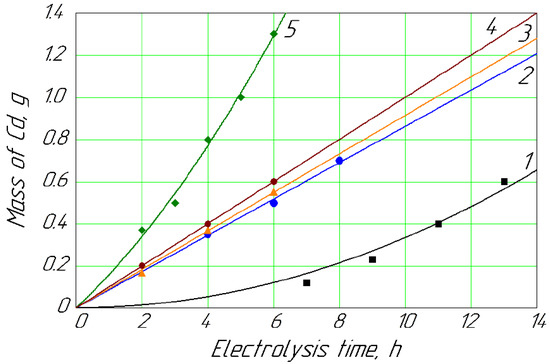
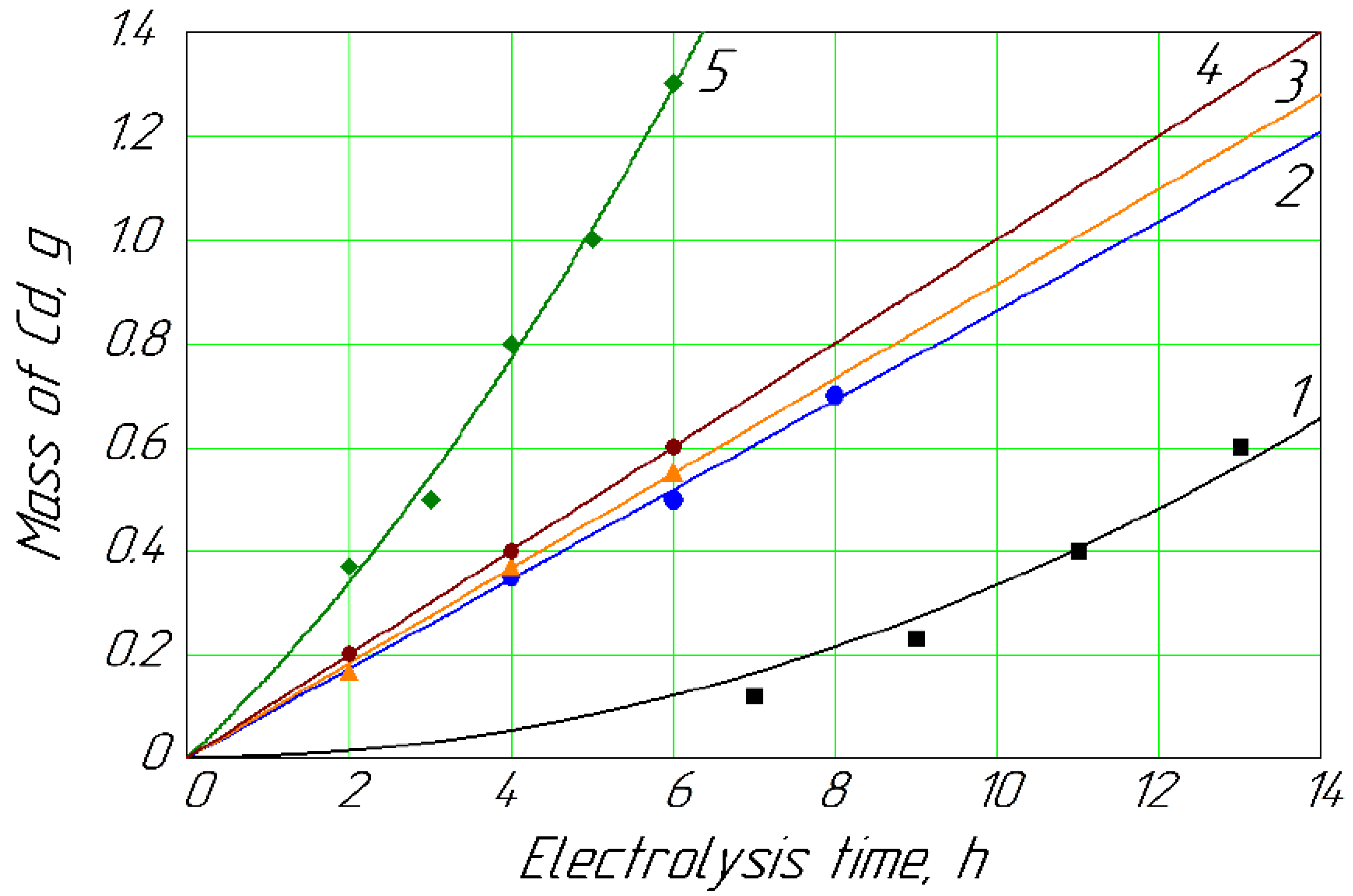
Figure 2.
Mass of Cd deposited on the cathode vs. time for different concentrations of Cd2+ in anolyte: 1, 0.013 mol/L; 2, 0.018 mol/L; 3, 0.022 mol/L; 4, 0.027 mol/L; 5, 0.089 mol/L.
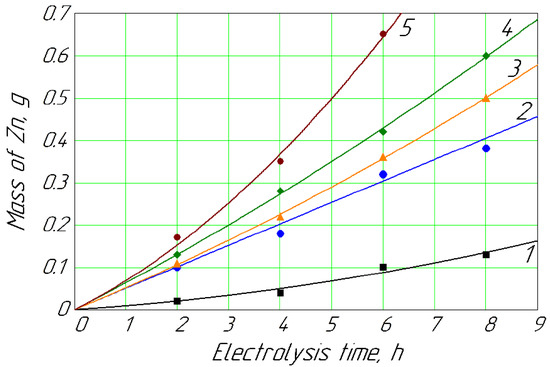
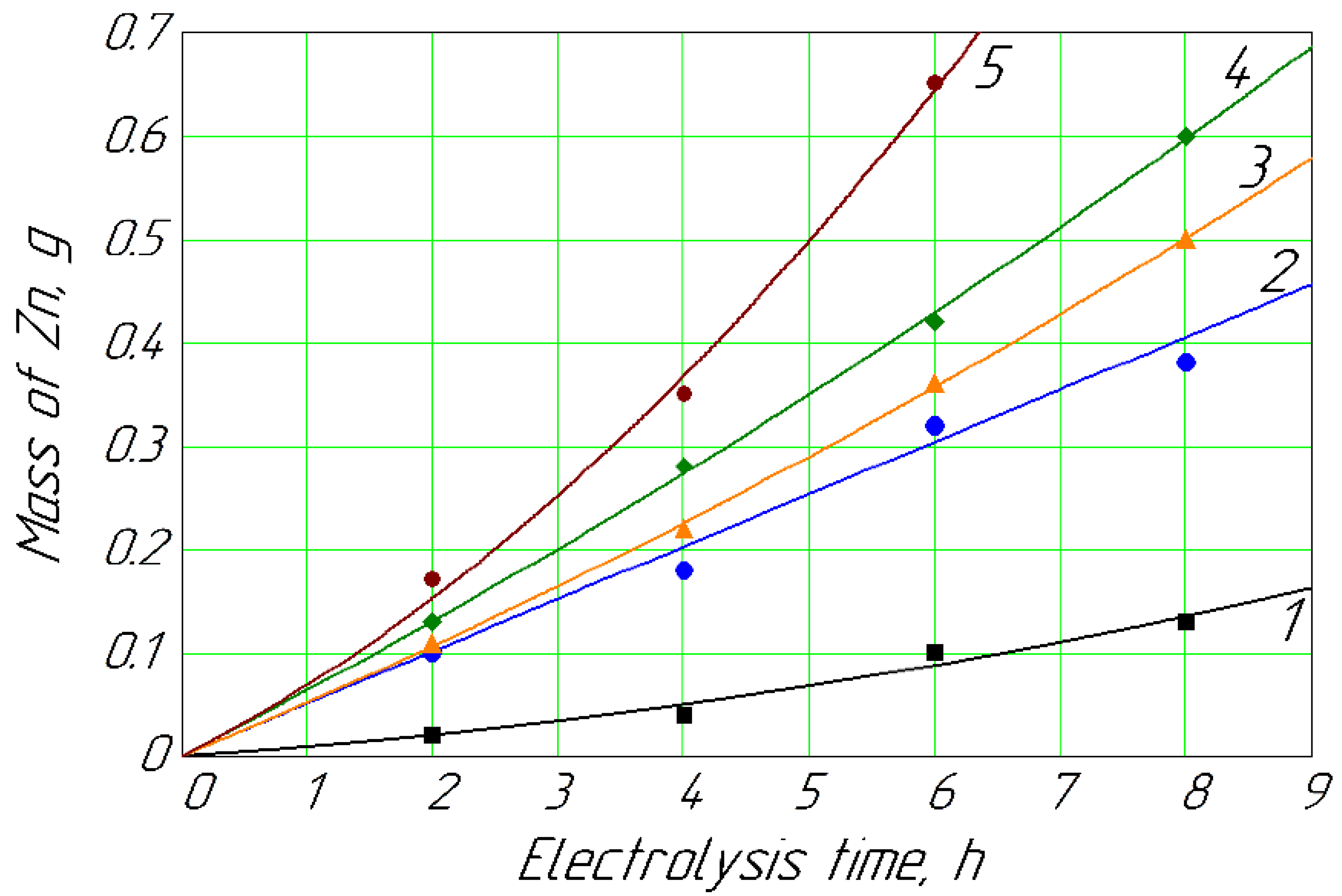
Figure 3.
Mass of Zn deposited on the cathode vs. time for different concentrations of Zn2+ in anolyte: 1, 0.013 mol/L; 2, 0.018 mol/L; 3, 0.022 mol/L; 4, 0.027 mol/L; 5, 0.089 mol/L.
Figure 2 shows that with a minimum concentration of Cd (about 1.5 g/L) in the anolyte, its deposition on the cathode was relatively slow in contrast to the maximum concentration (about 10.0 g/L). Simultaneously, the intensity of the Cd deposition was relatively stable at a concentration range of 2.0–3.0 g/L.
Figure 3 shows the dynamics of the release of metallic zinc at different concentrations in the anolyte. The content of 0.013 mol/L of Zn2+ ions in the anolyte solution naturally had the lowest dynamics of metallic zinc formation on the cathode (curve 1). Curves 2, 3, and 4 showed the dynamics of the release of cathode zinc at concentrations of 0.018, 0.022, and 0.027 mol/L, respectively. These curves were next to each other and indicated a natural gradual increase in the metal release at the cathode with an increase in its concentration in the anolyte solution. Curve 5 stood a little higher, not next to curve 4; this naturally occurred with a concentration of Zn2+ ions in the anolyte of 0.089 mol/L. That is, the release of zinc on the cathode (Figure 3) was similar to the cadmium release (Figure 2), which occured without applying mechanical stirring.
Mixing occurred due to the release of oxygen at the anode and the formation of a gas–liquid mixture, which was less dense than the density of the surrounding liquid. As a result, a local pressure difference ensued and solution convection occurred. The second mixing mechanism was electroconvection, which happened due to the uneven passage of the current through the membrane and its partial reflection from the non-conductive polypropylene frame of the heterogeneous membrane. The effect of the reflection of a part of the current was the repulsion of the liquid from the membrane and the formation of vortices near the anode.
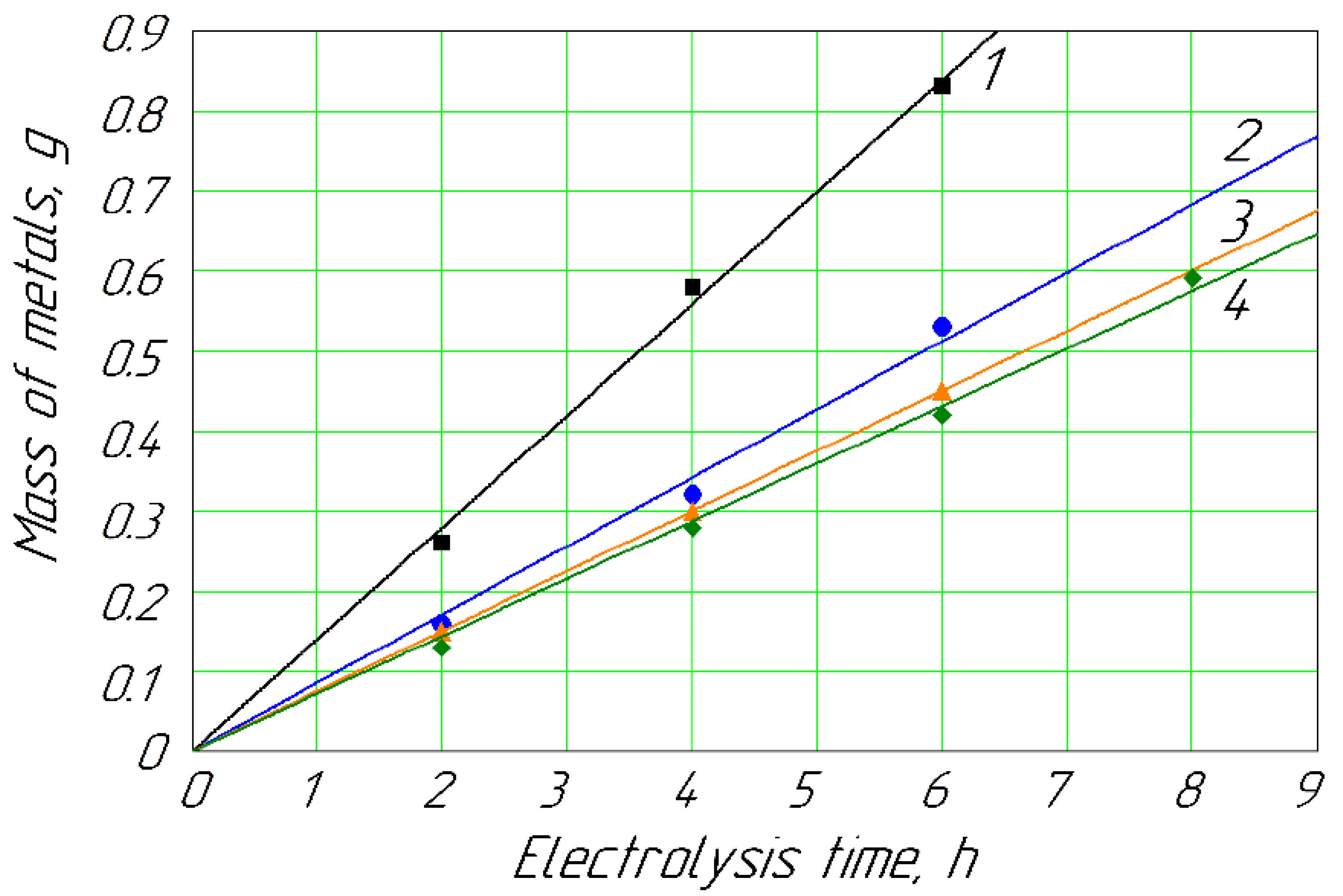
Remarkably, the mechanical mixing of the anolyte solutions significantly impacted the deposition of metals on the cathode (Figure 4 and Figure 5). The mechanical mixing of the passivating solutions helped to increase the electrolyte flow between the membrane and the anode. At the same time, the gel-like substances were removed from the surface of the membrane to a greater extent, decreasing the clogging of the membrane as well as the ion concentration on the membrane surface. These factors contributed to a reduction in concentration polarization on the outer membrane surface. Forced mixing also contributed to the better removal of gaseous oxygen from the anode and the intermembrane space. Figure 4 shows that mechanical stirring did not significantly affect the amount of zinc released when its concentration in the anolyte was 0.026 mol/L. When diluting a solution by a definite component, the ratio of the other ions can affect the processes of charge transfer in a complex multicomponent system. According to Formula (11), the averaged reaction rate constant for Cd equaled 0.056; for Zn, it was 0.062.
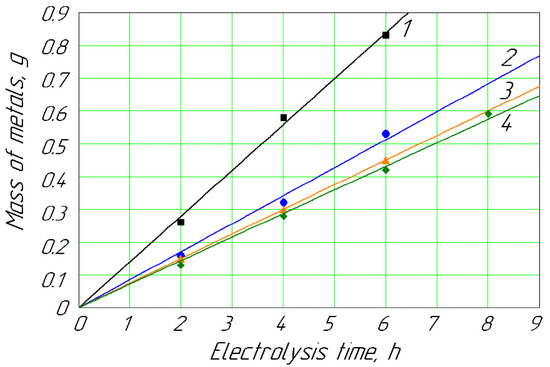
Figure 4.
Deposition of metals on the cathode vs. hydrodynamic mode for concentration of Cd2+ and Zn2+ ions of 0.026 mol/L: 1, Cd (Re = 2975); 2, Cd (Re = 392, without mixing); 3, Zn (Re = 2975); 4, Zn (Re = 392, without mixing).
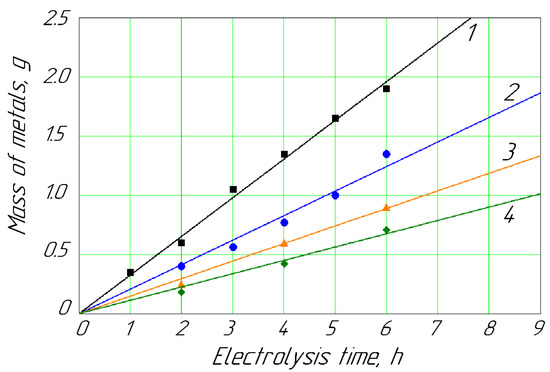
Figure 5.
Deposition of metals on the cathode vs. hydrodynamic mode for concentration of Cd2+ and Zn2+ ions of 0.089 mol/L: 1, Cd (Re = 2975); 2, Cd (Re = 392, without mixing); 3, Zn (Re = 2975); 4, Zn (Re = 392, without mixing).
The mass rate coefficients evaluated by Formulas (16) and (17) based on the experimental data presented in Figure 2, Figure 3, Figure 4 and Figure 5 are summarized in Table 1 and Table 2.

Table 1.
Reaction rate constants for Cd deposition on the cathode.

Table 2.
Reaction rate constants for Zn deposition on the cathode.
According to the correlation analysis, the values of the Pearson coefficient (r) of more than 0.9 for the data presented in Figure 2, Figure 3, Figure 4 and Figure 5 and Table 1 and Table 2 indicated a sufficient correlation of the obtained data. Moreover, the mean square analysis of these datasets showed that the maximum average relative error of the received data did not exceed 7.8%. This also proved the reliability of the proposed approach in the kinetics features of Cd and Zn cathodic formations during the membrane electrolysis process.
4. Discussion
Based on the conducted experimental studies of membrane electrolysis, the main criterion for the regeneration of the model solutions of passivation baths was the cathode output of the metal by the current. As is known, the metal output at the cathode directly shows the amount transferred through the membrane of the contaminant metal from the anolyte from the working process bath. To ensure the cathodic deposition of metals, the hydrogen index in the catholyte is maintained at the level of 1.5–1.8. At values of the hydrogen indicator in the electrolyte of the cathode chamber below 1.5, intensive hydrogen release occurs at the cathode. At hydrogen index values above 1.8, insoluble metal hydroxides begin to form near the cathode. The cathode current output, according to research, is directly affected by the concentration of pollutant metal ions in the anolyte, the current density on the membrane, the electrolytic system temperature, and the hydrodynamic conditions of the near-membrane zone of the electrolyte.
From Equations (11), (16) and (17), the cathodic deposition reaction constants of Zn and Cd based on the experimental data were evaluated. According to the calculations, the results of which are shown in Table 1, there was an increase in the reaction rate constants of the reactions with increasing concentrations of these metal ions in the anolyte and with the stirring of the membrane zone of the anolyte solution.
The Cd and Zn depositions depended on their concentration in the anolyte. The process was slow at their minimum concentrations (about 0.013 kg/mol). However, their deposition occurred more intensively at the maximum concentrations of Cd and Zn in the anolyte (about 0.089 kg/mol). This is of great practical importance in production conditions. Thus, by the cadmium or zinc amount formed on the cathode per unit of time, it is possible to judge the contamination degree of the passivation baths with the ions of these metals and to determine the need to use membrane electrolysis to clean passivating solutions.
The mechanical mixing process also impacted the cathodic deposition of the above metals, especially with an increase in the Reynolds number of the anolyte up to 2975. In particular, the Cd deposition rate on the cathode increased 2.2 times for a wide range of 0.026–0.089 kg/mol of Cd2+ ions in the anolyte. Simultaneously, the mechanical mixing of the anolyte solution with ions of Zn2+ led to an increase in the Zn deposition rate by 27%. The process of mechanical forced mixing is appropriate to use in the case of significant contamination of industrial passivating baths with Cd2+ or Zn2+ ions. In the case of heavy contamination, a membrane electrochemical device application and mechanical stirring help to quickly clean the passivating solution and restore functions.
The results of our experimental studies were implemented on the galvanic lines of electrochemical cadmium plating and electrochemical galvanizing of JSC “NASOSENERGOMASH Sumy” (Sumy, Ukraine). Implementing the created industrial electrochemical devices made it possible to reduce the consumption and discharge of sodium dichromate into wastewater by six times. Thanks to the use of these devices, the concentration of Cd2+, Zn2+, Cr3+, and CrO42- ions in wastewater decreased. Accordingly, the need for reagents for their neutralization decreased. That is, thanks to the reduction in economic costs and the decrease in the impact on the environment, the value of m2 cadmium and zinc galvanic coatings decreased by USD 0.51 and 1.03, respectively.
5. Conclusions
Membrane electrolysis is a complex process. The results of it depend on the influence of many different factors. A few factors are difficult to pinpoint due to their instability. Nevertheless, the concentration of Cd2+ and Zn2+ ions in the area near the membrane of an anolyte—in the outer, middle, and inner membrane layers (from the side of the cathode chamber)—is almost impossible to determine. Therefore, thanks to experimental studies, the effect of changes in the main factors on these electromembrane processes was studied; namely, the concentration and hydrodynamic conditions. As a result of this work, the well-known mathematical model was improved. Due to this, according to the conclusions of the experimental studies and the subsequent calculations, the constants of their instantaneous reaction rates of the cathodic deposition of the metals were obtained for the first time.
At high concentrations of Cd2+ and Zn2+ ions in the anolyte (about 0.089 mol/L), the mechanical mixing of their solutions significantly affected the processes of metal deposition on the cathodes. In particular, with an increase in the Reynolds number from 0 to 3·103, the Cd deposition rate significantly increased from 0.147 g/h to 0.327 g/h. Simultaneously, the intensity of the Zn deposition rate increased by 27%. The rate of the metal cathodic deposition with the increasing Reynolds numbers increased due to the reduction in the influence of concentration polarization, the reduction in adsorption on the membrane surface, the reduction in membrane pore clogging, and the destruction of the gel-like layer near the membrane.
Thus, it can be stated that at a given concentration of Cd ions in the anolyte, their deposition rate on the cathode could be increased 2.2 times using an active hydrodynamic environment (transient or turbulent hydrodynamic modes) obtained by the intense mixing of the anolyte.
Author Contributions
Conceptualization, V.S. (Vasyl Serdiuk) and S.B.; methodology, V.S. (Vasyl Serdiuk) and I.P.; software, S.W. and A.K.; validation, V.S. (Vsevolod Sklabinskyi), M.M. and Z.B.; formal analysis, M.O.; investigation, V.S. (Vasyl Serdiuk) and I.P.; resources, S.B. and V.S. (Vsevolod Sklabinskyi); data curation, Z.B. and M.O.; writing—original draft preparation, I.P., S.B., M.O. and A.K.; writing—review and editing, V.S. (Vsevolod Sklabinskyi) and M.M.; visualization, A.K. and M.M.; supervision, S.W.; project administration, M.O.; funding acquisition, Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Education (SBAD).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The scientific results were obtained within the scientific project “Creation of new granular materials for nuclear fuel and catalysts in the active hydrodynamic environment” (State reg. no. 0120U102036). The results were partially obtained within the research project “Fulfillment of tasks of the perspective plan of development of a scientific direction “Technical sciences, Sumy State University” funded by the Ministry of Education and Science of Ukraine (State reg. no. 0121U112684). The authors appreciate the support of the International Association for Technological Development and Innovations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Irannajad, M.; Haghighi, H.K.; Soleimanipour, M. Adsorption of Zn2+, Cd2+ and Cu2+ on zeolites coated by manganese and iron oxides. Physicochem. Probl. Miner. Proce 2016, 52, 2016–2894. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.-X.; Zhang, T.-A.; Zhu, X.-F. Simultaneous and clean separation of titanium, iron, and alumina from coal fly ash in one spot: Electrolysis-hydrolysis method. Sep. Purif. Technol. 2022, 294, 121247. [Google Scholar] [CrossRef]
- Kruglikov, S.S. Application of electromembrane processes in chromium electroplating technology. Pet. Chem. 2016, 56, 969–976. [Google Scholar] [CrossRef]
- Fischer, R.; Seidel, H.; Rahner, D.; Morgenstern, P.; Löser, C. Elimination of Heavy Metals from Leachates by Membrane Electrolysis. Eng. Life Sci. 2004, 4, 438–444. [Google Scholar] [CrossRef]
- Younesi, S.; Alimadadi, H.; Alamdari, E.K.; Marashi, S. Kinetic mechanisms of cementation of cadmium ions by zinc powder from sulphate solutions. Hydrometallurgy 2006, 84, 155–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Qiu, H.; Yang, W.; Zhao, Z.; Zhao, J.; Cui, G. Pursuit of reversible Zn electrochemistry: A time-honored challenge towards low-cost and green energy storage. NPG Asia Mater. 2020, 12, 7. [Google Scholar] [CrossRef]
- Kubáň, P.; Boček, P. The effects of electrolysis on operational solutions in electromembrane extraction: The role of acceptor solution. J. Chromatogr. A 2015, 1398, 11–19. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Y.; Zhang, X.; Liu, Y.; Qiao, J.; Zhang, J.; Wilkinson, D.P. Novel hierarchical SnO2 microsphere catalyst coated on gas diffusion electrode for enhancing energy efficiency of CO2 reduction to formate fuel. Appl. Energy 2016, 175, 536–544. [Google Scholar] [CrossRef]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M.G. Electrocatalytic oxidation of methanol on Ni and NiCu alloy modified glassy carbon electrode. Int. J. Hydrogen Energy 2008, 33, 4367–4376. [Google Scholar] [CrossRef]
- Plevová, M.; Hnát, J.; Žitka, J.; Pavlovec, L.; Otmar, M.; Bouzek, K. Optimization of the membrane electrode assembly for an alkaline water electrolyser based on the catalyst-coated membrane. J. Power Sources 2022, 539, 231476. [Google Scholar] [CrossRef]
- Chen, G.; An, Y.; Liu, S.; Sun, F.; Qi, H.; Wu, H.; He, Y.; Liu, P.; Shi, R.; Zhang, J.; et al. Highly accessible and dense surface single metal FeN4 active sites for promoting the oxygen reduction reaction. Energy Environ. Sci. 2022, 15, 2619–2628. [Google Scholar] [CrossRef]
- Halim, E.M.; Demir-Cakan, R.; Perrot, H.; El Rhazi, M.; Sel, O. Interfacial charge storage mechanisms of composite electrodes based on poly(ortho-phenylenediamine)/carbon nanotubes via advanced electrogravimetry. J. Chem. Phys. 2022, 156, 124703. [Google Scholar] [CrossRef]
- Luin, U.; Valant, M. Electrolysis energy efficiency of highly concentrated FeCl2 solutions for power-to-solid energy storage technology. J. Solid State Electrochem. 2022, 26, 929–938. [Google Scholar] [CrossRef]
- Serdiuk, V.O.; Sklavbinskyi, V.I.; Bolshanina, S.B.; Ivchenko, V.D.; Qasim, M.N.; Zaytseva, K.O. Membrane Processes during the Regeneration of Galvanic Solution. J. Eng. Sci. 2018, 5, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, L.N.; Gerhardt, M.R.; Weber, A.Z. Modeling Electrolyte Composition Effects on Anion-Exchange-Membrane Water Electrolyzer Performance. ECS Trans. 2019, 92, 767–779. [Google Scholar] [CrossRef]
- Ahmed, M.E.I.; Huang, K.-L.; Holsen, T.M. Nafion-117 Behavior during Cation Separation from Spent Chromium Plating Solutions. Ind. Eng. Chem. Res. 2009, 48, 6805–6810. [Google Scholar] [CrossRef]
- Tamburini, A.; La Barbera, G.; Cipollina, A.; Micale, G.; Ciofalo, M. CFD prediction of scalar transport in thin channels for reverse electrodialysis. Desalination Water Treat. 2015, 55, 3424–3445. [Google Scholar] [CrossRef]
- Liu, Y.; Vijayakumar, P.; Liu, Q.; Sakthivel, T.; Chen, F.; Dai, Z. Shining Light on Anion-Mixed Nanocatalysts for Efficient Water Electrolysis: Fundamentals, Progress, and Perspectives. Nano-Micro Lett. 2022, 14, 219–242. [Google Scholar] [CrossRef]
- Veerman, J.; Post, J.W.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reducing power losses caused by ionic shortcut currents in reverse electrodialysis stacks by a validated model. J. Membr. Sci. 2008, 310, 418–430. [Google Scholar] [CrossRef]
- Liang, Y.; Chapman, M.; Weihs, G.F.; Wiley, D. CFD modelling of electro-osmotic permeate flux enhancement on the feed side of a membrane module. J. Membr. Sci. 2014, 470, 378–388. [Google Scholar] [CrossRef]
- Vakal, V.; Pavlenko, I.; Vakal, S.; Hurets, L.; Ochowiak, M. Mathematical Modeling of Nutrient Release from Capsulated Fertilizers. Period. Polytech. Chem. Eng. 2020, 64, 562–568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).