Feeding of Plankton in a Turbulent Environment: A Comparison of Analytical and Observational Results Covering Also Strong Turbulence
Abstract
1. Introduction
2. Results
2.1. Analytical Results
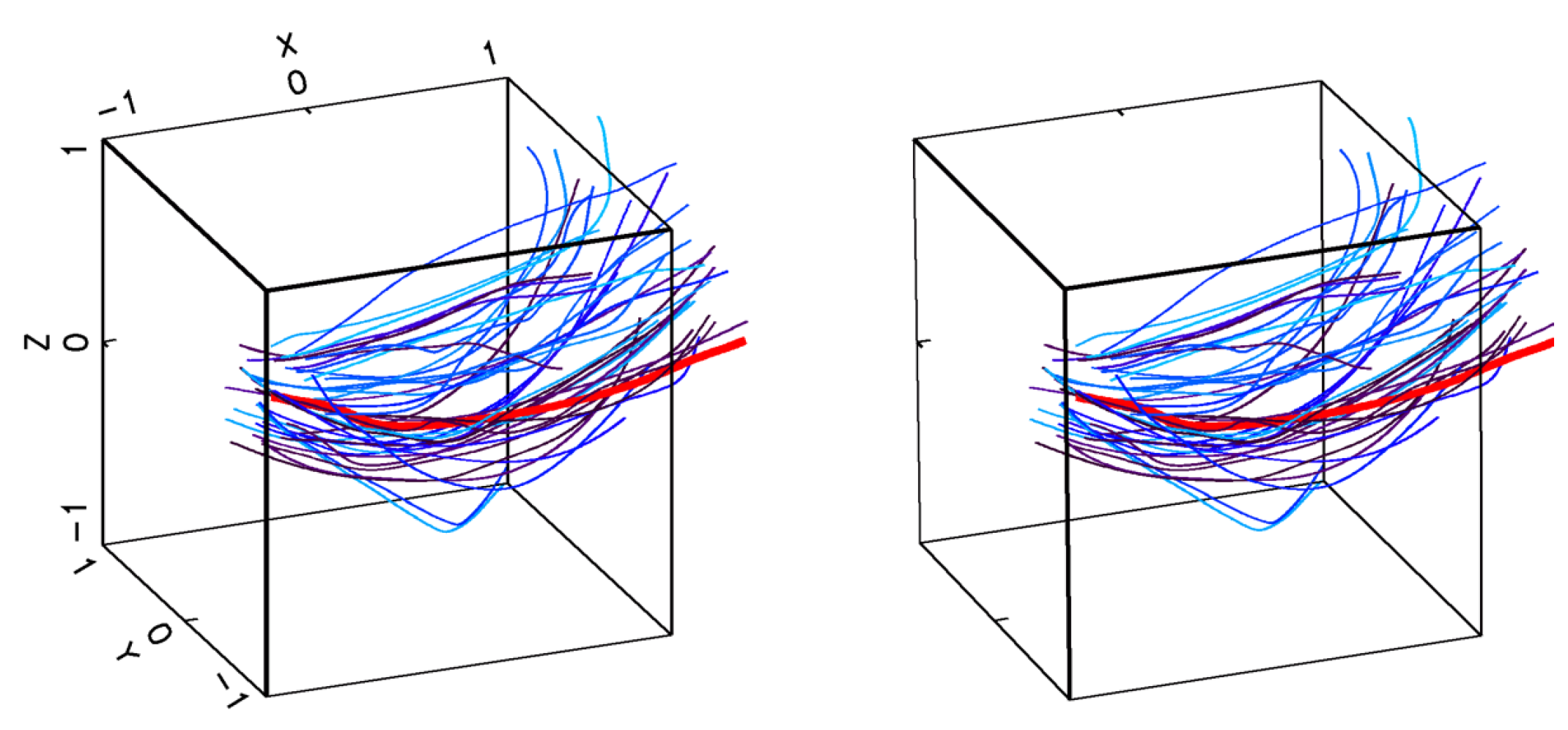
2.1.1. Encounter Rates in Turbulent Flows
2.1.2. Capture Probability in Turbulent Environments
2.1.3. Probability Density of Gut Content
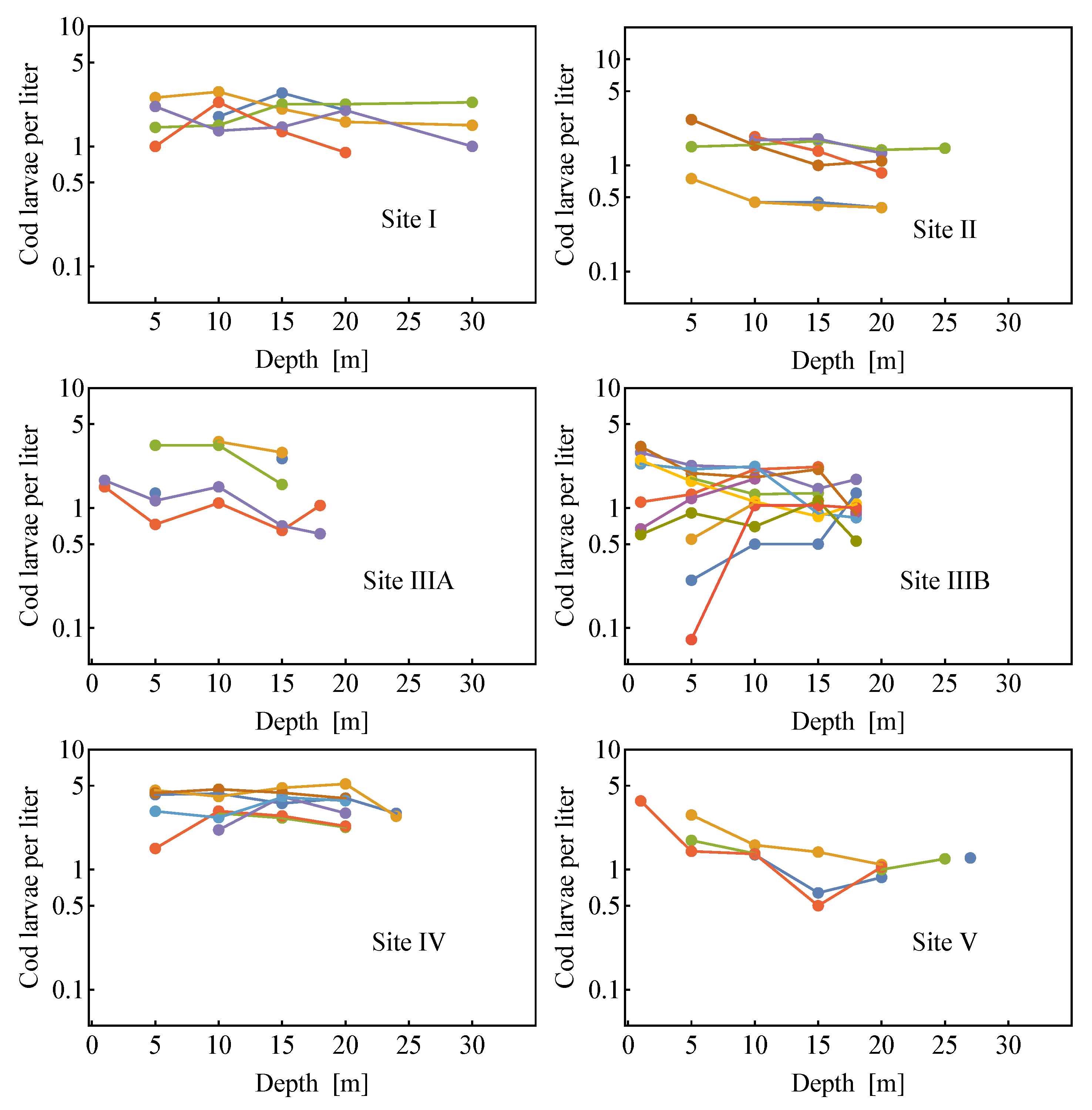
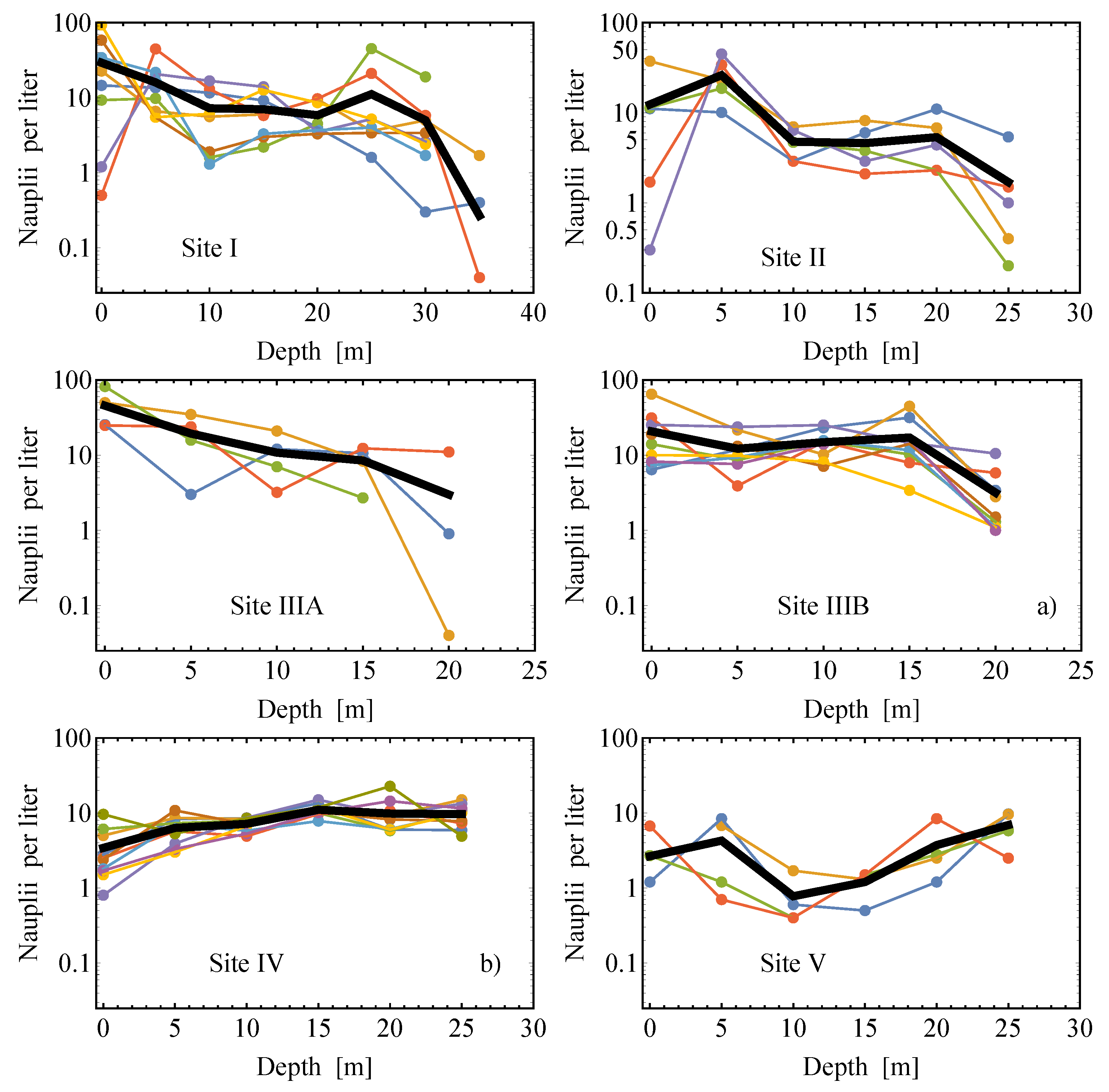
2.2. Field Data
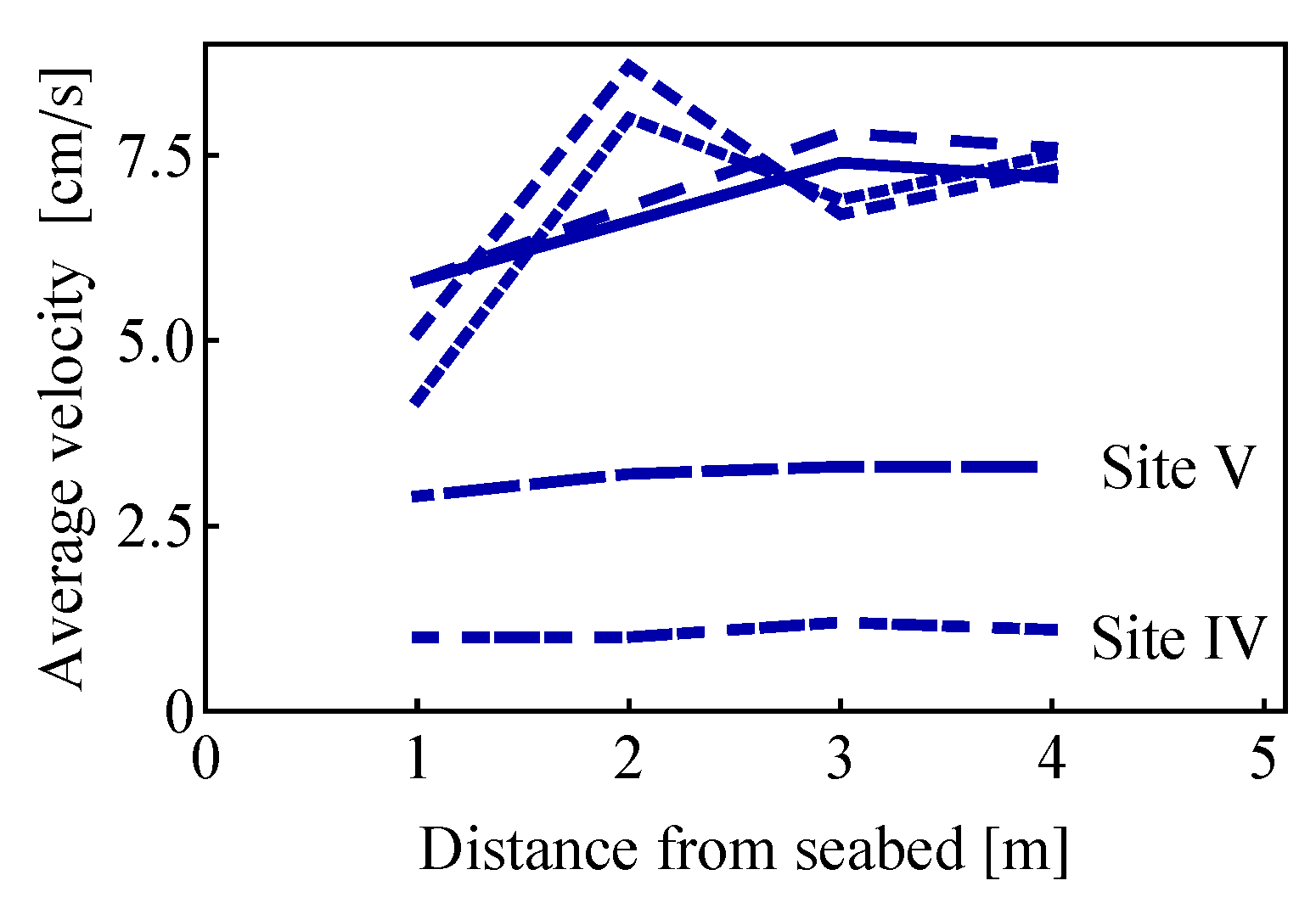
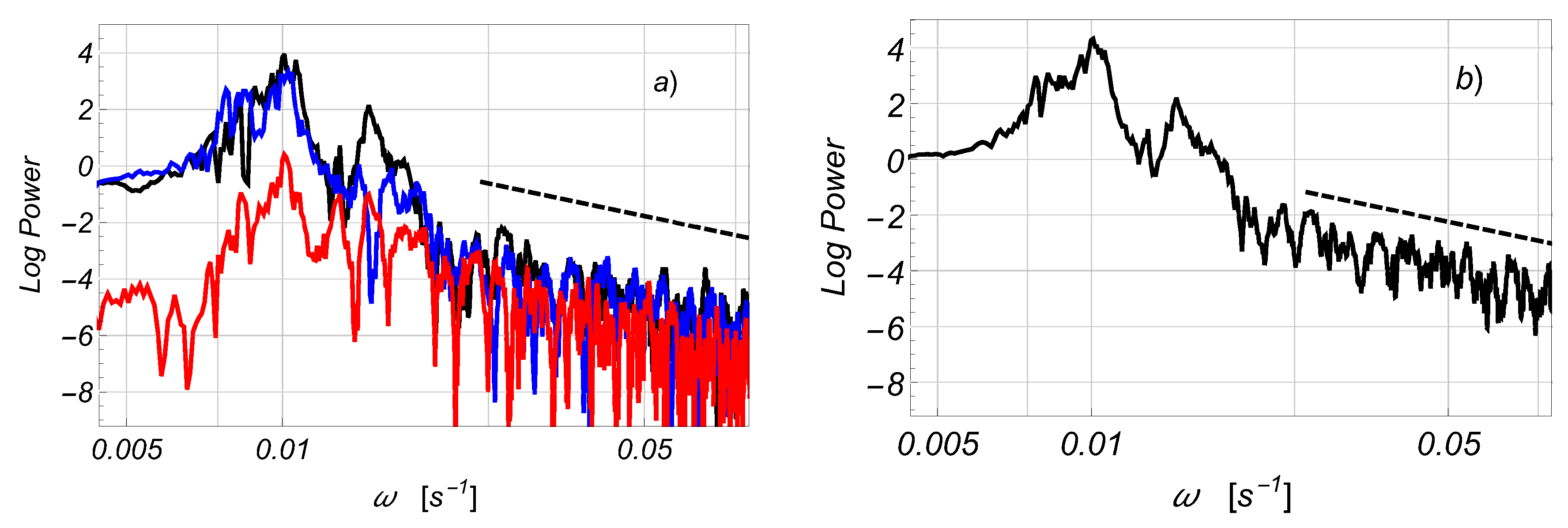
2.2.1. Estimates of the Average Specific Turbulent Energy Dissipation Rate
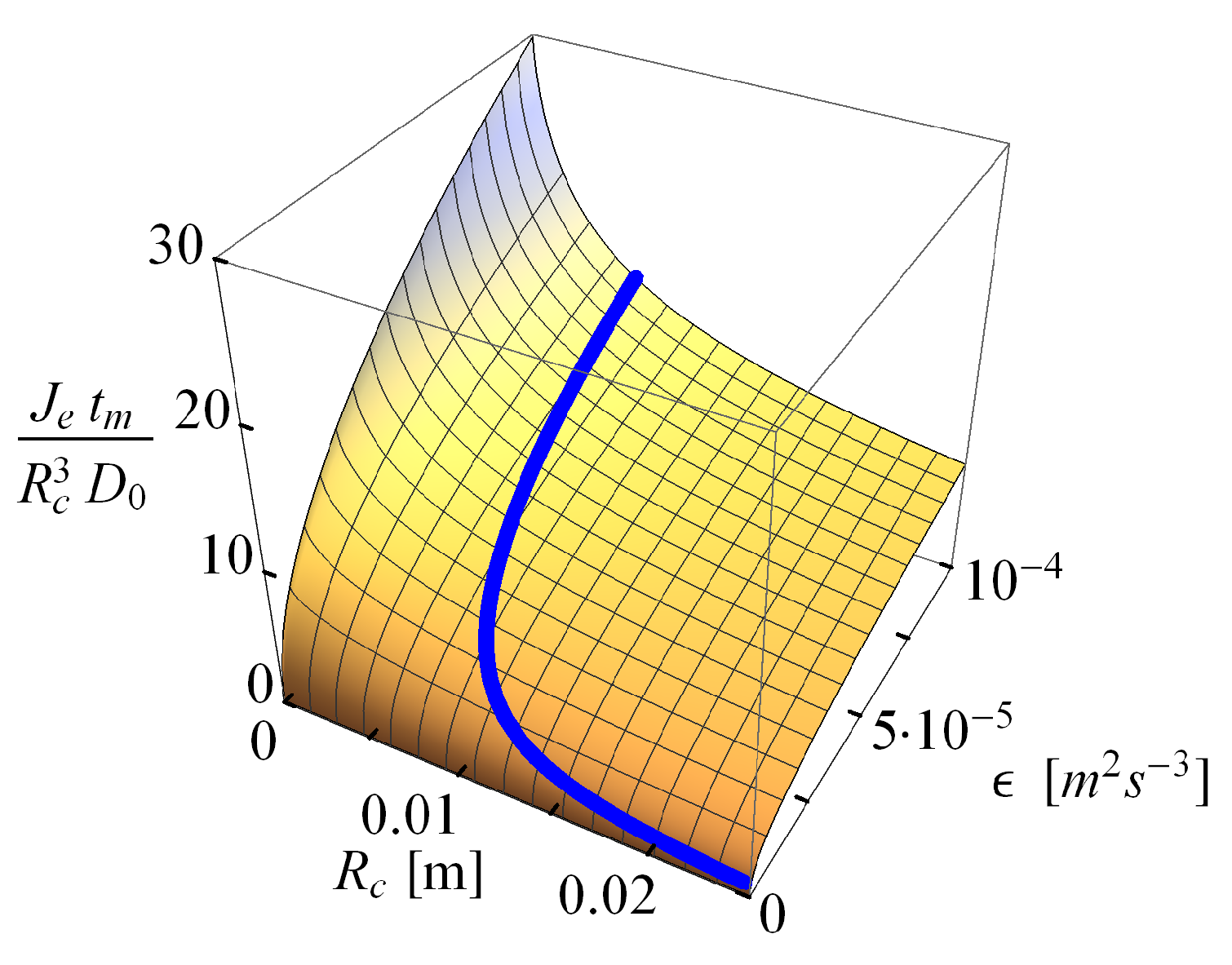
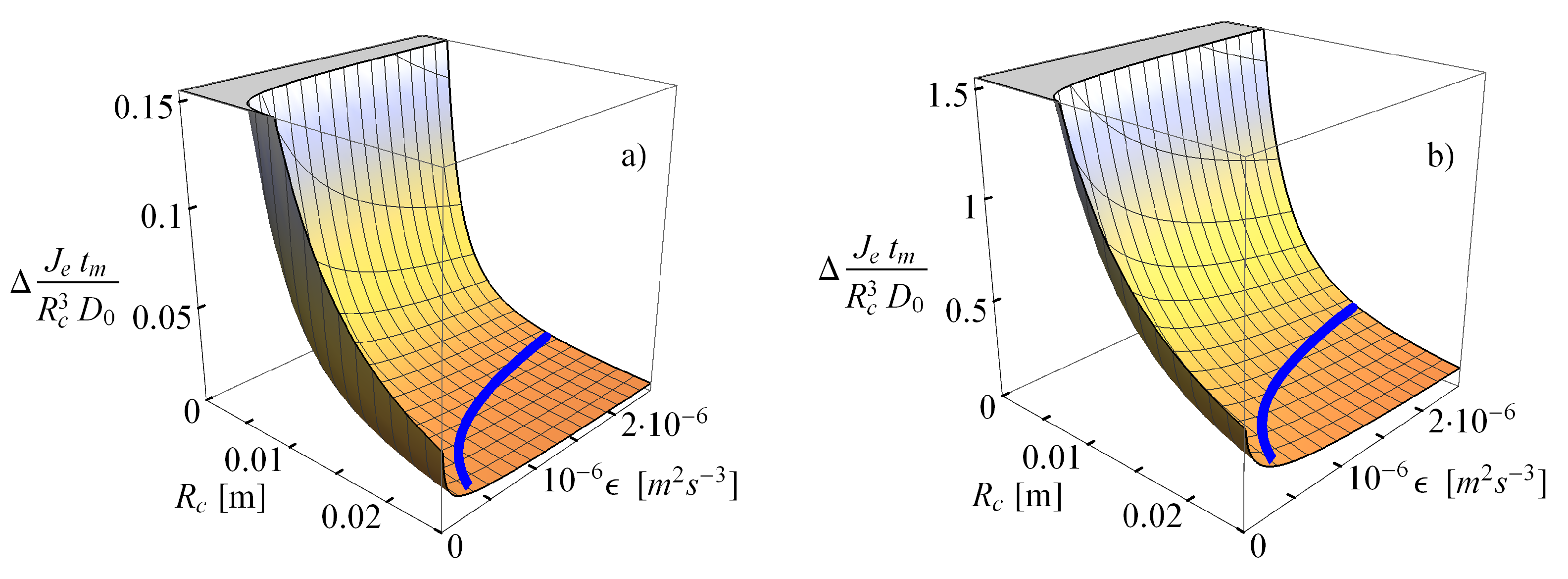
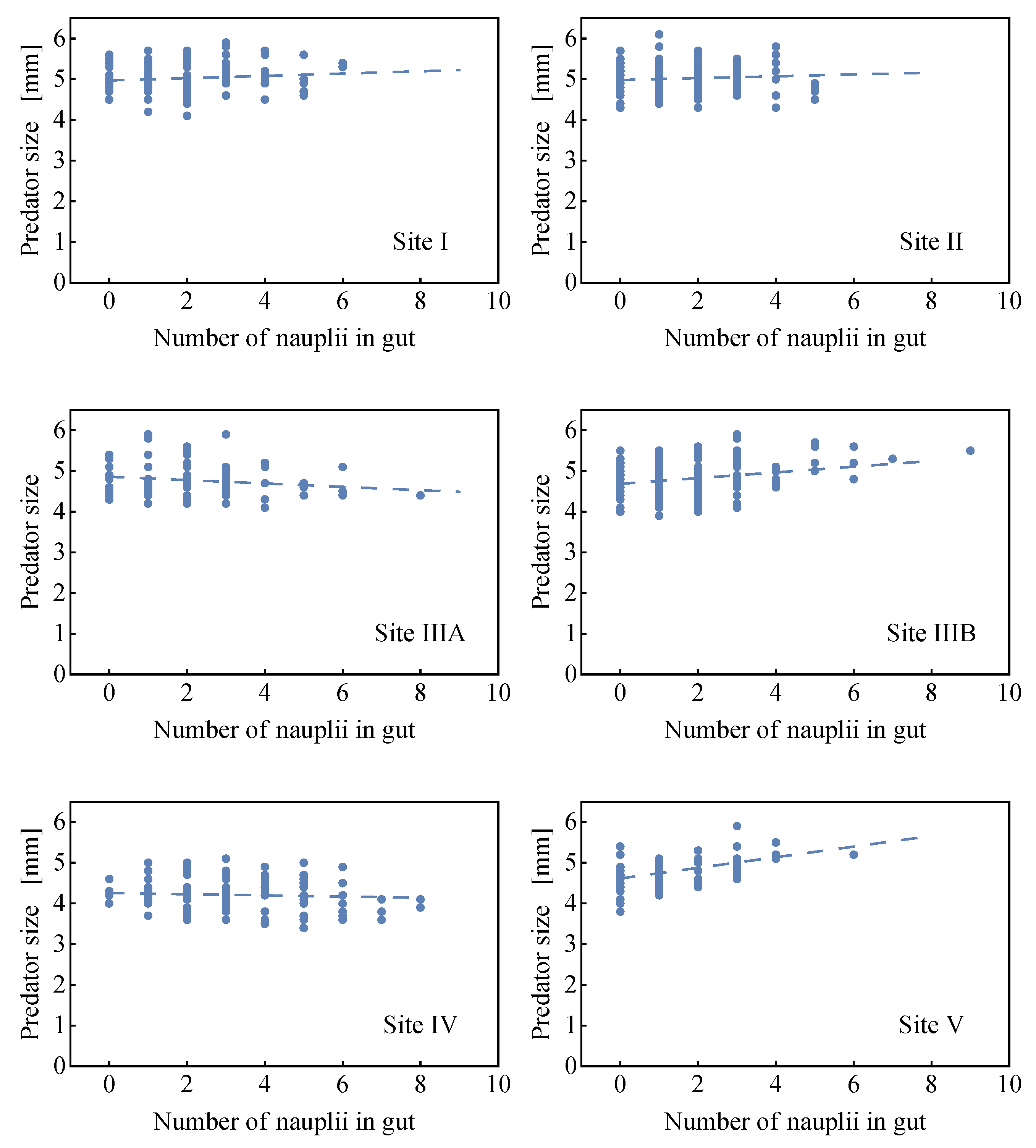
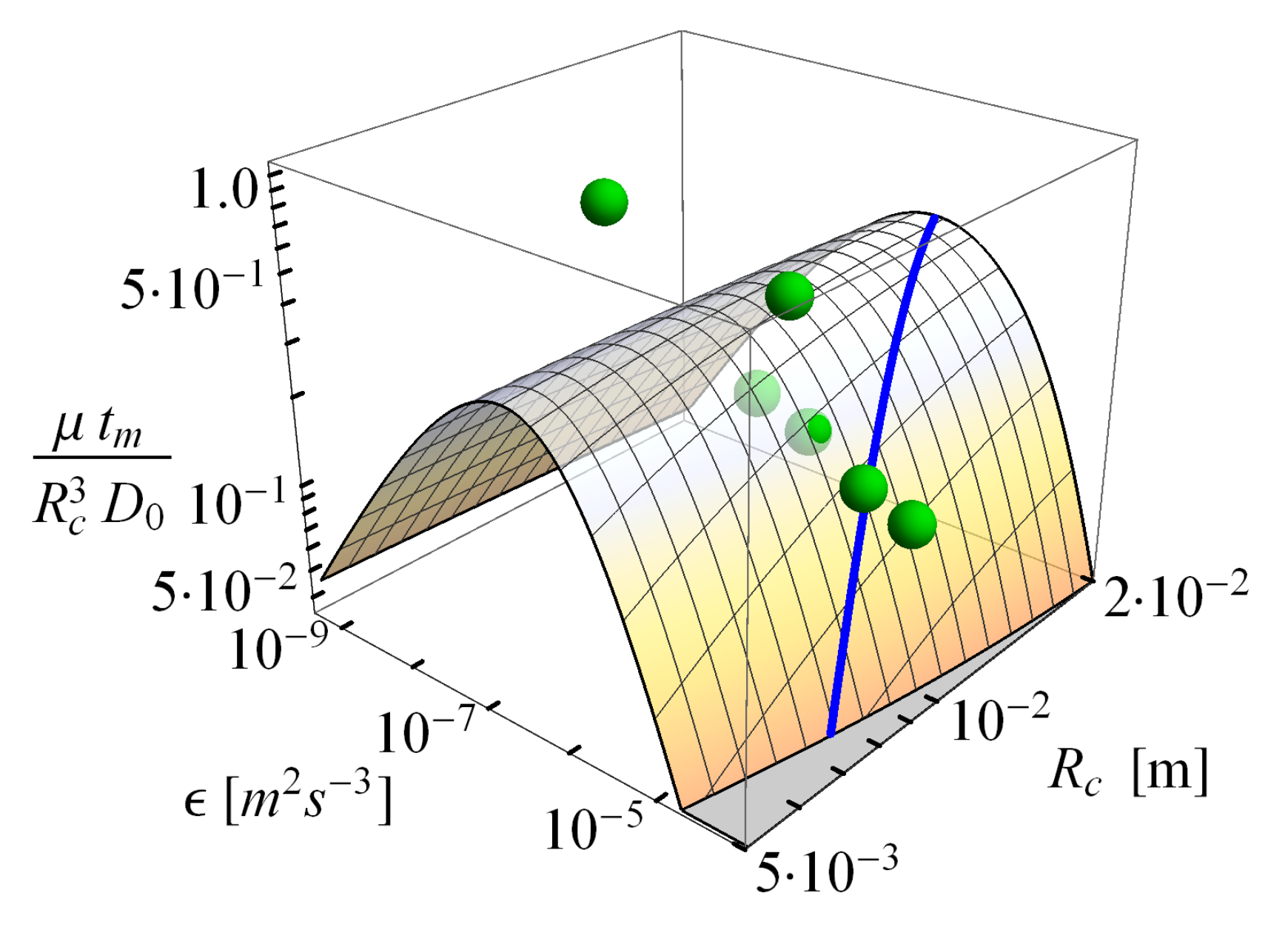
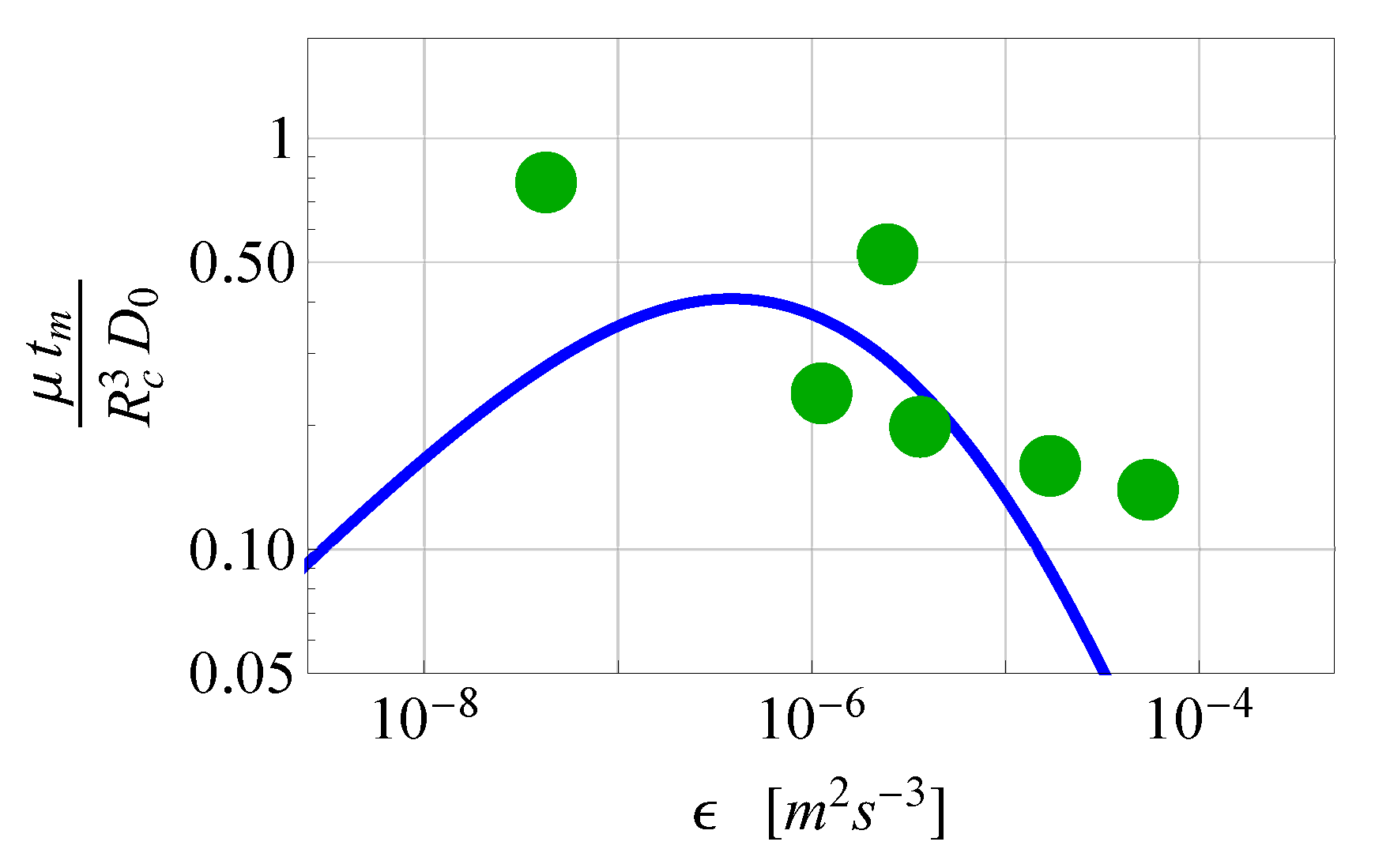
2.2.2. Comparison of the Analytical Model for Capture Rates and the Field Data Results
2.2.3. Consequences of Finite Sizes
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houde, E.D. Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 1987, 2, 17–29. [Google Scholar]
- Houde, E.D. Subtleties and episodes in the early life of fishes. J. Fish Biol. 1989, 35, 29–38. [Google Scholar] [CrossRef]
- Hinrichsen, H.H.; Möllmann, C.; Voss, R.; Köster, F.W.; Kornilovs, G. Biophysical modeling of larval Baltic cod (Gadus morhua) growth and survival. Can. J. Fish. Aquat. Sci. 2002, 59, 1858–1873. [Google Scholar] [CrossRef]
- Lough, R.G.; Buckley, L.J.; Werner, F.E.; Quinlan, J.A.; Pehrson Edwards, K. A general biophysical model of larval cod (Gadus morhua) growth applied to populations on Georges Bank. Fish. Oceanogr. 2005, 14, 241–262. [Google Scholar] [CrossRef]
- Rothschild, B.J.; Osborn, T.R. Small-scale turbulence and plankton contact rates. J. Plankton Res. 1988, 10, 465–474. [Google Scholar] [CrossRef]
- Osborn, T. The role of turbulent diffusion for copepods with feeding currents. J. Plankton Res. 1996, 18, 185–195. [Google Scholar] [CrossRef][Green Version]
- Hill, P.S.; Nowell, A.R.M.; Jumars, P.A. Encounter rate by turbulent shear of particles similar in diameter to the Kolmogorov scale. J. Mar. Res. 1992, 50, 643–668. [Google Scholar] [CrossRef][Green Version]
- Mann, J.; Ott, S.; Pécseli, H.L.; Trulsen, J. Turbulent particle flux to a perfectly absorbing surface. J. Fluid Mech. 2005, 534, 1–21. [Google Scholar] [CrossRef]
- Boffetta, G.; Pécseli, H.L.; Trulsen, J. Numerical studies of turbulent particle fluxes into perfectly absorbing spherical surfaces. J. Turbul. 2006, 7, N22. [Google Scholar] [CrossRef]
- Pécseli, H.L.; Trulsen, J. Turbulent particle fluxes to perfectly absorbing surfaces: A numerical study. J. Turbul. 2007, 8, N42. [Google Scholar] [CrossRef]
- Lewis, D.M.; Pedley, T.J. Planktonic contact rates in homogeneous isotropic turbulence: Theoretical predictions and kinematic simulations. J. Theor. Biol. 2000, 205, 377–408. [Google Scholar] [CrossRef] [PubMed]
- Pécseli, H.L.; Trulsen, J.; Fiksen, Ø. Predator-prey encounter rates in turbulent water: Analytical models and numerical tests. Prog. Oceanogr. 2010, 85, 171–179. [Google Scholar] [CrossRef]
- MacKenzie, B.R.; Miller, T.J.; Cyr, S.; Leggett, W.C. Evidence for a dome-shaped relationship between turbulence and larval fish ingestion rates. Limnol. Oceanogr. 1994, 39, 1790–1799. [Google Scholar] [CrossRef]
- Jenkinson, I.R. A review of two recent predation-rate models: The dome-shaped relationship between feeding rate and shear rate appears universal. ICES J. Mar. Sci. 1995, 52, 605. [Google Scholar] [CrossRef]
- MacKenzie, B.R.; Kiørboe, T. Larval fish feeding and turbulence: A case for the downside. Limnol. Oceanogr. 2000, 45, 1–10. [Google Scholar] [CrossRef]
- Fiksen, Ø.; Utne, A.; Aksnes, D.; Eiane, K.; Helvik, J.; Sundby, S. Modelling the influence of light, turbulence and ontogeny on ingestion rates in larval cod and herring. Fisheries Oceanog. 1998, 7, 355–363. [Google Scholar] [CrossRef]
- Vollset, K.; Folkvord, A.; Browman, H. Foraging behaviour of larval cod (Gadus morhua) at low light intensities. Mar. Biol. 2011, 158, 1125–1133. [Google Scholar] [CrossRef][Green Version]
- Pécseli, H.L.; Trulsen, J.K.; Stiansen, J.E.; Sundby, S.; Fossum, P. Feeding of plankton in turbulent oceans and lakes. Limnol. Oceanogr. 2019, 64, 1034–1046. [Google Scholar] [CrossRef]
- Solberg, T.; Tilseth, S. Growth, energy consumption and prey density requirements in first feeding larvae of cod (Gadus morhua L.). In Proceedings of the Propagation of Cod Gadus morhua L., Arendal, Noway, 14–17 June 1983. [Google Scholar]
- Sundby, S. Turbulence and ichthyoplankton: Influence on vertical distributions and encounter rates. Sci. Mar. 1997, 61, 159–176. [Google Scholar]
- Sundby, S.; Fossum, P. Feeding conditions of arcto-Norwegian cod larvae compared with the Rothschild-Osborn theory on small-scale turbulence and plankton contact rates. J. Plankton Res. 1990, 12, 1153–1162. [Google Scholar] [CrossRef]
- Sundby, S.; Ellertsen, B.; Fossum, P. Encounter rates between first-feeding cod larvae and their prey during moderate to strong turbulent mixing. ICES Mar. Sci. Symp. 1994, 393–405. [Google Scholar]
- Oakey, N.S.; Elliott, J.A. Dissipation within the surface mixed layer. J. Phys. Oceanogr. 1982, 12, 171–185. [Google Scholar] [CrossRef]
- Biferale, L.; Boffetta, G.; Celani, A.; Devenish, B.; Lanotte, A.; Toschi, F. Multifractal statistics of Lagrangian velocity and acceleration in turbulence. Phys. Rev. Lett. 2004, 93, 064502. [Google Scholar] [CrossRef] [PubMed]
- Biferale, L.; Boffetta, G.; Celani, A.; Lanotte, A.; Toschi, F. Particle trapping in three-dimensional fully developed turbulence. Phys. Fluids 2005, 17, 021701. [Google Scholar] [CrossRef]
- Tennekes, H.; Lumley, J.L. A First Course in Turbulence; The MIT Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Sreenivasan, K.R. On the universality of the Kolmogorov constant. Phys. Fluids 1995, 7. [Google Scholar] [CrossRef]
- Davidson, P.A. Turbulence. An Introduction for Scientists and Engineers; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Pécseli, H.L.; Trulsen, J.; Fiksen, Ø. Predator-prey encounter and capture rates for plankton in turbulent environments. Prog. Oceanogr. 2012, 101, 14–32. [Google Scholar] [CrossRef]
- Kiørboe, T. A Mechanistic Approach to Plankton Ecology; Princeton Univ. Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Pigolotti, S.; Jensen, M.H.; Vulpiani, A. Absorbing processes in Richardson diffusion: Analytical results. Phys. Fluids 2006, 18, 048104. [Google Scholar] [CrossRef]
- Lewis, D.M.; Pedley, T.J. The influence of turbulence on plankton predation strategies. J. Theor. Biol. 2001, 210, 347–365. [Google Scholar] [CrossRef]
- Lewis, D.M.; Bala, S.I. Plankton predation rates in turbulence: A study of the limitations imposed on a predator with a non-spherical field of sensory perception. J. Theor. Biol. 2006, 242, 44–61. [Google Scholar] [CrossRef]
- Mann, J.; Ott, S.; Pécseli, H.L.; Trulsen, J. Laboratory studies of predator-prey encounters in turbulent environments: Effects of changes in orientation and field of view. J. Plankton Res. 2006, 28, 509–522. [Google Scholar] [CrossRef][Green Version]
- MacKenzie, B.R.; Kiørboe, T. Encounter rates and swimming behaviour of pause-travel and cruise larval fish predators in calm and turbulent laboratory environments. Limnol. Oceonogr. 1995, 40, 1278–1289. [Google Scholar] [CrossRef]
- Reigada, R.; Hillary, R.M.; Bees, M.A.; Sancho, J.M.; Sagués, F. Plankton blooms induced by turbulent flows. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.M.; Climent, E.; Barry, M.; De Lillo, F.; Boffetta, G.; Cencini, M.; Stocker, R. Turbulence drives microscale patches of motile phytoplankton. Nat. Comm. 2013, 4, 2148. [Google Scholar] [CrossRef]
- Breier, R.E.; Lalescu, C.C.; Waas, D.; Wilczek, M.; Mazza, M.G. Emergence of phytoplankton patchiness at small scales in mild turbulence. Proc. Natl. Acad. Sci. USA 2018, 115, 12112–12117. [Google Scholar] [CrossRef] [PubMed]
- Pécseli, H.L.; Trulsen, J.K.; Fiksen, Ø. Predator-prey encounter and capture rates in turbulent environments. Limnol. Oceanog. Fluids Environ. 2014, 4, 85–105. [Google Scholar] [CrossRef]
- Dam, H.G.; Peterson, W.T. The effect of temperature on the gut clearance rate constant of planktonic copepods. J. Exp. Mar. Biol. Ecol. 1988, 123, 1–14. [Google Scholar] [CrossRef]
- Marrasé, C.; Costello, J.H.; Granata, T.; Strickler, J.R. Grazing in a turbulent environment: Energy dissipation, encounter rates, and efficacy of feeding currents in Centropages hamatus. Proc. Nat. Acad. Sci. USA 1990, 87, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Saiz, E.; Kiørboe, T. Predatory and suspension-feeding of the copepod Acartia-tonsa in turbulent environments. Mar. Ecol. Prog. Ser. 1995, 122, 147–158. [Google Scholar] [CrossRef]
- Kiørboe, T.; Saiz, E. Planktivorous feeding in calm and turbulent environments, with emphasis on copepods. Mar. Ecol. Prog. Ser. 1995, 122, 135–145. [Google Scholar] [CrossRef]
- Pécseli, H.L.; Trulsen, J.K. Plankton’s perception of signals in a turbulent environment. Adv. Phys. X 2016, 1, 20–34. [Google Scholar] [CrossRef]
- Kiørboe, T.; Visser, A.W. Predator and prey perception in copepods due to hydromechanical signals. Marine Ecol. Prog. Ser. 1999, 179, 81–95. [Google Scholar] [CrossRef]
- Abramowitz, M.; Stegun, I.A. Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables; Dover: New York, NY, USA, 1972. [Google Scholar]
- Buckingham, E. On physically similar systems; illustrations of the use of dimensional equations. Phys. Rev. 1914, 4, 345–376. [Google Scholar] [CrossRef]
- Gytre, T.; Nilsen, J.E.O.; Stiansen, J.E.; Sundby, S. Resolving small scale turbulence with acoustic Doppler and acoustic travel time difference current meters from an underwater tower. In Proceedings of the OCEANS 96 MTS/IEEE Conference Proceedings, The Coastal Ocean—Prospects for the 21st Century, Fort Lauderdale, FL, USA, 23–26 September 1996; pp. 442–450. [Google Scholar]
- Sharqawy, M.H.; Lienhard, J.H.; Zubair, S.M. Thermophysical properties of seawater: A review of existing correlations and data. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Sharqawy, M.H.; Lienhard, J.H.; Zubair, S.M. Erratum to Thermophysical properties of seawater: A review of existing correlations and data [Desalination and Water Treatment, Vol. 16 (2010) 354-380]. Desalin. Water Treat. 2012, 44, 361. [Google Scholar] [CrossRef]
- Wandel, C.F.; Kofoed-Hansen, O. On the Eulerian-Lagrangian transformation in the statistical theory of turbulence. J. Geophys. Res. 1962, 67, 3089–3093. [Google Scholar] [CrossRef]
- Shkarofsky, I.P. Turbulence in Fluids and Plasmas; Chapter “Analytic Forms for Decaying Space/Time Turbulence Functions”; Polytechnic Press: Brooklyn, NY, USA, 1969; pp. 289–301. [Google Scholar]
- Tennekes, H. Eulerian and Lagrangian time microscales in isotropic turbulence. J. Fluid Mech. 1975, 67, 561–567. [Google Scholar] [CrossRef]
- Wyngaard, J.C.; Clifford, S.F. Taylor’s hypothesis and highfrequency turbulence spectra. J. Atmos. Sci. 1977, 34, 922–929. [Google Scholar] [CrossRef]
- Stiansen, J.E.; Sundby, S. Improved methods for generating and estimating turbulence in tanks suitable for fish larvae experiments. Sci. Mar. 2001, 65, 151–167. [Google Scholar] [CrossRef]
- Trujillo, J.J.; Trabucchi, D.; Bischoff, O.; Hofsäß, M.; Mann, J.; Mikkelsen, T.; Rettenmeier, A.; Schlipf, D.; Kühn, M. Testing of frozen turbulence hypothesis for wind turbine applications with a Staring Lidar. Geophys. Res. Abstr. 2010, 12, 5410. [Google Scholar]
- Geng, C.; He, G.; Wang, Y.; Xu, C.; Lozano-Durán, A.; Wallace, J.M. Taylor’s hypothesis in turbulent channel flow considered using a transport equation analysis. Phys. Fluids 2015, 27, 025111. [Google Scholar] [CrossRef]
- Larsén, X.G.; Vincent, C.; Larsen, S. Spectral structure of mesoscale winds over the water. Quart. J. R. Meteorol. Soc. 2013, 139, 685–700. [Google Scholar] [CrossRef]
- Tilseth, S.; Ellertsen, B. Food consumption rate and gut evacuation processes of first-feeding cod larvae (Gadus Morhua L.). In Proceedings of the Propagation of Cod Gadus morhua L., Arendal, Noway, 14–17 June 1983. [Google Scholar]
- Maxey, M.R.; Riley, J.J. Equation of motion for a small rigid sphere in a nonuniform flow. Phys. Fluids 1983, 26, 883–889. [Google Scholar] [CrossRef]
- Pécseli, H.L.; Trulsen, J. Predator-prey encounter rates in turbulent environments: consequences of inertia effects and finite sizes. In From Leonardo to ITER: Nonlinear and Coherence Aspects, Proceedings of the AIP Conference; American Institute of Physics: Melville, NY, USA, 2009; Volume 1177, pp. 85–95. [Google Scholar]
- Batchelor, G.K.; Binnie, A.M.; Phillips, O.M. The mean velocity of discrete particles in turbulent flow in a pipe. Proc. R. Soc. Lond. 1955, B 68, 1095–1104. [Google Scholar] [CrossRef]
- Mikkelsen, T.; Larsen, S.E.; Pécseli, H.L. Diffusion of Gaussian puffs. Quart. J. R. Meteorol. Soc. 1987, 113, 81–105. [Google Scholar] [CrossRef]
- Misguich, J.H.; Balescu, R.; Pécseli, H.L.; Mikkelsen, T.; Larsen, S.E.; Xiaoming, Q. Diffusion of charged particles in turbulent magnetoplasmas. Plasma Phys. Contr. Fusion 1987, 29, 825–856. [Google Scholar] [CrossRef]
- Heisenberg, W. Zur statistische theorie der turbulenz. Z. Phys. 1948, 124, 628–657. [Google Scholar] [CrossRef]
- Pécseli, H.L. Low Frequency Waves and Turbulence in Magnetized Laboratory Plasmas and in the Ionosphere; IOP Publishing: Bristol, UK, 2016. [Google Scholar] [CrossRef]
- MacKenzie, B.R.; Leggett, W.C. Wind-based models for estimating the dissipation rates of turbulent energy in aquatic environments: empirical comparisons. Mar. Ecol. Prog. Ser. 1993, 94, 207–216. [Google Scholar] [CrossRef]
- Maar, M.; Visser, A.W.; Nielsen, T.G.; Stips, A.; Saito, H. Turbulence and feeding behaviour affect the vertical distributions of Oithona similis and Microsetella norwegica. Mar. Ecol. Prog. Ser. 2006, 313, 157–172. [Google Scholar] [CrossRef][Green Version]
- Tanaka, M. Changes in vertical distribution of zooplankton under wind-induced turbulence. Fluids 2019, 4, 195. [Google Scholar] [CrossRef]
- Oakey, N.S. Determination of the rate of dissipation of turbulent energy from simultaneous temperature and velocity shear microstructure measurements. J. Phys. Oceanogr. 1982, 12, 256–271. [Google Scholar] [CrossRef]
- Strand, K.O.; Vikebø, F.; Sundby, S.; Sperrevik, A.K.; Breivik, Ø. Subsurface maxima in buoyant fish eggs indicate vertical velocity shear and spatially limited spawning grounds. Limnol. Oceanog. 2019, 64, 1239–1251. [Google Scholar] [CrossRef]
- Esters, L.; Breivik, Ø.; Landwehr, S.; Ten Doeschate, A.; Sutherland, G.; Christensen, K.H.; Bidlot, J.R.; Ward, B. Turbulence scaling comparisons in the ocean surface boundary layer. J. Geophys. Res. Oceans 2018, 123, 2172–2191. [Google Scholar] [CrossRef]
- Ellertsen, B.; Fossum, P.; Solemdal, P.; Sundby, S.; Tilseth, S. A case study of the distribution of cod larvae and availability of prey organisms in relation to physical processes in Lofoten. In Proceedings of the Propagation of Cod Gadus morhua L., Arendal, Noway, 14–17 June 1983. [Google Scholar]
- Kristiansen, T.; Vollset, K.W.; Sundby, S.; Vikebø, F. Turbulence enhances feeding of larval cod at low prey densities. ICES J. Mar. Sci. 2014, 71, 2515–2529. [Google Scholar] [CrossRef]
- Greenberg, D.A. Modelling the mean barotropic circulation in the bay of Fundy and Gulf of Maine. J. Phys. Oceanogr. 1983, 13, 886–904. [Google Scholar] [CrossRef]








| Station | [mm] | [l] | [m] | [ms] | [mm] | [s] | [m s] | |
|---|---|---|---|---|---|---|---|---|
| I | 4.69 | 1.99 | 11.22 | 299 | 1.12 | 17.11 | 1.16 | 1.14 |
| II | 4.80 | 1.45 | 9.17 | 299 | 3.62 | 12.77 | 0.64 | 1.53 |
| IIIA | 4.72 | 2.09 | 19.74 | 245 | 5.44 | 6.49 | 0.17 | 3.01 |
| IIIB | 4.70 | 1.62 | 13.59 | 306 | 1.70 | 8.68 | 0.30 | 2.24 |
| IV | 4.22 | 3.34 | 7.90 | 214 | 4.25 | 38.80 | 5.94 | 5.03 |
| V | 4.69 | 1.26 | 3.25 | 263 | 2.46 | 14.06 | 0.78 | 1.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pécseli, H.L.; Trulsen, J.K.; Stiansen, J.E.; Sundby, S. Feeding of Plankton in a Turbulent Environment: A Comparison of Analytical and Observational Results Covering Also Strong Turbulence. Fluids 2020, 5, 37. https://doi.org/10.3390/fluids5010037
Pécseli HL, Trulsen JK, Stiansen JE, Sundby S. Feeding of Plankton in a Turbulent Environment: A Comparison of Analytical and Observational Results Covering Also Strong Turbulence. Fluids. 2020; 5(1):37. https://doi.org/10.3390/fluids5010037
Chicago/Turabian StylePécseli, Hans L., Jan K. Trulsen, Jan Erik Stiansen, and Svein Sundby. 2020. "Feeding of Plankton in a Turbulent Environment: A Comparison of Analytical and Observational Results Covering Also Strong Turbulence" Fluids 5, no. 1: 37. https://doi.org/10.3390/fluids5010037
APA StylePécseli, H. L., Trulsen, J. K., Stiansen, J. E., & Sundby, S. (2020). Feeding of Plankton in a Turbulent Environment: A Comparison of Analytical and Observational Results Covering Also Strong Turbulence. Fluids, 5(1), 37. https://doi.org/10.3390/fluids5010037






