Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles
Abstract
1. Introduction
2. Materials and Methods
2.1. Theory
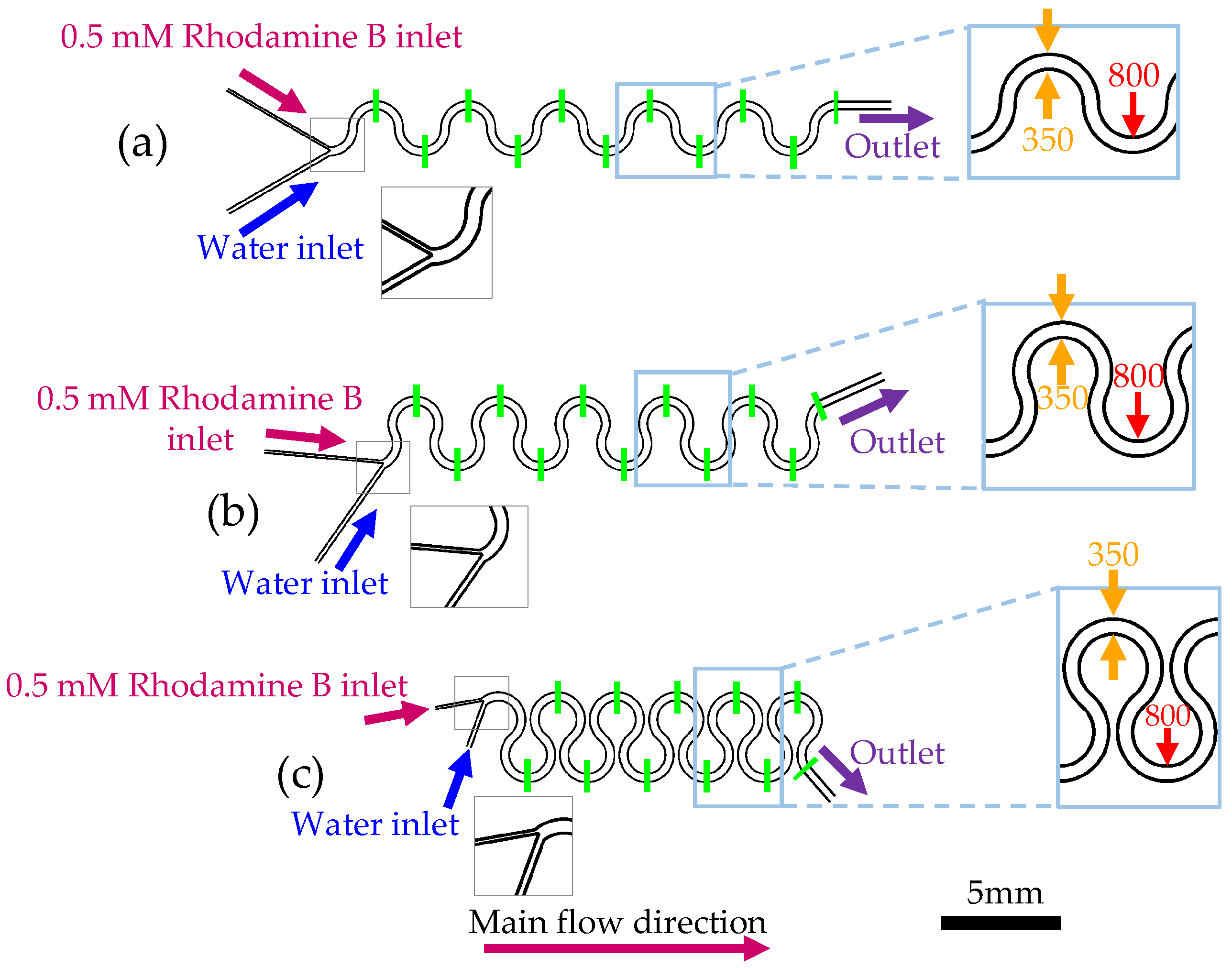
2.2. Micromixers Design
2.3. Micromixers Fabrication
2.4. Solution Preparation
2.5. Experimental Procedure
2.6. Image Processing
2.7. Numerical Simulations
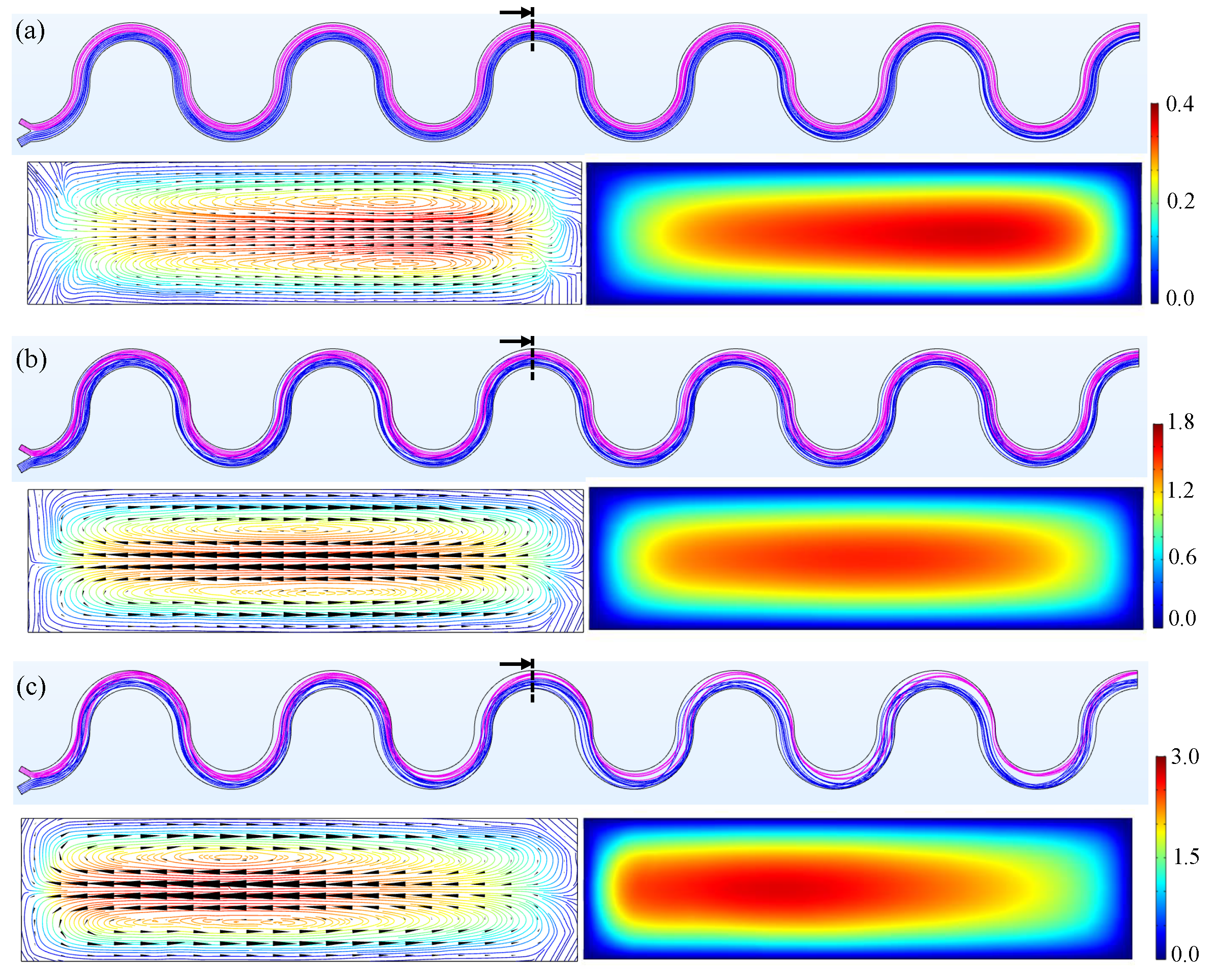
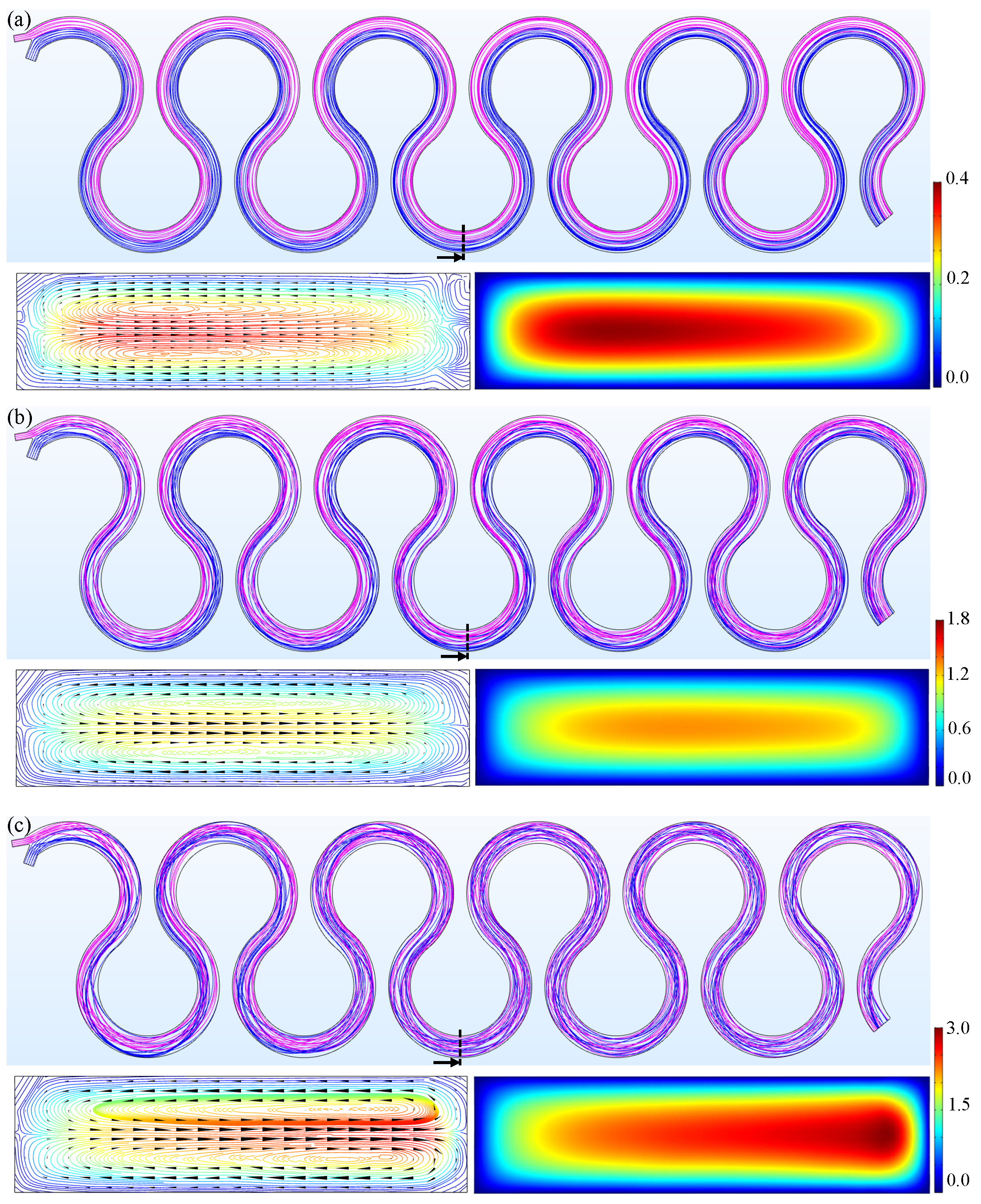
3. Results and Discussion
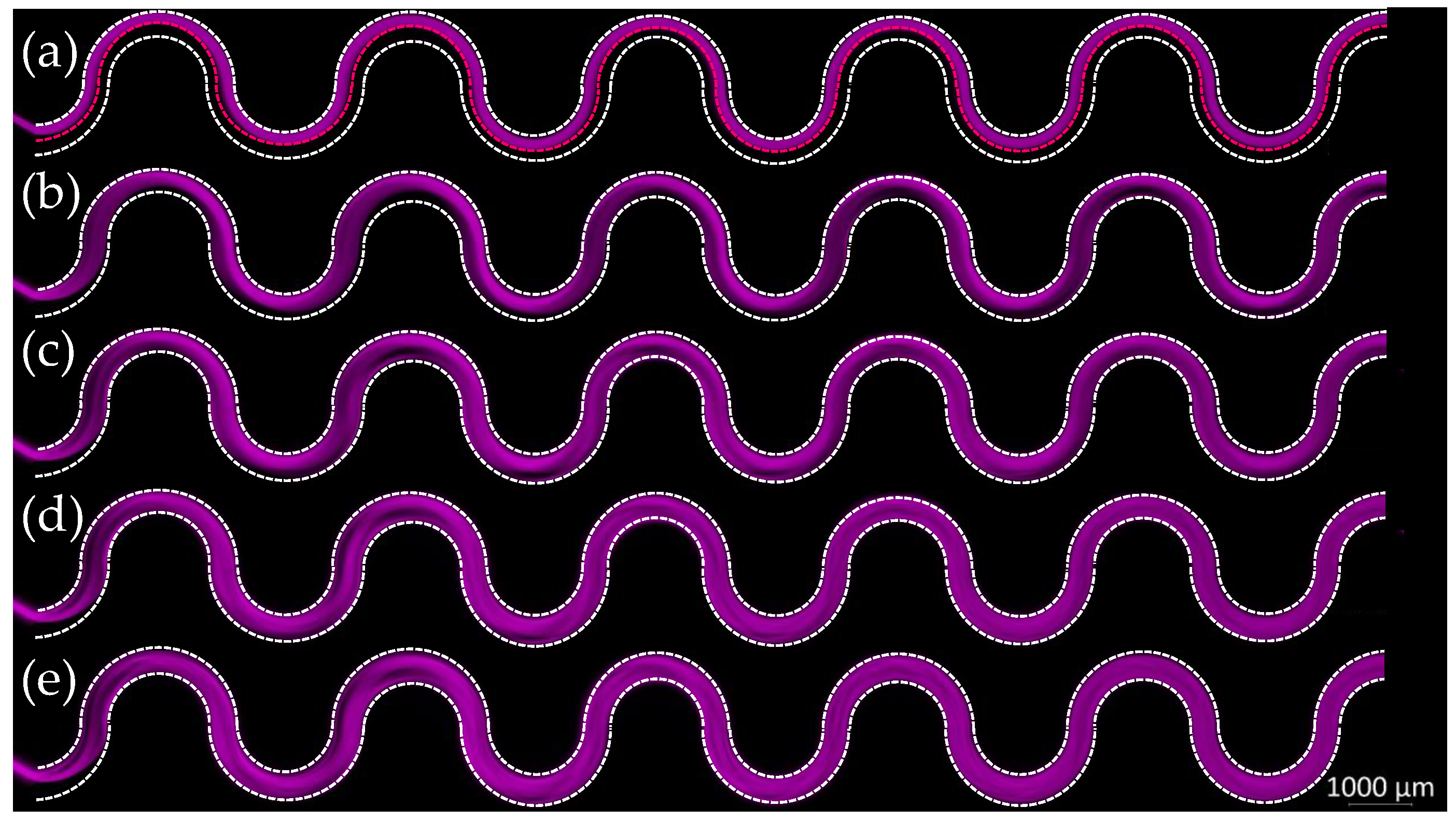
3.1. M180
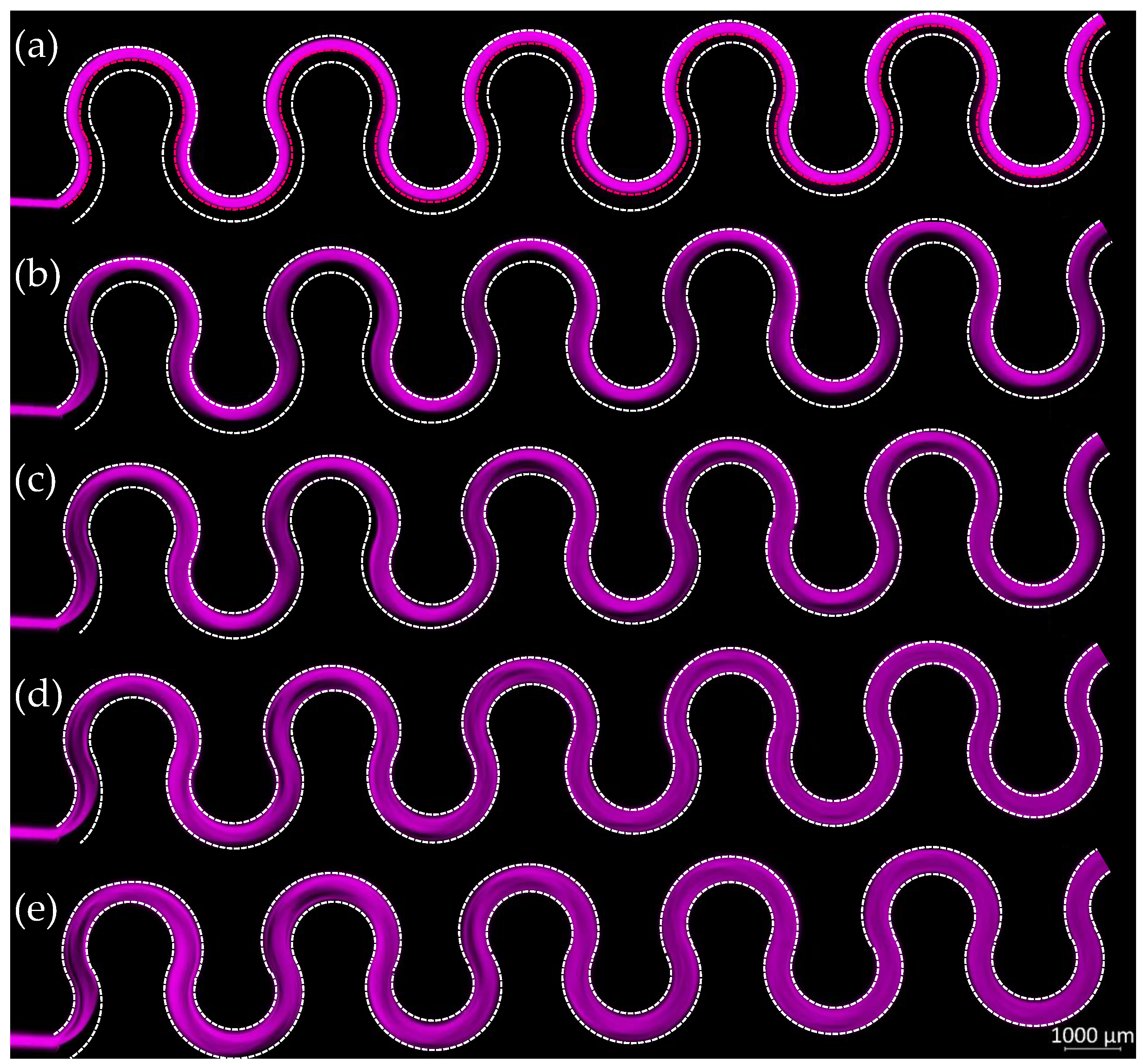
3.2. M230
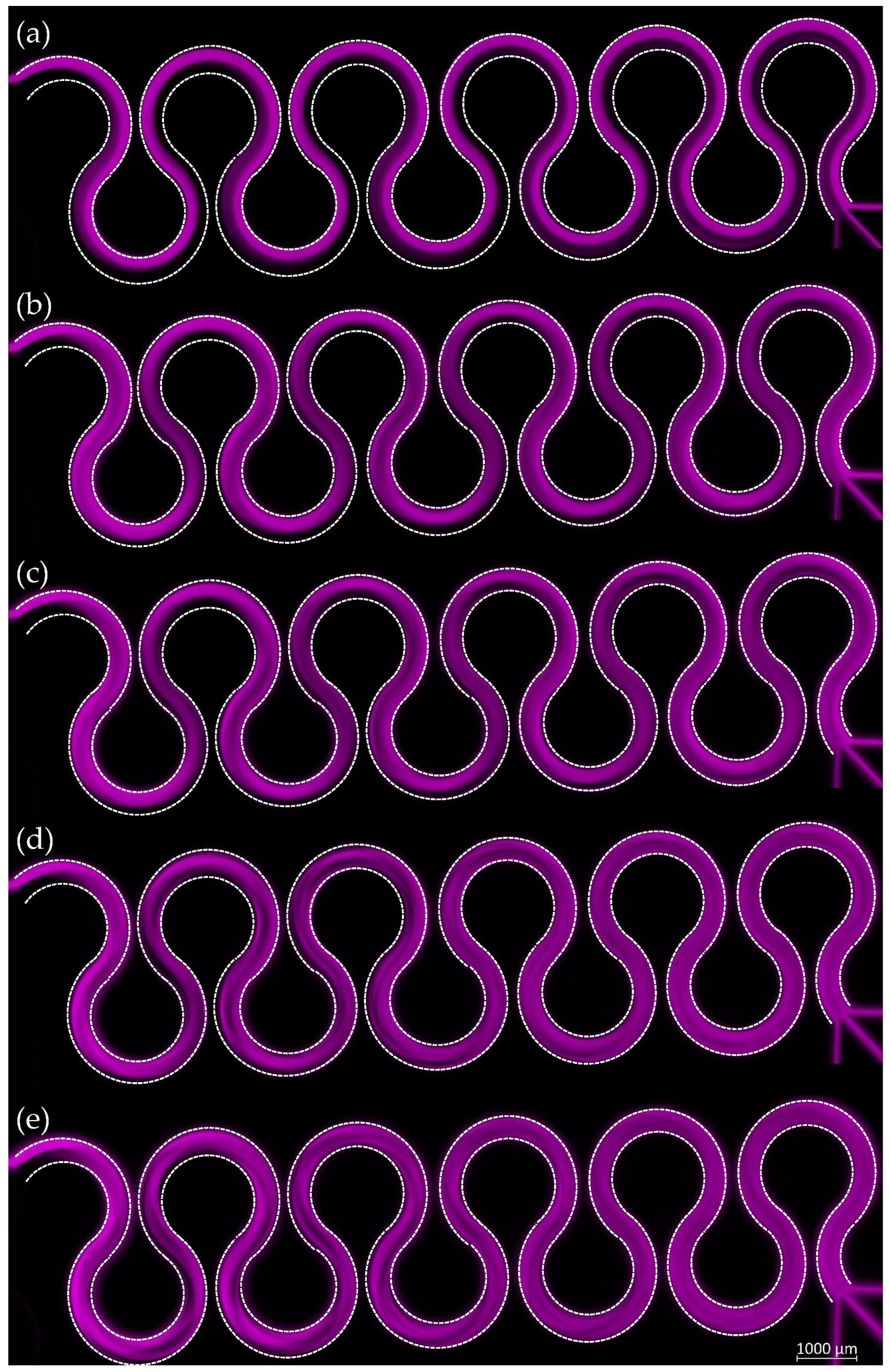
3.3. M280
3.4. Simulation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capretto, L.; Cheng, W.; Hill, M.; Zhang, X. Micromixing within microfluidic devices. Top. Curr. Chem. 2011, 304, 27–68. [Google Scholar] [PubMed]
- Lee, C.Y.; Fu, L.M. Recent advances and applications of micromixers. Sens. Actuators B Chem. 2018, 259, 677–702. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Munoz-Berbel, X.; Ortiz, P.; Büttgenbach, S.; Fernández-Sánchez, C.; Llobera, A. Self-validating lab-on-a-chip for monitoring enzyme-catalyzed biological reactions. Sens. Actuators B Chem. 2016, 237, 16–23. [Google Scholar] [CrossRef]
- Thiele, M.; Knauer, A.; Malsch, D.; Csáki, A.; Henkel, T.; Köhler, J.M.; Fritzsche, W. Combination of microfluidic high-throughput production and parameter screening for efficient shaping of gold nanocubes using Dean-flow mixing. Lab Chip 2017, 17, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, K.H.; Fang, W.F.; Tsai, S.H.; Fang, J.M.; Yang, J.T. Flash synthesis of carbohydrate derivatives in chaotic microreactors. Chem. Eng. J. 2011, 174, 421–424. [Google Scholar] [CrossRef]
- Ryu, M.; Kimber, J.; Sato, T.; Nakatani, R.; Hayakawa, T.; Romano, M.; Pradere, C.; Hovhannisyan, A.; Kazarian, S.; Morikawa, J. Infrared thermo-spectroscopic imaging of styrene radical polymerization in microfluidics. Chem. Eng. J. 2017, 324, 259–265. [Google Scholar] [CrossRef]
- Peng, G.; He, Q.; Lu, Y.; Huang, J.; Lin, J.M. Flow injection microfluidic device with on-line fluorescent derivatization for the determination of Cr (III) and Cr (VI) in water samples after solid phase extraction. Anal. Chim. Acta 2017, 955, 58–66. [Google Scholar] [CrossRef]
- Kastania, A.S.; Tsougeni, K.; Papadakis, G.; Gizeli, E.; Kokkoris, G.; Tserepi, A.; Gogolides, E. Plasma micro-nanotextured polymeric micromixer for DNA purification with high efficiency and dynamic range. Anal. Chim. Acta 2016, 942, 58–67. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hyun, K.A.; Kim, S.I.; Jung, H.I. An integrated microfluidic chip for one-step isolation of circulating tumor cells. Sens. Actuators B Chem. 2017, 238, 1144–1150. [Google Scholar] [CrossRef]
- Giuffrida, M.C.; Zanoli, L.M.; D’Agata, R.; Finotti, A.; Gambari, R.; Spoto, G. Isothermal circular-strand-displacement polymerization of DNA and microRNA in digital microfluidic devices. Anal. Bioanal. Chem. 2015, 407, 1533–1543. [Google Scholar] [CrossRef]
- Larrea, A.; Clemente, A.; Luque-Michel, E.; Sebastian, V. Efficient production of hybrid bio-nanomaterials by continuous microchannel emulsification: Dye-doped SiO2 and Au-PLGA nanoparticles. Chem. Eng. J. 2017, 316, 663–672. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A review on micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, M.; Nguyen, N.T. A rapid magnetofluidic micromixer using diluted ferrofluid. Micromachines 2017, 8, 37. [Google Scholar] [CrossRef]
- Luong, T.D.; Phan, V.N.; Nguyen, N.T. High-throughput micromixers based on acoustic streaming induced by surface acoustic wave. Microfluid. Nanofluid. 2011, 10, 619–625. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Y.; Tao, Y.; Hou, L.; Hu, Q.; Jiang, H. A novel micromixer based on the alternating current-flow field effect transistor. Lab Chip 2017, 17, 186–197. [Google Scholar] [CrossRef]
- Kunti, G.; Bhattacharya, A.; Chakraborty, S. Rapid mixing with high-throughput in a semi-active semi-passive micromixer. Electrophoresis 2017, 38, 1310–1317. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef]
- Cook, K.J.; Fan, Y.; Hassan, I. Mixing evaluation of a passive scaled-up serpentine micromixer with slanted grooves. J. Fluid. Eng. 2013, 135, 081102. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2004, 15, R1. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Fluid mixing in planar spiral microchannels. Lab Chip 2006, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Drese, K.S.; Hardt, S.; Küpper, M.; Schönfeld, F. Helical flows and chaotic mixing in curved micro channels. AIChE J. 2004, 50, 2297–2305. [Google Scholar] [CrossRef]
- Chen, J.J.; Chen, C.H.; Shie, S.R. Optimal designs of staggered Dean vortex micromixers. Int. J. Mol. Sci. 2011, 12, 3500–3524. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Tsai, R.T. Fluid mixing via multidirectional vortices in converging–diverging meandering microchannels with semi-elliptical side walls. Chem. Eng. J. 2013, 217, 320–328. [Google Scholar] [CrossRef]
- Alam, A.; Afzal, A.; Kim, K.Y. Mixing performance of a planar micromixer with circular obstructions in a curved microchannel. Chem. Eng. Res. Des. 2014, 92, 423–434. [Google Scholar] [CrossRef]
- Akgönül, S.; Özbey, A.; Karimzadehkhouei, M.; Gozuacik, D.; Koşar, A. The effect of asymmetry on micromixing in curvilinear microchannels. Microfluid. Nanofluid. 2017, 21, 118. [Google Scholar] [CrossRef]
- Clark, J.; Kaufman, M.; Fodor, P.S. Mixing Enhancement in Serpentine Micromixers with a Non-Rectangular Cross-Section. Micromachines 2018, 9, 107. [Google Scholar] [CrossRef]
- Geschke, O.; Klank, H.; Telleman, P. Microsystem Engineering of Lab-on-a-Chip Devices; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Marques, M.P.; Fernandes, P. Microfluidic devices: Useful tools for bioprocess intensification. Molecules 2011, 16, 8368–8401. [Google Scholar] [CrossRef]
- Rani, S.A.; Pitts, B.; Stewart, P.S. Rapid diffusion of fluorescent tracers into Staphylococcus epidermidis biofilms visualized by time lapse microscopy. Antimicrob. Agents Chemother. 2005, 49, 728–732. [Google Scholar] [CrossRef]
- Dean, W.; Hurst, J. Note on the motion of fluid in a curved pipe. Mathematika 1959, 6, 77–85. [Google Scholar] [CrossRef]
- Kockmann, N.; Kiefer, T.; Engler, M.; Woias, P. Convective mixing and chemical reactions in microchannels with high flow rates. Sens. Actuators B Chem. 2006, 117, 495–508. [Google Scholar] [CrossRef]
- Fan, L.L.; Zhu, X.L.; Zhao, H.; Zhe, J.; Zhao, L. Rapid microfluidic mixer utilizing sharp corner structures. Microfluid. Nanofluid. 2017, 21, 36. [Google Scholar] [CrossRef]
- Khaydarov, V.; Borovinskaya, E.; Reschetilowski, W. Numerical and Experimental Investigations of a Micromixer with Chicane Mixing Geometry. Appl. Sci. 2018, 8, 2458. [Google Scholar] [CrossRef]



| Mesh Size | No. of Elements | Average Element Quality | Discretization of Fluids | Degree of Freedom | Calculated U at the Fifth Segment (m/s) | Relative Error | |
|---|---|---|---|---|---|---|---|
| M180 | Fine | 144229 | 0.648 | P2 + P1 | 720272 | 0.19195 | 9.31% |
| P2 + P2 | 915632 | 0.19194 | 9.31% | ||||
| P3 + P2 | 2419838 | 0.19776 | 6.56% | ||||
| Finer | 450012 | 0.678 | P2 + P1 | 2112701 | 0.19740 | 6.73% | |
| P2 + P2 | 2691880 | 0.19740 | 6.73% | ||||
| P3 + P2 | 7235620 | 0.20131 | 4.88% | ||||
| M230 | Fine | 127368 | 0.663 | P2 + P1 | 655439 | 0.18759 | 11.37% |
| P2 + P2 | 832312 | 0.18759 | 11.37% | ||||
| P3 + P2 | 2182042 | 0.19504 | 7.84% | ||||
| Finer | 486913 | 0.673 | P2 + P1 | 2282180 | 0.19550 | 7.63% | |
| P2 + P2 | 2907900 | 0.19550 | 7.63% | ||||
| P3 + P2 | 7819197 | 0.20001 | 5.50% | ||||
| M280 | Fine | 213993 | 0.661 | P2 + P1 | 1062064 | 0.19066 | 9.91% |
| P2 + P2 | 1349960 | 0.19064 | 9.92% | ||||
| P3 + P2 | 3570536 | 0.19699 | 6.92% | ||||
| Finer | 638723 | 0.693 | P2 + P1 | 2994855 | 0.19614 | 7.33% | |
| P2 + P2 | 3815208 | 0.19614 | 7.33% | ||||
| P3 + P2 | 10253151 | 0.20040 | 5.31% |
| Inlet Velocity [m/s] | Outlet M | |||||
|---|---|---|---|---|---|---|
| M180 | M230 | M28 | ||||
| 0.21 | 8.4E + 04 | 30 | 12 | 0.19 ± 0.04 | 0.35 ± 0.08 | 0.66 ± 0.02 |
| 0.32 | 1.3E + 05 | 45 | 17 | 0.26 ± 0.09 | 0.38 ± 0.04 | 0.71 ± 0.05 |
| 0.48 | 1.9E + 05 | 68 | 26 | 0.36 ± 0.01 | 0.40 ± 0.03 | 0.79 ± 0.02 |
| 0.63 | 2.5E + 05 | 91 | 35 | 0.42 ± 0.08 | 0.36 ± 0.04 | 0.80 ± 0.02 |
| 0.79 | 3.2E + 05 | 114 | 44 | 0.63 ± 0.05 | 0.59 ± 0.07 | 0.87 ± 0.06 |
| 0.95 | 3.8E + 05 | 136 | 52 | 0.66 ± 0.06 | 0.74 ± 0.03 | 0.94 ± 0.02 |
| 1.11 | 4.4E + 05 | 159 | 61 | 0.80 ± 0.06 | 0.90 ± 0.01 | 0.94 ± 0.02 |
| 1.27 | 5.1E + 05 | 182 | 70 | 0.89 ± 0.04 | 0.93 ± 0.02 | 0.93 ± 0.01 |
| 1.43 | 5.7E + 05 | 205 | 78 | 0.92 ± 0.02 | 0.93 ± 0.02 | 0.92 ± 0.01 |
| 1.59 | 6.3E + 05 | 227 | 87 | 0.90 ± 0.02 | 0.92 ± 0.01 | 0.92 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alijani, H.; Özbey, A.; Karimzadehkhouei, M.; Koşar, A. Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles. Fluids 2019, 4, 204. https://doi.org/10.3390/fluids4040204
Alijani H, Özbey A, Karimzadehkhouei M, Koşar A. Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles. Fluids. 2019; 4(4):204. https://doi.org/10.3390/fluids4040204
Chicago/Turabian StyleAlijani, Hossein, Arzu Özbey, Mehrdad Karimzadehkhouei, and Ali Koşar. 2019. "Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles" Fluids 4, no. 4: 204. https://doi.org/10.3390/fluids4040204
APA StyleAlijani, H., Özbey, A., Karimzadehkhouei, M., & Koşar, A. (2019). Inertial Micromixing in Curved Serpentine Micromixers with Different Curve Angles. Fluids, 4(4), 204. https://doi.org/10.3390/fluids4040204






